Abstract
Estrogen signaling is required for the proliferation of normal breast epithelial cells. However, prophylactic inhibition of estrogen signaling fails to prevent 56% of human breast cancer cases. The underlying mechanism is not well understood. Aberrant activation of growth factor signaling is known to provide alternative proliferation pathways in breast cells that are fully transformed, but it is not known whether activation of growth factor signaling can substitute for estrogen signaling in causing aberrant proliferation in the normal breast epithelium. Here, we report that in a retrovirus-based somatic mouse model (replication-competent ALV-LTR splice acceptor/tumor virus A) that closely mimics the evolution of sporadic human breast cancers, mammary epithelial cells harboring PyMT or activated ErbB2 evolve into tumors independent of estrogen or other ovarian functions in contrast to previous observations of estrogen-dependent cancer formation in germ line mouse models of ErbB2 activation. Importantly, ErbB2 activation in normal mammary cells causes estrogen-independent proliferation in both estrogen receptor (ER)-negative cells as well as in normally quiescent ER-positive cells. Therefore, aberrant activation of growth factor signaling contributes to estrogen-independent proliferation of both preneoplastic and cancerous mammary cells, and prophylactic therapy against both growth factor signaling and estrogen signaling may need to be considered in women with increased risk of breast cancer.
Introduction
Estrogen receptor α (referred to as ER hereafter) is produced in approximately 15%of mammary epithelial cells in the human breast [1]. Estrogen and estrogen signaling through ER are required for the development of the mammary gland and proliferation of mammary epithelial cells in the normal breast. Paradoxically, however, proliferation is rarely found in ER+ cells, but rather in ER- cells. It is generally considered that the binding of estrogen, released primarily from the ovaries, causes ER to transactivate genes whose products act through a paracrine mechanism to stimulate proliferation of neighboring ER- cells [2,3]. ER is also expressed in at least a fraction of tumor cells in approximately 70% of human breast tumors [4]. In these tumors, ER+ cells are no longer quiescent but usually still depend on ovarian estrogen for proliferation.
Prophylactic suppression of estrogen signaling in high-risk women reduces the occurrence of breast cancer. However, treatment with the antiestrogens tamoxifen or raloxifene only decreases the incidence of breast tumors in these women by 44% [5]. Thus, in many patients, mutated breast cells can progress to cancer unhindered in the absence of estrogen signaling. The molecular mechanism underlying such a clinical observation is not well understood. ErbB2 (Her2, Neu) encodes a member of the epidermal growth factor receptor family of receptor tyrosine kinases and is amplified in 20% to 25% of human breast cancers [6]. Studies using mouse xenografts of cultured human breast cancer cell lines suggest that activation of ErbB2 or members of other growth factor signaling pathways can result in estrogen-independent growth of established breast tumor cells that normally require estrogen for proliferation [7–11], providing a mechanistic explanation for the failed prevention of some breast cancers using antiestrogens. However, it is not known whether activation of ErbB2 or other components of growth factor signaling in ER+ or ER- cells in the normal breast epithelium is sufficient to cause these normal cells to undergo aberrant proliferation and tumor evolution independent of estrogen signaling. If so, this could provide a complementary explanation and would suggest that estrogen-independent proliferation may occur at any stage of breast cancer evolution when growth factor signaling is activated.
In transgenic mice expressing ErbB2 from the mouse mammary tumor virus (MMTV) LTR, a ubiquitously active mammary-selective promoter, administration of tamoxifen starting at the age of 12 weeks delayed mammary tumor development [12]. In accord, ovariectomy at the age of 2 months also decreased the tumor incidence in this transgenic line [13]. These observations suggest that ErbB2-induced mammary tumorigenesis may still depend on estrogen signaling. However, it is not known whether suppression of clinical tumor formation in these models is due to an estrogen requirement for the initial aberrant proliferation of precancerous mammary cells or for expansion from nascent cancerous cells to a palpable tumor.
Further complicating the interpretation of these results, these and other genetically engineered models suffer oncogenic changes before pubertal mammary development [14], and the development of a normal ductal tree is impaired [15,16]. Besides, the widespread oncogenic expression in most mammary epithelial cells in these germ line models fails to closely mimic the initiation of sporadic human breast cancers, which arise in a few mutated cells among a field of normal breast cells in a normally developed breast, although most of these models eventually develop stochastic tumors. In addition, commonly used promoters for mammary transgenic expression, such as the MMTV LTR or the promoter driving the gene encoding whey acidic protein, are hormone-responsive [17–19]. Consequently, attenuation of estrogen signaling may decrease the expression levels of the transgenic oncogene, complicating the interpretation of these transgenic tumor studies.
We therefore asked whether tumorigenesis initiated by ErbB2 or the gene encoding polyoma virus middle T antigen (PyMT), which activates overlapping signaling pathways with ErbB2, still depends on estrogen signaling in a somatic mouse model that more closely mimics the evolution of sporadic human breast cancers. This somatic model was recently adapted by us from the tumor virus A (TVA)-mediated gene transfer technique for generating mouse models of breast cancer with both spatial and temporal controls [20–23]. Transgenic mice were made to express the gene encoding the retroviral receptor TVA in the mammary epithelium from the MMTV LTR. An avian retroviral vector (replication-competent ALV-LTR splice acceptor, RCAS) was used to infect and deliver oncogenes selectively into mammary epithelial cells producing TVA. Consequently, cancers evolve from one or a few oncogene-activated cells in an otherwise normal mammary epithelium in this model [22]. Because carcinogenesis in this model more closely recapitulates the evolution of sporadic human breast cancers from breast cells with somatically activated growth factor signaling, we used it in this report to test whether ErbB2 or PyMT-induced mammary tumorigenesis requires estrogen signaling for any phase of tumor formation.
Materials and Methods
Mice
The MMTV-tva transgenic line has been previously described [21]. All animals used in this study were of the FVB/N genetic background. Mice were kept in specific pathogen-free housing with abundant food and water according to National Institutes of Health guidelines.
Virus Preparation
The RCAS vector carrying PyMT (RCAS-PyMT) has been described [24]. RCAS-ErbB2 contains the rat ErbB2 DNA insert with a Val 664 point mutation to Glu and truncations in both the extracellular and intracellular domains [25]. This ErbB2 insert contains a double-HA tag as well as the ovalbumin tag SIINFEKL, which was not expected to cause any immune response in the FVB/N strain of mice used in this study. RCAS-β-actin was generated by inserting the HA-tagged β-actin gene [26] (a gift from Tom Kristie, National Institutes of Health) into RCAS. To produce RCAS viruses, DF-1 chicken fibroblasts [27,28] were transfected with RCAS vectors using Superfect (Qiagen, Hilden, Germany), and maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and penicillin-streptomycin in humidified 37°C incubators supplemented with 5% CO2. Viruses in the culture supernatant were concentrated 100-fold by centrifugation at 125,000g for 90 minutes, resuspended in Dulbecco modified Eagle medium containing 10% fetal bovine serum, and frozen in aliquots for titer determination and infection of cells and animals. Virus titers were determined by limiting dilution in DF-1 cells [29].
Tumor Latency Studies
RCAS-PyMT (104 IU) or RCAS-ErbB2 (1.5 x 106 IU) was delivered into the nos. 2, 3, and 4 mammary glands through intraductal injection [29] into pubertal (6 weeks) or adult (12-15 weeks) MMTV-tva mice. A tracking dye (0.1% bromophenol blue) was used to determine injection success. One week after infection, mice were randomized, and either ovariectomy or sham surgery was performed. These infected mice were palpated daily (PyMT) or three times weekly (ErbB2) for tumors. For tamoxifen studies, RCAS-PyMT or RCAS-ErbB2 was delivered into the nos. 2, 3, and 4 mammary glands through intraductal injection into pubertal (6 weeks) or adult (10-12 weeks) MMTV-tva mice, respectively. One week after infection, mice were randomized, and either tamoxifen or vehicle was introduced as previously described [30]. Mice infected with RCAS-PyMT were monitored for tumors daily, and mice infected with RCAS-ErbB2 were monitored for tumors three times per week.
Tissue Harvest and Analysis
Mammary glands and tumors were removed and either fixed in 10% buffered formalin overnight at 4°C or snap frozen in liquid nitrogen. Fixed tissues were embedded in paraffin, and 3-µm sections were used for hematoxylin and eosin staining and/or immunostaining. Western blot analysis was performed as previously described [30]. Scion Image (Scion Corporation, Frederick, MD) was used for Western blot quantitation. Primary antibodies used in these studies include rabbit immunoglobulin G (IgG) against ER (1:500, SC-452; Santa Cruz Biotechnology, Santa Cruz, CA), Ki67 (1:500, NCL-Ki67-P; Novacastra, Newcastle upon Tyne, UK), progesterone receptor (PR, 1:500, A0098; Dako, Glostrup, Denmark), phospho-p44/42 mitogen-activated protein kinase (MAPK; 1:1000, 9101; Cell Signaling, Danvers, MA), p44/42 MAPK (1:1000, 9102; Cell Signaling), phospho-AKT (1:1000, 9271; Cell Signaling), AKT (1:1000, 9272; Cell Signaling), HA (1:1000, PRB-101P; Covance, Princeton, NJ), and glyceraldehyde 2-phosphate dehydrogenase (GAPDH; 1:2000, sc25778; Santa Cruz Biotechnology); and mouse monoclonal antibodies against Ki67 (1:200, 556003; BD, Franklin Lanes, NJ), and HA (1:500, MMS-101P; Covance). For immunohistochemical staining, sections were deparaffinized and rehydrated in a graded xylene/ethanol series and then stained using the Vector ABC immunostaining kits (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. For immunofluorescent staining, deparaffinized slides were incubated with primary antibodies for 1 hour at room temperature. After three washes with Tris-buffered saline supplemented with 0.05% Tween 20 (TBST), slides were incubated with fluorophore-conjugated secondary antibodies for 30 minutes at room temperature, washed with Tris-buffered saline supplemented with 0.05% Tween 20, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to identify nuclei.
Statistical Analysis
A generalized Wilcoxon test was used in all tumor latency studies. Student's t tests were used for comparison of ER status of early lesions and tumors, tumor proliferation studies, and for Western blot analysis. Wilcoxon signed rank tests were used for comparisons of proliferation rates between early lesions and normal ducts. Wilcoxon rank sum tests were used for comparison of proliferation rates between intact and ovariectomized mice.
Results
RCAS-Mediated Gene Expression Is Detected in Both ER+ and ER- Mammary Epithelial Cells in MMTV-tva Mice; RCAS-PyMT Leads to ER+ Mammary Tumors in the Majority of Infected MMTV-tva Mice
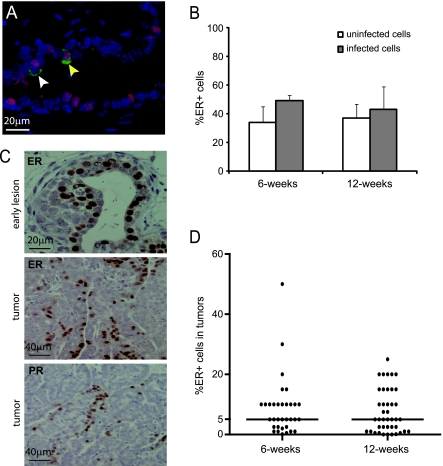
Our previous studies showed that in MMTV-tva mice, only 0.3% of mammary cells were infected after intraductal injection of 107 IU of RCAS virus [21]. Because ER- cells are the majority of mammary epithelial cells and are the subset of cells undergoing proliferation [1], a state that is preferred for infection by retroviruses such as RCAS [31], we first tested whether besides ER- cells, ER+ cells were also infected after intraductal injection of RCAS. RCAS-β-actin (containing the HA epitope) was injected into 6- and 12-week-old MMTV-tva mice (n = 4 and 9, respectively) at 106 IU per gland, and the infected glands were collected 4 days later for immunofluorescent staining for ER. ER+ mammary cells constituted 34% ± 11% of mammary epithelial cells at the age of 6 weeks and 37% ± 10% at the age of 12 weeks (Figure 1B). Among the infected cells identified by the HA tag, 49% ± 4% were also positive for ER at the age of 6 weeks (Figure 1, A and B), and 43 ± 16% were positive for ER at the age of 12 weeks (Figure 1B). These observations suggest that this virus infects both ER+ and ER- cells, although we cannot exclude the possibility that the ER status of some of the originally infected cells had changed by the time the mammary glands were collected for these assays.
Figure 1.
RCAS-mediated gene expression is detected in both ER+ and ER- mammary epithelial cells in MMTV-tva mice; RCAS-PyMT leads to ER+ mammary tumors in most infected MMTV-tva mice. (A and B) Pubertal (n = 4; 6 weeks) and adult (n = 9; 12 weeks) MMTV-tva mice were infected with RCAS-β-actin (HA-tagged) and killed 4 days later. Coimmunofluorescent staining for HA (green) and ER (red) was performed on the infected glands. A merged image that includes the DAPI nuclear counterstain is shown for an infected mammary gland from a 6-week-old mouse (A). A costained cell is indicated by a yellow arrow, and a non-costained cell is indicated by a white arrow. Bar graph (B) shows the percentage of ER+ cells in the general epithelium or among the infected cells (identified by staining for HA). (C) Six-week-old MMTV-tva mice (n = 5) were infected with RCAS-PyMT and killed 4 days later. Immunohistochemical staining for the indicated proteins was performed on the resulting early lesions as well as 33 tumors induced by infecting 6-week-oldmice with RCAS-PyMT (107 IU). (D) Dot plot showing the percentage of ER+ cells in these tumors as well as in tumors (n = 41) generated by intraductal injection of RCAS-PyMT (107 IU) into 12-week-old MMTV-tva mice. Horizontal bar indicates the median value.
We have previously induced mammary tumors using RCAS virus expressing the gene encoding PyMT (RCAS-PyMT) [21], a potent viral oncoprotein that activates MAPK and phosphoinositide 3-kinase pathways, both of which are activated by receptors associated with growth factor signaling [32]. Intraductal injection of RCAS-PyMT (107 IU) led to the formation of well-differentiated mammary tumors with a median latency of 12 days [21]. Because well-differentiated tumors in humans usually express ER [33–35], we asked whether preneoplastic lesions and tumors induced by RCAS-PyMT also produced ER. Six-week-old MMTV-tva mice (n = 5) were intraductally injected with RCAS-PyMT (107 IU per gland). Four days later, the infected glands were collected for immunohistochemical staining for ER. About 74%of the early lesions in these infected glands harbored at least 5%ER+ cells (Figure 1C), demonstrating that delivery of PyMTusing this virus induces predominately ER+ preneoplastic lesions. This is consistent with the finding that ER is produced in most premalignant breast lesions in human patients [4], regardless of the eventual tumor ER status, as well as in several transgenic and knockout mouse models of breast cancer [30,36–38].
We next examined the ER status of tumors induced by RCASPyMT infection (107 IU). ER was detected in the nuclei of 5% or more of tumor cells in 70% (23/33) of tumors from MMTV-tva mice infected at the age of 6 weeks (Figure 1, C and D) and in 61% (25/41) of tumors from mice infected at the age of 12 weeks (Figure 1D). Therefore, these tumors were defined as ER+. In addition to ER, these tumors also produced PR (Figure 1C and data not shown), a classic transcriptional target of ER, suggesting that estrogen signaling is intact in these ER+ tumor cells. Collectively, these studies establish that somatic introduction of PyMTusing this viral approach predominately induces ER+ mammary early lesions and tumors.
Estrogen-Independent Evolution of Tumors Induced by RCAS-PyMT
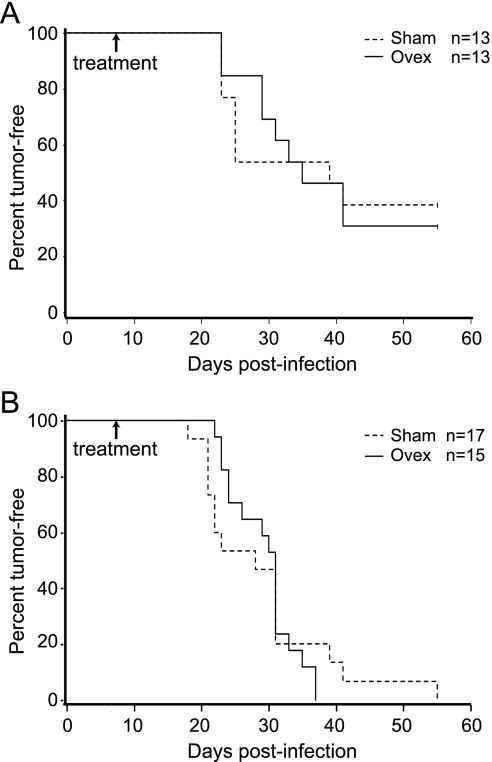
Having found that RCAS-PyMT induces predominately ER+ early lesions and tumors in the mammary gland, we next asked whether estrogen deprivation prevents or delays tumorigenesis in this somatic model. A cohort of twenty-six 6-week-old MMTV-tva mice was injected with RCAS-PyMT at a low dose (104 IU/gland) into one set of nos. 2, 3, and 4 glands. This dose was estimated to infect much fewer cells than the 107 IU used in our previous studies, thus allowing any potential differences in tumor latency caused by ovariectomy to be more pronounced. Seven days later, animals were randomized for ovariectomy (n = 13) or sham (n = 13) surgery. These mice were then palpated daily for tumor appearance. The Kaplan-Meier tumor-free survival plot is shown in Figure 2A. Sham-treated mice developed stochastic tumors with a median latency of 39 days. Tumors in ovariectomized mice arose with a median latency of 35 days, not significantly different from the control mice (P = .47).
Figure 2.
Estrogen-independent evolution of tumors induced by RCAS-PyMT. (A and B) MMTV-tva mice at the age of 6 (A) or 12 (B) weeks were infected with RCAS-PyMT (104 IU/gland, one set of nos. 2–4 glands per mouse). One week later, the infected mice were randomized and treated with ovariectomy or sham surgery. Tumor appearance was monitored by daily palpation.
In chemoprevention and treatment of human breast cancer, suppression of estrogen signaling is usually achieved by the administration of pharmacological inhibitors such as tamoxifen. Therefore, we also tested whether tamoxifen could prevent tumorigenesis induced by PyMT. A cohort of 47 pubertal mice was infected with RCAS-PyMT (107 IU/gland). One week after infection, the mice were treated with either tamoxifen or vehicle, and tumorigenesis was monitored daily. As with ovariectomy, tamoxifen was unable to prevent tumorigenesis in these mice (P = .2; Figure W1A). Collectively, these data suggest that the removal of ovarian hormones by ovariectomy or attenuation of estrogen signaling with tamoxifen cannot prevent or delay tumor evolution if PyMT is introduced somatically into mammary epithelial cells at puberty.
In the human breast, although initiating oncogenic mutations may start to accumulate at the onset of puberty, tumor initiation is expected to occur predominately during adulthood. By intraductal injection of RCAS-PyMT into the mammary glands of five adult (12 weeks old) MMTV-tva mice, we generated early lesions 4 days after infection. Of these early lesions, 54% were designated ER+ by immunohistochemistry (IHC; data not shown). We next tested whether tumor development in these adult mice depended on intact estrogen signaling. We infected thirty-two 12-week-old MMTV-tva mice with RCAS-PyMT (104 IU per gland; one set of nos. 2, 3, and 4 glands per mouse). One week later, mice were randomized, and either ovariectomy (n = 15) or sham surgery (n = 17) was performed. Mice were palpated daily for tumor appearance. Tumors appeared with a median latency of 28 and 31 days in the infected mice treated with sham surgery or ovariectomy, respectively (P = .55; Figure 2B). Thus, ovariectomy also does not affect tumor latency in adult mice after the gain of PyMT. Tumors arising in these ovariectomized mice were histologically similar to those arising in the intact mice (data not shown). The rate of ER+ tumor formation was also similar between these two groups of mice: 69% (9/13) were ER+ in the intact mice, whereas 61% (11/18) were ER+ in the ovariectomized mice (data not shown). PR was also detected in all of the ER+ subset of mammary tumors in intact mice (data not shown). As expected, most (15/18) of tumors from the ovariectomized mice were PR- (data not shown). These data suggest that estrogen is dispensable for the initiation and development of tumors somatically induced by PyMT.
Estrogen-Independent Evolution of Tumors Induced by RCAS-ErbB2
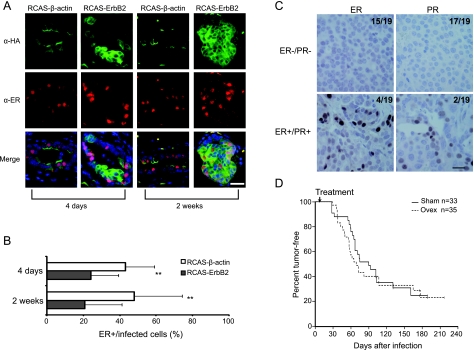
Having demonstrated estrogen-independent tumorigenesis induced by somatic introduction of PyMT, we next tested whether estrogen signaling was required for tumor evolution initiated by an oncogene responsible for many human breast cancers, ErbB2.We have previously reported that RCAS (107 IU) encoding an activated version of ErbB2 induced mammary tumors in adult MMTV-tva mice with a median latency of 6 months [21]. Here, a double HA-tagged (for ease of detection by IHC and immunofluorescence), activated version of ErbB2 was cloned into RCAS, and the resulting RCAS-ErbB2 virus was used for in vivo experiments.We first determined whether ER was still produced in mammary cells infected by RCAS-ErbB2 because there is often an inverse correlation with ErbB2 expression and ER in breast cancer [39], and ErbB2 has been reported to suppress ER expression in cultured breast cancer cell lines [40]. Adult MMTV-tva mice (12–14 weeks) were infected with RCAS-ErbB2 and RCAS-β-actin in contralateral glands and were killed either 4 days (n = 9) or 14 days (n = 4) later, when mild and more advanced hyperplasias were apparent, respectively. Coimmunofluorescent staining for ER and HA (Figure 3A) was used to determine the frequency of ER+ cells among infected cells. At day 4 after infection, ER was detected in 43% ± 16% of RCAS-β-actin-infected cells, but only in 24% ± 15% of RCAS-ErbB2-infected cells, an almost 50% reduction (P = .005; Figure 3B). At day 14, the percentage of ER+ cells did not change significantly in either group of infected cells—48% ± 26% RCAS-β-actin-infected cells and 21% ± 20% RCAS-ErbB2-infected cells—again an approximate 50% reduction (P = .006). These observations suggest that ErbB2 activation in normal mammary cells may suppress ER in at least a subset of these cells.
Figure 3.
Estrogen-independent evolution of tumors induced by RCAS-ErbB2. (A and B) MMTV-tva mice (12–14 weeks) were injected intraductally with RCAS-ErbB2 (HA-tagged) and with RCAS-β-actin (HA-tagged) in the contralateral glands. Mice were killed 4 (n = 9) or 14 days (n = 4) later. Coimmunofluorescence for HA and ER was performed on infected glands (A). Scale bar, 20 µm. Bar graph (B) shows percentage of HA+ cells that were also stained positive for ER. **P < .01. (C) IHC for ER and PR was performed on tumors arising from intact mice in (D). Representative images for ER-/PR- tumors and ER+/PR+ tumors are shown. The fractions of cases stained positive or negative for either ER or PR are indicated. Scale bar, 20 µm. (D) 68 MMTV-tva mice (12–15 weeks) were infected with RCAS-ErbB2 (1.5x 106 IU/gland, one set of nos. 2–4 glands per mouse). One week after infection, mice were randomized, and either ovariectomy or sham surgery was performed. Tumor appearance was monitored three times weekly to obtain tumor-free survival curves.
To determine the effect of ovariectomy on tumor induction by RCAS-ErbB2, 68 adult MMTV-tva mice (12–15 weeks) were infected with this virus (1.5 x 106 IU per gland, one set of nos. 2, 3, and 4 glands per mouse). One week later, they were randomized, and either ovariectomy (n = 35) or sham surgery (n = 33) was performed. Tumor development in these infected mice was monitored by palpation. In sham-treated mice, tumors developed with a median latency of 90 days, and the ovariectomized mice developed tumors with a median latency of 70 days (Figure 3D). Generalized Wilcoxon analysis found no difference in tumor latency between sham-treated and ovariectomized mice (P = .44). In addition, we asked whether tamoxifen could prevent tumorigenesis induced by somatic activation of ErbB2. One week after infection with RCAS-ErbB2, 28 adult MMTV-tva mice were randomized and were treated with either tamoxifen (n = 15) or vehicle (n = 13). As with ovariectomy, tamoxifen also failed to prevent or delay tumorigenesis in this model (P = .3; Figure W1B). Therefore, somatic activation of ErbB2 in mammary epithelial cells does not require estrogen signaling for tumorigenesis.
As predicted from the ubiquitous nature of the RCAS LTR, ErbB2 was made in similar amounts in RCAS-ErbB2-induced early lesions in both intact and ovariectomized mice (Figure W2A). Likewise, ErbB2 levels were similar between tumors arising in intact mice versus those arising in ovariectomized mice, based on Western blot analysis (P = .4), although the level varied among individual tumors within both groups of tumors, perhaps representing the different degrees of stroma infiltration (Figure W2, B and C). In accord, phosphorylation of Erk, a critical event downstream of ErbB2, was unaffected in these tumors by ovariectomy (Figure W3, A and B). Phosphorylation of Akt, another event downstream of ErbB2, is slightly reduced by ovariectomy (P = .05; Figure W3, C and D). Estrogen signaling has been reported to activate Akt through transcription-independent, membrane signaling [41–43]. Perhaps the loss of membrane signaling from ER in the ovariectomized mice led to this modest reduction of activated Akt in the tumors. Nevertheless, estrogen signaling does not seem to contribute much to the growth of these ErbB2-initiated tumor cells: ER was produced in 5% or more of tumor cells in 21% (4/19) of these tumors arising in intact mice, and half of these ER+ tumors were also found to be PR+ (Figure 3C), but the proliferation rate of the ER+ tumors was comparable to that of ER- tumors in these mice, based on Ki67 staining (P = .5; Figure W4).
RCAS-ErbB2 Induces Estrogen-Independent Proliferation of ER- Mammary Epithelial Cells as Well as of a Subset of Normally Quiescent ER+ Mammary Epithelial Cells
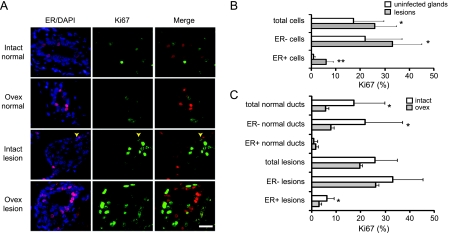
The lack of estrogen dependence in this RCAS-ErbB2 model suggests that aberrant activation of ErbB2 in normal mammary epithelial cells confers a proliferative advantage that is not compromised even after the loss of estrogen signaling.To test whether this is indeed the case, we infected 18 MMTV-tva mice (12–15 weeks) with RCAS-ErbB2 and performed ovariectomy on half of the infected mice and sham surgery on the other half 1 week after infection. Two weeks after surgery, the mice were killed, and immunofluorescent staining for Ki67 was performed on the paraffin sections of these infected glands (Figure 4A). As expected, cells in the early lesions exhibited a higher proliferation rate (26% ± 9%) than those in the normal ducts in contralateral noninfected glands (17% ± 12%; P = .04; Figure 4B). This higher proliferation rate in early lesions did not decrease significantly after ovariectomy (26% ± 9% in intact mice vs 20% ± 8% in ovariectomized mice, P = .14), whereas as expected, the percentage of proliferating cells in noninfectedmammary ducts was significantly reduced after ovariectomy (17% ± 12% in intact mice vs 6% ± 3% in ovariectomized mice, P = .03; Figure 4C). These observations suggest that ErbB2 activation in normal mammary cells causes estrogen-independent mammary cell proliferation and aberrant expansion.
Figure 4.
RCAS-ErbB2 induces estrogen-independent proliferation of ER- mammary epithelial cells as well as a subset of normally quiescent ER+ mammary epithelial cells. (A–C) MMTV-tva mice (12–16 weeks; n = 18) were infected with RCAS-ErbB2. One week later, mice were randomized, and ovariectomy or sham surgery was performed. After another 2 weeks, both infected and noninfected glands were collected. Coimmunofluorescence was performed for ER and Ki67 (A). A costained cell is indicated by a yellow arrow. Scale bar, 20 µm. Bar graphs show the proliferation rates in total cells in addition to the ER- and ER+ subsets of cells in uninfected glands and early lesions in intact mice (B), and the proliferation rates in the indicated subsets of cells in intact and ovariectomized mice (C). *P = .01 to .05; **P < .01.
To investigate whether ErbB2-induced estrogen-independent proliferation occurred in ER- cells, ER+ cells, or both, we performed coimmunofluorescence for ER and Ki67 in early lesions as well as in the normal ducts in the contralateral glands as a baseline control (Figure 4A). As expected, ovariectomy reduced the proliferation of normal ER- cells: 22% ± 15% were found to proliferate in the intact glands, but only 8% ± 4% were proliferative in the ovariectomized glands (P = .04; Figure 4C). Also as expected, normal ER+ cells in noninfected glands in both the intact and ovariectomized mice had very low proliferation rates: 0.8% ± 1.3% and 1.5% ± 4% were found in the intact glands and ovariectomized glands, respectively (P = .73; Figure 4C). In early lesions in intact mice, ER- cells proliferated at a rate of 33% ± 12%, much higher than the proliferation rate of the ER- cells (22% ± 15%) in contralateral uninfected glands (P = .04; Figure 4B), and this high rate was not significantly affected by ovariectomy (from 33% ± 12% to 26% ± 11%, P = .30; Figure 4C). These data suggest that aberrant ErbB2 signaling causes ER- mammary epithelial cells to undergo estrogen-independent proliferation.
In early lesions in intact mice, ER+ cells had a proliferation rate of 6% ± 3%, much higher than the approximately 0.8% observed in ER+ cells in the contralateral uninfected glands (P = .008; Figure 4B), demonstrating that ErbB2 activation in largely quiescent ER+ mammary epithelial cells can induce at least some of them to undergo proliferation. Ovariectomy reduced this aberrant proliferation in ER+ cells to 2.9% ± 2.0% (P = .02; Figure 4C); however, this rate was still higher than the low basal proliferation rate (0.8% ± 1.3%) detected in normal mammary epithelium in intact mice (P = .02), suggesting that at least some of the ErbB2-activated ER+ cells can undergo estrogen-independent proliferation.
Discussion
By delivering PyMTor activated ErbB2 into a small number of somatic mammary cells, we generated both ER+ and ER- mammary tumors in mice. ER was produced in most tumors induced by RCAS-PyMT in pubertal and adult mice and in a smaller proportion of tumors arising in adult mice infected by RCAS-ErbB2. These two models add to the relatively short list of mouse models of ER+ human breast cancers, which comprise approximately 70% of all human breast cancer cases [4]. These ER+ models may be useful for elucidating the molecular mechanisms of anti-hormone resistance found in a subset of human ER+ tumors. Moreover, 50% of ErbB2+ human breast cancers produce ER, but no ER has been reported in transgenic models of ErbB2+ human breast cancers. Therefore, our RCAS-ErbB2 model may be a valuable in vivo model for understanding how ErbB2 causes ER+ tumors and why these tumors are inherently resistant to antiestrogenic therapies.
Ovariectomy or tamoxifen treatment was unable to prevent or delay tumorigenesis induced by either PyMT or ErbB2 (Figures 2, 3, and W1), demonstrating that somatic activation of growth factor signaling through these two oncoproteins causes estrogen-independent breast tumor formation. This finding suggests that aberrant growth factor signaling causes normal mammary cells to undergo estrogen-independent proliferation. Indeed, at 3 weeks after the activation of ErbB2, estrogen-independent proliferation was detected in ER- cells and in at least some of the ER+ cells (Figure 4). These findings suggest that prophylactic antiestrogenic treatment fails to slow tumor evolution shortly after activation of growth factor signaling. Furthermore, estrogen signaling does not seem to provide a proliferation benefit to the resulting tumors (Figure W4). In accord, it has been reported that stable expression of ErbB2 in the ER+MCF7 human breast cancer cells causes them to become estrogen-independent or tamoxifen-resistant [8,44–46].
Whereas most epithelial cells in the normal breast epithelium became independent of estrogen for proliferation after they had activated ErbB2, a small subset of the ER+ cells remained dependent on estrogen for proliferation (Figure 4). However, these cells most likely did not contribute significantly to tumor formation in these ovariectomized or intact mice in our experiments because of the observed estrogen-independent nature of tumorigenesis and the similar proliferation rate between ER+ and ER- tumors arising in these mice. We do not yet know whether tumor evolution might be slowed by ovariectomy if ErbB2 could be selectively targeted to the ER+ subset of mammary epithelial cells. In addition, we do not know whether estrogen signaling may have contributed to ErbB2-induced tumor initiation in the first few days after ErbB2 activation, as ovariectomy or tamoxifen treatment was performed 1 week after infection—this length of time was chosen to ensure the completion of infection and thus the same rate of infection in the ovariectomized versus intact mice.
Our results demonstrating estrogen-independent tumor initiation from ErbB2-activated mammary cells differ from the outcomes of prevention experiments performed using MMTV-ErbB2 transgenic mice, in which tamoxifen or ovariectomy delayed tumor appearance if given before subclinical tumor formation [12,13]. There are several potential explanations for this difference. In MMTV-tva mice, ErbB2 was targeted by the RCAS vector to a small subset of mammary cells in developmentally normal mammary glands, whereas in MMTV-ErbB2 transgenic mice, oncogene expression occurs in most mammary epithelial cells and presumably starts before the onset of puberty [14]. It is unlikely that tumor initiation would occur before the onset of puberty in humans without hereditary mutations. Furthermore, MMTV-driven activation of ErbB2 results in impaired development of the mammary gland including incomplete filling of the mammary fat pad as late as the age of 13 weeks [15,16], suggesting that the normal cellular milieu in which human breast tumors usually originate is already compromised in this germ line transgenic model.
Alternatively, RCAS virus may have induced mammary tumors from a subset of mammary cells different from the cell origin of tumors in MMTV-ErbB2 transgenic mice. Although the same MMTV promoter is used both to drive ErbB2 in the transgenic model and to dictate the spectrum of cells that express tva and are susceptible to infection by RCAS in the our TVA model, the cell distribution patterns of MMTV-regulated transgenes in the mammary epithelium can vary between lines because of the different transgene integration sites. Mammary tumors induced by RCAS-PyMT contain heterogeneous cell types, implying a progenitor origin [21], but tumors arising in MMTV-PyMTand MMTV-ErbB2 models are more homogeneous and are thought to originate from more differentiated cells [21,47–51]. Because the cell origin of breast cancer has been reported to affect tumor histopathology, cellular heterogeneity, gene expression profiles, and the metastatic potential of the resulting tumors [52], the potential difference in the cellular origin between this somatic model and the transgenic models may also contribute to the difference in dependence on estrogen signaling. Indeed, if ErbB2 were selectively targeted to the subset of RCAS-ErbB2-infected ER+ cells whose proliferative capacity was reduced by ovariectomy, tumor development might be attenuated on estrogen withdrawal. It will also be interesting to test if estrogen dependence is observed if ErbB2 or PyMT is introduced through RCAS infection into differentiation stage-defined mammary cells using cell subtype-specific (such as stem, progenitor, or alveolar) transgenic promoters to control the expression of tva. Finally, it is of note that a truncated and activated ErbB2 was used in the RCAS vector. The wild type ErbB2 was used in the MMTV-ErbB2 line used for previous antiestrogenic experiments, but ErbB2 is, nevertheless, somatically mutated to an activated version in most of the tumors arising in this transgenic line [49].
Finally, in the transgenic tumor models, the transgenic promoter MMTV LTR is regulated by several hormones including progesterone [17,18]; thus, hormone manipulations such as ovariectomy or through the use of tamoxifen in these models may incidentally reduce the level of transgenic oncogene, jeopardizing the interpretation of the results from the use of these conventional models. Although it did not seem to affect progesterone signaling in cultured MCF7 cancer cells [53], tamoxifen has been shown to significantly reduce PR levels in normal mammary cells in vivo [54]. We are able to avoid this complication in our study: The MMTV-tva transgenic product is only required for the initial viral infection, and the expression of ErbB2 or PyMT in the infected cells is solely controlled by the proviral RCAS LTR, which we confirmed to be unaffected by ovariectomy (Figure W2).
In conclusion, this in vivo study in a somatic mouse model suggests that activation of ErbB2 or other growth factor signaling pathways causes normal ER- cells as well as some normal ER+ cells to undergo estrogen-independent proliferation and evolution to breast carcinomas. These data suggest that prophylactic estrogen deprivation therapy cannot prevent breast cancer initiation from mammary epithelial cells that have gained certain genetic alterations, especially those encoding components of growth factor signaling pathways, and that therapeutic agents that target growth factor signaling pathways may be an important complement to antiestrogens in achieving more effective prevention against both ER+ and ER- breast cancers in at-risk women.
Supplementary Material
Acknowledgments
The authors thank Amanda McGrath, Meghali Goswami, and the late Paul Maliakkal for technical assistance; Drs Jeffrey Rosen, Michael Lewis, and Gary Chamness for stimulating discussions and/or critical review of the article; the Pathology Core Facility at the Breast Center for tissue processing; and the Transgenic Mouse Facility at Baylor College of Medicine for animal husbandry.
Footnotes
This work was supported in part by funds from National Institutes of Health (CA113869 and CA124820 to Y.L. and AI45764 to J.F.) and from the United States Department of Defense (BC060332 to Y.L. and BC073703 to Y.L.).
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 2.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allred DC, Brown P, Medina D. The origins of estrogen receptor alpha-positive and estrogen receptor-negative human breast cancer. Breast Cancer Res. 2004;6:240–245. doi: 10.1186/bcr938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.El-Ashry D, Miller DL, Kharbanda S, Lippman ME, Kern FG. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene. 1997;15:423–435. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- 8.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, Hilsenbeck S, Schiff R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 9.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, el-Ashry D, Chen D, Ding IY, Kern FG. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 11.Miller DL, el-Ashry D, Cheville AL, Liu Y, McLeskey SW, Kern FG. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for a role of EGFR in breast cancer growth and progression. Cell Growth Differ. 1994;5:1263–1274. [PubMed] [Google Scholar]
- 12.Menard S, Aiello P, Tagliabue E, Rumio C, Lollini PL, Colnaghi MI, Balsari A. Tamoxifen chemoprevention of a hormone-independent tumor in the proto-neu transgenic mice model. Cancer Res. 2000;60:273–275. [PubMed] [Google Scholar]
- 13.Anisimov VN, Popovich IG, Alimova IN, Zabezhinski MA, Semenchenko AV, Yashin AI. Number of pregnancies and ovariectomy modify mammary carcinoma development in transgenic HER-2/neu female mice. Cancer Lett. 2003;193:49–55. doi: 10.1016/s0304-3835(02)00721-8. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Lee JS, Liang X, Zhang H, Zhu T, Zhang Z, Taylor ME, Zahnow C, Feigenbaum L, Rein A, et al. Hoxb7 inhibits transgenic HER-2/neu-induced mouse mammary tumor onset but promotes progression and lung metastasis. Cancer Res. 2008;68:3637–3644. doi: 10.1158/0008-5472.CAN-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee S, Louie SG, Campbell M, Esserman L, Shyamala G. Ductal growth is impeded in mammary glands of C-neu transgenic mice. Oncogene. 2000;19:5982–5987. doi: 10.1038/sj.onc.1203964. [DOI] [PubMed] [Google Scholar]
- 17.Cato AC, Henderson D, Ponta H. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 1987;6:363–368. doi: 10.1002/j.1460-2075.1987.tb04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ham J, Thomson A, Needham M, Webb P, Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988;16:5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittius CW, Sankaran L, Topper YJ, Hennighausen L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol Endocrinol. 1988;2:1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- 20.Reddy JP, Li Y. The RCAS-TVA system for introduction of oncogenes into selected somatic mammary epithelial cells in vivo. J Mammary Gland Biol Neoplasia. 2009;14:405–409. doi: 10.1007/s10911-009-9157-1. [DOI] [PubMed] [Google Scholar]
- 21.Du Z, Podsypanina K, Huang H, McGrath A, Toneff MJ, Bogoslovskaia E, Zhang X, Moraes RC, Fluck MM, Allred DC, et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci USA. 2006;103:17396–17401. doi: 10.1073/pnas.0608607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Z, Li Y. RCAS-TVA in the mammary gland: an in vivo oncogene screen and a high fidelity model for breast transformation? Cell Cycle. 2007;6:823–826. doi: 10.4161/cc.6.7.4074. [DOI] [PubMed] [Google Scholar]
- 23.Reddy JP, Peddibhotla S, Bu W, Zhao J, Haricharan S, Du YC, Podsypanina K, Rosen JM, Donehower LA, Li Y. Defining the ATM-mediated barrier to tumorigenesis in somatic mammary cells following ErbB2 activation. Proc Natl Acad Sci USA. 2010;107:3728–3733. doi: 10.1073/pnas.0910665107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland EC, Li Y, Celestino J, Dai C, Schaefer L, Sawaya RA, Fuller GN. Astrocytes give rise to oligodendrogliomas and astrocytomas after gene transfer of polyoma virus middle Tantigen in vivo. Am J Pathol. 2000;157:1031–1037. doi: 10.1016/S0002-9440(10)64615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargmann CI, Weinberg RA. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988;7:2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khurana B, Kristie TM. A protein sequestering system reveals control of cellular programs by the transcriptional coactivator HCF-1. J Biol Chem. 2004;279:33673–33683. doi: 10.1074/jbc.M401255200. [DOI] [PubMed] [Google Scholar]
- 27.Himly M, Foster DN, Bottoli I, Iacovoni JS, Vogt PK. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer-Klein J, Givol I, Barsov EV, Whitcomb JM, VanBrocklin M, Foster DN, Federspiel MJ, Hughes SH. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology. 1998;248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen D-A, Beeman N, Lewis M, Schaack J, Neville MC. Intraductal injection into the mouse mammary gland. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. New York, NY: Kluwer Academic; 2000. pp. 259–270. [Google Scholar]
- 30.Zhang X, Podsypanina K, Huang S, Mohsin SK, Chamness GC, Hatsell S, Cowin P, Schiff R, Li Y. Estrogen receptor positivity in mammary tumors of Wnt-1 transgenic mice is influenced by collaborating oncogenic mutations. Oncogene. 2005;24:4220–4231. doi: 10.1038/sj.onc.1208597. [DOI] [PubMed] [Google Scholar]
- 31.Hughes SH. The RCAS vector system. Folia Biol (Praha) 2004;50:107–119. doi: 10.14712/fb2004050030107. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Viciana P, Collins C, Fried M. Polyoma and SV40 proteins differentially regulate PP2A to activate distinct cellular signaling pathways involved in growth control. Proc Natl Acad Sci USA. 2006;103:19290–19295. doi: 10.1073/pnas.0609343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard PV, Davies CJ, Blamey RW, Elston CW, Johnson J, Griffiths K. Relationship between oestrogen-receptor content and histological grade in human primary breast tumours. Br J Cancer. 1978;38:745–748. doi: 10.1038/bjc.1978.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarty KS Jr, Barton TK, Fetter BF, Woodard BH, Mossler JA, Reeves W, Daly J, Wilkinson WE, McCarty KS Sr. Correlation of estrogen and progesterone receptors with histologic differentiation in mammary carcinoma. Cancer. 1980;46:2851–2858. doi: 10.1002/1097-0142(19801215)46:12+<2851::aid-cncr2820461424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Millis RR. Correlation of hormone receptors with pathological features in human breast cancer. Cancer. 1980;46:2869–2871. doi: 10.1002/1097-0142(19801215)46:12+<2869::aid-cncr2820461426>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT, Mohsin SK, Hilsenbeck SG, Lamph WW, Bissonette R, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 37.Green JE, Shibata MA, Yoshidome K, Liu ML, Jorcyk C, Anver MR, Wigginton J, Wiltrout R, Shibata E, Kaczmarczyk S, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–1027. doi: 10.1038/sj.onc.1203280. [DOI] [PubMed] [Google Scholar]
- 38.Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63:1067–1072. [PubMed] [Google Scholar]
- 39.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 40.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 41.Tsai EM, Wang SC, Lee JN, Hung MC. Akt activation by estrogen in estrogen receptor-negative breast cancer cells. Cancer Res. 2001;61:8390–8392. [PubMed] [Google Scholar]
- 42.Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A. Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol Endocrinol. 2003;17:818–830. doi: 10.1210/me.2002-0330. [DOI] [PubMed] [Google Scholar]
- 43.Stoica GE, Franke TF, Moroni M, Mueller S, Morgan E, Iann MC, Winder AD, Reiter R, Wellstein A, Martin MB, et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 44.Benz CC, O'Hagan RC, Richter B, Scott GK, Chang CH, Xiong X, Chew K, Ljung BM, Edgerton S, Thor A, et al. HER2/neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene. 1997;15:1513–1525. doi: 10.1038/sj.onc.1201331. [DOI] [PubMed] [Google Scholar]
- 45.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 46.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- 49.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 50.Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, Hinds PW. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 52.Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, Iglehart JD, Weinberg RA. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Yue W, Wang J, Savinov A, Brodie A. Effect of aromatase inhibitors on growth of mammary tumors in a nude mouse model. Cancer Res. 1995;55:3073–3077. [PubMed] [Google Scholar]
- 54.de Lima GR, Facina G, Shida JY, Chein MB, Tanaka P, Dardes RC, Jordan VC, Gebrim LH. Effects of low dose tamoxifen on normal breast tissue from premenopausal women. Eur J Cancer. 2003;39:891–898. doi: 10.1016/s0959-8049(02)00530-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.