Abstract
Gemcitabine is widely used as first-line chemotherapeutic drug in the treatment of pancreatic cancer. Our previous experimental chemotherapy studies have shown that treatment of human pancreatic carcinoma cells with 5-fluorouracil (5-FU) alters the cellular transporter expression profile and that modulation of the expression of multidrug resistance protein 5 (MRP5; ABCC5) influences the chemoresistance of these tumor cells. Here, we studied the influence of acute and chronic gemcitabine treatment on the expression of relevant uptake and export transporters in pancreatic carcinoma cells by reverse transcription-polymerase chain reaction (RT-PCR), quantitative RT-PCR, and immunoblot analyses. The specific role of MRP5 in cellular gemcitabine sensitivity was studied by cytotoxicity assays using MRP5-overexpressing and MRP5-silenced cells. Exposure to gemcitabine (12 nM for 3 days) did not alter the messenger RNA (mRNA) expression of MRP1, MRP3, MRP5, and equilibrative nucleoside transporter 1 (ENT1), whereas high dosages of the drug (20 µM for 1 hour) elicited up-regulation of these transporters in most cell lines studied. In cells with acquired gemcitabine resistance (up to 160 nM gemcitabine), the mRNA or protein expression of the gemcitabine transporters MRP5 and ENT1 was upregulated in several cell lines. Combined treatment with 5-FU and gemcitabine caused a 5- to 40-fold increase in MRP5 and ENT1 expressions. Cytotoxicity assays using either MRP5-overexpressing (HEK and PANC-1) or MRP5-silenced (PANC1/shMRP5) cells indicated that MRP5 contributes to gemcitabine resistance. Thus, our novel data not only on drug-induced alterations of transporter expression relevant for gemcitabine uptake and export but also on the link between gemcitabine sensitivity and MRP5 expression may lead to improved strategies of future chemotherapy regimens using gemcitabine in pancreatic carcinoma patients.
Introduction
Pancreatic cancer is the fourth most common cause of cancer-related death in the western world, with an estimated 35,000 deaths in 2009 in the United States [1]. Less than 20% of pancreatic cancer patients are diagnosed with resectable and potentially curable disease, whereas most patients have advanced disease at the time of diagnosis and hence a dismal prognosis [2]. Median survival of patients with advanced disease ranges around 6 months despite chemotherapy, mostly because of an almost complete chemoresistance.
Gemcitabine has been the standard systemic therapy for palliative treatment of pancreatic cancer for the last decade, although 1-year survival rates ranging around 18% remain unsatisfactory [3–5]. Despite the value of gemcitabine in improving clinical benefit, median survival, and 1-year survival, patients diagnosed with pancreatic carcinoma are still confronted with poor prognosis amounting to less than 5% survival beyond 5 years [2,3]. Treatment of pancreatic cancer patients with gemcitabine alone or in combination with other cytotoxic drugs is not curative, and the acquisition of resistance during chemotherapy may further limit the therapeutic success. Such resistance of human cells to the cytotoxic action of chemotherapeutic drugs can be the result of various mechanisms and cellular targets including microRNA, all of which have been identified to influence, as single factors or in combination, resistance to gemcitabine [6–12].
The chemotherapeutic effectiveness of gemcitabine requires not only efficient uptake of the drug into target cells and intracellular activation to its active triphosphatemetabolite before incorporation into DNA and RNA but also sufficient drug concentration and time before removal from intracellular compartments by export transporters. Cellular uptake of nucleosides such as the cytidine analog gemcitabine (2′,2′-difluorodeoxycytidine) across plasma membranes occurs primarily through specialized equilibrative or concentrative nucleoside transporters (ENTs or CNTs) [13]. Gemcitabine is taken up into cells with high affinity (Km = 18 µM) by CNT1 (gene symbol: SLC28A1) [14], by CNT3 (SLC28A3) [15], and also by ENT1 (SLC29A1) and ENT2 (SLC29A2) albeit with less affinity (ENT1, Km = 160 µM; ENT2, Km = 740 µM) [16]. In pancreatic tumor cells, ENT1 is expressed at high levels, whereas members of the CNT family are present only at negligible or at a low functional level [17]. Interestingly, both ENT1 and CNT1 expressions have previously been linked to gemcitabine resistance or sensitivity of pancreatic cancer cells [14,17–19]. Preliminary data indicated that export of gemcitabine or its phosphorylated metabolites into the extracellular space is mediated by multidrug resistance protein 5 (MRP5; gene symbol: ABCC5), a member of the ATP binding cassette (ABC) transporter family [20]. Among other ABC transporters, MRP3 (ABCC3), MRP4 (ABCC4), and MRP5 (ABCC5) are expressed in normal or diseased human pancreas [21–26] and have been demonstrated to confer resistance against chemotherapeutic drugs such as etoposide, 5-fluorouracil (5-FU), and gemcitabine [26–29]. Most importantly, recent studies showed that expression levels of the nucleoside uptake transporters ENT1 and CNT3 (SLC28A3) are predictive for patient survival times after gemcitabine treatment [30–33]. Conversely, the cellular expression profile of transporters can be altered by chemotherapeutic drugs [6,18,26,34]. Further, enhanced expression of ABC transporters seems to be characteristic for cells with cancer stem cell features [35]. We therefore investigated in this study whether acute or chronic treatment of pancreatic carcinoma cells with gemcitabine affects the expression levels of uptake and export transporters involved in gemcitabine action. Our results demonstrate 1) that gemcitabine alone or in combination with 5-FU at concentrations relevant for clinical chemotherapy regimens can alter uptake and export transporter expression in pancreatic cancer cells; 2) that acquired gemcitabine resistance is paralleled by upregulated or downregulated transporter expressions, dependent on the specific cell type; and 3) that MRP5 contributes to gemcitabine resistance, as demonstrated with MRP5-overexpressing and MRP5-silenced cells, that is, overexpression of MRP5 renders pancreatic cancer cells more resistant to gemcitabine, whereas silencing of MRP5 expression renders them more sensitive to gemcitabine.
Materials and Methods
Cells and Drugs
Parental HEK293 cells (HEK; ATCC, Manassas, VA) and established human pancreatic carcinoma cell lines (Capan-1, Capan-2, PANC-1, AsPC-1, BxPC-3, MiaPaCa-2, and PaTu-8902 (PaTu)) [36] were cultured in Dulbecco modified Eagle medium/Ham F-12 medium with l-glutamine (PAA, Pasching, Austria) and 10% fetal bovine serum (Invitrogen, Carlsbad, CA), gentamicin (50 µg/ml), and amphothericin B (0.25 µg/ml; PAA); in addition, the culture medium of MRP5-transfected, MRP5-overexpressing HEK293 cells (HEK/MRP5 cells; Millennium Pharmaceuticals, Cambridge, MA) contained geneticin (250 µg/ml). All cells were cultured at 37°C, 5% CO2, and 95% humidity. Gemcitabine was obtained from Eli Lilly (Indianapolis, IN), 5-FU was from Teva (Kirchzarten, Germany), doxycycline was from Sigma-Aldrich (St Louis, MO), and tetracycline-free fetal bovine serum was from Clontech (Mountain View, CA).
Generation of Overexpressing and Knockdown Cell Lines
PANC-1 cells stably overexpressing MRP5 (PANC-1/MRP5 cells) were obtained by transfection of parental PANC-1 cells with plasmid containing MRP5 complementary DNA (cDNA) using FuGENE HD transfection reagent (Roche, Mannheim, Germany) and hygromycin selection as reported [37]. The enhanced expression of MRP5 messenger RNA (mRNA) and protein in these PANC-1/MRP5 clones was checked by quantitative reverse transcription-polymerase chain reaction (QRT-PCR) and immunoblot, respectively [26]; MRP5 mRNA levels in PANC-1/MRP5 clones exceeded those of parental PANC-1 cells by more than 300-fold. For the targeted MRP5 knockdown, PANC-1 cells containing doxycycline-inducible short hairpin RNA (shRNA) targeting MRP5 mRNA (PANC-1/shMRP5 cells) were generated using the pSingle-tTS-shRNA vector (Clontech) containing one of three different oligonucleotides targeting MRP5 mRNA (target position within the MRP5 mRNA sequence [accession no. NM_005688]: 158–176, 368–386, or 547–565 bp). Selected PANC-1/shMRP5 clones were kept under geneticin (800 µg/ml) and were cultured in tetracycline-free medium. MRP5 silencing was checked by QRT-PCR using PANC-1/shMRP5 clones treated without or with doxycycline for 3 to 6 days with replenishment of doxycycline every 48 hours.
Drug Treatment of Cells
To establish gemcitabine-resistant cells, cells were adapted to increasing concentrations of gemcitabine (10–160 nM); for this, cells were exposed to each gemcitabine concentration for at least 14 days before the drug concentration was doubled in cultures of the surviving cells. For single short-time drug treatment, cells were treated with gemcitabine (12 nM) for 72 hours before RNA isolation. Alternatively, cells were incubated with gemcitabine (20 µM) for 1 hour, then the medium was replaced with fresh medium containing no gemcitabine, and RNA was isolated 72 hours later. In experiments comparing the effects of single or combined treatment of cells, the following schedules were applied: (a) 5-FU (30 µM) for 24 hours, then fresh drug-free medium; (b) gemcitabine (20 µM) for 1 hour, then fresh drug-free medium; and (c) 5-FU (30 µM) for 24 hours, then gemcitabine (20 µM) for 1 hour, then fresh drug-free medium. RNA isolation was performed at the times indicated in the Results section.
Antibodies
The rat monoclonal antibody M5I-1 against human MRP5 was from Kamiya (Seattle, WA), the mouse monoclonal antibody NCL-MRP3 against human MRP3 was from Novocastra (Newcastle, UK), and the mouse monoclonal anti-β-actin antibody (AC-15) was from Sigma-Aldrich. The secondary antibodies conjugated to horseradish peroxidase were obtained from Pierce (Rockford, IL) and Dianova (Hamburg, Germany).
Immunoblot
Membrane samples were prepared for electrophoresis as reported [26,38] and subjected to SDS-PAGE on 7.5% polyacrylamide gels. For one particular cell line, equal amounts of membrane proteins were loaded per lane. Protein determination was performed using BSA as standard [39]. After electrophoresis, the separated proteins were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA) and immunoblotted using chemiluminescence for detection (SuperSignal West Femto; Pierce). The primary antibodies were used as follows: anti-MRP5 (M5I-1) and anti-MRP3 (NCL-MRP3), 1:1000; anti-β-actin, 1:10,000. The horseradish peroxidase-conjugated secondary antibodies were used at a dilution of 1:2000. The half-life times of MRP5 and MRP3 protein in pancreatic cancer cells and HEK-MRP5 cells, respectively, were determined by immunoblot using membranes from cells treated with tunicamycin for 0 to 72 hours as reported [23]. Quantification and normalization of the immunoblot signals were performed by densitometric analysis on at least duplicate immunoblots each from two independent experiments using Alpha Imager software (Alpha Innotech Corporation, San Leandro, CA).
RT-PCR and QRT-PCR Analyses
Total RNA was isolated from cells using the RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Hilden, Germany) and analyzed by RT-PCR as reported [26]. QRT-PCR was performed in an Mx3000P (Stratagene, Amsterdam, the Netherlands) using ABsolute QPCR SYBR Green Mix (Abgene, Epsom, UK) in a total volume of 20 µl including 5 µl of the synthesized single-stranded cDNA with conditions and quality control of amplified products as described [26]. The relative amounts of target gene mRNA expression compared with the pancreatic housekeeping gene RPL13A as reference gene were calculated using the ΔΔCt method. Each QRT-PCR analysis was performed at least in duplicate technical replicas from at least duplicate biological samples. The primers specific for RPL13A, MRP1 (ABCC1), MRP3, and MRP5, their sequences, positions, and length of the respective amplified fragment were as reported [25,26]. Other specific sense and antisense primers used for amplification and designed using Primer3 software were as follows: ENT1 (gene symbol: SLC29A1; accession no. NM_004955), 5′-AGTGGCTCGGAGCTATCAGA-3′ and 5′ GTGCTCGAAGACCACAGTCA-3′ (588-bp fragment, bases 918-1505); CNT3 (gene symbol: SLC28A3; accession no. NM_022127), 5′-ATGAATTCAGCCCTGTCCTG-3′ and 5′-AAACGTGATGGCAGTTGATG-3′ (484-bp fragment, bases 1482–1965); MDR-1 (gene symbol: ABCB1; accession no. NM_000927), 5′-TGGAGGAGCAAAGAAGAAGAAC-3′ and 5′-GCAGCCAAAGTTCCCACCAC-3′ (150-bp fragment, bases 442–591). Specificity of PCR was checked by agarose gel electrophoretic analysis of amplicons.
Cytotoxicity Studies
Cells (parental HEK, HEK/MRP5, PANC-1, PANC-1/MRP5, or PANC-1/shMRP5 cells) were seeded into 96-well plates at a density of 3000 cells per well. One day later, medium was replaced by fresh medium containing gemcitabine at the indicated concentration (range, 0–10 µM). All experimental incubation conditions were performed with at least duplicate biological samples and triplicate technical samples. Cell viability was determined after 3 days (HEK cells) or 6 days (PANC-1 cells) of continuous drug treatment using the WST-1 cell proliferation assay (Roche) and evaluated as described [26]. For the determination of gemcitabine cytotoxicity in stably MRP5-silenced PANC-1/shMRP5 cells, cells were treated for 6 days without or with doxycycline (100 ng/ml), trypsinized, seeded into 96-well plates (3000 cells/well), and exposed to gemcitabine for another 6 days before WST-1 assay; during the whole experiment, doxycycline was replenished every 48 hours.
Cell Proliferation Rate
Population doubling time was determined during the exponential growth phase of unsynchronized monolayer cultures. Briefly, 2000 cells/well were seeded onto 96-well plates in growth medium. Cell proliferation was indirectly assessed using the WST-1 assay as indicated previously, measuring cell numbers every 24 hours for 3 days. Previous calibration of the assay had indicated a linear correlation between color development and cell numbers in the range of 100 to 20,000 cells.
Results and Discussion
Chemotherapy in human pancreatic carcinoma patients reaches plasma concentrations of gemcitabine approximating 20 to 90 µM sufficient to maximize the rate of intracellular gemcitabine triphosphate accumulation [40]. However, the sensitivity of human pancreatic carcinoma cell lines to the cytotoxic effect of gemcitabine in vitro has been reported to vary over a wide range of drug concentration, with half-maximal inhibitory concentration (IC50) values ranging from few nanomolars to several micromolars, even for one particular cell line (IC50 values: [a] gemcitabine continuously on cells for 72 hours: Capan-1 = 11.5 nM, Capan-2 = 12 nM, AsPC-1 = 14.6 nM, BxPC-3 = 18 nM, MiaPaCa-2 = 36–40 nM, PANC-1 = 50 nM; [b] gemcitabine for 1 hour on cells: MiaPaCa-2 = 11.0 µM, Capan-1 = 18.1 µM, PANC-1 = 160.5 µM) [41–44]. We hypothesized that treatment of pancreatic cancer cells with gemcitabine at a cytotoxic concentration might elicit a rescue response in the surviving cells allowing them to better tolerate gemcitabine. We incubated several pancreatic carcinoma cell lines (AsPC-1, BxPC-3, MiaPaCa-2, Capan-1, and PANC-1) with 12 nM gemcitabine, which represents a drug concentration in the IC50 range for some cell lines. Such gemcitabine treatment for 3 days did not elicit any significant alterations in the mRNA expression levels of exporters MRP1, MRP3, or MRP5 or the relevant uptake transporter ENT1 in the pancreatic carcinoma cell lines studied (Figure 1).
Figure 1.
Influence of continuous gemcitabine exposure on transporter expression. Cells were incubated with gemcitabine (12 nM) for 72 hours before isolation of RNA. The individual expression levels of the indicated transporters were analyzed by QRT-PCR, normalized to mRNA expression of RPL13A as pancreatic housekeeping gene, and shown as x-fold relative to the normalized expression of the respective target gene in untreated control cells (set as 1). Values are means ± SD from at least duplicate biological and technical samples.
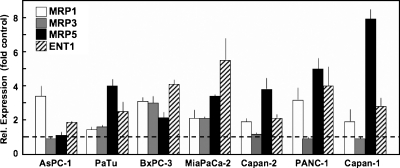
In contrast, short-time incubation of pancreatic carcinoma cells with gemcitabine in a setting more closely resembling the chemotherapy situation in patients in vivo (20 µM gemcitabine for 1 hour) caused up-regulation of most transporters studied (Figure 2). Interestingly, the most prominent up-regulation by gemcitabine in this regimen was observed for MRP5 and ENT1 (ca. six- to eight-fold, respectively), both of which are transporters mediating gemcitabine uptake or export of its metabolites in pancreatic carcinoma cells [16,17,20]. An analogous effect on pancreatic ENT1 expression has been demonstrated after 5-FU treatment [18,45]. In contrast, MRP3 expression either was not affected (AsPC-1, Capan-1, Capan-2, and PANC-1) or was only slightly upregulated (PaTu, MiaPaCa-2, and BxPC-3 cells; Figure 2). Thus, most pancreatic cancer cell lines studied reacted to the acute treatment with a high dose of gemcitabine in a manner that would, by increasing MRP5 expression, diminish the potential chemotherapeutic action of the drug by accelerating its elimination from intracellular compartments. Conversely, the concomitantly increased ENT1 expression would allow the enhanced uptake of nucleosides into these cells.
Figure 2.
Alterations in transporter mRNA expression after short-time exposure to gemcitabine. After gemcitabine treatment (20 µM for 1 hour), the indicated pancreatic carcinoma cell lines were grown for 72 hours in drug-free medium before isolation of RNA. Transporter mRNA expression was analyzed by QRT-PCR as in Figure 1. Values are means ± SD from at least duplicate biological and technical samples.
We next addressed the problem of acquired resistance that pancreatic tumors are known to develop during chemotherapy with gemcitabine [46,47]. To this end, we mimicked the clinical situation of drug-induced resistance by analyzing gemcitabine-resistant pancreatic cancer cell lines adapted to and maintained in increasing concentrations of the drug. Compared with the untreated parental cell line, some cells with such acquired resistance to 160 nM gemcitabine showed increased MRP5 and ENT1 expression (AsPC-1 and MiaPaCa-2; Figure 3), which is in line with earlier reports on ENT1 mRNA levels in different cell lines [6]. In other gemcitabine-resistant cells, we found only MRP5 (PaTu) or ENT1 (BxPC-3) increased, whereas gemcitabine-resistant Capan-1 cells demonstrated no change in MRP5 and even a down-regulation of ENT1 expression (Figure 3). Because mRNA expression does not necessarily correspond to the functional protein level of a gene transcript, we checked by immunoblot the MRP5 protein expression in parental cells and in cells adapted to 40 and 160 nM gemcitabine (Figure 4); the half-life time of MRP5 and MRP3 protein in pancreatic cancer cells was likewise determined to be 35 and 40 hours, respectively. In line with the mRNA expression data (Figure 3), MRP5 protein levels normalized to β-actin were slightly upregulated in gemcitabine-resistant Capan-1 cells, were unaffected or downregulated in correspondingly treated BxPC-3 cells, and were 3- to 3.5-fold upregulated in AsPC-1 and PaTu cells grown in medium containing 160 nM gemcitabine (Figure 4). Thus, similar to the observed heterogeneous and cell line-specific alterations in transporter expression of gemcitabine-resistant pancreatic cells in vitro, individual human pancreatic tumors in vivo may react very differently to acute gemcitabine treatment and during development of gemcitabine resistance with respect to expression of relevant transporters involved in gemcitabine transport.
Figure 3.
MRP5 and ENT1 mRNA expression in cells with acquired gemcitabine resistance. RNA was extracted from untreated control cells (open bars) or from cells stepwise adapted to and cultured for at least 14 days in medium containing 160 nM gemcitabine (filled bars). Normalized mRNA expression levels for MRP5 (upper panel) and ENT1 (lower panel) were analyzed by QRT-PCR as in Figure 1. Values are means from at least duplicate biological and technical samples.
Figure 4.
MRP5 protein expression in cells with acquired gemcitabine resistance. Indicated pancreatic cancer cell lines were adapted to increasing concentrations of gemcitabine as described in the Materials and Methods section. After culturing the cells at the indicated gemcitabine concentration for at least 14 days, membrane fractions were isolated from these cells and subjected to immunoblot analysis for MRP5 and β-actin. MRP5 protein expression was normalized to the corresponding immunoblot signal for β-actin on the same PVDF membrane. Representative immunoblot analysis each performed twice on samples from two independent experiments.
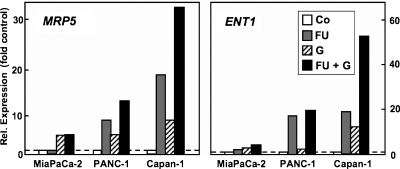
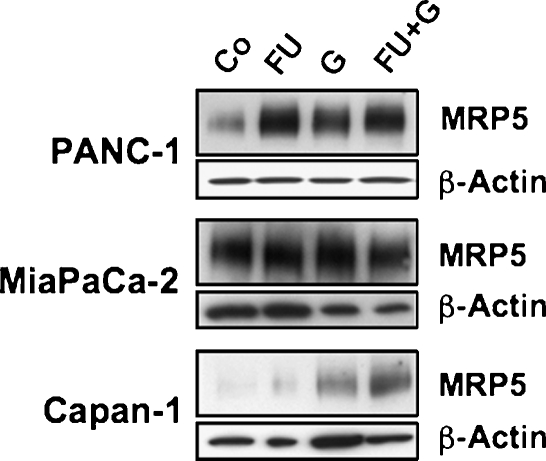
Several chemotherapy regimens using gemcitabine together with other drugs have been developed, some of them combining gemcitabine with 5-FU and resulting in improved benefit [48–50]. Interestingly, studies with human pancreatic cancer cells in vitro [18] or in a murine xenograft model [45] demonstrated an improved therapeutic effect when 5-FU was administered before gemcitabine. Further, treatment of cells with 5-FU alters the expression of nucleoside and ABC transporters [18,26,51]. We were thus interested to see how the transporter profile of pancreatic cancer cells was influenced by the combination of these chemotherapeutic drugs. Because MRP5 and ENT1 have been suggested or characterized as export and import transporters of 5-FU and gemcitabine or their metabolites [14,20,52], we analyzed the expression of these two transporters. Our QRT-PCR analyses demonstrated that sequential treatment of three different pancreatic cancer cell lines by 5-FU followed by gemcitabine elicited a marked additive up-regulation of both MRP5 and ENT1 within 4 days (Figure 5). As shown for MRP5 in PANC-1 cells, the increase in transporter expression was noticeable 2 days after start of drug treatment and was maximal within 4 days (Figure 6). Whereas single drug treatment with 5-FU or gemcitabine alone also elicited the enhanced expression of MRP5 and ENT1 (Figure 5), this up-regulation was highest in all cases after combined drug administration and was most prominent in Capan-1 and PANC-1 cells: in Capan-1 cells, the drug combination caused a 32.8 ± 10.2-fold (mean ± SEM) increase of MRP5 and a 52.4 ± 11.7-fold rise in ENT1 expression compared with control cells (Figure 5); interestingly, the expression of the nucleoside transporter CNT3 increased more than 100-fold in 5-FU/gemcitabine-treated Capan-1 cells, whereas concomitantly MDR-1 was elevated only about 5-fold (data not shown). In PANC-1 cells, MRP5 was elevated 13.7 ± 4.3-fold, and ENT1 was elevated 17.4 ± 8.7-fold after 5-FU/gemcitabine treatment (Figure 5). MiaPaCa-2 cells also reacted correspondingly albeit weaker to this drug regimen, with MRP5 expression rising 5.2 ± 0.1-fold and ENT1 by 1.6 ± 0.3-fold compared with untreated control cells (Figure 5). We also checked whether the observed enhanced transporter mRNA expression after 5-FU and gemcitabine resulted in elevated cellular protein levels of the drug transporters. As demonstrated by MRP5 immunoblot, the up-regulation of MRP5 mRNA expression was paralleled by a correspondingly enhanced expression of MRP5 protein, which again was most prominent in PANC-1 and Capan-1 cells after single or combined drug treatment (Figure 7). Thus, our data indicate that the cytotoxic action of gemcitabine, 5-FU, and a combination of both drugs elicits a strongly increased expression of the export pump MRP5, which diminishes the chemotherapeutic efficiency of these drugs by allowing a faster drug detoxification through enhanced excretion by MRP5 from pancreatic carcinoma cells. However, the concomitant up-regulation of the uptake transporters CNT3 and ENT1 may support cell survival by improving their import capacity for physiologic nucleobases and nucleosides but can also improve the bioavailability and thus the chemotherapeutic efficacy of gemcitabine by modulation the expression of its main uptake transporter ENT1 [53]. This interpretation is in line with an earlier study that had demonstrated superior chemotherapeutic benefit by using 5-FU administration followed by gemcitabine treatment in mice in vivo [45]. Further studies will investigate the potential signaling pathways involved in this drug-evoked up-regulation of transporter expression such as altered transcription factor levels or promoter induction through epigenetic mechanisms such as DNA demethylation or histone deacetylation [54,55].
Figure 5.
Individual and combined effects of 5-FU and gemcitabine on transporter expression. Treatment of indicated pancreatic cancer cells was as follows: FU: 30 µM5-FU, 24 hours (gray bars); G: 20 µM gemcitabine, 1 hour (hatched bars); FU + G: 30 µM 5-FU, 24 hours, followed by 20 µM gemcitabine, 1 hour (black bars). RNA from treated or untreated control cells (Co; empty bars) was extracted 4 days after start of 5-FU or 3 days after gemcitabine treatment, respectively. Expression of MRP5 (left panel) and ENT1 mRNA (right panel) was analyzed and normalized by QRT-PCR as in Figure 1. Data represent mean values from three independent experiments, each with treatment conditions performed in duplicate, and all technical samples are analyzed at least in duplicate.
Figure 6.
Time course of MRP5 induction by 5-FU and gemcitabine in PANC-1 cells. Cells were exposed to 30 µM 5-FU for 1 day, followed by treatment with 20 µM gemcitabine for 1 hour. RNA was extracted from cells before treatment or at the indicated time points after start of drug treatment. Expression of MRP5 mRNA was analyzed and normalized by QRT-PCR as in Figure 1. Insert shows agarose gel electrophoretic analyses and ethidium bromide staining of amplicons for MRP5 and RPL13A after RT-PCR.
Figure 7.
MRP5 protein expression after 5-FU and gemcitabine treatment in pancreatic cancer cells. Immunoblot detection of MRP5 and β-actin was performed with membranes from untreated control cells (Co) or from cells treated with 5-FU (FU), gemcitabine (G), or with 5-FU followed by gemcitabine (FU + G) as described in Figure 5. Representative immunoblot analysis each performed twice on samples from two independent experiments.
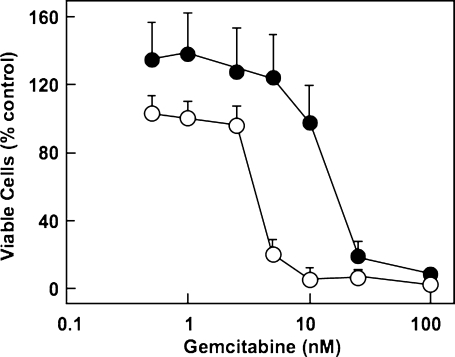
Because drug export through MRP transporters has been demonstrated to contribute to cellular resistance against various chemotherapeutic compounds [26–29,56–58], we tested the suggested ability of MRP5 to affect chemoresistance against gemcitabine [20,29,59]. To this end, we compared the gemcitabine sensitivity of parental HEK cells with transfected HEK cells stably overexpressing human MRP5 (HEK/MRP5 cells). The endogenous MRP5 protein expression in parental HEK cells is low, whereas HEK/MRP5 cells contain at least 10-fold more MRP5 protein, as estimated from the immunoblot signal. QRT-PCR analysis indicated that MRP5 mRNA expression in HEK/MRP5 cells is increased approximately 410 ± 49-fold (mean ± SD) compared with that in parental HEK cells (control), whereas the expressions of ENT1 and CNT3 are almost identical in these two cell lines (ENT1 = 0.9- and CNT3 = 1.4-fold control). Our cytotoxicity studies with these cells showed that MRP5-overexpressing HEK/MRP5 cells indeed showed increased resistance against gemcitabine (IC50 = 45 nM; Figure 8, upper panel), compared with parental HEK cells (IC50 = 15 nM; Figure 8, upper panel). Such altered gemcitabine resistance due to different MRP5 expression levels was also observed in PANC-1 cells overexpressing MRP5 (PANC-1/MRP5 cells; Figure 8, lower panel). Compared with parental PANC-1 cells (IC50 = 9 nM), the IC50 value for gemcitabine in PANC-1/MRP5 cells amounts to ca. 200 nM (Figure 8). Because the cytotoxic action of gemcitabine depends on cell proliferation, we determined whether the gemcitabine-resistant PANC-1/MRP5 cells differ in their growth rate from parental PANC-1 cells. However, no significant difference in the population doubling time between PANC-1 and PANC-1/MRP5 cells was observed when cells were grown as unsynchronized monolayer cultures (population doubling time: 40 and 38 hours for PANC-1 and PANC-1/MRP5 cells, respectively). Thus, the marked difference in gemcitabine sensitivity between PANC-1 and PANC-1/MRP5 cells is not due to a corresponding difference in cell proliferation rates.
Figure 8.
MRP5 and gemcitabine chemosensitivity of HEK and PANC-1 cells. Parental HEK cells (open triangles, upper panel) and MRP5-overexpressing HEK/MRP5 cells (filled triangles, upper panel) or PANC-1 cells (open circles, lower panel) and MRP5-overexpressing PANC-1/MRP5 cells (filled circles, lower panel) were subjected to gemcitabine (HEK: 0–100 nM for 4 days; PANC-1: 0–10 µM for 6 days) before cytotoxicity assay using WST-1. Values are means from two independent experiments performed with each condition in triplicate; bars indicate SD.
Next, we investigated the role of MRP5 in gemcitabine resistance by silencing this transporter in PANC-1 cells by specific RNA interference. We used three different shRNA oligonucleotides, each targeting specific regions of the MRP5 mRNA sequence. Judging from the QRT-PCR analyses, we achieved at best an 80% knockdown of MRP5 mRNA in stably transfected PANC-1/shMRP5 cells after doxycycline-induced silencing. Such MRP5-silenced, doxycycline-treated PANC-1/shMRP5 cells indeed showed an increased chemosensitivity toward gemcitabine (IC50 = 4 nM; Figure 9) compared with their untreated counterpart (PANC-1/shMRP5 cells: IC50 = 20 nM). Thus, our experimental data analyzing gemcitabine sensitivity in cells with either increased or diminished expression of MRP5 suggest that this ABC transporter contributes to gemcitabine resistance in pancreatic cancer cells. This is in line with data from gemcitabine-sensitive lung cancer cells showing a correlation between MRP5 expression and gemcitabine action [29].
Figure 9.
Gemcitabine chemosensitivity in MRP5-silenced PANC-1/shMRP5 cells. PANC-1 cells containing doxycycline-inducible shRNA targeting MRP5 mRNA (PANC-1/shMRP5 cells) were treated with doxycycline (100 ng/ml; open circles) or vehicle (filled circles) for 6 days and exposed to indicated gemcitabine concentrations for another 6 days before determination of cell viability using WST-1 assay. Values are means from triplicate samples; bars indicate SD.
In conclusion, our studies showed 1) that chemotherapeutic treatment of pancreatic cancer cells with gemcitabine alone or in combination with 5-FU at concentrations relevant for chemotherapy regimens can change the expression levels of transporters that are involved in gemcitabine uptake or elimination; 2) that acquired gemcitabine resistance is paralleled by upregulated or downregulated transporter expressions, dependent on the specific cell type; and 3) that the multidrug resistance protein MRP5 represents an additional and novel entity among the various cellular factors influencing gemcitabine resistance of pancreatic cancer. Thus, the efficacy of future chemotherapeutic regimens using gemcitabine and/or 5-FU may benefit from taking into account the observed alterations of relevant transporter expression levels induced by the chemotherapeutic drugs. Particularly, the development and use of specific inhibitors of MRP5 may be an important aim for this purpose. This may hold true especially for pancreatic tumor cells with known gemcitabine resistance, where, for example, inhibition of nuclear factor κB was shown to be ineffective [60]. In addition, the observed heterogeneous response of different human pancreatic cancer cells regarding their MRP5 transporter expression after gemcitabine treatment may reflect the heterogeneous response pattern in vivo and would argue for an individualized screening test before chemotherapy in patients to improve the efficacy of the intended treatment.
Acknowledgments
The authors thank the excellent technical assistance of Vera Mickler, Inga Kohler, and Swetlana Sander-Naderi. The MRP5 cDNA was kindly provided by Dr Gabriele Jedlitschky, Greifswald.
Abbreviations
- ABC
ATP binding cassette
- CNT
concentrative nucleoside transporter
- ENT
equilibrative nucleoside transporter
- 5-FU
5-fluorouracil
- MRP
multidrug resistance protein
- (Q)RT-PCR
(quantitative) reverse transcription-polymerase chain reaction
Footnotes
This study was supported by a grant from the Dr Hans und Lore Graf Stiftung (J.M.L.).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Löhr M. Is it possible to survive pancreatic cancer? Nat Clin Pract. 2006;3:236–237. doi: 10.1038/ncpgasthep0469. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin Oncol. 2007;34:347–353. doi: 10.1053/j.seminoncol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly EM. Pancreatic adenocarcinoma: new strategies for success. Gastrointest Cancer Res. 2009;3:S11–S15. [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liau SS, Whang E. HMGA1 is a molecular determinant of chemoresistance to gemcitabine in pancreatic adenocarcinoma. Clin Cancer Res. 2008;14:1470–1477. doi: 10.1158/1078-0432.CCR-07-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu WS, Yan HJ, Qin RY, Tian R, Wang M, Jiang JX, Shen M, Shi CJ. siRNA directed against survivin enhances pancreatic cancer cell gemcitabine chemosensitivity. Dig Dis Sci. 2009;54:89–96. doi: 10.1007/s10620-008-0329-4. [DOI] [PubMed] [Google Scholar]
- 9.Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 10.Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 11.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 12.Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010;70:1130–1140. doi: 10.1158/0008-5472.CAN-09-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26:85–110. doi: 10.1007/s10555-007-9044-4. [DOI] [PubMed] [Google Scholar]
- 14.Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, Crawford CR, Cass CE. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349–4357. [PubMed] [Google Scholar]
- 15.Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, Ritzel RG, Mowles DA, Carpenter P, Chen XZ, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside co-transporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) J Biol Chem. 2001;276:2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- 16.Mackey JR, Yao SY, Smith KM, Karpinski E, Baldwin SA, Cass CE, Young JD. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst. 1999;91:1876–1881. doi: 10.1093/jnci/91.21.1876. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2′,2′-difluorodeoxycytidine-induced cytotoxicity. Clin Cancer Res. 2003;9:5000–5008. [PubMed] [Google Scholar]
- 18.Rauchwerger DR, Firby PS, Hedley DW, Moore MJ. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000;60:6075–6079. [PubMed] [Google Scholar]
- 19.Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3′-deoxy-3′-fluorothymidine. Biochem Pharmacol. 2010;79:587–595. doi: 10.1016/j.bcp.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JD, Ma L, Iverson PW, Lesoon A, Jin S, Horwitz L, Gallery M, Slapak CA. Human multi-drug resistance protein 5 (MRP5) confers resistance to gemcitabine. Proc Am Assoc Cancer Res. 2002;43:3868. [Google Scholar]
- 21.Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3) FEBS Lett. 1998;433:149–152. doi: 10.1016/s0014-5793(98)00899-0. [DOI] [PubMed] [Google Scholar]
- 22.König J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29:1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 23.Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 25.König J, Hartel M, Nies AT, Martignoni ME, Guo J, Büchler MW, Friess H, Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115:359–367. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 26.Hagmann W, Jesnowski R, Faissner R, Guo C, Löhr JM. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9:136–144. doi: 10.1159/000178884. [DOI] [PubMed] [Google Scholar]
- 27.Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P. Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3) J Biol Chem. 2001;276:46400–46407. doi: 10.1074/jbc.M107041200. [DOI] [PubMed] [Google Scholar]
- 28.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, III, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 29.Oguri T, Achiwa H, Sato S, Bessho Y, Takano Y, Miyazaki M, Muramatsu H, Maeda H, Niimi T, Ueda R. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther. 2006;5:1800–1806. doi: 10.1158/1535-7163.MCT-06-0025. [DOI] [PubMed] [Google Scholar]
- 30.Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 31.Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, Orlandini C, Boggi U, Campani D, Del Chiaro M, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–3935. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 32.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 33.Marechal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, Cass CE, Young J, Salmon I, Deviere J, et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913–2919. doi: 10.1158/1078-0432.CCR-08-2080. [DOI] [PubMed] [Google Scholar]
- 34.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 35.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 36.Löhr M, Schmidt C, Ringel J, Kluth M, Müller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 37.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 38.Hagmann W, Nies AT, König J, Frey M, Zentgraf H, Keppler D. Purification of the human apical conjugate export pump MRP2. Reconstitution and functional characterization as substrate-stimulated ATPase. Eur J Biochem. 1999;265:281–289. doi: 10.1046/j.1432-1327.1999.00735.x. [DOI] [PubMed] [Google Scholar]
- 39.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 41.Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–362. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 42.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307–2318. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- 43.Giovannetti E, Mey V, Danesi R, Mosca I, Del Tacca M. Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res. 2004;10:2936–2943. doi: 10.1158/1078-0432.ccr-03-0520. [DOI] [PubMed] [Google Scholar]
- 44.Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, Fujii Y, Endo I, Togo S, Danenberg PV, et al. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007;17:1201–1205. [PubMed] [Google Scholar]
- 45.Tsujie M, Nakamori S, Nakahira S, Takeda S, Takahashi Y, Hayashi N, Okami J, Nagano H, Dono K, Umeshita K, et al. Schedule-dependent therapeutic effects of gemcitabine combined with uracil-tegafur in a human pancreatic cancer xenograft model. Pancreas. 2006;33:142–147. doi: 10.1097/01.mpa.0000226882.48204.26. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 48.El-Rayes BF, Philip PA. Systemic therapy for advanced pancreatic cancer. Expert Rev Anticancer Ther. 2002;2:426–436. doi: 10.1586/14737140.2.4.426. [DOI] [PubMed] [Google Scholar]
- 49.Kulke MH. Advanced pancreatic cancer: is there a role for combination therapy? Expert Rev Anticancer Ther. 2003;3:729–739. doi: 10.1586/14737140.3.5.729. [DOI] [PubMed] [Google Scholar]
- 50.Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Metaanalysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pressacco J, Wiley JS, Jamieson GP, Erlichman C, Hedley DW. Modulation of the equilibrative nucleoside transporter by inhibitors of DNA synthesis. Br J Cancer. 1995;72:939–942. doi: 10.1038/bjc.1995.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratt S, Chen V, Perry WI, III, Starling JJ, Dantzig AH. Kinetic validation of the use of carboxydichlorofluorescein as a drug surrogate for MRP5-mediated transport. Eur J Pharm Sci. 2006;27:524–532. doi: 10.1016/j.ejps.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Torras S, Garcia-Manteiga J, Mercade E, Casado FJ, Carbo N, Pastor-Anglada M, Mazo A. Adenoviral-mediated overexpression of human equilibrative nucleoside transporter 1 (hENT1) enhances gemcitabine response in human pancreatic cancer. Biochem Pharmacol. 2008;76:322–329. doi: 10.1016/j.bcp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Kantharidis P, El-Osta A, de Silva M, Wall DM, Hu XF, Slater A, Nadalin G, Parkin JD, Zalcberg JR. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin Cancer Res. 1997;3:2025–2032. [PubMed] [Google Scholar]
- 55.Hauswald S, Duque-Afonso J, Wagner MM, Schertl FM, Lubbert M, Peschel C, Keller U, Licht T. Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clin Cancer Res. 2009;15:3705–3715. doi: 10.1158/1078-0432.CCR-08-2048. [DOI] [PubMed] [Google Scholar]
- 56.König J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 57.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflügers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y, König J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- 59.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 60.Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, Lopez-Berestein G, Vivas-Mejia PE, Sood AK, McConkey DJ, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]