SUMMARY
CD8+ T cells are selected via low affinity interaction with MHC class I molecules on thymic epithelial cells (TECs). However, compromised T cell receptor signaling was proposed to force CD8+ T cell selection on hematopoietic cells through a SLAM-associated protein (SAP)-dependent mechanism similar to NKT cells. The outcome is an unconventional CD8+ T cell with phenotypic and functional characteristics of innate lymphocytes. Here we showed that Id3−/ − CD8+ T cells had an innate-like phenotype and required SAP for their development. However, like conventional CD8+ T cells, Id3−/ − CD8+ thymocytes were selected on TECs. The requirement for SAP and the innate-like phenotype was not intrinsic to Id3−/ − CD8+ thymocytes. Rather, an expanded population of NKT-like cells induced the innate phenotype on CD8+ T cells through production of interleukin-4. Our findings reveal that accumulation of NKT-like cells promotes conventional CD8+ thymocytes to acquire innate lymphocyte characteristics.
CD8+ T cells play a major role in cell-mediated immunity by recognizing pathogen-infected cells via peptides displayed on MHC class I. During their development CD8 T cells are tested, by interaction of their T cell receptor (TCR) with MHC class I proteins expressed on thymic epithelial cells, to ensure that only cells with sufficient affinity for MHC undergo positive selection (Klein et al., 2009). During positive selection, the strength of TCR signaling influences the CD4 versus CD8 cell fate decision (Bosselut, 2004). Sustained or strong signaling supports development toward the CD4 lineage whereas transient or weak signaling promotes CD8 T cell development (Singer et al., 2008). However, very strong TCR signaling results in negative selection whereas insufficient TCR signaling leads to “death by neglect” (Singer et al., 2008). Studies in mice bearing mutations in mediators of TCR signaling raised the hypothesis that the “strength” of TCR signaling may influence the effector fate of CD8 T cells (Prince et al., 2009; Schwartzberg et al., 2009). Mice that lack the Tec family kinases Itk and Rlk fail to activate the Mitogen Activated Protein (MAP) kinases Erk1 and Erk2 in response to TCR signaling and therefore show a reduced capacity for positive selection (Liao and Littman, 1995; Lucas et al., 2002; Schaeffer et al., 2000). The CD8 T cells in these mice have an activated or memory phenotype including expression of the common β chain of the IL-2, IL-7 and IL-15 receptor (CD122), high expression of CD44 and low expression of CD62L and CD24 (HSA) and expression of the T-box transcription factor Eomesodermin in the absence of T-bet (Atherly et al., 2006; Broussard et al., 2006; Hu et al., 2007; Takemoto et al., 2006). Itk−/ − and Itk−/ −Rlk−/ − CD8 T cells also have characteristics of cells of the innate immune system including rapid production of interferon-γ (IFN-γ ) without prior antigen exposure and expression of NKR-P1C (NK1.1). The prevailing hypothesis to explain this phenotype had been that compromised TCR signaling allows TCRs with strong MHC binding affinity to escape negative selection and develop innate-like features with superior effector potential.
A recent study of Itk−/ − CD8 T cells suggested that these cells undergo positive selection through a fundamentally different mechanism than conventional CD8 T cells (Horai et al., 2007). The innate-like phenotype of CD8 T cells appeared to be a consequence of a selection process similar to that used by invariant natural killer (iNK) T cells and some other innate-like T cell lineages that involves the SLAM associated adapter protein (SAP) (Bendelac et al., 2007; Detre et al., 2010). iNKT cells, in contrast to conventional thymocytes, are selected by glycolipids presented on CD1d molecules expressed on hematopoietic cells (Bendelac et al., 2007; Coles and Raulet, 2000; Urdahl et al., 2002). An innate-like CD4 population was described in mice expressing the MHC class II activator transcription factor (CIITA) in CD4+ thymocytes further implicating selection on DP thymocytes as a requirement for development of innate-like characteristics (Li et al., 2005; Li et al., 2007b). Positive selection of iNKT cells requires many of the same signaling pathways as conventional T cells. However, iNKT cells also have unique signaling requirements, in particular, the SLAM family of receptors (CD150 and Ly108) and their downstream effectors SAP, FynT and protein kinase C theta (Veillette et al., 2007). SLAM receptor expression is restricted to hematopoietic cells and these proteins function in homotypic interactions suggesting that selection of iNKT cells on DP thymocytes might lead to activation of the SLAM-SAP pathway. How the SLAM-SAP pathway and TCR signals converge to generate innate-like lymphocytes is currently an unresolved issue. However, induction of the PLZF transcription factor is necessary and sufficient for many of the innate-like properties of iNKT cells including the ability to produce both IFN-γ and interleukin-4 (IL-4) (Kovalovsky et al., 2008; Savage et al., 2008). PLZF is also expressed in a subset of γ δ T cells that resemble iNKT cells, termed γ δ NKT cells (Alonzo et al., 2009; Kreslavsky et al., 2009; Verykokakis et al., 2010). In contrast to iNKT cells, until recently, there were no signaling molecules known to be required selectively for development of conventional T cells. However, the Tec kinase-Erk MAP kinase pathway is required for development of conventional CD8 T cells and their absence causes CD8 T cells to adopt an innate-like fate (Atherly et al., 2006; Broussard et al., 2006).
Id3, an antagonist of E protein transcription factors, is induced by the Erk MAP kinase pathway in T cells (Bain et al., 2001; Engel et al., 2001). Deletion of Id3 leads to altered positive and negative selection similar to that previously described for mice lacking Itk and Rlk (Liao and Littman, 1995; Liu et al., 2006; Lucas et al., 2002; Rivera et al., 2000; Schaeffer et al., 2000). Therefore, we hypothesized that Id3 is the essential transcriptional regulator of the Tec kinase-Erk Map kinase pathway that controls the fate of CD8 T cells. This hypothesis was further supported by the recent observation that deletion of the E proteins E2A and HEB in DP thymocytes augments the generation of conventional CD8 T cells even in the absence of a functional TCR (Jones and Zhuang, 2007). Therefore, inhibition of E protein activity is sufficient for development of conventional CD8 T cells. However, an essential role for Id3 in development of conventional CD8 T cells has not been demonstrated.
To further examine the requirement for Id3 in T cell development we investigated the phenotype and function of CD8 T cells in Id3−/ − mice. We found that Id3−/ − CD8 thymocytes had many of the characteristics of innate-like lymphocytes. Surprisingly however, although development of the innate-like phenotype was SAP-dependent, we found that Id3−/ − CD8 T cells were not selected on hematopoietic cells. In contrast, like conventional CD8 T cells, Id3−/ − CD8 T cells undergo positive selection on MHC class Ia expressed on non-hematopoietic cells. The innate-like phenotype of Id3−/ − CD8 T cells was not an intrinsic property of CD8 T cells. We demonstrated here that the innate-like phenotype was imprinted on CD8 T cells by IL-4 produced by an expanded population of SAP- and PLZF-dependent γ δ and α β NKT cells.
RESULTS
Id3−/ − CD8+ thymocytes have an innate-like phenotype
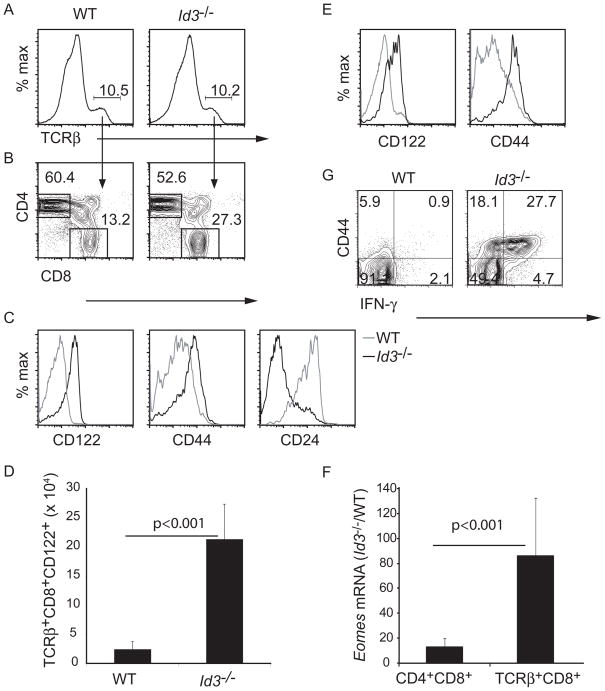
Id3 is a well-characterized target of TCR and Erk MAP kinase signaling in thymocytes (Bain et al., 2001). Therefore, we hypothesized that Id3 is an essential regulator of conventional CD8 T cell development and, as observed in Itk−/ − mice, Id3−/ − CD8 T cells should adopt an innate-like fate (Atherly et al., 2006; Broussard et al., 2006). To test this hypothesis we undertook a rigorous analysis of TCRβ + CD8 thymocytes in Id3−/ − mice. The frequency of CD8 thymocytes in Id3−/ − mice was higher than in wild-type (WT) mice due to an expanded population of γ δ NKT cells, many of which expressed CD8 (Verykokakis et al., 2010). However, the frequency of CD8 cells among TCRβ + thymocytes was also higher in Id3−/ − mice as compare to WT (Figure 1A, B). In contrast to WT cells, Id3−/ − CD8 thymocytes showed high expression of CD122 and CD44 but low expression of CD24, a surface marker of immature thymocytes (Figure 1C). The total number of CD122+TCRβ + CD8 thymocytes was approximately 10-fold higher than in WT mice. (Figure 1D). Elevated expression of CD122 and CD44 was also observed on CD8 T cells in the spleen of Id3−/ − mice (Figure 1E). Id3−/ − TCRβ + CD8 thymocytes expressed Eomes mRNA, which codes for the transcription factor Eomesodermin, (Figure 1F), which is associated with the innate-like phenotype in CD8 T cells (Atherly et al., 2006; Fukuyama et al., 2009). Eomes mRNA was increased in Id3−/ − DP thymocytes indicating that it’s induction occurred during thymic development rather than as a consequence of activation in peripheral tissues (Figure 1F). The hypothesis that CD8 T cells acquired the innate phenotype during development was further suggested by the expression of CD122 on Id3−/ − CD8 thymocytes isolated from 1 week old mice (Figure S1). At this age it is unlikely that mature T cells have had time to become activated in the periphery and circulate back to the thymus. Therefore, Id3−/ − CD8 T cells had an innate-like phenotype similar to that described for CD8 T cells in mice lacking the Tec family kinases Itk, and Rlk and the co-activator protein CBP (Atherly et al., 2006; Broussard et al., 2006; Fukuyama et al., 2009; Horai et al., 2007).
Figure 1.
Id3−/ − CD8 thymocytes have an innate-like phenotype. (A) WT and Id3−/ − thymocytes were analyzed by flow cytometry for TCRβ . The frequency of TCRβ + cells is indicated. (B) CD4 and CD8 expression on TCRβ + cells. The frequency of CD4+ or CD8+ cells among TCRβ + cells is indicated. (C) Expression of CD122, CD44 or CD24 on WT (grey) or Id3−/ − (black) TCRβ +CD8+ thymocytes. (D) Number of TCRβ +CD8+CD122+ thymocytes in WT and Id3−/ − mice. n = 13 for each genotype. Graphs show mean +/− s.d. (E) Expression of CD122 or CD44 on WT (grey) or Id3−/ − (black) TCRβ +CD8 splenocytes. Data are representative of 10 experiments. (F) Relative expression of Eomes mRNA in Id3−/ − CD4+CD8+ or TCRβ +CD8+ thymocytes compared to WT. Graphs show mean +/− s.d. Representative of 3 experiments (G) Expression of CD44 and intracellular IFNγ in WT or Id3−/ − CD8 thymocytes 5 hours after stimulation with PMA + ionomycin. Data is representative of 3 experiments. For data on neonates see Figure S1.
To further examine the innate-like characteristics of Id3−/ − CD8 T cells we stimulated thymocytes in vitro with PMA and ionomycin for 5 hours and examined their ability to produce IFN-γ . Less than 2% of WT CD8 thymocytes made IFN-γ under these conditions at this time point (Figure 1G). In contrast, greater than 27.7% of Id3−/ − CD8 thymocytes produced IFNγ and the responding cells were CD44hi (Figure 1G). Therefore, the CD8 T cells in Id3−/ − mice responded rapidly to TCR-mimicking stimuli as expected for innate-like lymphocytes.
Positive Selection of Id3−/ − CD8+ T cells required MHC class I on thymic epithelial cells
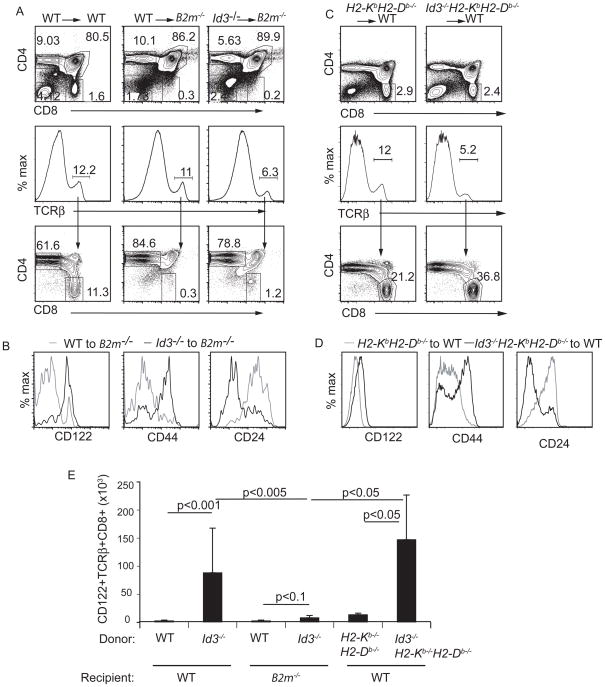
Positive selection of conventional CD8 T cells requires MHC class Ia on TECs (Klein et al., 2009); however innate-like CD8 T cells in Itk−/ − mice were reported to be selected on hematopoietic cells via MHC class 1b (Broussard et al., 2006; Horai et al., 2007). Importantly, the frequency of TCRβ + thymocytes was reduced in the thymus of Id3−/ −H2-KbH2-Db−/ − mice compared to both WT and H2-KbH2-Db−/ − mice (Figure 2A). In addition, the frequency of CD8 cells among TCRβ + thymocytes was extremely low in Id3−/ −H2-KbH2-Db−/ − mice and in H2-KbH2-Db−/ − mice (Figure 2B). Therefore, the total number of TCRβ + CD8 T cells was dramatically reduced in the thymus of both Id3−/ −H2-KbH2-Db−/ − and H2-KbH2-Db−/ − mice indicating that Id3−/ − CD8 T cells were selected on MHC class Ia (Figure 2D). Moreover, although the small number of TCRβ + CD8 T cells present in the thymus of Id3−/ −H2-KbH2-Db−/ − mice expressed CD122 they did not express CD44 and had high expression of CD24 indicating that they did not have the full innate-like phenotype (Figure 2C). Therefore, the innate-like CD8 T cells in Id3−/ − mice are selected on MHC class Ia.
Figure 2.
Id3−/ − innate-like CD8 T cells were selected on MHC class Ia. (A) WT, H2-KbH2-Db−/ − or Id3−/ −H2-KbH2-Db−/ − thymocytes were analyzed by flow cytometry for TCRβ . The frequency of TCRβ + cells is indicated. (B) CD4 and CD8 expression on TCRβ + cells. The frequency of CD4+ or CD8+ cells among TCRβ + cells is indicated. (C) Expression of CD122, CD44 or CD24 on H2-KbH2-Db−/ − (grey) or Id3−/ − H2-KbH2-Db−/ − (black) TCRβ +CD8 thymocytes. Data are representative of >5 experiments. (D) Average number (+ standard deviation) of TCRβ +CD8+ cells in the thymus of WT, Id3−/ −, H2- KbH2-Db−/ − or Id3−/ −H2-KbH2-Db−/ −mice. n > 5 for each genotype, p-values are shown for the comparison of populations at the end of each line.
We next tested whether development of innate-like CD8 T cells required MHC class Ia on TECs or hematopoietic cells. To address the requirement for MHC class Ia on TECs we reconstituted lethally irradiated B2m−/ − mice with bone marrow from Id3−/ − mice. In these chimeric animals TECs lacked β 2M and therefore MHC class I; however β 2M was expressed on Id3−/ − hematopoietic cells. As expected, six weeks after transplantation, WT → B2m−/ − chimeras showed a reduced frequency of CD8 thymocytes compared to WT → WT chimeras (Figure 3A). Surprisingly however, and in contrast to what was reported for Itk−/ − CD8 thymocytes, there were very few TCRβ + CD8 T cells in Id3−/ − → B2m−/ − chimeras (Figure 3A). The few TCRβ + CD8 T cells that develop in Id3−/ − → B2m−/ − chimeras had high expression of CD122 and CD44 and low expression of CD24 in contrast to those in WT → B2m−/ − chimeras (Figure 3B). Nonetheless, the total number of CD122+ TCRβ + CD8 T cells in Id3−/ − → B2m−/ − chimeras was less than 5% of Id3−/ − → WT chimeras (Figure 3E). Importantly, the majority of CD8 thymocytes in Id3−/ − → WT chimeras had an innate-like phenotype when analyzed 6 weeks after transplantation (Figure S2). Taken together, these data demonstrate that selection of Id3−/ − CD8 T cells required MHC class I molecules on TECs.
Figure 3.
Id3−/ − innate-like CD8 T cells were selected on non-hematopoietic cells. (A–D) Flow cytometric analysis of thymocytes derived from chimeric mice 6 weeks after transplantation. (A and C) CD4 and CD8 expression on total thymocytes (upper panels), TCRβ expression on total thymocytes (middle panels) and CD4 and CD8 expression on TCRβ + thymocytes (lower panels). Expression of CD122, CD44 or CD24 on TCRβ +CD8 thymocytes in (B) WT to B2m−/ − chimeras (grey) or Id3−/ − to B2m−/ − chimeras (black) or (D) H2-KbH2-Db−/ − to WT (grey) or Id3−/ −H2-KbH2-Db−/ − to WT chimeras. (E) Average number (+ standard deviation) of CD122+TCRβ +CD8 thymocytes in chimeric mice from the indicated donor and recipient combinations. n> 3 for each combination. P-values are shown for populations at the end of each line. Three independent experiments were performed. See Figure S2 for analysis of the innate-like phenotype on Id3−/ − to WT chimeras.
The innate-like CD8 T cells in Id3−/ − mice were selected on TECs; however, our experiments did not rule out the possibility that these cells might also require interaction with MHC class Ia on hematopoietic cells. To address this issue we created chimeric mice in which Id3−/ − hematopoietic cells lack MHC class Ia but non-hematopoietic cells express MHC class Ia. As expected, in H2-KbH2-Db−/ − → WT chimeric mice CD8 T cells developed at frequencies similar to that in WT → WT chimeras (Figure 3A, D). Importantly, in Id3−/ −H2-KbH2-Db−/ − → WT chimeras TCRβ + CD8 thymocytes developed at least as well as they did in H2-KbH2-Db−/ − → WT chimeras (Figure 3C). The frequency and total number of TCRβ + CD8 thymocytes with the innate-like phenotype in Id3−/ −H2-KbH2-Db−/ − → WT chimeras was similar to, or slightly greater than that in Id3−/ − mice (Figure 3D, E). These findings confirm that selection of innate-like CD8 T cells in Id3−/ − mice, like conventional CD8 T cells, required MHC class Ia on TECs but not hematopoietic cells.
Development of Id3−/ − innate-like CD8 thymocytes required SLAM-Associated Protein
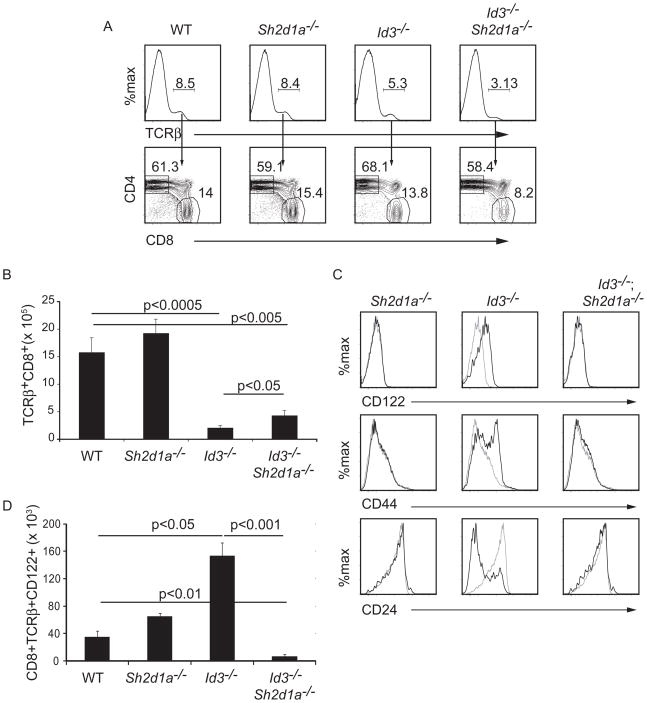
A previous study showed that the innate-like CD8 T cells that develop in Itk−/ − mice and innate-like CD4 cells in CIITA-transgenic mice, like NKT cells, are selected through a SAP-dependent pathway (Griewank et al., 2007; Horai et al., 2007; Li et al., 2007a). SAP is an adapter protein that organizes signaling from the SLAM family receptors, whose expression is restricted to hematopoietic cells (Detre et al., 2010). Therefore, although SAP is required when thymocytes are selected on hematopoietic cells (Veillette et al., 2007), a requirement for SAP in thymocytes undergoing positive selection on TECs is unprecedented. Given this, we tested whether development of innate-like CD8 T cells in Id3−/ − mice required SAP by creating Id3−/ −Sh2d1a−/ − mice. Deletion of Sh2d1a, which encodes SAP, resulted in an increase in the total number of thymocytes in WT and Id3−/ − mice, as we noted previously (Verykokakis et al., 2010). Importantly, although deletion of Sh2d1a had a minor effect on the frequency of TCRβ + CD8 T cells in the thymus of Id3−/ − mice, the number of TCRβ + CD8 T cells was higher in Id3−/ −Sh2d1a−/ − mice than in Id3−/ − mice (Figure 4A, B). However, the TCRβ + CD8 thymocytes in Id3−/ −Sh2d1a−/ − mice did not have an innate-like phenotype (Figure 4C). Indeed, the total number of CD122+ TCRβ + CD8 T cells in Id3−/ −Sh2d1a−/ − mice was approximately 1% of the number in Id3−/ − mice (Figure 4D). Taken together, these data demonstrated that the adaptor protein SAP was not required for selection of CD8 T cells in Id3−/ − mice but was required for development of the innate-like phenotype on these cells.
Figure 4.
SAP was required for the innate-like phenotype of Id3−/ − CD8 thymocytes. (A) WT, Id3−/ − or Sh2d1a−/ − and Id3−/ −Sh2d1a−/ − thymocytes were analyzed by flow cytometry for TCRβ (upper) and TCRβ + cells were analyzed for CD4 and CD8 (lower). The frequency of TCRβ +cells and CD4+ or CD8+ cells among TCRβ + cells is indicated. (B) Average number of TCRβ + CD8 thymocytes in the thymus of mice with the indicated genotype. n > 6 for each genotype, p-values are shown for the comparison of populations at the end of each line. (C) Expression of CD122, CD44 or CD24 on TCRβ +CD8 thymocytes from WT (grey) or mutant mice (black). (D) Average number of CD122+TCRβ + CD8 thymocytes in the thymus of mice with the indicated genotype. n > 5 for each genotype, p-values are shown for the comparison of populations at the end of each line. Data are representative of > 5 experiments.
SAP was required for development of an expanded population of PLZF+ thymocytes in Id3−/ − mice
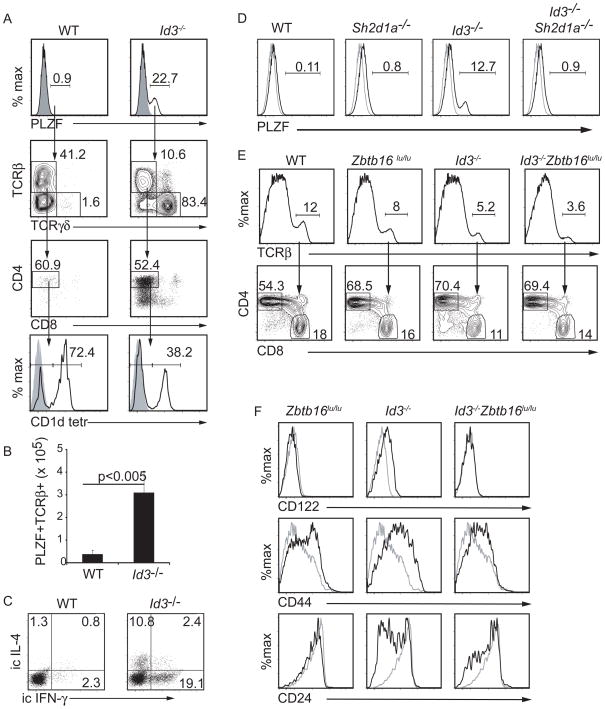
Given that SAP was not required for positive selection of Id3−/ − CD8 T cells but was required for the innate-like phenotype we considered the possibility that this phenotype was an indirect consequence of the SAP-dependent lymphocytes in Id3−/ − mice. We and others, have identified an expanded population of PLZF+ SAP-dependent γ δ NKT cells in Id3−/ − mice (Alonzo et al., 2009; Ueda-Hayakawa et al., 2009; Verykokakis et al., 2010). However, in Id3−/ − mice the increase in PLZF+ cells extended beyond the γ δ NKT cell subset. We found approximately 25-fold more PLZF+ thymocytes in Id3−/ − mice than in WT mice (Figure 5A). Although a subset of these cells expressed γ δ TCR, there were approximately 10-fold more TCRβ + PLZF+ cells and the majority of these cells expressed CD4 but not CD8 (Figure 5A, B). Moreover, many of the CD4+ cells failed to bind CD1d tetramer indicating that they were not iNKT cells, which are the major CD4+TCRβ +PLZF+ cells in the WT thymus (Figure 5A). Importantly, like the γ δ NKT cells described in these mice (Alonzo et al., 2009; Verykokakis et al., 2010), a subset of TCRβ +CD4+ cells produced both IFN-γ and IL-4 after a 5 hour stimulation with PMA plus ionomycin (Figure 5C). Importantly, development of this expanded population of PLZF+ cells was SAP-dependent (Figure 5D). These data demonstrated that there was an expanded population of SAP-dependent PLZF+ α β and γ δ NKT cells in Id3−/ − mice that was poised to produce IFN-γ and IL-4.
Figure 5.
SAP-dependent PLZF+ T cells were required for the innate-like CD8 phenotype in Id3−/ − mice. (A) WT and Id3−/ − thymocytes were examined by flow cytometry for intracellular PLZF (upper, grey histogram is isotype control) and PLZF+ cells were analyzed for TCRβ and TCRγ δ . PLZF+TCRβ + cells were analyzed for CD4 and CD8. PLZF+TCRβ +CD4+ cells analyzed for staining with CD1d tetramer loaded with PBS57 (lower, grey histogram is unloaded tetramer). (B) Average number (+ standard deviation) of PLZF+TCRβ + cells in the thymus of WT and Id3−/ − mice. n > 5 for each genotype. (C) CD4 thymocytes were analyzed for intracellular expression of IL4 and IFNγ 5 hours after stimulation with PMA + ionomycin. (D) Intracellular staining for PLZF (black line) in mice of the indicated genotype. Isotype control (grey). (E) WT, Zbtb16lu/lu, Id3−/ − and Id3−/ −Zbtb16lu/lu thymocytes were analyzed by flow cytometry for TCRβ (upper) and TCRβ + cells were analyzed for CD4 and CD8 (lower). The frequency of TCRβ + cells and CD4 or CD8 cells among TCRβ + cells is indicated. (E) Expression of CD122, CD44 or CD24 on TCRβ +CD8 thymocytes from WT (grey) or mutant mice (black). Data is representative of 3 experiment.
PLZF was required for the innate-like CD8 phenotype
Our data suggest that the expanded population of SAP-dependent PLZF+ lymphocytes in Id3−/ − mice was required for the innate-like phenotype of CD8 T cells. Many of the unique features of SAP-dependent NKT cells are regulated by PLZF, which is sufficient to confer the ability to produce both Th1 and Th2 cell cytokines such as IFN-γ and IL-4 (Kovalovsky et al., 2008; Savage et al., 2008). To demonstrate that NKT cells were necessary for the innate-like phenotype on CD8 thymocytes we created mice that lacked Id3 and carried a point mutation in the gene that encodes PLZF (Zbtb16) that results in the loss of functional PLZF (Green, 1961; Savage et al., 2008). The frequency of CD8 cells among TCRβ + thymocytes was similar between Id3−/ −Zbtb16lu/lu mice and WT, Zbtb16lu/lu and Id3−/ − mice. (Figure 5E). Zbtb16lu/lu CD8 T cells did not express CD122 but had slightly increased expression of CD44 compared to WT CD8 cells, although this did not reach the levels seen in Id3−/ − CD8 cells. Importantly however, CD8 thymocytes in Id3−/ −Zbtb16lu/lu mice resembled those in Zbtb16lu/lu mice in that they did not express CD122, had intermediate expression of CD44 and few cells were CD24lo (Figure 5F). Given that PLZF was not expressed in Id3−/ − CD8 thymocytes, our findings demonstrated a requirement for NKT cells in establishing the innate-like phenotype of CD8 T cells.
The innate-like CD8 phenotype was not CD8 T cell intrinsic
The observations that the innate-like CD8 thymocytes in Id3−/ − mice were not selected on hematopoietic cells where SLAM receptors are expressed and yet their development required SAP and PLZF suggested that this phenotype was not an intrinsic property of Id3−/ − CD8 cells. To address this question we established chimeric mice in which a majority of thymocytes (>85%) were Id3−/ − and a minority (<15%) were WT and investigated the phenotype of WT CD8 T cells that developed in an Id3−/ − hematopoietic environment (Figure 6A). As expected, in control mice transplanted with a mix of CD45.2 and CD45.1 WT bone marrow, the CD8 T cells derived from the majority and minority population had a conventional CD8 T cell phenotype (Figure S3). In chimeric mice with a majority of Id3−/ − thymocytes and a minority of WT thymocytes the majority Id3−/ − CD8 T cells had an innate-like phenotype (Figure 6B). However, in these chimeric animals the minority WT CD8 T cells also had an innate-like phenotype with high expression of CD122 and CD44 and low expression of CD24 (Figure 6B). In addition, in chimeric mice where WT thymocytes were the majority and Id3−/ − thymocytes were the minority, neither the WT nor the Id3−/ − CD8 T cells had an innate-like phenotype (Figure 6A, B). In contrast to the innate-like phenotype on CD8 cells, the heightened expression of PLZF was an intrinsic property of the Id3−/ − thymocytes because WT thymocytes, when present as a minority population in the presence of Id3−/ − thymocytes, did not acquire PLZF expression (Figure S3). In contrast, when Id3−/ − thymocytes were in the minority they continued to have an elevated frequency of PLZF+ cells (Figure S3). Importantly, Id3−/ − thymocytes, whether in the majority or the minority population, like WT thymocytes, showed low expression MHC class II indicating that aberrant expression of MHC class II was not the reason for the increased number of PLZF+ CD4 cells, as was observed in human fetal and perinatal thymocytes (Lee et al., 2010). Taken together, these data demonstrated that the innate-like phenotype was not intrinsic to CD8 T cells but rather, was imprinted on these cells by a distinct subset of hematopoietic cells that lack Id3.
Figure 6.
Unequal chimeras reveal that the innate-like phenotype was not CD8 cell autonomous. (A) Mixed bone marrow chimeras were established using an excess of Id3−/ − cells (>85%, CD45.2) and a minority of WT cells (<15%, CD45.1) (left) or an excess of WT cells (>90%, CD45.1) and a minority of Id3−/ − cells (<10%, CD45.2) (right). Cells were injected into irradiated CD45.2 mice. (B) TCRβ +CD8 thymocytes (black line) from the majority and minority populations were analyzed for CD122 (upper), CD44 (middle) and CD24 (lower). Isotype control (grey). (C) Mixed bone marrow chimeras were established using an excess of WT cells (>70%, CD45.2) and a minority of Sh2d1a−/ − cells (<30%, CD45.1) (left) or an excess of Id3−/ − cells (>75%, CD45.1) and a minority of Sh2d1a−/ − cells (<25%, CD45.2) (right). (D) TCRβ +CD8 thymocytes (black) from the majority and minority populations were analyzed for CD122 (upper), CD44 (middle) and CD24 (lower). Isotype control (grey). Data are representative of > three experiements. See Figure S3 for experimental controls and analysis of PLZF expression.
We hypothesized that the requirement for SAP in development of innate-like CD8 thymocytes reflected a role for SAP in selection of PLZF+ NKT cells and was not due to an intrinsic requirement for SAP in CD8 thymocytes. To test this hypothesis we established unequal chimeras in which Id3−/ − thymocytes (or WT thymocytes for the control) were in the majority and Sh2d1a−/ − thymocytes were in the minority (Figure 6C). Under these conditions, a large population of SAP-dependent PLZF+ cells will develop from the majority Id3−/ − hematopoietic cells but SAP cannot function in the minority Sh2d1a−/ − CD8 thymocytes. When Id3−/ − thymocytes but not WT thymocytes were in the majority, we found that CD8 T cells in the minority Sh2d1a−/ − population acquired the innate-like phenotype even though they lacked the adaptor protein SAP (Figure 6D). Therefore, the requirement for SAP signaling is not an intrinsic property of innate-like CD8 cells and reflects a requirement for SAP in the hematopoietic cells of Id3−/ − mice.
IL-4 is required for the innate-like CD8 phenotype
Our data demonstrate that NKT cells are responsible for the innate-like phenotype of CD8 thymocytes in Id3−/ − mice. One of the unique features of NKT cells, which is a consequence of PLZF expression, is their ability to produce both IFN-γ and IL-4 (Kovalovsky et al., 2008; Savage et al., 2008). Indeed, a previous study in Itk−/ − mice revealed a pathogenic consequence of IL-4 production by γ δ NKT cells in these mice, resulting in hyper-IgE production (Felices et al., 2009). Therefore, we considered the possibility that cytokines produced by NKT cells might be responsible for their ability to confer the innate-like phenotype on CD8 thymocytes. Flow cytometric analysis of Id3−/− CD8 thymocytes revealed expression of the receptor for IL-4 (IL-4Rα ), which was not expressed detectably on WT CD8 thymocytes (Figure 7A). Moreover, IL-4Rα was induced on WT CD8 T cells in chimeric mice where Id3−/ − hematopoietic cells were in the majority (Figure. 7B). The expression of IL-4Rα on Id3−/ − CD8 T cells required PLZF indicating that it is the NKT cells in Id3−/ − mice that promote the upregulation of IL-4Rα (Figure 7A). Because Il4ra is upregulated in response to IL-4, we hypothesized that heightened production of IL-4 in vivo was the mechanism by which NKT cells impose the innate-like phenotype on CD8 T cells. To test the requirement for IL-4 we created Id3−/ −Il4−/ − mice and examined the phenotype of CD8 thymocytes. Importantly, IL4Rα was not expressed detectably on the CD8 T cells from Id3−/ −Il4−/ − mice indicating that IL4Rα was induced in response to IL-4 (Figure 7A). Moreover, although PLZF+ NKT cells were still present in Id3−/ −Il4−/ − mice (Figure 7C), the CD8 T cells in these animals did not have an innate-like phenotype (Figure 7D, E). Taken together, our data indicate that development of innate-like CD8 T cells was a consequence of IL4 produced by SAP- and PLZF-dependent NKT cells.
Figure 7.
IL4 was necessary for the innate-like phenotype on Id3−/ − CD8 thymocytes. (A) FACS analysis for IL4Rα on CD8 thymocytes from Id3−/ − (left), Id3−/ − Zbtb16lu/lu (middle) and Id3−/ −Il4−/ − (right) mice compared to WT (grey). (B) IL4Rα expression on WT minority cells that developed with a majority of WT (left) or Id3−/ − (right). Isotype control (shaded histogram). (C) Intracellular PLZF in CD8 thymocytes from mice of the indicated genotype compared to isotype control (shaded). (D) WT, IL4−/ −, Id3−/ − and Id3−/ −IL4−/ − thymocytes were analyzed for TCRβ (upper) and CD4 versus CD8 on TCRβ + thymocytes (lower). (E) CD8 thymocytes from mice of the indicated genotype (black) or WT mice (grey) were analyzed for CD122 (upper), CD44 (middle) or CD24 (lower). Grey histogram is WT CD8 thymocytes. Data are representative of > 4 experiments.
DISCUSSION
We showed here that during thymocyte development Id3 was required to prevent CD8 T cells from adopting an innate-like fate characterized by expression of a memory surface marker phenotype, expression of the transcription factor Eomesodermin, the ability to produce IFNγ rapidly after TCR-mimicking stimuli, and a dependence on the SAP signaling pathway. These observations appeared consistent with the hypothesis that Id3 is the essential transcriptional effector of the Itk-Erk-MAP kinase signaling pathway that is initiated by TCR ligation since a similar phenotype has been reported in Itk−/ − and Itk−/ −Rlk−/ − mice (Atherly et al., 2006; Broussard et al., 2006; Horai et al., 2007). However, in contrast to what was reported for Itk−/ − mice, CD8 T cells in Id3−/ − mice underwent positive selection via interaction with MHC class I on TEC. Importantly, we found that the innate-like phenotype of Id3−/ − CD8 T cells was not intrinsic to the CD8 cells but was a consequence of SAP-dependent PLZF+ NKT cells. Moreover, we localized the mechanism by which NKT cells function to the production of IL-4, because IL-4 was necessary for the innate-like phenotype of Id3−/ − CD8 thymocytes. Interestingly, the innate-like CD8 phenotype in Itk−/ − and Klf2−/ − mice shows a similar dependence on PLZF+ cells indicating that multiple mutant mouse strains with increased NKT cell numbers develop an innate-like population of CD8 T cells (Weinreich et al., 2010). Therefore, our data reveal that the innate-like versus conventional fate of CD8 T cells was instructed by IL-4 produced by NKT cells.
The development of innate-like CD8 cells in Itk−/ − and Itk−/ −Rlk−/ − mice was proposed to occur as a consequence of alterations in TCR signal strength during selection of CD8 T cells (Prince et al., 2009). This explanation seems unlikely given that WT CD8 thymocytes adopt the innate-like phenotype when they develop in a hematopoietic environment with a preponderance of Id3−/ − lymphocytes. Nonetheless, Id3−/ − thymocytes, as well as Itk−/ − and Rlk−/ − thymocytes do have alterations in perceived TCR signal strength because these mice show less efficient positive and negative selection in transgenic and super-antigen deletion models (Liao and Littman, 1995; Lucas et al., 2002; Rivera et al., 2000; Schaeffer et al., 2000). However, expression of CD5, a frequently used indicator of TCR signal strength, differs between Id3−/ − and Itk−/ − or Itk−/ −Rlk−/ − thymocytes. Compared to wild-type controls, Itk−/ − and Itk−/ −Rlk−/ − DP thymocytes express lower CD5 whereas Id3−/ − DP thymocytes have increased expression of CD5 (Lucas et al., 2002; Rivera et al., 2000), and data not shown). Therefore, deletion of Itk or Rlk and Id3 may have different effects on TCR signaling pathways indicating that they may not function in a linear pathway.
We, and others, have shown that the absence of Id3 leads to a profound expansion of γ δ NKT cells expressing a canonical Vγ 1.1Vδ 6.3 TCR and PLZF, which require SAP for their survival and rapidly produce both IFNγ and IL-4 after stimulation (Alonzo et al., 2009; Verykokakis et al., 2010). A similar subset of γ δ NKT cells exists in Itk−/ − mice and IL-4 production from these cells was shown to result in increased serum IgE (Felices et al., 2009). We demonstrated that the absence of Id3 led to the accumulation of a diverse population of PLZF+ cells including cells with α β or γ δ TCRs. The majority of the Id3−/ − TCRβ + PLZF+ cells were not conventional (Vα 14+) iNKT cells as determined by binding of CD1d tetramer loaded with PBS57; however, the identity of the majority of Id3−/ − TCRβ + NKT cells remains to be fully explored. Nonetheless, it is important to note that the innate-like TCRβ + CD8 cells in Id3−/ − mice are distinct from NKT cells in that they do not express PLZF and they do not produce IL-4 after stimulation (data not shown).
How does Id3 deficiency lead to expansion of PLZF+ cells? Our data do not support a role for Id3 in repression of PLZF because, at least in γ δ T cells, PLZF remains restricted to cells with canonical receptors that are normally associated with expression of PLZF (Verykokakis et al., 2010). This finding suggests that cells with the appropriate TCR specificity are either expanding preferentially or surviving better than in WT mice. γ δ NKT cells do not hyper-proliferate in Id3−/ − mice leading us to propose that E proteins promote survival of γ δ NKT cells and, by analogy, other PLZF+ cell types. It was shown for γ δ NKT cells that PLZF expression is a consequence of strong TCR signaling and the cells that express PLZF are generally reactive with endogenous ligands (Azuara et al., 1997; Kreslavsky et al., 2009). However, while TCR cross-linking of immature CD24high γ δ T cells results in induction of PLZF, cross-linking of mature splenic CD24lo γ δ T cells led to the loss of these cells from culture (Kreslavsky et al., 2009). This observation suggests that high affinity ligand recognition in the thymus could induce PLZF but subsequent ligand recognition in the periphery may lead to cell death. Therefore, it is tempting to speculate that the failure to induce Id3 downstream of strong TCR signaling may abrogate the death incurred by mature self-reactive NKT cells thereby allowing an accumulation of these cells in vivo.
PLZF is sufficient to confer an innate-like phenotype on CD4 and CD8 T cells including expression of a memory surface marker phenotype and the ability to produce both IFNγ and IL4 (Kovalovsky et al., 2008; Raberger et al., 2008; Savage et al., 2008). However, the mechanisms leading to PLZF induction have not been fully elucidated. Previous studies showed that expression of CIITA, a transcriptional activator of MHC Class II, in DP thymocytes results in PLZF expression and the innate-like phenotype on CD4 thymocytes (Li et al., 2005). Development of these innate-like CD4 cells also requires SAP signaling indicating that positive selection of thymocytes on DP cells allows engagement of SLAM family receptors and acquisition of an alternative developmental fate (Li et al., 2007a). However, the relationship between SLAM-SAP and PLZF is not known. Activation of this pathway is a physiological process for NKT cells that undergo selection on CD1d, which is expressed on DP thymocytes (Coles and Raulet, 2000; Park et al., 1998; Wei et al., 2005), and in humans, MHC class II is expressed on DP thymocytes during embryonic development resulting in a population of MHC class II restricted PLZF+ CD4 cells with innate characteristics (Kovalovsky et al., 2008; Lee et al., 2010; Li et al., 2005; Savage et al., 2008). One explanation for the expanded population of TCRβ + CD4+ PLZF+ T cells in Id3−/ − mice, therefore, would be that MHC class II is expressed on DP thymocytes. However, if this were the case we would expect that the minority WT population in our mixed bone marrow chimeras, where Id3−/ − cells are the majority, would also develop a significant population of PLZF+ T cells; however, we showed that this was not the case. Therefore, the Id3−/ − DP cells do not express sufficient MHC class II to cause selection of WT CD4 T cells into the NKT pathway. In addition, Id3−/ − thymocytes did not show any obvious increase in MHC class II expression.
We showed that IL-4 was the critical NKT cell product that induced the innate-like phenotype on CD8 thymocytes. CD8 T cells in Id3−/ −Il4−/ − mice did not have an innate-like phenotype even though these mice had an expanded population of NKT cells. Therefore, IL-4 was not required for the NKT cell expansion, in contrast to SAP. IL-4 can induce Eomes in CD8 T cells and Eomesodermin is involved in the transcription of Il2rb and Ifng (Intlekofer et al., 2005; Pearce et al., 2003; Takemoto et al., 2006), three of the defining characteristics of the innate-like phenotype. However, although IL-4 is clearly necessary for the innate-like CD8 phenotype we cannot rule out the possibility that other NKT-derived products might also affect the phenotype of these cells.
The development of innate-like CD8 T cells is a common feature of mutant mouse strains that develop expanded populations of PLZF+ T cells (Felices et al., 2009; Odumade et al., 2010; Weinreich et al., 2010). However, innate-like CD8 T cells are readily detected in WT Balb/c mice, where their development is dependent on iNKT cells (Weinreich et al., 2010), and they are likely present in humans during embryonic and perinatal stages (Lee et al., 2010). Because these cells are able to produce IFN-γ rapidly after TCR stimulation without prior antigen exposure they may provide a rapid first line of defense against invading pathogens. However, mature CD122+ CD8 cells have been shown to segregate into two distinct functional groups based on the expression PD1 and production of IL-10 (Dai et al., 2010), the PD1+ IL-10 producing subset having properties similar to regulatory T cells (Dai et al., 2010; Endharti et al., 2005; Rifa’i et al., 2008). These observations raise the intriguing possibility that innate-like CD8 T cells might function to modulate the immune response. Because deletion of Id3 reduces perceived strength of TCR signaling the CD8 cells that arise in these mice could have an altered repertoire, possibly including cells with higher affinity for self, making their selection into a regulatory pathway beneficial. Future studies will be necessary to fully address the function of innate-like CD8 cells.
Our results also raise the question of whether activation of NKT cells during an immune response might alter the phenotype and function of bystander CD8 T lymphocytes. Activation of CD8 T cells has been observed rapidly after injection of mice with the NKT cell ligand α GalCer suggesting the plausibility of such an effect (Carnaud et al., 1999). Such bystander activation could have implications for NKT cell based immunotherapies and for CD8 T cell function during immune responses that elicit strong NKT cell activation. In Itk−/ −, Itk−/ −Rlk−/ −, and Id3−/ − mice the innate-like phenotype of CD8 T cells is initiated already within the DP population suggesting that NKT cells are influencing thymocytes during positive selection. However, this does not exclude the possibility that CD8 T cells in the periphery could acquire the innate-like phenotype in the presence of activated NKT cells. Indeed, peripheral CD8 T cells in Id3−/ − mice do have the innate-like phenotype suggesting that they are either exposed to NKT cell-derived signals, or that the innate-like phenotype, once acquired, can not be reversed. Further studies will be needed to determine whether NKT cells can induce distinct functional properties to CD8 T cells during an immune response. Nonetheless, our data demonstrate that expansion of NKT cells or hyper-activation of these cells is the cause of the innate-like or unconventional fate of CD8 T cells.
MATERIALS AND METHODS
Mice and genotyping
Id3−/ −, β 2m−/ −, H2-KbH2-Db−/ −Zbtb16lu/ (Luxoid, B6.C3-Zbtb16Lu/J) and Il4−/ − mice on a C57Bl/6J background were purchased from The Jackson Laboratory and have been described elsewhere (Atherly et al., 2006; Broussard et al., 2006; Buaas et al., 2004; Pan et al., 1999). Id3−/ − SJL (CD45.1) were generated in house. Sh2d1a−/ − mice were a gift from C. Terhorst. Mice were housed at The University of Chicago Animal Resource Center and experiments were performed according to the guidelines of The University of Chicago Institutional Animal Care and Use Committee. Id3−/ − mice were genotyped by PCR using the following primers: Id3KO: 5’-GCCAGAGGCCACTTGTGTAG-3’, Id3Com: 5’-CATTCTCGGAAAAGCCAGTG-3’ and Id3WT: 5’-CTCCCTCGCTCTTCTCTCCT-3’. The Sh2d1a wild-type allele was detected using SAPWTF1: 5’-GATGATCAAACCCAGACAAGTTTGAAGC-3’ and SAPWTR1: 5’-TCTTTGATGGGCCCAGAAGAAGGATGG-3’, the Sh2d1a mutation was detected using SAPKOF1: 5’-CCTTGCAGCCATGTTCTGCTGTGG-3’ and SAPKOR1: 5’-GCAGGCATGCTGGGGATGCGG-3’. Luxoid mice were genotyped with the following primers: Zbtb16 for geno: 5’-CCAGGCATCTGATGACAATG-3’ and Zbtb16rev geno: 5’-CCCCTCTTTGCTCCTCTCTT-3’. The PCR product was purified and sequenced to identify the lu mutation with the following primers: Zbtb16 for seq: 5’-AGAGGACCGTAAGGCTCGAT-3’ or Zbtb16 rev seq: 5’-CCATCCCTCCTGAGATGCTA-3’. Il4−/ − mice were genotyped using the following primers: IL4 WT: 5’-GCACAGAGCTATTGATGGGTC-3’, IL4 Com: 5’-GCTGTGAGGACGTTTGGC-3’ and IL4 KO: 5’-TCAGGACATAGCGTTGGC-3’. H2- KbH2-Db−/ − mice were genotyped by flow cytometry after staining peripheral blood with anti-H2-Kb-FITC (BD Biosciences).
Antibodies, Flow Cytometry and Cell Sorting
Thymuses and spleens from four to eight week old mice, or one week old neonates, were stained with anti-FcγR prior to staining with antibodies conjugated to biotin, FITC, PE, PE-Cy7 or APC, acquired in a FACS Canto machine and analysed with FLOWjo software. Dead cells were excluded with PI. Cells were sorted on a FACSAria. Anti-CD4, -CD8β , -TCRβ , -TCRγ δ , -CD122, -CD44, -CD24, -IFNγ, -IL4, -CD124, -MHCII were purchased from BD Biosciences or eBiosciences. α-PLZF antibody was from Santa-Cruz (clone D9, Cat No: sc-28319). And was conjugated with Pacific Blue using the Pacific Blue Monoclonal Antibody Labeling Kit (Invitrogen, Cat No: P30013). APC conjugated Cd1d tetramers, unloaded or loaded with PBS57, were from the NIH Tetramer Facility at Emory University.
Cell Culture, IFNγ production and intracellular staining
Treatment of thymocytes or splenocytes with phorbol 12-myristate 13-acetate (PMA) plus ionomycin and intracellular staining for IFNγ were described previously (Atherly et al., 2006). Intracellular staining for IFNγ and IL4 was performed with the BD Biosciences Cytofix/Cytoperm kit (Cat No: 51-2090KZ).
Adoptive transfers of bone marrow
Bone marrow was prepared from femur and tibiae. Recipient mice were lethally irradiated (1000 rad) at least 5h prior to i.v. injection of 5x106 cells. Thymocytes and splenocytes were analyzed 6, 8 or 12 weeks after transfer. For mixed bone marrow chimeras, bone marrow cells were mixed in the proportions mentioned prior to transfer of 5X106 cells into recipient mice. Thymocytes analyzed 6–8 weeks after transfer.
RNA isolation and Real-time Quantitative (Q)PCR
Total RNA was extracted and DNAase-treated from sorted TCRβ +CD4 and TCRβ +CD8 thymocytes using the RNAeasy mini kit (QIAGEN) and was reverse-transcribed using Superscript III (Invitrogen). QPCR was performed with gene-specific primers in a iCycler (BioRad), using the iQ SYBR Green Supermix (BioRad). Results were normalized and quantified with the manufacturer’s software (MyiQ, Biorad). A standard curve was included for every primer set. Eomesodermin primers: EomesFor: 5’-TCATCACCAAACAGGGCA GG-3’ and EomesRev: 5’-CGAAAACATTGTAGTGGGCGG-3’. Hprt primers were reported previously (Boos et al 2007).
Supplementary Material
Acknowledgments
We thank members of the Kee and Bendelac labs for helpful discussions, C. Spaulding for excellent technical help, and C. Terhorst for generously providing the Sh2d1a−/ − mice. We also thank K. Hogquist for communicating data prior to publication. This work was supported by the NIH (R01 CA99978) and a Leukemia and Lymphoma Society Scholar award to B.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant’angelo DB. Development of Promyelocytic Zinc Finger and ThPOK-Expressing Innate {gamma}{delta} T Cells Is Controlled by Strength of TCR Signaling and Id3 3. J Immunol. 2009 doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–540. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting Edge: Programmed Death-1 Defines CD8+CD122+ T Cells as Regulatory versus Memory T Cells. J Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010 doi: 10.1007/s00281-009-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe K, Suzuki H. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med. 2001;194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Mol Cell Biol. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MC. The position of luxoid in linkage group II of the mouse. J Hered. 1961;52:297–300. doi: 10.1093/oxfordjournals.jhered.a107103. [DOI] [PubMed] [Google Scholar]
- Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207:237–246. S231–237. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–386. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007a;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sofi MH, Yeh N, Sehra S, McCarthy BP, Patel DR, Brutkiewicz RR, Kaplan MH, Chang CH. Thymic selection pathway regulates the effector function of CD4 T cells. J Exp Med. 2007b;204:2145–2157. doi: 10.1084/jem.20070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Liu G, Grifman M, Keily B, Chatterton JE, Staal FW, Li QX. Mineralocorticoid receptor is involved in the regulation of genes responsible for hepatic glucose production. Biochem Biophys Res Commun. 2006;342:1291–1296. doi: 10.1016/j.bbrc.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Atherly LO, Berg LJ. The absence of Itk inhibits positive selection without changing lineage commitment. J Immunol. 2002;168:6142–6151. doi: 10.4049/jimmunol.168.12.6142. [DOI] [PubMed] [Google Scholar]
- Odumade OA, Weinreich MA, Jameson SC, Hogquist KA. Kruppel-like factor 2 regulates trafficking and homeostasis of gammadelta T cells. J Immunol. 2010;184:6060–6066. doi: 10.4049/jimmunol.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19:5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Prince AL, Yin CC, Enos ME, Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol Rev. 2009;228:115–131. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberger J, Schebesta A, Sakaguchi S, Boucheron N, Blomberg KE, Berglof A, Kolbe T, Smith CI, Rulicke T, Ellmeier W. The transcriptional regulator PLZF induces the development of CD44 high memory phenotype T cells. Proc Natl Acad Sci U S A. 2008;105:17919–17924. doi: 10.1073/pnas.0805733105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of gd Lineage during thymopoiesis. J Immunol. 2009 doi: 10.4049/jimmunol.0804249. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Dong Z, Latour S. Consequence of the SLAM–SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007;27:698–710. doi: 10.1016/j.immuni.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, Kee BL. Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent “innate” gammadelta T cells. PLoS One. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8(+) T cells. Nat Immunol. 2010 doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.