Abstract
The preferential metastasis of prostate cancer cells to bone disrupts the process of bone remodeling and results in lesions that cause significant pain and patient morbidity. Although prostate-specific antigen (PSA) is an established biomarker in prostate cancer, it provides only limited information relating to bone metastases and the treatment of metastatic bone disease with bisphosphonates or novel noncytotoxic targeted or biological agents that may provide clinical benefits without affecting PSA levels. As bone metastases develop, factors derived from bone metabolism are released into blood and urine, including N- and C-terminal peptide fragments of type 1 collagen and bone-specific alkaline phosphatase, which represent potentially useful biomarkers for monitoring metastatic bone disease. A number of clinical trials have investigated these bone biomarkers with respect to their diagnostic, prognostic, and predictive values. Results suggest that higher levels of bone biomarkers are associated with an increased risk of skeletal-related events and/or death. As a result of these findings, bone biomarkers are now being increasingly used as study end points, particularly in studies investigating novel agents with putative bone effects. Data from prospective clinical trials are needed to validate the use of bone biomarkers and to confirm that marker levels provide additional information beyond traditional methods of response evaluation for patients with metastatic prostate cancer.
Introduction
In the United States and Europe, an estimated 192,000 and 346,000 new cases of prostate cancer are diagnosed each year, respectively [1,2]. For advanced prostate cancer, the standard first-line treatment is androgen deprivation therapy using medical or surgical castration [3]. Although most patients initially respond to castration therapy, prostate cancer eventually progresses despite castrate levels of androgens (<50 ng/ml; termed castration-resistant prostate cancer [CRPC]). Early clinical manifestations of progressive CRPC include a rising prostate-specific antigen (PSA) concentration and substantial pain in 90% and 35%patients, respectively [4]. As CRPC progresses, approximately 90% patients will develop bone metastases and 20% will develop soft-tissue metastases, most commonly in the lung, liver, or lymph nodes [4,5]. Currently, prostate cancer with bone metastases is not considered curable [6]. The median survival of patients with bone metastases is approximately 20 months [7] and is increasing with chemotherapy and other novel agents. Management of quality of life in this patient group is therefore especially important and is likely to improve with the emergence of new bone-specific therapies, such as inhibitors of receptor activator of nuclear factor κB ligand (RANKL), and the increasing focus on patients with bone metastases in the overall management of prostate cancer. Other agents in clinical development for CRPC that have potential activity against bone metastases include inhibitors of SRC kinase or endothelin A receptor.
The aim of this review was to discuss the role of bone biomarkers in the management of CRPC and development and optimization of bone-targeted therapy.
Biology of Bone Metastases
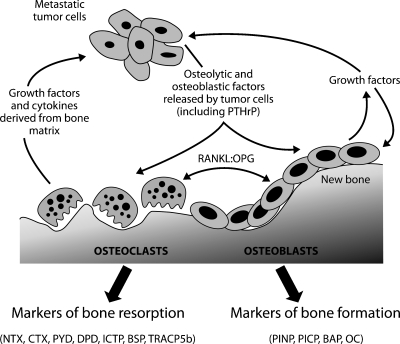
Bone metastases are most commonly situated in well-vascularized areas of the skeleton, such as the vertebral column, ribs, skull, and proximal ends of the long bones [8]. In normal bone, the remodeling process is in equilibrium; however, the presence of tumor cells disrupts the process, causing bone lesions that are detectable by radiographic imaging or radionuclide bone scan. Lesions result from an imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Osteoclasts adhere to the bone surface and resorb the bone matrix by secreting proteases and acid, and in turn, osteoblasts secrete collagen fibrils that become mineralized, eventually forming bone [9,10]. However, cross talk between tumor cells and the bone microenvironment causes disequilibrium in the remodeling process, leading to a pathologic “vicious cycle.” Specifically, tumor cells secrete factors that stimulate osteoclast-mediated bone destruction, and factors that were immobilized within the bone matrix are released, stimulating the growth of cancer cells and promoting further bone destruction (Figure 1) [11]. On the basis of radiographic appearance, bone lesions are typically referred to as “osteolytic” (reflecting net bone loss), “osteoblastic” or “sclerotic” (reflecting bone formation), or “mixed” [12]. In prostate cancer, bone metastases are primarily osteoblastic; however, high bone turnover and consequent excess bone resorption are also characteristic features [13].
Figure 1.
The vicious cycle of bone metastases (reproduced and adapted from Guise et al. [121], with permission from the American Association for Cancer Research). The production of cytokines and growth factors by tumor cells, particularly parathyroid hormone-related peptide (PTH-rP), stimulates osteoblasts to produce RANKL, a key mediator of osteoclastogenesis that is inhibited by OPG. In turn, osteoclast-mediated bone resorption releases growth factors, such as transforming growth factor β, platelet-derived growth factor (PDGF), and insulin-like growth factors (IGFs), which promote tumor growth [9,10]. Osteoblast and osteoclast activity results in the release of proteins, protein fragments, or mineral components directly involved in bone structure or metabolism into the blood and urine. Biomarkers of bone formation include BAP, PICP/PINP, and OC. Biomarkers of bone resorption include CTX/NTX, PYD, and DPD; ICTP; BSP; and TRACP5b.
Skeletal-Related Events
Because of their high frequency in CRPC, bone metastases are responsible for a considerable proportion of patient morbidity [14], primarily through complications known as skeletal-related events (SREs). Tumor-associated bone lacks the structural integrity of normal bone and is therefore intrinsically weaker, resulting in a high risk of pathologic fracture. Bone metastases can also cause intermittent or constant bone pain. Metastatic sites in the vertebral columnmay also cause spinal cord/nerve root compression. Patients with bone metastases often require palliative radiation therapy or surgery to bone. Unlike other malignancies such as breast cancer, hypercalcemia is rare among patients with prostate cancer. SREs significantly reduce health-related quality of life and result in high medical costs [15], and patients who develop pathologic fractures have decreased survival compared with patients without fractures [16]. In a study of 442 men with advanced prostate cancer, approximately one-third experienced an SRE before study entry, 49% experienced at least one SRE while on study, and 31% experienced two or more SREs [17], highlighting that patients are at high risk of experiencing multiple SREs and the importance of reducing the occurrence of SREs [18].
Treatment Options for Patients with Bone Metastatic CRPC
Because progression of prostate cancer to CRPC signifies incurable disease, the goals of treatment are to reduce symptoms and extend survival. Current treatment options therefore include agents that decrease tumor growth and/or decrease the morbidity of metastatic bone disease.
Chemotherapy
Chemotherapy is used in patients with CRPC who have radiologic evidence of nodal, bone, or visceral metastases. Docetaxel, an inhibitor of microtubule function and cell division, became the standard of care for patients with CRPC after two landmark phase 3 studies that demonstrated a survival benefit in patients with progressive metastatic CRPC [19–21]. In the TAX327 study, 1006 patients were randomized to receive docetaxel or mitoxantrone, each combined with low-dose prednisolone. Compared with the mitoxantrone arm, the docetaxel arm had longer survival, enhanced quality of life, a lower need for pain control, and higher rates of objective tumor response and PSA decline [21]. The survival advantage with docetaxel-based chemotherapy was confirmed in the Southwest Oncology Group (SWOG) 9916 study, in which 770 men received mitoxantrone plus prednisone or estramustine phosphate plus docetaxel [20].
Bone-Directed Therapy
Although chemotherapeutic agents such as docetaxel can lead to a reduction in bone pain, treatments that specifically target bone disease are an important component of therapy. Bisphosphonates (BPs), which inhibit osteoclast-mediated bone resorption at sites of active bone remodeling including bone metastases, have become an integral part of managing metastatic bone disease [22]. In patients with advanced CRPC and bone metastases, placebo-controlled studies have shown that the BP zoledronic acid significantly reduces the incidence and delays the onset of SREs [17,23,24]. Zoledronic acid, administered as a 4-mg infusion every 3 to 4 weeks in conjunction with standard anticancer therapy, is the only BP approved for use in CRPC; however, zoledronic acid treatment is not recommended in patients with severe renal impairment [25] and it has been associated with osteonecrosis of the jaw [26], although this is a rare complication and is now known to also occur with newer therapies that inhibit bone resorption such as denosumab [27].
Improved understanding of the biology of bone metastases has led to the development of novel bone-specific agents, which, in some cases, are in advanced clinical trials and approaching registration. These are likely to have a major effect on the management of metastatic bone disease in prostate cancer and are considered in detail later in this review.
Systemically administered radiotherapeutic agents (traditionally phosphorus 32 and strontium 89) have been used for palliation of skeletal metastases. Recently, novel agents that preferentially localize to osteoblastic metastases have been developed, which combine chelating agents with radionuclides that have shorter half-lives and lower-energy particle emissions. In a phase 2 study of patients with CRPC and bone metastases, radium 223 was well tolerated with minimal myelotoxicity. Median overall survival was 65.3 weeks for radium 223 compared with 46.4 weeks for placebo (P = .066) [28]. Radium223 is currently in phase 3 trials. Similarly, combination therapy with samarium 153, and docetaxel was well tolerated in phase 1 and 2 studies and provided sustained pain relief [29,30].
Bone Biomarkers in Prostate Cancer
A biomarker is defined as a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. If a biomarker is to be used to monitor treatment response, it should be specific to the relevant disease and correlate closely with aspects of the disease that influence quality of life and survival. Biomarkers should be sensitive, have no overlap between untreated and healthy patients, and show little variation within the general population. Biomarkers should also be predictive, changing rapidly in response to specific treatments, and sufficiently different from control values to indicate disease severity and prognosis. Finally, biomarkers should be robust and accessible, that is, unaffected by unrelated conditions and reliably quantified in clinical samples. Although biomarkers can be tissue- or fluid-based, difficulty in obtaining tissue for direct tumor analysis means fluid-based biomarkers, such as those found in blood and urine, are most common. Together, the criteria listed represent an ideal biomarker. Although it is very unlikely that any clinical biomarker could attain all of these characteristics, biomarkers already play significant roles in clinical practice and will likely become increasingly important. This is especially true of prostate cancer, where PSA is already the most commonly used biomarker in cancer, and bone biomarkers are showing increasing potential in clinical management.
The clinical value of PSA, a 34-kDa glycoprotein normally found almost exclusively in prostate cells and seminal fluid, has been extensively investigated. Because serum PSA levels roughly correlate with the extent of the disease [31] and can be easily and reproducibly evaluated, PSA has been used as a diagnostic, prognostic, and predictive tool [32–34]. Importantly, a rise in PSA while a patient is receiving androgen deprivation therapy potentially signals a transition from hormone-sensitive prostate cancer to CRPC. However, castration levels of serum testosterone must be demonstrated before castration resistance is confirmed. Alternative biomarkers that may identify progression to castration resistance at an early stage are currently under investigation [35].
PSA, however, has several restrictions as a biomarker [36]. Its use in monitoring CRPC is limited, for example, in the context of novel non-cytotoxic treatments that may have little effect on PSA levels [37,38]. Importantly, PSA levels do not provide accurate information regarding the extent of bone metastasis or bone-specific effects of treatment, meaning that alternative biomarkers are required for this specific purpose.
Biomarkers of Bone Turnover
During bone turnover, active bone resorption and formation results in the release of bone-associated proteins, protein fragments, or mineral components into the blood and urine, which represents a rich source of potential biomarkers (Table 1) [13,39–58]. In metastatic bone disease, the disruption of normal bone turnover leads to abnormally high levels of these biomarkers. Bone biomarkers are usually classified as either “formation markers” or “resorption markers” depending on which side of the process they arise.
Table 1.
| Marker | Tissue of Origin | Specimen | Evaluation Method | Known Limitations |
| Markers of bone formation | ||||
| PICP/PINP | Bone; soft tissue; skin | Serum | RIA, ELISA [40–42] | Concentrations may increase with increased turnover of nonskeletal collagen |
| BAP | Bone | Serum | Colorimetric, electrophoresis, precipitation, IRMA, EIA [43,44] | Significant cross-reactivity (±15%) with liver alkaline phosphatase may occur during assaying |
| OC | Bone; platelets | Serum | RIA, IRMA, ELISA [45] | Not a pure marker of osteoblast function because some OC may be derived from bone resorption |
| Detection may be confounded by high lipid levels that bind OC [45] | ||||
| Measurement is complicated by the presence of several immunoreactive fragments and a lack of standardization in methodology | ||||
| Markers of bone resorption | ||||
| PYD | Bone; cartilage; tendon; blood vessels | Urine, serum | HPLC, ELISA [46,47] | - |
| DPD | Bone; dentin | Urine, serum | HPLC, ELISA [47,48] | - |
| CTX | All tissues containing type 1 collagen | Urine (α and ʲ), serum (β) | ELISA, RIA [49,50] | Limited assay precision at low urinary concentrations |
| NTX | All tissues containing type 1 collagen | Urine, serum | ELISA, CLIA, RIA [51,52] | - |
| ICTP | Bone; skin | Serum | RIA [53] | Might be derived from newly synthesized collagen |
| BSP | Bone; dentin; hypertrophic cartilage | Serum | RIA, ELISA [54] | May reflect cell-related processes rather than the release of degradation products |
| Binds to complement factor H and this complex must be disrupted for accurate measurement [55] | ||||
| OPG | Bone; cardiovascular, gastrointestinal and neurologic tissue | Plasma, serum | ELISA [56] | - |
| TRACP5b | Bone; blood | Plasma, serum | Colorimetry, RIA, ELISA [57,58] | - |
CLIA indicates chemiluminescent assay; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography; IRMA, immunoradiometric assay; RIA, radioimmunoassay.
Biomarkers of Bone Formation
Type 1 collagen constitutes approximately 90% of the bone matrix and is synthesized as a procollagen that has amino-terminal (PINP) and carboxy-terminal (PICP) propeptides. Before fibril formation, PINP and PICP are cleaved off and released into the circulation in equimolar amounts and are cleared by the liver. Blood concentrations of PINP and PICP are indicators of ongoing type 1 collagen synthesis and early bone formation, and concentrations increase during osteoblast proliferation [59,60]. Bone-specific alkaline phosphatase (BAP) is a marker of the middle stage of bone formation, appearing during the matrix maturation phase [60]. Alkaline phosphatase isoforms originate mainly from liver and bone, with the bone isoform accounting for 40% to 50% in normal adults [61]. The bone isoform is a specific marker for osteogenesis, and concentrations are elevated in metastatic bone disease, Paget disease, and osteomalacia [39]. Osteocalcin (OC) is a noncollagenous marker of late bone formation, appearing during the mineralization phase, that is produced by osteoblasts and is also detectable in blood [60]. Osteoprotegerin (OPG) is produced by osteoblast and stromal cells and binds to RANKL, preventing interaction with RANK and thereby inhibiting osteoclastogenesis and bone resorption [62]. Bone metastases from prostate cancer stimulate a profound elevation in OPG and decrease in the RANKL/OPG ratio [63].
Biomarkers of Bone Resorption
Several markers of bone resorption are degradation products of type 1 collagen. For example, N- and C-terminal peptide fragments of type 1 collagen (NTX and CTX) are released into the circulation and urine during osteoclast-mediated bone resorption [11]. The NTX peptide exists in α1 and α2 isoforms, with the latter primarily derived from bone. Similarly, CTX exists as α and β isoforms, with the β isoform found primarily derived from bone [39]. Strands of type 1 collagen are connected between lysine and hydroxylysine residues by chemical crosslinks, including pyridinoline (PYD) and deoxypyridinoline (DPD), which mechanically stabilize the collagen molecule by bridging several collagen peptides. During collagen degradation, PYD and DPD are released at a ratio of approximately 3:1 and are detectable in the circulation and urine [64], with urinary excretion closelymirroring the rate of bone resorption. Although DPD is relatively specific for bone, PYD is also found in articular cartilage, ligaments, and tendons, although bone is the main reservoir and has a higher turnover than connective tissues. C-terminal cross-linked telopeptides (ICTPs) are also released into the circulation after enzymatic degradation of type 1 collagen by metalloproteinases. These are distinct to CTX peptides, which are generated through a distinct collagenolytic pathway [65].
Bone sialoprotein (BSP) is a noncollagenous glycoprotein found within the bone matrix that is produced by osteoclasts and may promote osteoclast attachment to bone and osteoclastogenesis [39,66]. In patients with localized prostate cancer, high BSP expression is associated with biochemical disease progression [67]. BSP activity is regulated by tartrate-resistant acid phosphatase type 5b (TRACP5b) [39], which itself is a marker of osteoclastic activity and bone resorption secreted by osteoclasts after attaching to bone [68]. After entering the circulation, TRACP5b is inactivated and degraded, so that levels of catalytically active enzyme reflect bone-resorptive activity [39].
Clinical Value of Bone Biomarkers
Bone biomarkers complement information derived from radiographic assessment and can be measured easily, serially, relatively noninvasively, and inexpensively. Because changes in bone biomarkers occur rapidly, they provide information on the status of bone metastases. By contrast, radiographic methods report accumulated effects; therefore, response to treatment may not become evident for several months. Samples for bone marker analysis are generally sent to laboratories; however, there is increasing interest in developing point-of-care devices to enable clinical teams to make more rational management decisions at the time of patient visit.
The diagnostic, prognostic, and predictive values of bone biomarkers have been assessed in several clinical studies.
Bone Biomarkers in Diagnosis
In retrospective studies, levels of bone biomarkers have been found to correlate with the presence of bone metastases, with higher levels of BAP, PINP, PICP, and urinary CTX indicating an increased extent of bone disease [60,69,70]. PINP levels have also been shown to be significantly higher in patients with bone metastases than in patients without metastases or with lymph node metastases only [71,72], and in a separate study, preliminary analyses suggested that CTX most sensitively reflected accelerated bone resorption induced by bone metastases [73]. However, it is important to note that these were not prospective studies and the use of bone biomarkers in screening or diagnosing bone progression remains unvalidated.
Prognostic Value of Bone Biomarkers
The prognosis associated with high levels of bone biomarkers in clinical trials of patients with CRPC and metastatic bone disease has been analyzed retrospectively. In a study including 117 patients with prostate cancer, of which 44 had bone metastases, survival was significantly shorter in patients who had high concentrations of PINP, BAP, total alkaline phosphatase, OPG, NTX, TRACPb5, and CTX than in patients with lower concentrations [74]. In placebo-treated patients with either metastatic CRPC or non-small cell lung cancer enrolled in two phase 3 trials of zoledronic acid, higher levels of urinary NTX (uNTX) and BAP, both at baseline and on study, were associated with an increased risk of SREs and death than low uNTX levels, and uNTX was a stronger prognostic indicator of negative outcome than BAP [75]. In an analysis of data from a randomized trial, zoledronic acid-treated patients with CRPC and bone metastases who had high uNTX levels at baseline or on study had a 5.72-fold increased risk of death than patients with low levels (P<.001) [76]. In a separate analysis of this patient cohort, serum BAP significantly correlated with uNTX (correlation coefficient = 0.674, 95% confidence interval [CI] = 0.628–0.715, P < .0001). Furthermore, an elevated level of serum BAP but not uNTX was independently associated with a shorter overall survival after controlling for other variables [77]. In a separate study of patients with CRPC receiving zoledronic acid, elevated uNTX, serum PSA, and performance status were all independent prognostic factors for overall survival. In patients with uNTX with 20 nmol/mM creatinine or higher or less than 20 nmol/mM creatinine, median overall survival was 12 months (95% CI = 10–16 months) and 25 months (95% CI = 21–34 months), respectively [7]. In another study, baseline PINP, BAP, and CTX levels were all elevated in patients with metastatic prostate cancer, but only PINP was an independent predictor of survival [78]. Similarly, in a study by the same group, serum levels of PINP, BAP, and CTX measured serially were higher in men with metastatic CRPC compared with healthy men, and in a univariate analysis, each marker was significantly associated with reduced survival at 6 months; however, in a multivariate analysis, only high serum PINP was significantly associated with reduced survival [79].
Predictive Value of Bone Markers
The effects of zoledronic acid on bone biomarkers and their relationship with clinical outcome have been investigated retrospectively using data from large, randomized, double-blind, phase 3 trials. In the subset of patients with CRPC, normalization of uNTX after 3 months of treatment with zoledronic acid, compared with persistent elevation of uNTX, correlated with improved overall survival, with the risk of death reduced by 59% (relative risk = 0.41; 95% CI = 0.29–0.59; P < .0001). In addition, SRE-free survival was increased by 49% (P = .0009). Regardless of baseline uNTX level, a 40% reduction in uNTX at 3 months resulted in a significant reduction in the risk of death [80]. In a smaller study of 71 patients with bone metastases from various cancers who received zoledronic acid, patients with an initial elevation in NTX with treatment had a significantly higher rate of bone disease progression compared with those who had an initial decline (67% vs 19%, P = .001) [81]. In a study of 117 men with CRPC (56 with an SRE and 61 without), serial measurements of BAP, PINP, NTX, ICTP, CTX, and PSA were taken at baseline and every 12 weeks for 60 weeks after administration of zoledronic acid. Except for ICTP, bone markers decreased to 20% to 80% of baseline values at week 12, and except for NTX, declines were higher in the non-SRE group. At all time points, higher and increasing concentrations of bone markers were observed in patients with SREs than those without. The study showed that changes in PINP, ICTP, CTX, and NTX during treatment were significant predictors of SRE, although after multivariate analysis, onlyNTXremained independently predictive [82,83].
The Role of Bone Biomarkers in the Development of Novel Therapies
Although bone biomarkers are not yet in use for routine clinical management of CRPC, they are well established as surrogate end points in phase 1/2 studies of new bone-targeted therapies (Tables 2 and 3) [84–97]. In phase 3 trials, SRE measurements remain the criterion standard for assessing efficacy; however, studies have alsomeasured bone biomarkers, providing supportive data for correlative analyses.
Table 2.
Effects of Novel Agents on Bone Markers.
| Agent | Patient Population | N | Treatment and Dosage | Treatment Efficacy | Bone Biomarker Effects |
| Atrasentan | Metastatic CRPC [84,85] | 288 | Atrasentan 2.5 or 10 mg QD vs placebo | Median time to progression: 183 days (10 mg atrasentan) vs 137 days (placebo); P = .13 | No significant decrease from baseline with atrasentan |
| Median time to PSA progression: 155 days (10 mg atrasentan) vs 71 days (placebo); P = .002 | Smaller elevations in NTX, CTX and DPD from baseline with 10 mg atrasentan vs placebo | ||||
| Metastatic CRPC [86] | 809 | Atrasentan 10 mg QD vs placebo | No reduction in risk of disease progression | Mean BAP increased by 13.2 ng/ml (atrasentan) vs 33.9 ng/ml (placebo); P < .001 | |
| Longer time to PSA progression with atrasentan | Median time to BAP progression: 505 days (atrasentan) vs 254 days (placebo); P<.01 | ||||
| Metastatic prostate cancer [87] | 44 | Atrasentan 10 mg QD (n = 22) or atrasentan 10 mg QD plus zoledronic acid Q4W (n = 22) | SD (no PSA or radiographic progression) at 12 weeks in 3/22 (14%) in both arms | No significant difference in BAP changes between arms Mean serum NTX increased by 32.4% ± 14.5% (atrasentan), decreased by 34.4% ± 6.9% (combination); P < .001 | |
| Metastatic CRPC [88] | 31 | Atrasentan 10 mg QD plus docetaxel 60, 70 or 75 mg/m2 Q21D | PR in 2/13 (15%); unconfirmed SD in 10/13 (77%) | Median BAP decreased from 18 µg/l to 12.2 µg/l (P = .0003) | |
| PSA response (>30% decline from baseline within 3 months) in 35% | Median NTX value decreased from 12.8 nM BCE to 10.9 nM BCE; P = .04 | ||||
| Zibotentan | Metastatic CRPC [89] | 16 | Zibotentan 10–200 mg QD (escalating doses) | No objective responses | Considerable intrapatient and interpatient variability in BAP, PINP, CTX, and NTX |
| Dasatinib | Metastatic CRPC [90] | 47 | Dasatinib 100 mg BID (n = 25) and 70 mg BID (n = 22) | Lack of progression by RECIST and bone scan in 20/47 (43%) patients at week 12 and 9/47 (19%) patients at week 24 | 51% of patients had a ≥40% reduction in urine NTX and 60% had a reduction in BAP |
| Metastatic CRPC [91] | 48 | Dasatinib 100 mg QD | 12 patients (25%) had disease control (PSA response; SD, CR or PR by RECIST; improved bone scan) | 51% of patients had a ≥40% reduction in urine NTX and 59% had a reduction in BAP | |
| Metastatic CRPC [92] | 46 | Dasatinib 50–120 mg QD and docetaxel 65 or 75 mg/m2 Q21D (n = 46) | PR in 17/30 patients (57%); SD ≥18 weeks in 5/30 (17%) | 49% of patients had a =35% decrease in urine NTX and 73% had a reduction in BAP | |
| Reduction in size and number of bone lesions at ≥6 weeks in 13/45 (29%) | |||||
| PSA response (>50% decline sustained for ≥6 weeks) in 26/44 (59%) | |||||
| Saracatinib | Healthy volunteers [93] | 59 | Single-dose saracatinib 60–250 mg, followed 7–10 days later with daily doses for 10–14 days | N/A | Reduction from baseline in serum CTX (88%), uNTX (67%) and TRACPb5 (11%) with 250 mg (maximum tolerated dose) |
| Advanced CRPC [94] | 28 | Saracatinib 175 mg QD | Transient PSA reduction in five patients | Not reported | |
| Denosumab | Solid tumors and bone metastases, with or without prior BP therapy [95] | 366 | IV BP Q4W (n = 80); denosumab 30 mg QW4 (n = 42), 120 mg QW4 (n = 42), 180 mg QW4 (n = 81), 60 mg Q12W (n = 42) or 180 mg Q12W (n = 79) | N/A | Median uNTX decrease of 75% (BP-naive) or 80% (previous IV therapy); median TRACP5b decrease of 73% |
| Solid tumors and bone metastases, with prior BP therapy (subanalysis) [96] | 111 | IV BP Q4W (n = 37); denosumab 180 mg Q12W (n = 36) or 180 mg Q4W (n = 38) | N/A | Median uNTX decrease of 78%; reductions in serum CTX, PINP, TRACP5b, BAP, and OC | |
| Prostate cancer and bone metastases, with previous BP therapy (subanalysis) [97] | 50 | IV BP Q4W (n = 17); denosumab 180 mg Q4W (n= 17) or 180 mg Q12W (n = 16) | N/A | 69% of patients had uNTX <50 nM; median uNTX decrease of 84% |
BCE, bone collagen equivalent; BID, twice daily; CR, complete response; IV, intravenous; N/A, not applicable; PR, partial response; Q4W, every 4 weeks; Q12W, every 12 weeks; Q21D, every 21 days; QD, once daily; RECIST, response criteria in solid tumors; SD, stable disease.
Denosumab
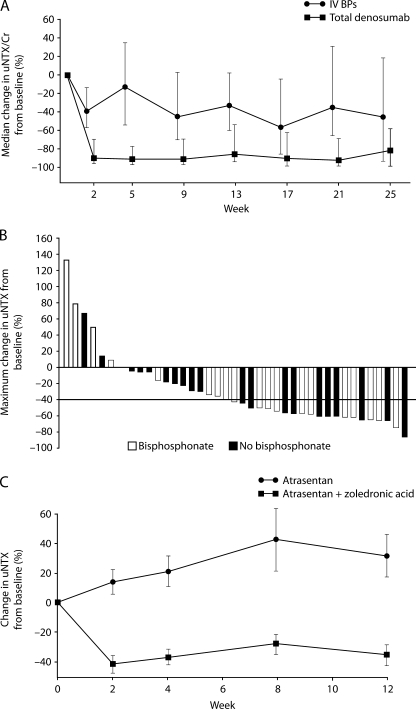
RANKL, together with its receptor, RANK, and the endogenous soluble RANKL inhibitor, OPG, play direct and essential roles in the formation, function, and survival of osteoclasts. Denosumab, a fully human monoclonal antibody against RANKL, has been shown to inhibit osteoclast-mediated bone resorption [96]. A phase 2 trial of denosumab was performed in patients with solid tumors (including prostate cancer) and bone metastases who had elevated uNTX levels despite ongoing BP therapy. In the prostate cancer subset, bone resorption was suppressed in a higher percentage of patients treated with denosumab compared with zoledronic acid, as demonstrated by the number of patients with uNTX levels less than 50 nM (22 [69%] of 32 vs 3 [19%] of 16, respectively) and the median percentage reduction in uNTX levels from baseline (84% vs 32%, respectively; Figure 2A). In addition, a lower proportion of patients treated with denosumab than zoledronic acid experienced an on-study SRE (1 [3%] of 33 vs 3 [19%] of 16, respectively) [97]. Bone biomarkers may therefore be valuable in individual patients for determining when to change to a different bone-targeted therapy. Primary data from a randomized phase 3 trial of denosumab in patients with metastatic CRPC are expected in 2010, with early reports indicating that compared with zoledronic acid, denosumab significantly delayed time to first SRE and significantly reduced first-and-subsequent SREs [98].
Figure 2.
Modulation of NTX in patients with metastatic CRPC after treatment with novel therapies. (A) Percentage change in uNTX during weeks 1 to 25 in patients treated with denosumab or zoledronic acid, demonstrating rapid denosumab-induced suppression of bone resorption (reprinted from Fizazi et al. [97], Copyright 2009, with permission from Elsevier). (B) Waterfall plot of maximal percentage change in uNTX from baseline in patients treated with 70 or 100mg of dasatinib twice daily. Of 41 patients, 33 (80%) had a decrease in uNTX while on study (reproduced and adapted from Yu et al. [90], with permission from the American Association for Cancer Research). (C) Percentage change in uNTX during weeks 1 to 12 in patients treated with atrasentan or atrasentan plus zoledronic acid. Mean serum NTX increased by 32.4% ± 14.5% with atrasentan and decreased by 34.4% ± 6.9% in the combination group (P < .001; reproduced and adapted from Michaelson et al. [87], with permission from John Wiley and Sons).
SRC Inhibitors
SRC and SRC family kinases have a key role in tumor cell processes, such as growth, invasion, and metastasis, in addition to normal and pathologic bone activity. SRC also acts in concert with the macrophage colony-stimulating factor receptor (FMS) to potentiate osteoclast activation. Dasatinib is a potent inhibitor of SRC plus SRC family kinases and FMS, which also has activity against platelet-derived growth factor receptor, c-KIT and ABL [99,100]. In vitro studies have found that dasatinib has antiosteoclast activity in addition to antitumor and antimetastatic activities against prostate cancer cell lines [101–103]. In two clinical studies in metastatic CRPC, dasatinib treatment has resulted in decreased levels of bone markers. In a study of dasatinib monotherapy administered in a twice-daily schedule, a 40% decrease in uNTX or higher or a decrease from baseline in BAP were observed in 51% (21/41) and 60% (24/40) patients, respectively (Figure 2B) [90], and similar results were observed in patients who received dasatinib once daily [91]. In a phase 1/2 dose-finding study of patients dasatinib plus docetaxel, 49% (18/37) had a 35% decrease in uNTX or higher and 73% (25/34) had a decrease in BAP from baseline [92]. The potential clinical benefits of combination treatment with dasatinib and docetaxel in patients with CRPC are currently being evaluated in a phase 3 trial.
Saracatinib (AZD0530), a highly selective oral SRC/ABL kinase inhibitor, inhibits osteoclast-mediated bone resorption and the growth of prostate cancer cells in vitro and in vivo [104,105]. In a healthy volunteer study, large decreases of serumCTX and uNTX were observed in response to daily dosing during a 14-day period. After treatment was stopped, there was a gradual return toward pretreatment levels, although both markers were still significantly reduced compared with baseline 12 days after the final dose [93].
Endothelin Inhibitors
Endothelin 1 is normally expressed in the prostate epithelium. Patients with metastatic prostate cancer have elevated levels of plasma endothelin 1 compared with patients with organ-confined cancer. Activation of endothelin A, the receptor for endothelin 1, is thought to promote the osteoblastic activity characteristic of prostate cancer bone metastases [106]. Atrasentan, a highly selective endothelin A receptor inhibitor, has antiosteoblast and antitumor activity in vitro [107]. In a phase 2 study of men with metastatic CRPC, significantly lower serum NTX levels were observed after treatment with atrasentan plus zoledronic acid compared with atrasentan alone (P <.001; Figure 2C). Reductions in serum BAP levels were not significantly different between the groups, and no objective responses were observed in either group [87]. A phase 3 study comparing atrasentan with placebo in men with metastatic CRPC was closed early because of an excess of early progression, mainly on bone scan, although an analysis of the 809 patients accrued demonstrated a nonsignificant trend toward increased time to progression in favor of atrasentan. In addition, at the final assessment, themean increase in BAP frombaselinewas 13.2 ng/mlwith atrasentan compared with 33.9 ng/ml with placebo [86]. In a phase 1/2 study with atrasentan plus docetaxel, serum NTX and BAP levels decreased significantly compared with baseline (BAP: P = .0003; NTX: P = .04) [88]. An ongoing phase 3 study is comparing the survival of patients with CRPC and bone metastases treated with docetaxel plus atrasentan with patients treated with docetaxel alone.
In a phase 1 study of zibotentan (ZD4054), an alternative endothelin A receptor antagonist, bone marker assessment of BAP, PINP, CTX, and NTX demonstrated considerable intrapatient and interpatient variability, and because of the small number of patients (n = 16), it was impossible to make any definitive conclusions [89]. In a randomized phase 2 trial, although there was no significant difference in progression-free survival, ZD4054 improved overall survival. Phase 2 results regarding the effects of zibotentan on bone metastases, however, are yet to be reported [108]. A large phase 3 trial (Enthuse M1C) is currently assessing the safety and efficacy of zibotentan in combination with docetaxel in patients with metastatic CRPC.
Limitations of Bone Biomarkers
Because of the substantive ongoing research into whether bone biomarker levels can guide treatment decisions for patients with metastatic CRPC, it is important that any potential limitations are considered. For example, bone remodeling follows a diurnal rhythm and may change with the season of the year, posture, exercise, and extremes of diet. In addition, all bone biomarkers follow a circadian rhythm, with highest levels in the early morning. However, circadian variations with PINP and PICP are negligible, and the longer half-life of BAP makes it less sensitive to circadian variation [61]. With other markers of bone formation and resorption, day-to-day variations of approximately 10% or 20%, respectively, have been observed, which can be reduced by consistency in sampling times. NTX, for example, is usually assessed on the second voided urine sample of the day.
Bone marker levels can also be affected by concurrent disease. For example, because BAP is cleared by the liver and NTX, CTX, PYD, and DPD are cleared by the kidneys, conditions that affect liver or renal function can influence marker concentrations [109]. Similarly, because biomarker levels are often normalized with respect to urine creatinine, increased excretion of urine creatinine due to renal damage can affect the results of urinary assays. After fracture, marker concentrations increase by 20%to 60%and remain high for 6months or longer, whereas with prolonged bed rest, markers may increase by 40%to 50%. Patterns of change vary between markers. Because the process of resorption is quicker, levels of resorption markers decrease faster than markers of formation (in 2–12 weeks compared with 3-6 months, respectively). Well-validated assays for assessment of bone biomarkers are now available, with typical intra-assay and interassay variations of less than 10% [39,109].
Discovery of Novel Bone Biomarkers to Predict Bone Metastases
The biomarkers discussed here are already proving valuable in developing new agents and have the potential to provide useful information in managing the skeletal complications of metastatic prostate cancer. However, they cannot predict the individual risk of developing metastatic bone disease; therefore, alternative markers, perhaps in association with PSA, are required. Some current approaches are discussed below.
Proteomics
Proteomics presents an exciting opportunity in novel biomarker discovery and has the advantage of examining functional end units (proteins) [110]. In biological fluids, challenges lie in the large dynamic range of protein concentrations and the dominance of a small number of abundant proteins. To address this, biomarker discovery generally incorporates prefractionation and/or multidimensional approaches [111].
Proteomic approaches remain relatively unexploited in bone metastasis [112], with only a few studies reported in prostate cancer [113,114] and multiple myeloma [115]. Using serum from patients with advanced prostate cancer, the clinical utility of surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) to identify serumbiomarker patterns associated with prostate cancer progression was investigated. A specific biomarker profile captured with SELDI-TOF MS was associated with biochemical relapse and was prognostic for long-term survival independent of clinical PSA status [113]. Similarly, SELDI-TOF MS profiling identified a group of serum amyloid A proteins that predominated in the spectra obtained from patients with prostate cancer and bone metastases [114]. These provide a proof of principle that proteomic profiling has potential for the discovery of novel biomarkers associated with prostate cancer bone metastases.
Circulating Tumor Cells
Circulating tumor cells (CTCs) provide a fluid-based biomarker that is being investigated as a potential prognostic marker in metastatic CRPC. CTCs are shed from either the primary tumor or the metastases and circulate in peripheral blood. Patients with bone metastases have higher CTC numbers than patients with soft-tissue metastases [116,117]. In a study of 120 patients with progressive CRPC, higher baseline CTC numbers were strongly associated with reduced survival [116]. Posttreatment CTC numbers can also predict survival. In a prospective study of 231 patients with metastatic CRPC, CTC number at various time points up to 20 weeks after treatment was a stronger independent predictor of overall survival than PSA reduction [118]. Similarly, in patients with metastatic CRPC receiving first-line chemotherapy, although high CTC number and PSA before treatment were both associated with increased risk of death, increases in CTC number after treatment were more strongly associated with risk of death than increases in PSA [119]. As a result, the US Food and Drug Administration has approved assessment of CTC using the CellSearch assay for clinical use in monitoring treatment response and predicting survival in prostate, breast, and colorectal cancers [118]. Assays of molecular markers expressed within CTCsmay be similarly predictive of response. A study quantifying PSA and human kallikrein 2 messenger RNA to determine the CTC level in 76 patients with CRPC produced results comparable with the CellSearch assay [117]. However, one limitation of using CTC count as a biomarker and a source of data for correlative studies in CRPC is that levels of detectable cells are low in the prechemotherapy setting [120]. Prospective measurement of CTC number in randomized phase 3 trials is warranted to assess how changes in CTC number can be used to guide the choice of treatment.
Conclusions
In patients with CRPC, bone metastases cause SREs that are responsible for substantial morbidity, and assessing bone health is an important aspect of clinical monitoring. PSA is the most widely used and best-characterized biomarker in CRPC; however, it does not provide accurate information regarding the extent of bone metastasis. Studies of patients with bone metastases have found an association between levels of bone biomarkers, both at baseline and during treatment, and long-term outcomes such as SREs and survival. As a result, bone biomarkers are increasingly recognized as potential surrogate end points. Although the most recent Prostate Cancer Working Group guidelines do not include bone biomarkers as validated end points for clinical trials of CRPC, various data suggest that bone biomarkers provide important information regarding effects of treatment on bone metastases. In particular, the correlation between bone biomarkers and patient outcomes in BP trials suggest that changes in biomarker levels may indicate treatment failure and/or the need for more aggressive treatment. Furthermore, recent studies with noncytotoxic targeted agents suggest bone effects and other clinical benefits may occur independent of changes in PSA level. However, the suggestion that bone biomarkers provide additional predictive information beyond traditional assessments requires further investigation and validation in prospective trials. In addition, it should be noted that diurnal and day-to-day variability occurs in bone biomarker levels, and assay results can vary considerably between laboratories, even if they use identical methodology, meaning that stringent standardization will be needed before assessment is incorporated into clinical practice. Overall, available data suggest that bone biomarkers hold future promise for monitoring and optimizing therapies targeted to the bone microenvironment.
Abbreviations
- BAP
bone-specific alkaline phosphatase
- BCE
bone collagen equivalent
- BP
bisphosphonate
- BSP
bone sialoprotein
- CRPC
castration-resistant prostate cancer
- CTC
circulating tumor cell
- CTX
carboxy-terminal telopeptide of type I collagen
- DPD
deoxypyridinoline
- ELISA
enzyme-linked immunosorbent assay
- FMS
macrophage colony-stimulating factor receptor
- HPLC
high-performance liquid chromatography
- ICTP
carboxy-terminal cross-linked telopeptides of type I collagen
- IRMA
immunoradiometric assay
- IV
intravenous
- NTX
amino-terminal telopeptide of type I collagen
- OC
osteocalcin
- OPG
osteoprotegerin
- PICP
carboxy-terminal propeptide of type I procollagen
- PINP
amino-terminal propeptide of type I procollagen
- PR
partial response
- PSA
prostate-specific antigen
- RANKL
receptor activator of nuclear factor κB ligand
- RECIST
response criteria in solid tumors
- RIA
radioimmunoassay
- SD
stable disease
- SELDI-TOF MS
surface-enhanced laser desorption/ionization time-of-flight mass spectrometry
- SRE
skeletal-related event
- TRACP5b
tartrate-resistant acid phosphatase serum type 5b
- uNTX
urinary NTX
Footnotes
J.E.B. is grateful to Cancer Research UK for the award of a Clinician Scientist Fellowship. The authors take full responsibility for the content of this article and confirm that it reflects their viewpoint and medical expertise. StemScientific, funded by Bristol-Myers Squibb, provided writing and editing support. Bristol-Myers Squibb did not influence the content of the article, nor did the authors receive financial compensation for authoring the article.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN. Systemic chemotherapy and new experimental approaches in the treatment of metastatic prostate cancer. Ann Oncol. 2008;19:vii91–vii95. doi: 10.1093/annonc/mdn473. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 6.Cheville JC, Tindall D, Boelter C, Jenkins R, Lohse CM, Pankratz VS, Sebo TJ, Davis B, Blute ML. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer-specific survival. Cancer. 2002;95:1028–1036. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- 7.Rajpar S, Massard C, Laplanche A, Tournay E, Gross-Goupil M, Loriot Y, Di PM, Bossi A, Escudier B, Chauchereau A, et al. Urinary N-telopeptide (uNTx) is an independent prognostic factor for overall survival in patients with bone metastases from castration-resistant prostate cancer. Ann Oncol. 2010 doi: 10.1093/annonc/mdq037. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Carlin BI, Andriole GL, The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–2994. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 10.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 11.Fohr B, Dunstan CR, Seibel MJ. Clinical review 165: markers of bone remodeling in metastatic bone disease. J Clin Endocrinol Metab. 2003;88:5059–5075. doi: 10.1210/jc.2003-030910. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005;2:504–517. doi: 10.1038/ncponc0320. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 15.Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, Schulman KA. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16:579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 16.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 17.Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 18.Major PP, Cook RJ, Chen BL, Zheng M. Survival-adjusted multiple-event analysis for the evaluation of treatment effects of zoledronic acid in patients with bone metastases from solid tumors. Support Cancer Ther. 2005;2:234–240. doi: 10.3816/SCT.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 19.Basch EM, Somerfield MR, Beer TM, Carducci MA, Higano CS, Hussain MH, Scher HI. American Society of Clinical Oncology endorsement of the Cancer Care Ontario Practice Guideline on nonhormonal therapy for men with metastatic hormone-refractory (castration-resistant) prostate cancer. J Clin Oncol. 2007;25:5313–5318. doi: 10.1200/JCO.2007.13.4536. [DOI] [PubMed] [Google Scholar]
- 20.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 21.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 22.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone- refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 25.Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, Antras L, Smith M, Neary MP, Duh MS. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer. 2007;109:1090–1096. doi: 10.1002/cncr.22504. [DOI] [PubMed] [Google Scholar]
- 26.Ortega C, Faggiuolo R, Vormola R, Montemurro F, Nanni D, Goia F, Aglietta M. Jaw complications in breast and prostate cancer patients treated with zoledronic acid. Acta Oncol. 2006;45:216–217. doi: 10.1080/02841860500341173. [DOI] [PubMed] [Google Scholar]
- 27.Kyrgidis A, Toulis KA. Denosumab-related osteonecrosis of the jaws. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1177-6. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, Lennernas B, Petersson U, Johannessen DC, Sokal M, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 29.Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, Di PM, Escudier B, Theodore C, Loriot Y, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castrationresistant prostate cancer. J Clin Oncol. 2009;27:2429–2435. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 30.Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, Rathkopf D, Gignac GA, Solit D, Schwartz L, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:2436–2442. doi: 10.1200/JCO.2008.20.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith DC, Dunn RL, Strawderman MS, Pienta KJ. Change in serum prostate-specific antigen as a marker of response to cytotoxic therapy for hormonerefractory prostate cancer. J Clin Oncol. 1998;16:1835–1843. doi: 10.1200/JCO.1998.16.5.1835. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, Eisenberger M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–3970. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 33.Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G, Akdas AM, Small EJ, Donnelly BJ, Sundram SK, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group trials 9346 (intergroup study 0162) and 9916. J Clin Oncol. 2009;27:2450–2456. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 35.van Gils MP, Stenman UH, Schalken JA, Schroder FH, Luider TM, Lilja H, Bjartell A, Hamdy FC, Pettersson KS, Bischoff R, et al. Innovations in serum and urine markers in prostate cancer current European research in the P-Mark project. Eur Urol. 2005;48:1031–1041. doi: 10.1016/j.eururo.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Ramiah V, George DJ, Armstrong AJ. Clinical endpoints for drug development in prostate cancer. Curr Opin Urol. 2008;18:303–308. doi: 10.1097/MOU.0b013e3282fb7807. [DOI] [PubMed] [Google Scholar]
- 37.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 38.Dror Michaelson M, Regan MM, Oh WK, Kaufman DS, Olivier K, Michaelson SZ, Spicer B, Gurski C, Kantoff PW, Smith MR. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman R, Brown J, Terpos E, Lipton A, Smith MR, Cook R, Major P. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat Rev. 2008;34:629–639. doi: 10.1016/j.ctrv.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melkko J, Niemi S, Risteli L, Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin Chem. 1990;36:1328–1332. [PubMed] [Google Scholar]
- 41.Melkko J, Kauppila S, Niemi S, Risteli L, Haukipuro K, Jukkola A, Risteli J. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996;42:947–954. [PubMed] [Google Scholar]
- 42.Orum O, Hansen M, Jensen CH, Sorensen HA, Jensen LB, Horslev-Petersen K, Teisner B. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone. 1996;19:157–163. doi: 10.1016/8756-3282(96)00165-2. [DOI] [PubMed] [Google Scholar]
- 43.Gomez B, Jr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem. 1995;41:1560–1566. [PubMed] [Google Scholar]
- 44.Woitge HW, Seibel MJ, Ziegler R. Comparison of total and bonespecific alkaline phosphatase in patients with nonskeletal disorder or metabolic bone diseases. Clin Chem. 1996;42:1796–1804. [PubMed] [Google Scholar]
- 45.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37:432–446. doi: 10.1177/000456320003700402. [DOI] [PubMed] [Google Scholar]
- 46.Arbault P, Grimaux M, Pradet V, Preaudat C, Seguin P, Delmas PD. Assessment of urinary pyridinoline excretion with a specific enzyme-linked immunosorbent assay in normal adults and in metabolic bone diseases. Bone. 1995;16:461–467. doi: 10.1016/8756-3282(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 47.Vesper HW, Audain C, Woolfitt A, Ospina M, Barr J, Robins SP, Myers GL. High-performance liquid chromatography method to analyze free and total urinary pyridinoline and deoxypyridinoline. Anal Biochem. 2003;318:204–211. doi: 10.1016/s0003-2697(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 48.Robins SP, Woitge H, Hesley R, Ju J, Seyedin S, Seibel MJ. Direct, enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorption. J Bone Miner Res. 1994;9:1643–1649. doi: 10.1002/jbmr.5650091019. [DOI] [PubMed] [Google Scholar]
- 49.Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem. 1994;40:2022–2025. [PubMed] [Google Scholar]
- 50.Bonde M, Fledelius C, Qvist P, Christiansen C. Coated-tube radioimmunoassay for C-telopeptides of type I collagen to assess bone resorption. Clin Chem. 1996;42:1639–1644. [PubMed] [Google Scholar]
- 51.Gertz BJ, Clemens JD, Holland SD, Yuan W, Greenspan S. Application of a new serum assay for type I collagen cross-linked N-telopeptides: assessment of diurnal changes in bone turnover with and without alendronate treatment. Calcif Tissue Int. 1998;63:102–106. doi: 10.1007/s002239900497. [DOI] [PubMed] [Google Scholar]
- 52.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–1258. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 53.Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin Chem. 1993;39:635–640. [PubMed] [Google Scholar]
- 54.Karmatschek M, Maier I, Seibel MJ, Woitge HW, Ziegler R, Armbruster FP. Improved purification of human bone sialoprotein and development of a homologous radioimmunoassay. Clin Chem. 1997;43:2076–2082. [PubMed] [Google Scholar]
- 55.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–4066. [PubMed] [Google Scholar]
- 56.Chen D, Sarikaya NA, Gunn H, Martin SW, Young JD. ELISA methodology for detection of modified osteoprotegerin in clinical studies. Clin Chem. 2001;47:747–749. [PubMed] [Google Scholar]
- 57.Kraenzlin ME, Lau KH, Liang L, Freeman TK, Singer FR, Stepan J, Baylink DJ. Development of an immunoassay for human serum osteoclastic tartrate-resistant acid phosphatase. J Clin Endocrinol Metab. 1990;71:442–451. doi: 10.1210/jcem-71-2-442. [DOI] [PubMed] [Google Scholar]
- 58.Lau KH, Onishi T, Wergedal JE, Singer FR, Baylink DJ. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: potential use to assess bone resorption. Clin Chem. 1987;33:458–462. [PubMed] [Google Scholar]
- 59.Jukkola A, Tahtela R, Tholix E, Vuorinen K, Blanco G, Risteli L, Risteli J. Aggressive breast cancer leads to discrepant serum levels of the type I procollagen propeptides PINP and PICP. Cancer Res. 1997;57:5517–5520. [PubMed] [Google Scholar]
- 60.Koizumi M, Yonese J, Fukui I, Ogata E. The serum level of the amino-terminal propeptide of type I procollagen is a sensitive marker for prostate cancer metastasis to bone. BJU Int. 2001;87:348–351. doi: 10.1046/j.1464-410x.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 61.Bergmann P, Body JJ, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman JM, Reginster JY, Gangji V. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2009;63:19–26. doi: 10.1111/j.1742-1241.2008.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 63.Mountzios G, Dimopoulos MA, Bamias A, Papadopoulos G, Kastritis E, Syrigos K, Pavlakis G, Terpos E. Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor-κB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol. 2007;46:221–229. doi: 10.1080/02841860600635870. [DOI] [PubMed] [Google Scholar]
- 64.Seibel MJ. Molecular markers of bone turnover: biochemical, technical and analytical aspects. Osteoporos Int. 2000;11:S18–S29. doi: 10.1007/s001980070003. [DOI] [PubMed] [Google Scholar]
- 65.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaisse JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 66.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 67.Waltregny D, Bellahcene A, Van Riet I, Fisher LW, Young M,, Fernandez P, Dewe W, de Leval J, Castronovo V. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J Natl Cancer Inst. 1998;90:1000–1008. doi: 10.1093/jnci/90.13.1000. [DOI] [PubMed] [Google Scholar]
- 68.Halleen JM, Alatalo SL, Janckila AJ, Woitge HW, Seibel MJ, Vaananen HK. Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem. 2001;47:597–600. [PubMed] [Google Scholar]
- 69.Garnero P. Markers of bone turnover in prostate cancer. Cancer Treat Rev. 2001;27:187–192. doi: 10.1053/ctrv.2000.0213. [DOI] [PubMed] [Google Scholar]
- 70.Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klepzig M, Jonas D, Oremek GM. Procollagen type 1 amino-terminal propeptide: a marker for bone metastases in prostate carcinoma. Anticancer Res. 2009;29:671–673. [PubMed] [Google Scholar]
- 72.Koopmans N, de Jong IJ, Breeuwsma AJ, van der Veer E. Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J Urol. 2007;178:849–853. doi: 10.1016/j.juro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 73.Leeming DJ, Koizumi M, Byrjalsen I, Li B, Qvist P, Tanko LB. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:32–38. doi: 10.1158/1055-9965.EPI-05-0492. [DOI] [PubMed] [Google Scholar]
- 74.Jung K, Lein M, Stephan C, Von HK, Semjonow A, Sinha P, Loening SA, Schnorr D. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 75.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 76.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 77.Cook RJ, Coleman R, Brown J, Lipton A, Major P, Hei YJ, Saad F, Smith MR. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12:3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 78.Brasso K, Christensen IJ, Johansen JS, Teisner B, Garnero P, Price PA, Iversen P. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 79.Johansen JS, Brasso K, Iversen P, Teisner B, Garnero P, Price PA, Christensen IJ. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007;13:3244–3249. doi: 10.1158/1078-0432.CCR-06-2616. [DOI] [PubMed] [Google Scholar]
- 80.Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, Brown JE, Coleman RE. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 81.Pectasides D, Nikolaou M, Farmakis D, Kanakis I, Gaglia A, Kountourakis P, Karamanos NK, Economopoulos T, Raptis SA. Clinical value of bone remodelling markers in patients with bone metastases treated with zoledronic acid. Anticancer Res. 2005;25:1457–1463. [PubMed] [Google Scholar]
- 82.Lein M, Wirth M, Miller K, Eickenberg HU, Weissbach L, Schmidt K, Haus U, Stephan C, Meissner S, Loening SA, et al. Serial markers of bone turnover in men with metastatic prostate cancer treated with zoledronic acid for detection of bone metastases progression. Eur Urol. 2007;52:1381–1387. doi: 10.1016/j.eururo.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 83.Lein M, Miller K, Wirth M, Weissbach L, May C, Schmidt K, Haus U, Schrader M, Jung K. Bone turnover markers as predictive tools for skeletal complications in men with metastatic prostate cancer treated with zoledronic acid. Prostate. 2009;69:624–632. doi: 10.1002/pros.20917. [DOI] [PubMed] [Google Scholar]
- 84.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 85.Nelson JB, Nabulsi AA, Vogelzang NJ, Breul J, Zonnenberg BA, Daliani DD, Schulman CC, Carducci MA. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003;169:1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 86.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormonerefractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 87.Michaelson MD, Kaufman DS, Kantoff P, Oh WK, Smith MR. Randomized phase II study of atrasentan alone or in combination with zoledronic acid in men with metastatic prostate cancer. Cancer. 2006;107:530–535. doi: 10.1002/cncr.22043. [DOI] [PubMed] [Google Scholar]
- 88.Armstrong AJ, Creel P, Turnbull J, Moore C, Jaffe TA, Haley S, Petros W, Yenser S, Gockerman JP, Sleep D, et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008;14:6270–6276. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 89.Schelman WR, Liu G, Wilding G, Morris T, Phung D, Dreicer R. A phase I study of zibotentan (ZD4054) in patients with metastatic, castrate-resistant prostate cancer. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9318-5. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Morris MJ, Hudes G, Calabro F, Cheng S, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu EY, Massard C, Gross M, Wilding G, Posadas E, Culine S, Carducci M, Trudel G, Paliwal P, Sternberg C. A phase II study of once-daily dasatinib for patients with castration-resistant prostate cancer (CA180085) J Clin Oncol. 2009;27:270s. Abstract 5147. [Google Scholar]
- 92.Araujo J, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, Gallick G, Trudel GC, Paliwal P, Logothetis CJ. Dasatinib and docetaxel combination treatment for patients with metastatic castration-resistant prostate cancer (CRPC): analysis of study CA180-086. Eur J Cancer. 2009;7:415S. Abstract 7028. [Google Scholar]
- 93.Hannon RA, Clack G, Rimmer M, Swaisland A, Lockton JA, Finkelman RD, Eastell R. Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double-blind, placebo-controlled, multiple ascending dose phase I trial. J Bone Miner Res. 2010;25:463–471. doi: 10.1359/jbmr.090830. [DOI] [PubMed] [Google Scholar]
- 94.Lara PN, Jr, Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, Posadas E, Stadler W, Gandara DR. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20:179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, Fizazi K. Effects of denosumab in patients with bone metastases, with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 96.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 97.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol. 2009;182:509–515. doi: 10.1016/j.juro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 98.Amgen Press Release, author. Denosumab demonstrated superiority over zometa in pivotal phase 3 head-to-head trial in prostate cancer patients with bone metastases. 2010. [March 2010]. Available at http://www.amgen.com/media/media_pr_detail.jsp?releaseID=1385163.
- 99.Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb NJ. Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor. Leukemia. 2009;23:590–594. doi: 10.1038/leu.2008.237. [DOI] [PubMed] [Google Scholar]
- 100.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, et al. Discovery of N-(2-chloro-6- methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 101.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 102.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 103.Vandyke K, Dewar AL, Farrugia AN, Fitter S, Bik TL, Hughes TP, Zannettino AC. Therapeutic concentrations of dasatinib inhibit in vitro osteoclastogenesis. Leukemia. 2009;23:994–997. doi: 10.1038/leu.2008.356. [DOI] [PubMed] [Google Scholar]
- 104.de Vries TJ, Mullender MG, van Duin MA, Semeins CM, James N, Green TP, Everts V, Klein-Nulend J. The Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclasts. Mol Cancer Res. 2009;7:476–488. doi: 10.1158/1541-7786.MCR-08-0219. [DOI] [PubMed] [Google Scholar]
- 105.Yang JC, Ok JH, Busby JE, Borowsky AD, Kung HJ, Evans CP. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 2009;69:151–160. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97:779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 108.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, Dawson NA. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebocontrolled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 109.Watts NB. Clinical utility of biochemical markers of bone remodeling. Clin Chem. 1999;45:1359–1368. [PubMed] [Google Scholar]
- 110.Conrads TP, Hood BL, Petricoin EF, III, Liotta LA, Veenstra TD. Cancer proteomics: many technologies, one goal. Expert Rev Proteomics. 2005;2:693–703. doi: 10.1586/14789450.2.5.693. [DOI] [PubMed] [Google Scholar]
- 111.Tang HY, li-Khan N, Echan LA, Levenkova N, Rux JJ, Speicher DW. A novel four-dimensional strategy combining protein and peptide separation methods enables detection of low-abundance proteins in human plasma and serum proteomes. Proteomics. 2005;5:3329–3342. doi: 10.1002/pmic.200401275. [DOI] [PubMed] [Google Scholar]
- 112.Bhattacharyya S, Byrum S, Siegel ER, Suva LJ. Proteomic analysis of bone cancer: a review of current and future developments. Expert Rev Proteomics. 2007;4:371–378. doi: 10.1586/14789450.4.3.371. [DOI] [PubMed] [Google Scholar]
- 113.Kohli M, Siegel E, Bhattacharya S, Khan MA, Shah R, Suva LJ. Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) for determining prognosis in advanced stage hormone relapsing prostate cancer. Cancer Biomark. 2006;2:249–258. doi: 10.3233/cbm-2006-2603. [DOI] [PubMed] [Google Scholar]
- 114.Le L, Chi K, Tyldesley S, Flibotte S, Diamond DL, Kuzyk MA, Sadar MD. Identification of serum amyloid A as a biomarker to distinguish prostate cancer patients with bone lesions. Clin Chem. 2005;51:695–707. doi: 10.1373/clinchem.2004.041087. [DOI] [PubMed] [Google Scholar]
- 115.Bhattacharyya S, Epstein J, Suva LJ. Biomarkers that discriminate multiple myeloma patients with or without skeletal involvement detected using SELDI-TOF mass spectrometry and statistical and machine learning tools. Dis Markers. 2006;22:245–255. doi: 10.1155/2006/728296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 117.Helo P, Cronin AM, Danila DC, Wenske S, Gonzalez-Espinoza R, Anand A, Koscuiszka M, Vaananen RM, Pettersson K, Chun FK, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with Cell Search assay and association with bone metastases and with survival. Clin Chem. 2009;55:765–773. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castrationresistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 119.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armstrong AJ, Febbo PG. Using surrogate biomarkers to predict clinical benefit in men with castration-resistant prostate cancer: an update and review of the literature. Oncologist. 2009;14:816–827. doi: 10.1634/theoncologist.2009-0043. [DOI] [PubMed] [Google Scholar]
- 121.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]