Influenza A virus (IAV) (family Orthomyxoviridae) is a highly infectious respiratory pathogen of birds and mammals, including human beings and horses (Palese and Shaw 2007). The virus is classified into different subtypes based on the antigenic properties of the haemagglutinin (HA) and neuraminidase (NA) proteins. Sixteen HA subtypes (H1 to H16) and nine NA subtypes (N1 to N9) have been identified (Fouchier and others 2005). Two subtypes, H3N8 and H7N7, have been isolated from horses. The H7N7 subtype was first isolated from a horse in Czechoslovakia in 1956 (Prague/56) (Sovinova and others 1958), and the H3N8 subtype was first isolated from a horse in Miami, USA, in 1963 (Waddell and others 1963). The H7N7 subtype has not been isolated from horses for three decades and is presumed to be extinct (Webster 1993). The H3N8 subtype is currently a common cause of disease in horses worldwide. In horses, influenza is characterised by an abrupt onset of pyrexia, depression, coughing and nasal discharge, and is often complicated by secondary bacteria infections that can lead to pneumonia and death (Hannant and Mumford 1996). Although H3N8 is a major cause of morbidity in horses throughout the world, information on the seroprevalence of IAV in horses and other domestic animals in Mexico is limited.
West Nile virus (WNV) (family Flaviviridae) is maintained in nature in an enzootic transmission cycle that primarily involves mosquitoes and birds (Hayes and others 2005, Blitvich 2008). Human beings and horses are incidental hosts in the natural transmission cycle. The clinical signs of infection include fever, aseptic meningitis and/or encephalitis. WNV has been responsible for over 29,000 cases of human illness and at least 26,000 cases of equine encephalitis in the USA in the past decade. Surprisingly, however, there have been few reports of WNV-associated illness in Latin America, despite serological evidence of widespread WNV activity in this region (Komar and Clark 2006, Blitvich 2008). Antibodies to WNV have previously been detected in asymptomatic vertebrate animals in the Yucatán Peninsula of Mexico (Farfan-Ale and others 2004, 2006, Loroño-Pino and others 2003). The reasons for the low incidence of WNV-associated illness in vertebrates in Mexico and elsewhere in Latin America are not known.
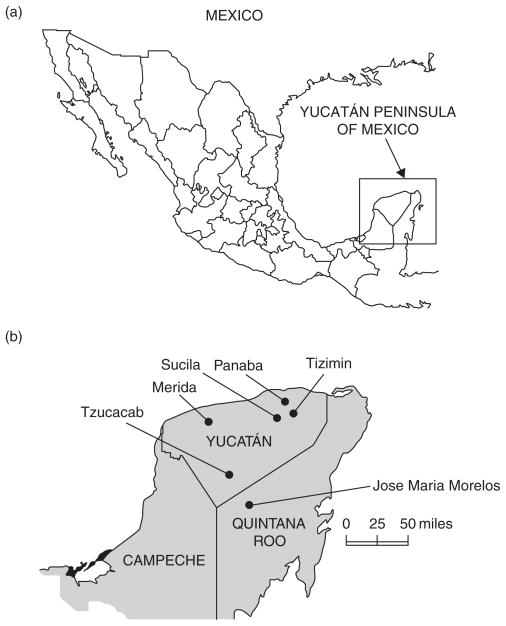
Because the impact of IAV and WNV on the health of horses in Mexico is poorly understood, a serological investigation was undertaken to obtain information on the seroprevalence of these viruses in domesticated animals in the Yucatán Peninsula of Mexico. Samples of serum were collected from 266 animals (186 horses, 38 sheep, 37 chickens and five turkeys) at 26 study sites, all on privately owned ranches or farms, between September 2007 and October 2008. The study sites were located in six municipalities, five of which (Panaba, Tizimin, Sucila and Tzucacab) are in Yucatán State and one (Jose Maria Morelos) in Quintana Roo State (Fig 1). The horses were from the municipalities of Panaba, Tizimin, Sucila and Jose Maria Morelos. The sheep and chickens were from Merida, and the turkeys were from Tzucacab. None of the animals had ever been outside the Yucatán Peninsula, and none had been vaccinated against IAV or WNV. All of the animals were regularly monitored (usually daily) by their keepers for signs of illness. Six horses were showing clinical signs at the time of serum collection (Table 1).
FIG 1.
Geographical location of (a) the Yucatán peninsula and (b) the study sites
TABLE 1.
Details of horses that displayed signs of illness at the time of serum collection
| Horse | Sampling date | Study site | Town/city | Municipality | Clinical signs |
|---|---|---|---|---|---|
| 1 | 08/2007 | La Guajira | Chankeken | Tizimin | Facial paralysis, encephalitis, then death |
| 2 | 09/2007 | La Central | Loche | Panaba | Fever, lethargy, depression |
| 3 | 11/2007 | San Jose | Yaxchenku | Tizimin | Lethargy |
| 4 | 09/2008 | Santa Martha | Panhatoro | Tizimin | Fever, ataxia, then death |
| 5 | 09/2008 | Santa Martha | Panhatoro | Tizimin | Posterior ataxia |
| 6 | 09/2008 | Santa Martha | Panhatoro | Tizimin | Posterior ataxia |
The serum samples were tested for antibodies to IAV and WNV by an epitope-blocking ELISA (bELISA). The protocol for the WNV-specific bELISA has been described previously by Blitvich and others (2003). The IAV-specific bELISA utilises the IAV nucleoprotein-specific monoclonal antibody clone A1 (Millipore), and recombinant IAV nucleoprotein (Imgenex) (Sullivan and others 2009). The IAV nucleoprotein is well conserved (Gorman and others 1990) and the bELISA can therefore detect antibodies to all IAV subtypes. A subset of sera positive for antibodies to IAV by bELISA was further tested by the haemagglutination inhibition (HI) test and neuraminidase inhibition (NI) tests at the National Veterinary Service Laboratories (NVSL) in Ames, Iowa, USA. HI tests were performed using the influenza reference strains A/equine/Kentucky/1/81 (H3N8), A/equine/Miami/1/63 (H3N8) and A/equine/Prague/1/56 (H7N7). NI tests were performed using standard reference reagents for N1 to N7 and N9, and N8 equine/Miami/63 reference reagent.
Forty-seven (25 per cent) of the 186 horses sampled had evidence of IAV-specific antibody by bELISA. Ten serum samples with bELISA antibodies to IAV were examined by the HI and NI tests, and all had antibodies to the H3N8 subtype. The HI antibody titres were at least fourfold higher to the Kentucky/81 strain than to the Miami/63 strain. Twenty-one of the seropositive horses were from the municipality of Panaba, and 26 were from Tizimin (Table 2). The seroprevalence was higher (41 per cent) in Tizimin. Three seropositive horses (horses 4, 5 and 6; Table 1) were symptomatic at the time of serum collection, although none had signs typically associated with IAV infections, such as nasal discharge or coughing. The youngest seropositive horse was a two-year-old filly sampled in Tizimin in September 2008, suggesting that the most recent IAV infection had occurred during or after 2006. No antibodies to IAV were detected in the sheep, chickens or turkeys.
TABLE 2.
Seroprevalence of influenza A virus (IAV) and West Nile virus (WNV) in horses in four municipalities of the Yucatán Peninsula, Mexico
| Municipality | Number of horses sampled | Number (%) seropositive for | ||
|---|---|---|---|---|
| IAV | WNV | IAV and WNV | ||
| Jose Maria Morelos | 11 | – | – | – |
| Panaba | 109 | 21 (19) | 18 (17) | 6 (6) |
| Sucila | 2 | – | 1 (50) | – |
| Tizimin | 64 | 26 (41) | 9 (14) | 7 (11) |
| Total | 186 | 47 (25) | 28 (15) | 13 (7) |
Twenty-eight (15 per cent) of the 186 horses had antibodies to WNV by bELISA: 18 from Panaba, nine from Tizimin and one from Sucila (Table 2). Of the municipalities in which more than 10 horses were sampled (Panaba and Tizimin), the rate of seropositivity was higher (17 per cent) in Panaba. All of the horses that were seropositive for WNV were asymptomatic at the time of sampling, and none had a history of WNV-like illness. The youngest seropositive horse was an 18-month-old filly sampled in Tizimin in October 2007, suggesting that the most recent WNV infection had occurred in or after 2006.
Thirteen (7 per cent) of the horses had antibodies to both IAV and WNV. Of these, seven were from Tizimin and six were from Panaba. Antibodies to WNV were detected in four chickens and five turkeys; all the sheep were negative for antibodies to WNV.
Two of the horses (horses 1 and 4) that showed neurological signs at the time of sample collection subsequently died. Cerebellar tissue was taken from horse 4 and tested by RT-PCR using flavivirus-, alphavirus- and rabies virus-specific primers, and by virus isolation in African green monkey kidney (Vero) cells. The specimen was negative in all tests (data not shown). A tissue sample was not obtained from horse 1 because of late notification of its death.
In summary, antibodies to IAV and WNV were detected in horses in the Yucatán Peninsula of Mexico. The seroprevalence for IAV in horses sampled in this study was reasonably high (25 per cent). Similar rates of seropositivity have been reported in other studies performed in regions where horses are not routinely vaccinated against IAV (Ataseven and Daly 2007). The seroprevalence for WNV in horses in the present study was 15 per cent. None of the seropositive horses had signs of WNV-like illness before or at the time of serum collection. These observations are similar to those reported in other studies of WNV in tropical regions (Dupuis and others 2003, Komar and others 2003, Farfan-Ale and others 2006, Morales-Betoulle and others 2006).
Acknowledgments
The authors thank Ruben Loroño for providing serum samples and Rosa Cetina for technical help. This study was supported by grant 5R21AI067281-02 from the National Institutes of Health, and in part by a cooperative agreement with the United States Department of Agriculture (reference number 0874880712CA).
Footnotes
Provenance: not commissioned; externally peer reviewed
Contributor Information
M. A. Loroño-Pino, Laboratorio de Arbovirologia, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’, Universidad Autónoma de Yucatán, Mérida, Yucatán, CP 97000, Mexico.
J. A. Farfan-Ale, Laboratorio de Arbovirologia, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’, Universidad Autónoma de Yucatán, Mérida, Yucatán, CP 97000, Mexico.
J. E. Garcia-Rejon, Laboratorio de Arbovirologia, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’, Universidad Autónoma de Yucatán, Mérida, Yucatán, CP 97000, Mexico.
M. Lin, Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA.
E. Rosado-Paredes, Laboratorio de Arbovirologia, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’, Universidad Autónoma de Yucatán, Mérida, Yucatán, CP 97000, Mexico.
F. I. Puerto, Laboratorio de Arbovirologia, Centro de Investigaciones Regionales ‘Dr Hideyo Noguchi’, Universidad Autónoma de Yucatán, Mérida, Yucatán, CP 97000, Mexico.
A. Bates, Centro Médico Veterinario del Oriente, Tizimin, Yucatán, CP 97702, Mexico.
J. J. Root, United States Department of Agriculture, Wildlife Services, National Wildlife Research Center, Fort Collins, CO 80521, USA.
A. B. Franklin, United States Department of Agriculture, Wildlife Services, National Wildlife Research Center, Fort Collins, CO 80521, USA.
H. J. Sullivan, United States Department of Agriculture, Wildlife Services, National Wildlife Research Center, Fort Collins, CO 80521, USA.
B. J. Blitvich, Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011, USA.
References
- ATASEVEN VS, DALY JM. Seroepidemiology of equine influenza virus infection in Turkey. Turkish Journal of Veterinary and Animal Sciences. 2007;31:199–202. [Google Scholar]
- BLITVICH BJ. Transmission dynamics and changing epidemiology of West Nile virus. Animal Health Research Reviews. 2008;9:71–86. doi: 10.1017/S1466252307001430. [DOI] [PubMed] [Google Scholar]
- BLITVICH BJ, BOWEN RA, MARLENEE NL, HALL RA, BUNNING ML, BEATY BJ. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. Journal of Clinical Microbiology. 2003;41:2676–2679. doi: 10.1128/JCM.41.6.2676-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPUIS AP, 2nd, MARRA PP, KRAMER LD. Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerging Infectious Diseases. 2003;9:860–863. doi: 10.3201/eid0907.030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARFAN-ALE JA, BLITVICH BJ, LOROÑO-PINO MA, MARLENEE NL, ROSADO-PAREDES EP, GARCIA-REJON JE, et al. Longitudinal studies of West Nile virus infection in avians, Yucatan State, Mexico. Vector-Borne and Zoonotic Diseases. 2004;4:3–14. doi: 10.1089/153036604773082942. [DOI] [PubMed] [Google Scholar]
- FARFAN-ALE JA, BLITVICH BJ, MARLENEE NL, LOROÑO-PINO MA, PUERTO-MANZANO F, GARCIA-REJON JE, et al. Antibodies to West Nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. American Journal of Tropical Medicine and Hygiene. 2006;74:908–914. [PubMed] [Google Scholar]
- FOUCHIER RA, MUNSTER V, WALLENSTEN A, BESTEBROER TM, HERFST S, SMITH D, RIMMELZWAAN GF, OLSEN B, OSTERHAUS AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. Journal of Virology. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORMAN OT, BEAN WJ, KAWAOKA Y, WEBSTER RG. Evolution of the nucleoprotein gene of influenza A virus. Journal of Virology. 1990;64:1487–1497. doi: 10.1128/jvi.64.4.1487-1497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNANT D, MUMFORD JA. Equine influenza. In: Studdert MJ, editor. Virus Infections of Equines. Elsevier Science Publishers; 1996. pp. 285–293. [Google Scholar]
- HAYES EB, KOMAR N, NASCI RS, MONTGOMERY SP, O’LEARY DR, CAMPBELL GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infectious Diseases. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMAR N, CLARK GG. West Nile virus activity in Latin America and the Caribbean. Revista Panamericana de Salud Pública. 2006;19:112–117. doi: 10.1590/s1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- KOMAR O, ROBBINS MB, KLENK K, BLITVICH BJ, MARLENEE NL, BURKHALTER KL, GUBLER DJ, GONZÁLVEZ G, PEÑA CJ, PETERSON AT, KOMAR N. West Nile virus transmission in resident birds, Dominican Republic. Emerging Infectious Diseases. 2003;9:1299–1302. doi: 10.3201/eid0910.030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOROÑO-PINO MA, BLITVICH BJ, FARFAN-ALE JA, PUERTO FI, BLANCO JM, MARLENEE NL, ROSADO-PAREDES EP, GARCIA-REJON JE, GUBLER DJ, CALISHER CH, BEATY BJ. Serologic evidence of West Nile virus infection in horses, Yucatan State, Mexico. Emerging Infectious Diseases. 2003;9:857–859. doi: 10.3201/eid0907.030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORALES-BETOULLE ME, MORALES H, BLITVICH BJ, POWERS AM, DAVIS EA, KLEIN R, CORDÓN-ROSALES C. West Nile virus in horses, Guatemala. Emerging Infectious Diseases. 2006;12:1038–1039. doi: 10.3201/eid1206.051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALESE P, SHAW ML. Orthomyxoviridae: the virus and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven; 2007. pp. 1647–1689. [Google Scholar]
- SOVINOVA O, TUMOVA B, POUSKA F, NEMEC J. Isolation of a virus causing respiratory disease in horses. Acta Virologica. 1958;2:52–61. [PubMed] [Google Scholar]
- SULLIVAN HJ, BLITVITCH BJ, VANDALEN K, BENTLER KT, FRANKLIN AB, ROOT JJ. Evaluation of an epitope-blocking enzyme-linked immunosorbent assay for the detection of antibodies to influenza A virus in domestic and wild avian and mammalian species. Journal of Virological Methods. 2009;161:141–146. doi: 10.1016/j.jviromet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- WADDELL GH, TEIGLAND MB, SIGEL MM. A new influenza virus associated with equine respiratory disease. Journal of the American Veterinary Medical Association. 1963;143:587–590. [PubMed] [Google Scholar]
- WEBSTER RG. Are equine 1 influenza viruses still present in horses? Equine Veterinary Journal. 1993;25:537–538. doi: 10.1111/j.2042-3306.1993.tb03009.x. [DOI] [PubMed] [Google Scholar]