Abstract
Objectives:
This study compares the effects of an integrated yoga program with brief supportive therapy on distressful symptoms in breast cancer outpatients undergoing adjuvant radiotherapy.
Materials and Methods:
Eighty-eight stage II and III breast cancer outpatients were randomly assigned to receive yoga (n = 44) or brief supportive therapy (n = 44) prior to their radiotherapy treatment. Intervention consisted of yoga sessions lasting 60 min daily while the control group was imparted supportive therapy once in 10 days during the course of their adjuvant radiotherapy. Assessments included Rotterdam Symptom Check List and European Organization for Research in the Treatment of Cancer—Quality of Life (EORTC QoL C30) symptom scale. Assessments were done at baseline and after 6 weeks of radiotherapy treatment.
Results:
A GLM repeated-measures ANOVA showed a significant decrease in psychological distress (P = 0.01), fatigue (P = 0.007), insomnia (P = 0.001), and appetite loss (P = 0.002) over time in the yoga group as compared to controls. There was significant improvement in the activity level (P = 0.02) in the yoga group as compared to controls. There was a significant positive correlation between physical and psychological distress and fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, and constipation. There was a significant negative correlation between the activity level and fatigue, nausea and vomiting, pain, dyspnea, insomnia, and appetite loss.
Conclusion:
The results suggest beneficial effects with yoga intervention in managing cancer-and treatment-related symptoms in breast cancer patients.
Keywords: Breast cancer, meditation, stress, symptom distress, yoga
INTRODUCTION
The diagnosis and treatment of breast cancer can pose a considerable amount of physical, psychological, and emotional distress,[1‐3] and affects about 80% of the patients during initial stages of their treatment.[3] Though advancements in the treatment of cancer have improved survival rates in cancer patients, they have to endure distressing symptoms for a longer time than ever before. Patients with breast cancer normally receive multimodal treatment over a long period of time[4,5] and experience a multitude of symptoms that grossly affect their overall quality of life and survival. As patients live longer with cancer, concern is growing about both the health-related quality of life of those diagnosed with cancer and the quality of care they receive. Primary care providers, specialists, other health care providers, patients, and families all have an important role in symptom management throughout the course of cancer. Therefore, using interventions that help alleviate distressful symptoms and improve quality of life as an add-on to conventional treatments are recommended as a cohesive strategy to mitigate this problem.[6]
Symptoms are perceived indicators of a change in healthy functioning as experienced by patients.[7] They are multidimensional, having subjective, perceptional, and experiential characteristics.[8,9] These characteristics include both the physiologic sensations that signal patients that some internal condition is different and the interpretive processes that motivate patients to construct meanings for the symptoms and decide how to respond to them.[10] The perceived discomfort experienced in relation to the symptom can lead to anxiety and depression if patients are unable to cope with this distress. This can significantly impact patient outcomes in terms of quality of life, functional and emotional status, compliance to treatment, self-care and management, mortality, and morbidity.[11‐13] Therefore adopting symptom management strategies that reduce symptom experience can significantly benefit the patient’s activity, functioning, and health outcomes. Although research is producing new insights into the causes of and cures for cancer, efforts to manage the symptoms of the disease and its treatment have not kept pace. Evidence suggests that pain is frequently undertreated. Patients and health care providers have reported depression and a persistent lack of energy as the aggressiveness of therapy has been increased and/or the underlying malignancy has worsened. The challenge is to increase awareness of the importance of recognizing and actively addressing cancer-related symptoms when they occur. Specifically, we need to be able to identify who is at risk for cancer-related pain, depression, and/or fatigue; what treatments work best to address these symptoms when they occur; and how best to deliver interventions across the continuum of care.[6]
Various psychosocial interventions and behavioral approaches have been used over the decades to alleviate distress and reduce distressing side effects or symptoms in these populations. [14‐19] Preliminary findings suggest beneficial effects for yoga in improving mood, coping, adjustment, and decrease in distressful symptoms such as loss of appetite and fatigue in cancer patients. However, subjects in these studies were a heterogeneous mix of cancer patients in varying stages of disease and treatment. Since cancer patients experience a multitude of symptoms of varying clusters at various stages of their disease and conventional treatment, it is difficult to ascertain if yoga intervention is beneficial in reducing a group of symptoms that are specific to the treatment and stage of their disease. It has also been shown that most of these symptoms may persist even after completion of treatment thereby impacting their quality of life. Managing these symptoms effectively during the early course of treatment has been shown to improve patient outcomes during later stages of their disease.
In this study, we compare the effects of “integrated yoga program” with “brief supportive therapy” on symptom control in early operable breast cancer patients undergoing adjuvant radiotherapy.
MATERIALS AND METHODS
Though the primary objective in our study was to evaluate effects of yoga on quality of life, the secondary objective was to evaluate its effects on symptom control. In this paper, we focus on effects of yoga in managing distressful symptoms in early breast cancer patients receiving adjuvant radiotherapy.
Subjects
Eighty-five recently diagnosed women with stage II and III breast cancer from two different urban cancer centers were recruited for this study over a 2-year period from January 2004 to June 2006. All subjects had undergone primary treatment as surgery and were receiving adjuvant radiotherapy. Patients were eligible to participate in this study if they met the following selection criteria at the start of the study: (i) women with recently diagnosed operable breast cancer, (ii) age between 30 and 70 years, (iii) Zubrod’s performance status 0-2 (ambulatory >50% of time), (iv) high-school education, and (v) written consent to participate in the study. Subjects were excluded if they had (i) any concurrent medical condition that was likely to interfere with the treatment, (ii) major psychiatric, neurological illness or autoimmune disorders, and (iii) any known metastases. Each study participant was prescribed adjuvant radiotherapy with a cumulative dose of 50.4 Gy with fractionations spread over 6 weeks. The details of the study were explained to the participants and their informed consent was obtained in writing.
Randomization
Of the 103 eligible participants, 88 (85.4%) consented to participate and were randomized to receive yoga (n = 44) or supportive therapy (n = 44) initially before intervention (prior to radiotherapy) using computer-generated random numbers. Randomization was performed using concealed allocation protocol into two study arms. Personnel who had no part in the trial performed randomization.
Sample size
The primary hypothesis of the study was to evaluate the effects of yoga intervention on the improvement in quality-of-life measures. Earlier study with Mindfulness-Based Stress Reduction Program (MBSR) had shown a modest effect size (ES = 0.38) on EORTC QoL C30 global quality-of-life measure. We used G power to calculate the sample size with α = 0.05 and β = 0.2, and the above-mentioned effect size of 0.38 for repeated-measures ANOVA between factor effects. The sample size thus required was n = 44 in each group.
Among the 88 participants, 75 (yoga, n = 42; control, n = 33) completed their prescribed radiation therapy of 6 weeks and follow-up assessment. There were 13 dropouts in the study [Figure 1]. The reasons for dropouts were migration to other hospitals (n = 4), use of other complementary therapies (e.g., homeopathy or ayurveda) (n = 2), refusal to continue the study (n = 2), time constraints (n = 4), and other concurrent illnesses such as infections delaying radiotherapy and intervention (n = 1).
Figure 1.
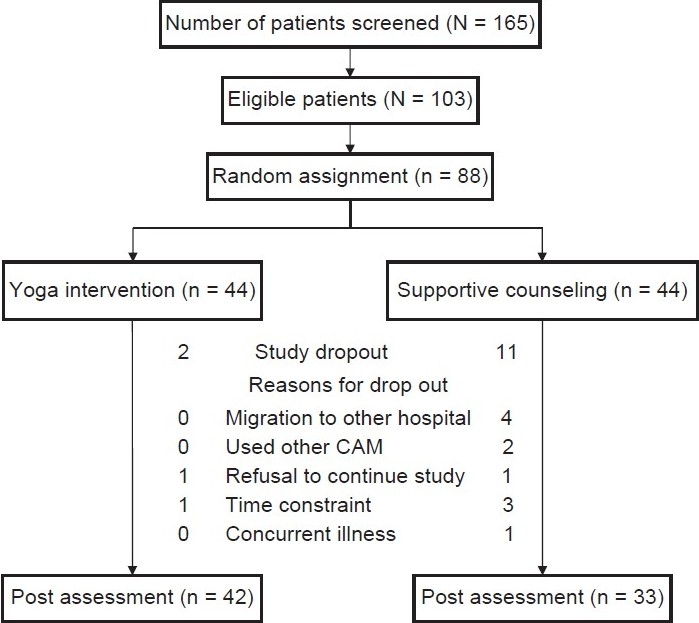
Trial profile
Measures
Before randomization demographic information, medical history, and clinical data were ascertained from all consenting participants.
Rotterdam symptom check list
Participants completed the Rotterdam Symptom Check List (RSCL) that measures symptoms that cause physical distress, psychological distress, and impairment in the activities of daily living. The RSCL is a 39-item scale which has been widely used as a brief measure of quality of life in cancer patients. It covers important domains of psychological distress, physical status (disease and treatment items), functional status, and global quality of life. Evidence of its reliability and validity has been found in a number of research settings. It comprises three subscales and includes one global question: How would you describe your quality of life during the past week? Responses range from “extremely poor” to “excellent” on a seven-point scale. The psychological symptom subscale contains eight symptoms. Respondents are asked to indicate the frequency with which they have experienced each symptom in the past week on a four-point scale, ranging from “not at all” (0) to “very much” (3). Possible scores on this scale therefore range from 0 to 24. The physical symptom subscale contains 22 symptoms; scores on this scale range from 0 to 66. The third subscale assesses whether respondents are able to perform eight activities, given their condition in the past week. Responses range from “unable” (0) to “without help” (3), and the possible range of scores on this subscale is from 0 to 24 with lower scores representing better levels of functioning.
The reliability of this scale has been reported to range from 0.68 to 0.90 for physical and psychological distress and from 0.42 to 0.89 for the activity subscale.[20]
Treatment-related symptoms were assessed using the European Organization for the Research and Treatment of Cancer—Quality of Life (EORTC QoL C30 questionnaire, version 1).[21] Though this 30-item questionnaire provides a measure on the dimensions of global health status, physical role, emotional, cognitive, social functioning, and cancer-related symptomatology, we report results of only cancer-related symptomatology. Assessments were carried out before and after radiotherapy treatment.
Interventions
The intervention group received the integrated yoga program and the control group received brief supportive therapy intervention, each imparted as a one-to-one session with the therapist. The yoga intervention consisted of a set of asanas (postures done with awareness), breathing exercises, pranayama (voluntarily regulated nostril breathing), meditation, and yogic relaxation techniques with imagery (mind-sound resonance technique and cyclic meditation). These practices were based on principles of attention diversion, mindful awareness, and relaxation to cope with day-to-day stressful experiences. Participants were required to attend a minimum of at least three in-person sessions/week for 6 weeks during their adjuvant radiotherapy treatment in the hospital with self-practice as homework on the remaining days. Each of these sessions lasted 1 h and was administered by a trained yoga therapist either before or after radiotherapy. These sessions started with a few easy yoga postures, breathing exercises, and pranayama (voluntarily regulated nostril breathing), and yogic relaxation. After this preparatory practice for about 20 min, the subjects were guided through any one of the meditation practices for the next 30 min, which included focusing awareness on sounds and chants from Vedic texts,[22] or breath awareness and impulses of touch emanating from palms and fingers while practicing yogic mudras, or a dynamic form of meditation that involved practicing, with eyes closed, of four yoga postures interspersed with relaxation while supine, thus achieving a combination of both “stimulating” and “calming” practices.[23] The control intervention consisted of brief supportive therapy with education as a component that is routinely offered to patients as a part of their care in this center. We chose to have this as a control intervention mainly to control for the nonspecific effects of the yoga program that may be associated with factors such as attention, support, and a sense of control as described in our earlier study.[24] Subjects and their caretakers underwent counseling by a trained social worker (once in 10 days, 15-min sessions) during their hospital visits for adjuvant radiotherapy. The control group received 3-4 such counseling sessions during a 6-week period, where as the intervention group received anywhere between 18 and 24 yoga sessions. While the goals of yoga intervention were stress reduction and appraisal changes, the goals of supportive therapy were education, reinforcing social support, and coping preparation.
Data analysis
Data were analyzed using Statistical Package for Social Sciences, version 10.0. Descriptive statistics were used to summarize the data. A GLM repeated-measures ANOVA was done with the within-subjects factor being time/assessments at two levels and between-subjects factor being groups at two levels (yoga vs. supportive therapy). Both group-by-time interaction effects and between-subjects and within-subjects effects were assessed. Post-hoc tests were done using Bonferroni's correction for changes at different time points between groups. Intention-to-treat analysis was also done on the initially randomized sample (n = 88) with the baseline measure and postradiotherapy measure (post-RT) for all participants. Baseline value was carried forward for participants who did not have a post-RT measure (study dropouts). Pearson’s correlation analyses were used to study the bivariate relationships between quality-of-life domains and treatment related symptoms.
RESULTS
A total of 74 participants (yoga, n = 40; control, n = 34) completed the prescribed radiotherapy regimen. All participants were ambulatory and had a Zubrod's performance status score of 0-2. All patients had mastectomy as primary treatment; 16 subjects received radiotherapy following mastectomy and 57 subjects received radiotherapy following mastectomy and three cycles of chemotherapy. Participants in both groups were comparable with respect to sociodemographic and medical characteristics [Table 1].
Table 1.
Demographic and medical characteristics of the initially randomized sample
| All subjects |
Yoga group |
Control group |
||||
|---|---|---|---|---|---|---|
| n = 88 (%) | n = 44 (%) | n = 44 (%) | ||||
| Stage of breast cancer | ||||||
| I | 5 | 5.7 | 2 | 4.5 | 3 | 6.8 |
| II | 18 | 20.4 | 11 | 25.0 | 7 | 15.9 |
| III | 65 | 73.9 | 31 | 70.5 | 34 | 77.3 |
| Grade of breast cancer | ||||||
| I | 1 | 1.1 | 1 | 2.3 | 0 | 0 |
| II | 33 | 37.5 | 21 | 51.1 | 10 | 22.7 |
| III | 54 | 61.4 | 22 | 47.7 | 34 | 77.3 |
| Menopausal status | ||||||
| Pre | 48 | 54.5 | 26 | 59.1 | 23 | 52.3 |
| Post | 40 | 45.5 | 18 | 40.9 | 21 | 47.7 |
| Regimen | ||||||
| After chemotherapy | 68 | 77.3 | 32 | 72.7 | 37 | 84 |
| After surgery | 20 | 22.7 | 12 | 27.3 | 7 | 15.9 |
| Marital status | ||||||
| Single | 2 | 2.2 | 1 | 2.3 | 1 | 2.2 |
| Married | 86 | 97.8 | 43 | 97.7 | 43 | 97.8 |
Psychological distress
A repeated-measures analysis of variance was done on psychological distress scores. Results showed significant group-by-time interaction effects [F (1, 72) = 7.64, P = 0.01] and between-subject effects [F (1, 72) = 7.91, P = 0.01]. Post-hoc tests using Bonferroni's correction showed significant differences between yoga and control groups in post-RT measures alone (mean difference ± SE, 3.72±1.07; P = 0.001). There was a significant within-subjects difference (post-and pre-RT measure) in the yoga group alone (mean difference ± SE, 2.95±0.66;P< 0.001; 95% CI 1.6-4.3) but not in the control group (0.26±0.71; P = 0.71; 95% CI−1.1 to 1.6) [Table 2]. Intention-to-treat analysis on the initially randomized sample showed significant improvement in psychological distress between groups following intervention (−3.47±0.73, P< 0.001, −4.91 to −2.03) [Table 3].
Table 2.
Comparison of scores for distress, activity, and symptom scale of European organization for research in the treatment of cancer QoLC30 scores using GLM repeated-measures ANOVA between yoga and control groups
| Outcome variables | Yoga (y) (n = 42) |
Control (c) (n = 33) |
Adjusted mean (y-c) (95% CI) | Effect size | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Psychological | 6.90 | 4.15 | 7.83 | 7.37 | −3.22 | 0.39 | |
| distress | (3.36) | (3.28)** | (3.55) | (3.65) | (−4.81 to −1.63) | ||
| Physical | 13.52 | 9.98 | 14.23 | 14.89 | − 4.91 | 0.33 | |
| distress | (6.16) | (7.04)** | (7.70) | (8.36) | (−8.45 to −1.37) | ||
| Activity level | 20.20 | 20.35 | 18.23 | 17.11 | 3.24 | 0.14 | |
| (5.78) | (5.35)* | (6.19) | (6.47) | (0.52-5.95) | |||
| Fatigue | 44.76 | 31.37 | 50.46 | 52.09 | −20.72 | 0.33 | |
| (22.89) | (21.79)** | (22.41) | (24.24) | (−31.40 to −10.04) | |||
| Pain | 33.74 | 23.17 | 42.47 | 41.52 | −18.36 | 0.14 | |
| (26.74) | (27.10)* | (28.49) | (32.57) | (−32.39 to −4.32) | |||
| Dyspnea | 8.13 | 5.69 | 12.12 | 10.13 | −4.44 | 0.01 | |
| (17.92) | (12.69) | (18.29) | (17.63) | (−11.47 to 2.59) | |||
| Insomnia | 47.15 | 21.14 | 34.34 | 35.44 | −14.31 | 0.47 | |
| (34.14) | (26.62)* | (25.66) | (32.11) | (−27.91 to −0.69) | |||
| Nausea and | 13.41 | 6.91 | 11.11 | 6.69 | 0.22 | 0.05 | |
| vomiting | (21.48) | (13.93) | (14.23) | (13.09) | (−6.10 to 6.55) | ||
| Appetite loss | 21.95 | 15.45 | 20.20 | 33.39 | −17.95 | 0.38 | |
| (24.28) | (19.86)** | (26.27) | (28.79) | (−29.25 to −6.65) | |||
| Diarrhea | 3.25 | 0.81 | 3.03 | 4.07 | −3.26 | 0.01 | |
| (12.48) | (5.21) | (12.81) | (13.83) | (−7.92 to 1.41) | |||
| Constipation | 6.50 | 6.50 | 7.07 | 8.11 | −1.61 | 0.14 | |
| (20.03) | (20.03) | (21.66) | (22.08) | (−11.38 to 8.17) | |||
P values < 0.05 and
P values < 0.01, for post-hoc tests comparing groups at pre-and postradiotherapy using Bonferroni's correction.
Table 3.
Comparison of scores between yoga and control groups at baseline and following intervention on intention-to-treat analysis using RMANOVA in the initially randomized sample (n = 88)
| Outcome variables | Yoga (44) |
Control (44) |
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Psychological | 6.75 | 4.25 | 8.09 | 7.72 |
| distress | (3.43) | (3.34)** | (3.32) | (3.43) |
| Physical | 14.05 | 10.82 | 14.47 | 15.00 |
| distress | (7.22) | (8.19)* | (7.52) | (8.06) |
| Activity level | 20.11 | 20.25 | 18.70 | 17.79 |
| (6.05) | (5.69) | (5.91) | (6.23) | |
| Fatigue | 45.48 | 33.26 | 49.32 | 50.52 |
| (24.08) | (23.82)** | (20.72) | (22.31) | |
| Pain | 34.07 | 24.44 | 42.04 | 41.38 |
| (27.96) | (28.56)** | (25.79) | (28.96) | |
| Dyspnea | 8.89 | 6.67 | 11.36 | 9.86 |
| (19.33) | (15.24) | (17.52) | (16.96) | |
| Insomnia | 48.15 | 24.44 | 37.12 | 37.93 |
| (35.22) | (30.48)* | (27.10) | (31.74) | |
| Nausea and | 15.55 | 9.64 | 13.26 | 9.97 |
| vomiting | (24.46) | (19.62) | (17.45) | (17.35) |
| Appetite loss | 22.96 | 17.03 | 21.21 | 31.10 |
| (26.42) | (23.16)* | (26.01) | (28.16) | |
| Diarrhea | 2.96 | 0.74 | 3.03 | 3.80 |
| (11.94) | (4.97) | (12.07) | (12.87) | |
| Constipation | 8.15 | 8.15 | 8.33 | 9.11 |
| (23.74) | (23.74) | (21.71) | (21.98) | |
P values < 0.05 and
P values < 0.01 for post-hoc tests comparing groups at different time points using Bonferroni's correction
Physical distress
A repeated-measures analysis of variance was done on physical distress scores. There was no significant group-by-time interaction effect [F (1, 72) = 3.86, P = 0.05] or between-subjects effect [F (1, 72) = 1.49, P = 0.23]. Post-hoc tests using Bonferroni’s corrections also did not show significant differences between yoga and control groups in post-RT measures (mean difference ± SE, −3.31 ± 1 .77; P= 0.07). But there was a significant within-subjects difference (post-and pre-RT measure) in the yoga group alone (mean difference ± SE, 3.20 ± 1.04; P< 0.01, 95% CI 1.1-5.2) but not in the control group (0.18 ± 1.13, P= 0.88, -2.0 to 2.4) [Table 2]. Intention-to-treat analysis on the initially randomized sample showed significant improvement in physical distress between groups following intervention (−4.18 ± 1.74, P = 0.02, −7.65 to −0.72) [Table 3.]
Activity level
A repeated-measures analysis of variance was done on activity level scores. There was no significant group-by-time interaction effect [F (1, 72) = 1.82, P = 0.18] but a significant between-subjects effect [F (1, 72) = 5.31, P = 0.02]. Post-hoc tests using Bonferroni's corrections showed significant differences between yoga and control groups in post-RT measures alone (mean difference ± SE, 3.65 ± 1.37;P = 0.01). There was no significant within-subjects effect in yoga or control groups following intervention [Table 2]. Intention-to-treat analysis did not show any significant changes between groups in the activity level [Table 3].
European organization for research in the treatment of cancer QoL C30 symptom scale
A repeated-measures analysis of variance was done on symptom scores. Results showed significant group-by-time interaction effects for fatigue [F (1, 72) = 7.74, P = 0.007], insomnia [F (1, 72) = 16.24, P = 0.001] and appetite loss [F (1, 72) = 10.41, P = 0.002] and significant between-subjects effects for fatigue [F (1, 72) = 8.26, P = 0.01] but not for insomnia [F (1, 72) = 0.02, P = 0.90] and appetite loss [F (1, 72) = 2.72, P = 0.10]. Post-hoc tests using Bonferroni's correction showed significant differences between yoga and control groups in post-RT measures alone for fatigue (mean difference ± SE, −20.72±5.36; P = 0.001), insomnia (−14.31 ± 6.83, P = 0.04), and appetite loss (−17.95 ± 5.67, P = 0.002). There was a significant decrease in fatigue (mean difference ± SE, 13.39 ± 3.61; P < 0.001; 95% CI 6.2-20.5) and insomnia (26.02 ± 4.49; P< 0.001; 17.0-34.9) in the yoga group alone; there was a significant increase in loss of appetite in the control group (−13.19 ± 4.54, P = 0.005, −22.2 to −4.1) [Table 2]. Intention-to-treat analysis on the initially randomized sample showed significant improvement in fatigue (−17.26 ± 4.89, P = 0.001, −26.99 to −7.53), pain (16-.93 ± 6.09, P = 0.007, −29.05 to −4.82), insomnia (−13.49 ± 6.60, P = 0.04, -26.60 to −0.38), and appetite loss (−14.07 ± 5.46, P = 0.01, −24.93 to −3.22) between groups following intervention [Table 3]. Other symptoms such as pain, dyspnea, nausea and vomiting, diarrhea, and constipation did not change significantly with time in both groups, and only pain showed significant between-subjects effects [F (1, 72) = 5.20, P = 0.03]. Post-hoc tests using Bonferroni's correction showed significant differences between yoga and control groups in pain scores in the post-RT measure (mean difference ± SE, −18.36 ± 7.04; P = 0.01). There was a significant decrease in pain (mean difference ± SE, 10.57±4.34; P = 0.02, 95% CI 1.9-19.2) and nausea and vomiting (6.50 ± 2.77, P = 0.02, 0.9-12.0) in the yoga group alone following intervention [Table 2]. Intention-to-treat analysis did not show any significant changes between groups in nausea and vomiting, dyspnea, constipation, and diarrhea [Table 3].
Bivariate relationships
Bivariate relationships were determined between the outcome measures. There was a significant positive correlation between physical and psychological distress and fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, and constipation. There was a significant negative correlation of activity levels with fatigue, nausea and vomiting, pain, dyspnea, insomnia, and appetite loss [Table 4].
Table 4.
Pearson correlation (r-values) between symptoms on European Organization for Research in the treatment of Cancer QoL C30 and distress subscales on the RSCL
| Physical distress | Psychological distress | Activity | |
|---|---|---|---|
| Fatigue | 0.68** | 0.62** | −0.40** |
| Nausea and vomiting | 0.48** | 0.27* | −0.39** |
| Pain | 0.34** | 0.42** | −0.06 |
| Dyspnea | 0.09 | 0.16 | −0.16 |
| Insomnia | 0.35** | 0.36** | −0.20 |
| Appetite loss | 0.23* | 0.40** | −0.03 |
| Constipation | 0.38** | 0.27* | −0.37** |
| Diarrhea | 0.19 | -0.05 | −0.38** |
P values < 0.05 and
P values < 0.01 for Pearson' correlation
Adherence to intervention
Adherence to intervention was good with 29.7% attending 10-20 supervised sessions, 56.7% attending 20-25 supervised sessions, and 13.7% attending > 25 supervised sessions over a 6-week period. The level of adherence did not seem to affect symptom scores (results not shown).
DISCUSSION
This study evaluated the effects of integrated approach of yoga therapy on symptom distress among early-stage breast cancer patients undergoing adjuvant radiotherapy. A significant reduction was observed in psychological distress, physical distress, a significant increase in the activity level on the RSCL and a significant reduction in fatigue, pain, insomnia, nausea and vomiting on the EORTC QoL symptom subscale following yoga intervention as compared to controls.
These results offer further support for preliminary findings that have shown beneficial effects of yoga intervention in reducing distressful symptoms such as fatigue, pain, loss of appetite, and insomnia and improving mood and quality-of-life concerns in cancer patients. Though beneficial effects are seen with yoga on symptom management, it should be noted that subjects in these studies were in various stages of treatment and disease and had varied diagnosis contributing to generalizability of findings.[25] In our study, the cohort sample were patients with stage II and III breast cancer receiving adjuvant therapy and our results support benefits of yoga in modulating distressful symptoms related to adjuvant radiotherapy. However, adherence did not seem to influence the outcome measures possibly due to the fact that an improved adherence created an “overall floor effect” thereby not influencing the outcome measures. The effect size was high (Cohen’s f >3) for fatigue, insomnia, psychological distress, and physical distress and moderate for pain and activity.
There was a significant positive correlation between symptoms and physical and psychological distress and negative correlation between these and activity. The results indicate that yoga intervention was helpful in reducing these distressing symptoms and that reduction in these could have reduced physical and psychological distress and improved activity. Patients with cancer often have multiple symptoms and symptoms can often cluster together in a systematic way whereby treatment of one can influence the treatment of other symptoms as well.[26] Though studying the effects of intervention on symptom clusters is not the main aim of this study, its effects on a multitude of symptoms such as pain, fatigue, nausea, insomnia, and loss of appetite could have nevertheless contributed to improvement in health outcomes. Moreover, most of these symptoms persist throughout the course of disease, and the effective treatment of these in initial stages is known to affect health outcomes later even after the completion of treatment.[27] It may be hypothesized that yoga could serve to be a prophylactic intervention in the initial stages of treatment affecting quality-of-life outcomes in future. This is more so important in today’s scenario where efforts are being directed at producing new insights into the causes of and cures for cancer, rather than managing the symptoms due to the disease and their treatment.
Evidence suggests that pain, fatigue, and depression are frequently undertreated. Patients and health care providers have reported depression and a persistent lack of energy as the aggressiveness of therapy has been increased and/or the underlying malignancy has worsened.[6] Cancer symptom management would benefit if an integrated intervention plan existed for a cluster of symptoms based on a clear understanding of which symptoms are likely to cluster, when clustering is likely to occur, and how a symptom cluster affects patient outcomes at different stages of treatment. Most of these symptom clusters are influenced by patient’s perception, awareness, education, and mood states, and can be explained through various biologic, psychological, behavioral, and sociocultural mechanisms that constitute a symptom interaction network and symptom experience.[28] The experience of multiple simultaneous symptoms has a synergistic effect on symptom distress.[29]
Management of symptoms therefore requires a holistic approach that integrates behavioral and mind–body strategies; this is more so emphasized in earlier studies that have shown several stress reduction and mind–body approaches to reduce distressful symptoms and manage mood states in cancer patients. The results of our study reinforce findings of our earlier study that has shown beneficial effects of yoga intervention in managing chemotherapy-related nausea and vomiting.[24] This simple intervention can be imparted to nurses and cancer care givers especially in developing countries where access and resources for supportive care rarely exist.
There are several limitations to our study; one among them could be inequality in contact time for interventions. However, it should be noted that supportive therapy[30] interaction was only used to negate the confounding variables such as instructor-patient interaction, education, and attention that could have significantly reduced distress in these patients.[31] Secondly, it was not possible to mask the yoga intervention from the study participants. Blinding in yoga studies is a topic of intense discussion in yoga research. As yet there has been no perfect method for blinding yoga therapy from the participants because of the nature of the therapy itself, which involves the patients being asked to perform asanas as well as a spiritual component that includes the knowledge that they are performing yoga.
In conclusion, our yoga intervention shows beneficial findings in managing cancer-related symptoms in early breast cancer patients taking recourse to adjuvant radiotherapy. More robust measures to assess individual symptoms must be attempted in future studies. Future studies should unravel putative mechanisms of action of our intervention for each of these symptoms.
Acknowledgments
This research was supported by a grant from Central Council for Research in Yoga and Naturopathy, Ministry of Health and Family Welfare, Govt. of India. We are thankful to Dr. Jayashree and Ms. Jayalakshmi for imparting the yoga intervention.
REFERENCES
- 1.Stehlin JS, Beach KH. Psychological aspects of cancer therapy: A surgeon's viewpoint. JAMA. 1966;197:100–4. [PubMed] [Google Scholar]
- 2.Thomas BC, Pandey M, Ramdas K, Nair MK. Psychological distress in cancer patients: Hypothesis of a distress model. Eur J Cancer Prev. 2002;11:179–85. doi: 10.1097/00008469-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Hughes J. Emotional reactions to diagnosis and treatment of early breast cancer. J Psychosom Res. 1982;26:277–83. doi: 10.1016/0022-3999(82)90047-2. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 5.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–62. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 6.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: Pain, depression, and fatigue, July 15-17, 2002 J Natl Cancer Inst Monogr. 2004;32:9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 7.Hegyvary ST. Patient care outcomes related to management of symptoms. Ann Rev Nursing Res. 1993;11:145–68. [PubMed] [Google Scholar]
- 8.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–76. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 9.Teel CS, Meek P, McNamara AM, Watson L. Perspectives unifying symptom interpretation. Image J Nurs Sch. 1997;29:175–81. doi: 10.1111/j.1547-5069.1997.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 10.de Vito Dabbs A, Hoffman LA, Swigart V, Happ MB, Iacono AT, Dauber JH. Using conceptual triangulation to develop an integrated model of the symptom experience of acute rejection after lung transplantation. ANS Adv Nurs Sci. 2004;27:138–49. doi: 10.1097/00012272-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. The influence of symptoms, age, comorbidity and cancer site on physical functioning and mental health of geriatric women patients. Women Health Psychol. 1999;29:1–12. doi: 10.1300/J013v29n03_01. [DOI] [PubMed] [Google Scholar]
- 12.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symp Manage. 1995;10:423–31. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 13.Fu MR, LeMone P, McDaniel RW. An integrated approach to an analysis of symptom management in patients with cancer. Oncol Nursing Forum. 2004;31:65–70. doi: 10.1188/04.ONF.65-70. [DOI] [PubMed] [Google Scholar]
- 14.Blake-Mortimer J, Gore-Felton C, Kimerling R, Turner-Cobb JM, Spiegel D. Improving the quality and quantity of life among patients with cancer: A review of the effectiveness of group psychotherapy. Eur J Cancer. 1999;35:1581–6. doi: 10.1016/s0959-8049(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 15.Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. J Consult Clin Psychol. 1992;60:552–68. doi: 10.1037//0022-006x.60.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham AJ. Adjuvant psychological therapy for cancer patients: Putting it on the same footing as adjunctive medical therapies. Psychooncology. 2000;9:367–71. doi: 10.1002/1099-1611(200009/10)9:5<367::aid-pon473>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: A meta-analysis of randomized experiments. Health Psychol. 1995;14:101–8. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
- 18.Owen JE, Klapow JC, Hicken B, Tucker DC. Psychosocial interventions for cancer: Review and analysis using a three-tiered outcomes model. Psychooncology. 2001;10:218–30. doi: 10.1002/pon.509. [DOI] [PubMed] [Google Scholar]
- 19.Targ EF, Levine EG. The efficacy of a mind -body-spirit group for women with breast cancer: A randomized controlled trial. Gen Hosp Psychiatry. 2002;24:238–48. doi: 10.1016/s0163-8343(02)00191-3. [DOI] [PubMed] [Google Scholar]
- 20.de Haes JC, Olschewski M. Quality of life assessment in a cross-cultural context: Use of the Rotterdam Symptom Checklist in a multinational randomised trial comparing CMF and Zoladex (Goserlin) treatment in early breast cancer. Ann Oncol. 1998;9:745–750. doi: 10.1023/a:1008282806910. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Telles S, Nagarathna R, Nagendra HR. Autonomic changes while mentally repeating two syllables -one meaningful and another neutral. Indian J Physiol Pharmacol. 1998;42:57–63. [PubMed] [Google Scholar]
- 23.Telles S, Reddy SK, Nagendra HR. Oxygen consumption and respiration following two yoga relaxation techniques. Appl Psychophysiol Biofeedback. 2000;25:221–7. doi: 10.1023/a:1026454804927. [DOI] [PubMed] [Google Scholar]
- 24.Raghavendra RM, Nagarathna R, Nagendra HR, Gopinath KS, Srinath BS, Ravi BD, et al. Effects of an integrated yoga programme on chemotherapy-induced nausea and emesis in breast cancer patients. Eur J Cancer Care. 2007;16:462–74. doi: 10.1111/j.1365-2354.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 25.Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165–71. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- 26.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34:971–80. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 27.Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 28.Parker KP, Kimble LP, Dunbar SB, Clark PC. Symptom interactions as mechanisms underlying symptom pairs and clusters. J Nurs Scholarsh. 2005;37:209–15. doi: 10.1111/j.1547-5069.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 29.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS Adv Nurs Sci. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Telch CF, Telch MJ. Group coping skills instruction and supportive group therapy for cancer patients: A comparison of strategies. J Consult Clin Psychol. 1986;54:802–8. doi: 10.1037//0022-006x.54.6.802. [DOI] [PubMed] [Google Scholar]
- 31.Greer S, Moorey S, Baruch JD, Watson M, Robertson BM, Mason A, et al. Adjuvant psychological therapy for patients with cancer: A prospective randomised trial. BMJ. 1992;304:675–80. doi: 10.1136/bmj.304.6828.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


