Abstract
An inability to maintain abstinence is a key indicator of tobacco dependence. Unfortunately, little evidence exists regarding the ability of the major tobacco dependence measures to predict smoking cessation outcome. This paper used data from four placebo-controlled smoking cessation trials and one international epidemiologic study to determine relations between the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991), the Heaviness of Smoking Index (HSI; Kozlowski et al., 1994), the Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al. 2004) with cessation success. Results showed that much of the predictive validity of the FTND could be attributed to its first item, time to first cigarette in the morning, and this item had greater validity than any other single measure. Thus, the time to first cigarette item appears to tap a pattern of heavy, uninterrupted, and automatic smoking and may be a good single-item measure of nicotine dependence.
Introduction
Improvements in smoking cessation treatment require a better understanding of nicotine dependence and of other factors that impede the ability of smokers to abstain from tobacco use. Several important questions exist with respect to the relation between nicotine dependence and ability to quit smoking. For instance, there is some ambiguity as to which tobacco dependence measures show the strongest associations with cessation success (e.g., Etter, 2005; Piper et al., 2006). In addition, while some dependence measures do predict ability to quit smoking, there is little understanding of this relation: i.e., the nature of the mechanism(s) via which dependence influences the ability to quit. If cessation ability can be accurately predicted by such measures, they may be used to adjust treatment (e.g., less able individuals would receive stronger treatments). In addition, such measures might be relevant to genetics research. An index of inability to cease tobacco use might be an important phenotypic candidate for genetic mapping. Finally, an accurate prognosticator of cessation success might illuminate the nature of tobacco dependence.
At present, there is ambiguity regarding the relative predictive validities of various dependence measures. Some measures [e.g., the Fagerström Test of Nicotine Dependence (FTND); Heatherton et al., 1991] have not performed consistently across studies, predicting outcomes in some studies but not others (Etter, 2005; Ferguson et al., 2003; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994; Piper et al., 2006). In addition, there are new measures that have not yet been adequately validated e.g., the Nicotine Dependence Syndrome Scale (the NDSS; Shiffman et al., 2004), the Tobacco Dependence Screener (TDS; a self-report of DSM symptoms; Kawakami, Takatsuka, Inaba, & Shimiz, 1999) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al. 2004).
To date, the original Fagerström Tolerance Questionnaire (FTQ; Fagerström, 1978) and its derivatives, the FTND (Heatherton et al., 1991) and the Heaviness of Smoking Index (HSI; Kozlowski et al., 1994) have been the most widely studied. Compared with the FTQ, the FTND has demonstrated better psychometric properties such as internal consistency (Haddock et al., 1999), and ability to predict cessation outcomes in some studies (Alterman et al., 1999; Campbell et al., 1996; Patten et al., 2001; Westman et al., 1997). The HSI, a scale comprising two FTND items (those that assess the time to smoke the first cigarette of the day after awakening and the number of cigarettes smoked per day), accounts for much of the predictive validity of the FTND (e.g., Heatherton et al., 1989). The HSI predicts both behavioral and biochemical indices of smoking (Breslau & Johnson, 2000; Heatherton et al., 1989; 1991; Kozlowski et al., 1994; Prokhorov et al., 2000) and has been shown to reflect a highly heritable component of dependence (Lessov et al., 2004), although the latency to smoke the first cigarette in the morning may be the most highly heritable item (Haberstick et al., 2007). There is evidence that the full FTND is multifactorial (e.g., Haddock et al. 1999) rather than unidimensional; i.e., comprising two or more distinct factors. Thus, it is possible that only a subset of items predicts cessation success.
Since the development of the FTQ-based measures, two multifactorial measures of nicotine dependence have emerged, both of which were developed based on a multidimensional conceptualization of dependence (Piper et al., 2004; Shiffman et al., 2004). The first, the NDSS (Shiffman et al., 2004), predicts dependence criteria such as number of cigarettes smoked per day, withdrawal elements (e.g., urge intensity), and latency to return to smoking. However, the extent to which the NDSS and its subscales can predict relapse is unknown. The second new measure, the WISDM (Piper et al., 2004) has 13 subscales, and only one study has examined each subscale's ability to predict relapse (Piper et al., 2004).
This paper presents data derived from three large clinical trials (including two with focused, real-time process measures) conducted in Madison and Milwaukee, WI, one clinical trial conducted in New Haven, CT, and one large international epidemiologic study. These data sets were collected and analyzed by the Transdisciplinary Tobacco Use Research Centers (TTURCs) at the University of Wisconsin, Yale University, and Roswell Park Cancer Institute. The use of multiple large data sets, and different types of smoker samples (nationally representative samples as well as treatment-seekers), should permit greater generalizability of these results. Moreover, the use of multiple dependence measures and real-time data acquisition strategies advances the construct validation of the dependence measures. This report: (a) compares the ability of the various dependence measures (the FTND, HSI, NDSS, and WISDM) and their subscales to predict early (1-week post-quit) and late (6-months post-quit) cessation outcomes, (b) identifies which elements of the instruments account for their predictive validity, and (c) examines mechanisms that may account for the relation of the measures with the criterion of cessation success.
Methods
Clinical Trials
University of Wisconsin TTURC
The first three clinical trials were conducted by the University of Wisconsin TTURC. Participants for each of these studies were recruited by media advertisements and met identical eligibility criteria. In all three studies, the psychosocial counseling provided focused on coping, problem solving, and intra-treatment social support (Fiore et al., 2000). Table 1 provides detail regarding the study designs and samples.
Table 1.
Design and demographic summaries for the five studies
| Study | Inclusion/Exclusion criteria | Design | Treatment | Population | Assessment | Attrition |
|---|---|---|---|---|---|---|
| UW Electronic Diary Study (N = 463) | Inclusion:
|
|
|
|
|
|
| UW Bupropion-Gum Study (N = 608) |
|
|
|
|
|
|
| UW Quit Line Study (N = 410) |
|
|
|
|
|
|
| Yale Naltrexone Augmentation of Nicotine Patch Study (O'Malley et al., 2006) | Inclusion:
|
|
|
|
|
|
| Roswell Park Predictors of Quitting from the International Tobacco Control Policy Evaluation Surveys (Hyland et al., 2006) | Inclusion:
|
Wave 1 – n = 9,058 (2,214 in Canada, 2,401 in the U.K., 2,138 in the U.S., and 2,305 in Australia) Wave 2 – n = 6,762 (approx. 6 months later) | N/A | Results broken out by country:
|
|
75% completed both surveys |
Cpd = cigarettes per day, CO = carbon monoxide, ppm = parts per million, FTND = Fagerstrom Test of Nicotine Dependence, NDSS = Nicotine Dependence Syndrome Scale, TDS = Tobacco Dependence Screener, WISDM = Wisconsin Inventory of Smoking Dependence Motives, EMA=ecological momentary assessment of withdrawal symptoms, affect and life events, SCID = Structured Clinical Interview for DSM-IV Axis I Disorders (First, Spitzer, Gibbon & Williams, 1996), Prime-MD = measure for diagnosing mental disorders in primary care, CES-D = Center for Epidemiologic Studies Depression questionnaire
The Electronic Diary Study comprised 463 smokers who were randomly assigned to receive: a) sustained-release (SR) bupropion + individual counseling (n = 113); b) bupropion SR + no counseling (n = 116); c) placebo + individual counseling (n = 121); or d) placebo + no counseling (n = 113).
The Bupropion-Gum Study comprised 608 participants who were randomized, in a double-blind fashion using blocked randomization within cohorts, to one of the three treatment groups: active bupropion SR (300 mg/day) + active 4-mg nicotine gum (n = 228); active bupropion SR + placebo nicotine gum (n = 224); or placebo bupropion SR + placebo gum (n = 156).
The Quit Line Study comprised 410 participants who were randomly assigned to receive: a) nicotine lozenge + Quit Line services (n = 106); b) nicotine lozenge + self-help brochure (n = 101); c) nicotine gum + Quit Line services (n = 101); or d) nicotine gum + self-help brochure (n = 102).
Yale University TTURC
The Yale Naltrexone Augmentation of Nicotine Patch Study (N = 385) was a double-blind, placebo-controlled clinical trial for smoking cessation examining whether naltrexone augments the efficacy of the nicotine patch (O'Malley et al., 2006). Participants were recruited through newspaper and radio advertisements, press releases, and mailings to physicians. Eligible participants were randomly assigned to receive placebo or 25, 50, or 100 mg naltrexone hydrochloride, and all participants received open label 21 mg nicotine patch. See Table 1 for design and study sample details.
Common outcome and predictor variables
In all four trials, participants who reported 7 days of abstinence at their 6- or 12-month follow-up were asked to provide a breath sample for CO testing (abstinent if CO ≤ 10 ppm). According to the intent-to-treat principle, subjects who could not be located for follow-up and those who did not provide a breath sample for CO testing, were considered to be smoking. In all four clinical trials, participants completed the FTND (Heatherton et al., 1991), the HSI (Kozlowski et al., 1994), and a smoking history form that included cigarettes smoked per day. The Wisconsin TTURC studies also administered the NDSS (not in the Quit Line Study; Shiffman et al., 2004), the Prime-MD (a self-report measure developed for diagnosing mental illness in primary care settings; Spitzer et al., 1994), the Center for Epidemiologic Studies Depression questionnaire (CES-D; Radloff, 1977), the TDS (Kawakami Takatsuka, Inaba, & Shimizu, 1999) and the WISDM (Piper et al., 2004).
Population-based Study
Roswell Park Cancer Institute TTURC: Predictors of Quitting from the International Tobacco Control Policy Evaluation Surveys
Wave 1 of the International Tobacco Control (ITC) 4-country survey was conducted between October 2002 and December 2002, using random digit dialing to recruit current smokers (smoked 100 cigarettes in lifetime and smoked within the last month; see Table 1). A total of 9,058 respondents completed the Wave 1 main survey, which included 2,214 in Canada, 2,401 in the U.K, 2,138 in the U.S, and 2,305 in Australia. Among these, 8,930 respondents reported that they were still smoking at the time of the main interview.
The Wave 2 follow-up survey was conducted between May 2003 and August 2003 among respondents who completed the Wave 1 survey. A total of 6,762 respondents completed the Wave 2 survey (75%). Respondents included in the present study were current smokers in the Wave 1 main survey who completed Wave 2 follow-up and responded to at least 80% of the survey (N = 6,682; 1,665 in Canada, 1,329 in the U.S., 1,837 in the U.K. and 1,851 in Australia). The follow-up completion rate in each country was: 76% in Canada, 63% in the U.S., 78% in the U.K. and 81% in Australia (see Hyland et al., 2006).
Outcome Measures
The outcomes assessed in this study were: 1) quit attempts (‘Have you made any attempts to stop smoking since we last talked with you in [MONTH OF LAST INTERVIEW]?’); 2) successful quitting among those who made a quit attempt (no smoking or smoking less than once per month); and 3) quitting among the entire baseline sample. All data were based on self-report and were not biochemically confirmed. The present report focuses on cessation success among individuals making a quit attempt.
Core Predictor Variables
The following core set of predictor variables was examined in this study (see Table 1 for both predictor and dependent variables):
Nicotine Dependence Variables: Time to first cigarette (<=5 minutes, 6-30 minutes, 31-60 minutes, >60 minutes); and baseline smoking frequency (daily smoker, less than daily smoker)
Socio-demographic Variables: country (Australia, Canada, UK, and US); age at recruitment, in years (18-24, 25-39, 40-54, 55 and older); gender (female, male); education (low, moderate, high); income (low, moderate, high); and identified minority group
Beliefs About Quitting Variables: intention to quit (in next month, in next 6 months, beyond 6 months, not planning to quit); and self-efficacy of quitting
Motivational Variables: outcome expectancy of quitting; worries about health and quality of life; favorable attitudes about smoking; overall attitude about smoking
Past Quitting History Variables: tried to quit within last year (yes, no); and longest time off smoking (never, 1 week or less, between 1 week and 6 months, 6 months or more).
Results
Analytic Summary
The analyses in this paper are sequentially determined (i.e., progressively driven by questions raised by the results of previous analyses). Therefore, they are relatively complex. This analytic summary is offered to enhance accessibility to the rationale for the following analyses.
Initial analyses examined relations amongst the various dependence measures (i.e., the FTND, NDSS, TDS, and WISDM), and then examined which measures were most highly related to abstinence status in clinical samples at 1-week and 6-months post-quit. Because the FTND showed the strongest relations with cessation outcomes, a subsequent series of analyses sought to determine the elements of FTND that were predictive. An initial step in this effort was to explore the predictive validities of each FTND item via a series of logistic regression models using clinical samples from both Wisconsin and Yale. FTND TTFC (time to first cigarette, which elicits information on latency to smoke in the morning: see Table 2) was found to be an especially strong predictor of abstinence outcomes, with the strongest results found in the Wisconsin data sets. Generalizability to non-treatment-seeking populations was then demonstrated as FTND TTFC significantly predicted abstinence outcomes in nationally representative samples from 4 countries (Roswell Park Cancer Institute TTURC). Next, a series of analyses related FTND TTFC response with latency to lapse, relapse, and the interval between lapse and relapse. In addition, FTND TTFC was related to morning report of smoking and urge level as assessed via real-time data acquisition. Finally a series of correlational and logistic analyses related FTND TTFC scores to a variety of measures that themselves predicted abstinence outcomes. Regression analyses then showed which predictors of abstinence had validities that were, and were not, orthogonal with FTND TTFC. These analyses suggested why FTND TTFC predicted relapse, and which dependence features are most determinant of quitting likelihood.
Table 2.
FTND items and scoring
| Item | Scoring |
|---|---|
| 1. How soon after waking do you smoke your first cigarette? (TTFC) | • within 5 minutes • 6-30 minutes • 31-60 minutes • after 60 minutes |
| 2. Do you find it difficult to refrain from smoking in places where it is forbidden? | • Yes • No |
| 3. Which cigarette would you hate to give up? | • The first one in the morning • All the others |
| 4. How many cigarettes do you smoke? | • 10 or less • 11-20 • 21-30 • 31 or more |
| 5. Do you smoke more frequently during the first hours after waking than during the rest of the day? | • Yes • No |
| 6. Do you smoke if you are so ill you are in bed most of the day? | • Yes • No |
Basic validity information
Correlations among the full scales, as well as their correlation with DSM tobacco dependence criteria (as reflected on a continuous scale ranging from 0-10 by the TDS), show that the various dependence measures tend to be only moderately related to one another (WISDM-NDSS r = .57; WISDM-FTND r = .47; WISDM-TDS r = .39; NDSS-FTND r = .53; NDSS-TDS r = .37; FTND-TDS r = .26; all p-values < .01). The relation of the FTND with the TDS is especially modest.
Using Wisconsin TTURC data, univariate logistic regression analyses showed that the WISDM and the FTND significantly predicted both 1-week and 6-month point-prevalence abstinence while the TDS and the NDSS did not (Table 3). (The NDSS was not used in the Quit Line Study, resulting in a lower N for that instrument.) Table 3 shows that the FTND yielded fairly large effect sizes in the prediction of 1-week and 6-month abstinence data. Logistic regressions (no demographic or treatment group covariates) were then conducted in the combined Wisconsin sample with simultaneous entry of all full-scale dependence scores as predictors and with smoking status at 1 week and 6 months as the dependent variables (See Table 4). In this multivariate regression, only the FTND was significantly related to smoking status at either time point. Results were essentially the same with active versus placebo treatment entered as a covariate: e.g., only the FTND predicted smoking outcome (Walds = 16.37 at 1 week and 10.69 at 6 months). It should be noted that in univariate logistic regression analyses, based on data from the two placebo-controlled Wisconsin studies, the FTND predicted outcome both in individuals who received placebo medication (Walds = 17.19 at 1 week and 5.02 at 6 months) and those who received active medication (Walds = 28.40 at 1 week and 5.57 at 6 months).
Table 3.
Predictors of 1-Week & 6-month smoking status in separate univariate analyses: Data are from Wisconsin unless otherwise indicated (no covariates)
| β | SE | Wald | Df | P | OR | |
|---|---|---|---|---|---|---|
| FTND: 1 Wk | 1.02 | .17 | 36.42 | 1 | .000 | 2.78 |
| FTND: 6 Mo | .98 | .19 | 27.66 | 1 | .000 | 2.67 |
| FTND: 1 Wk - Yale | .22 | .06 | 14.19 | 1 | .000 | 1.24 |
| FTND: 6 Mo - Yale | .12 | .06 | 3.64 | 1 | .057 | 1.13 |
| NDSS: 1 Wk | .20 | .14 | 2.03 | 1 | .154 | 1.22 |
| NDSS: 6 Mo | .27 | .16 | 3.05 | 1 | .08 | 1.31 |
| TDS: 1 Wk | .54 | .31 | 3.04 | 1 | .08 | 1.72 |
| TDS: 6 Mo | .48 | .34 | 2.01 | 1 | .157 | 1.61 |
| WISDM 1 Wk | .216 | .06 | 11.88 | 1 | .001 | 1.24 |
| WISDM 6 Mo | .137 | .07 | 4.08 | 1 | .043 | 1.15 |
N's = 836 & 853 for the NDSS analyses; 1,246 – 1,263 for all others; 370 for Yale
Table 4.
Predictors of 1-week and 6-month smoking status with simultaneous entry of dependence instruments using Wisconsin TTURC data (no covariates)
| β | SE | Wald | df | P | OR | |
|---|---|---|---|---|---|---|
| 1-week | ||||||
| FTND | .82 | .23 | 12.83 | 1 | .000 | 2.27 |
| NDSS | -.18 | .18 | .97 | 1 | .32 | .83 |
| TDS | .02 | .40 | .004 | 1 | .95 | 1.02 |
| WISDM | .07 | .10 | .56 | 1 | .46 | 1.07 |
| 6-months | ||||||
| FTND | .81 | .25 | 10.24 | 1 | .001 | 2.24 |
| NDSS | -.01 | .21 | .004 | 1 | .95 | .987 |
| TDS | .45 | .44 | 1.05 | 1 | .31 | 1.56 |
| WISDM | -.05 | .11 | .26 | 1 | .61 | .95 |
N = 1,063 for 1-week analyses, N = 1,069 for 6-month analyses
The above analyses were conducted on a merged data set, using Wisconsin data. However, results were consistent across the individual data sets. Across all three Wisconsin TTURC studies, only the FTND predicted follow-up smoking status significantly with simultaneous entry of predictors (unadjusted analyses). Moreover, it was a significant predictor in each data set. In fact, there was only one occasion where the FTND did not predict smoking status significantly (1-week outcome in the Electronic Diary study), and no scale predicted outcome in that analysis. The NDSS was not used in the Quit Line study so conclusions regarding this instrument rely upon the other two Wisconsin data sets.
Construction of best-fitting models
In order to explore further the predictive validity of the FTND, we computed logistic models using the Wisconsin Electronic Diary and Bupropion-Gum Studies (Quit Line data were not used so that the same subjects were used in the comparisons of the various instruments) with forward-stepping entry with all full scale and subscale scores (NDSS & WISDM) as candidate predictors. In these and other multivariate models, there was no evidence of collinearity as judged from such indicators as unusual changes in the regression coefficient or standard error terms (cf. Hosmer & Lemeshow, 2000).
Using the combined Wisconsin data set, the FTND was the strongest predictor of abstinence at Week 1 (data not shown). However, at 6-months post-quit the WISDM Tolerance subscale displaced the FTND as the sole significant predictor (Wald = 15.93, p < .001, OR = 1.26). We also tested the relation of the HSI (FTND TTFC & Item 4 – number of cigarettes per day). A forward stepwise analysis revealed that the HSI (with Items 1 & 4 entered as a set) was the sole predictor of 1-week and 6-month outcomes when it was used as a predictor in the logistic models (Model χ2= 40.19; df = 2, p < .001, for 6-months). However, while this pair of items significantly predicted the 6-month smoking outcome, the effect depended upon FTND TTFC (Wald = 30.84, p < .001, OR = 1.56): FTND Item 4 (cigs/day) was not significantly related (p = .31). Follow-up analyses showed that FTND TTFC also predicted 1-week outcome, (Wald = 44.7, p < .001, OR = 1.59), and that FTND Item 4 did not predict this outcome if FTND TTFC was also entered in the analysis (p > .30). For the 6-month time point, when FTND TTFC was entered in the logistic regression equation, no other scale or subscale predicted outcome (p's > .05). The same test on the Week 1 data revealed that FTND TTFC showed by far the strongest relations with outcome, but that the WISDM Social and Environmental Goads subscale also modestly predicted smoking status (Wald = 5.02, p = .03, OR = 1.09).
In sum, while other scales did possessed predictive validity with respect to relapse (e.g., the WISDM Tolerance and Social and Environmental Goads subscales), FTND TTFC displayed the best overall predictive validity at both the 1-week and 6-month time points.
Prediction with FTND items
We next explored the predictive validity of all of the FTND items to determine if any item possessed predictive validity beyond FTND TTFC (see Table 2 for FTND items and scoring; Breslau & Johnson, 2000; Heatherton, et al., 1989; Lichtenstein & Mermelstein, 1986). From this point on, all analyses using Wisconsin data are based on a combined sample of all three data sets unless otherwise noted. Table 5 depicts the intercorrelations of the FTND items and reveals associations that range from slight to moderate.
Table 5.
Intercorrelations of FTND items using Wisconsin and Yale TTURC data
| FTND items | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | .22 | .38 | .36 | .39 | .29 | |
| 2 | .28 | .06 | .24 | .11 | .26 | |
| 3 | .31 | .03 | .12 | .39 | .11 | |
| 4 | .27 | .27 | .12 | .20 | .22 | |
| 5 | .26 | .02 | .39 | .07 | .13 | |
| 6 | .30 | .21 | .07 | .13 | .15 |
Note. Intercorrelations above the diagonal are based on Wisconsin TTURC data and intercorrelations below the diagonal in italics are based on Yale TTURC data.
N's for Wisconsin data = 1,464 to 1,475 and N's for Yale data = 373 to 379.
We examined the prediction of Week 1 and Month 6 smoking status via each FTND item using univariate logistic regression. Unless otherwise indicated, TTFC & Item 4 were coded as ordinal level variables with four response options (Heatherton et al., 1989). Results of the analyses are depicted in Table 6. These analyses show that most items possessed significant predictive validity at both 1-week and 6-months post-quit. However, in general, FTND TTFC showed the strongest predictive relations across time points and data sets (Wisconsin and Yale). The only exception to this pattern is that Item 2 in the Yale data set had a stronger relation with the 6-month outcome. This is unusual since this item was associated neither with the 6-month outcome in the Wisconsin data set, nor with the 1-week outcome in the Yale data set. This pattern of outcomes remained essentially the same when treatment coding was used as a covariate.
Table 6.
Prediction of 1 week and 6 month post-quit smoking status by individual FTND items using Wisconsin and Yale TTURC data
| Item | Time | β | SE | Wald | P | OR |
|---|---|---|---|---|---|---|
| 1 | 1 wk | .46 | .07 | 44.71 | <.01 | 1.59 |
| .51 | .15 | 11.64 | <.01 | 1.67 | ||
| 6 mo | .47 | .08 | 39.45 | <.01 | 1.61 | |
| .28 | .15 | 3.59 | .06 | 1.33 | ||
| 2 | 1 wk | .41 | .15 | 7.60 | .01 | 1.50 |
| -.14 | .24 | .35 | .55 | .87 | ||
| 6 mo | .13 | .16 | .67 | .40 | 1.14 | |
| -.70 | .32 | 4.82 | .03 | .49 | ||
| 3 | 1 wk | .45 | .13 | 12.50 | <.01 | 1.56 |
| -.06 | .25 | .05 | .82 | .95 | ||
| 6 mo | .31 | .14 | 4.96 | .03 | 1.37 | |
| .15 | .30 | .27 | .60 | 1.17 | ||
| 4 | 1 wk | .21 | .08 | 7.08 | .01 | 1.23 |
| .46 | .15 | 9.89 | <.01 | 1.58 | ||
| 6 mo | .26 | .09 | 9.01 | <.01 | 1.30 | |
| .12 | .17 | .48 | .49 | 1.13 | ||
| 5 | 1 wk | .39 | .12 | 9.90 | <.01 | 1.47 |
| -.59 | .23 | 6.80 | .01 | .56 | ||
| 6 mo | .40 | .14 | 8.43 | <.01 | 1.49 | |
| -.10 | .26 | .15 | .70 | .90 | ||
| 6 | 1 wk | .20 | .12 | 2.70 | .10 | 1.22 |
| -.42 | .24 | 3.15 | .08 | .66 | ||
| 6 mo | .29 | .14 | 4.54 | .03 | 1.34 | |
| -.36 | .26 | 1.90 | .17 | .70 |
Note. The top results in normal font are based on Wisconsin TTURC data and the results in the second line in italics are based on Yale TTURC data.
There were no covariates in these analyses.
N for Wisconsin analyses = 1,481 and N=379
Next, using Wisconsin TTURC data, we built best-fitting models using FTND items to expose optimal predictors at 1-week and 6-months post-quit (using forward and backwards entry with decision rules consistent with Hosmer & Lemeshow, 2000). The best-fitting models for 1-week and 6-month smoking status comprised only one item: TTFC (p's < .001). No other FTND item significantly incremented the predictive validity at either follow-up time point (p's > .25).
Data from the Roswell Park Cancer Institute TTURC 4-Country Survey were then used to address whether FTND TTFC also showed predictive validity in nationally representative samples of smokers. Such smokers tend to differ from treatment seekers on multiple dimensions (Fiore et al., 1990; Hughes, 2004; Hughes, Giovino, Klevers & Fiore, 1997). Multivariate logistic regression was used to examine the association between cessation outcomes and all intrinsic predictor variables entered into the model such that the relative risks presented for a given variable are adjusted for all other covariates in the model (see the Table 7 Note for a list of the covariates). The interactions between country and other independent variables were also examined. Table 7 shows the predictors of successful quitting among 2,289 smokers who made a quit attempt between Waves 1 and 2 of the survey. Focusing on the time to first cigarette variable as the measure of dependence (FTND TTFC), similar results were obtained in the U.S. sample as were obtained in the overall 4-country sample: i.e., a strong inverse association between time to first cigarette in the morning and quit rate. In the U.S. sample, the quit rate was highest in those smoking 60 or more minutes after waking (36%) and lowest among those who smoked within 5 minutes of waking (8%, RR = 0.22, p < 0.01). We next sought to determine the relations of FTND TTFC with conceptually distinct stages of the relapse process.
Table 7.
Predictors of quitting by Wave 2 among baseline current smokers who made serious quit attempts between Waves 1 and 2: data from the Rowell Park ITC.
| Entire sample | U. S. | |||||||
|---|---|---|---|---|---|---|---|---|
| N | % quit | RR | p-value | N | % quit | RR | p-value | |
| Time to First cigarette | ||||||||
| > 60 minutes | 556 | 35% | Ref | 113 | 36% | Ref | ||
| 31 to 60 minutes | 437 | 24% | 0.77 | 0.10 | 92 | 24% | 0.69 | 0.32 |
| 6 to 30 minutes | 925 | 21% | 0.71 | 0.02 | 166 | 19% | 0.57 | 0.13 |
| ≤ 5 minutes | 371 | 18% | 0.66 | 0.04 | 83 | 8% | 0.22 | <0.01 |
NOTE: Adjusted for age, gender, education, income, race/ethnicity, intention to quit, past quit attempts, longest past quit attempt, smoking frequency, opinion about smoking, self efficacy, worries about health and QOL, and favorable attitudes about smoking.
Exploring the nature of the predictive validity of FTND TTFC
Relation with maintenance of abstinence
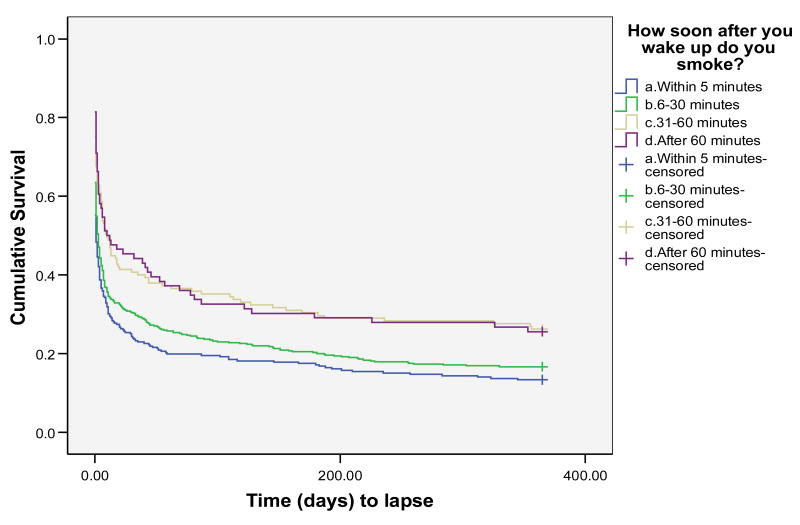
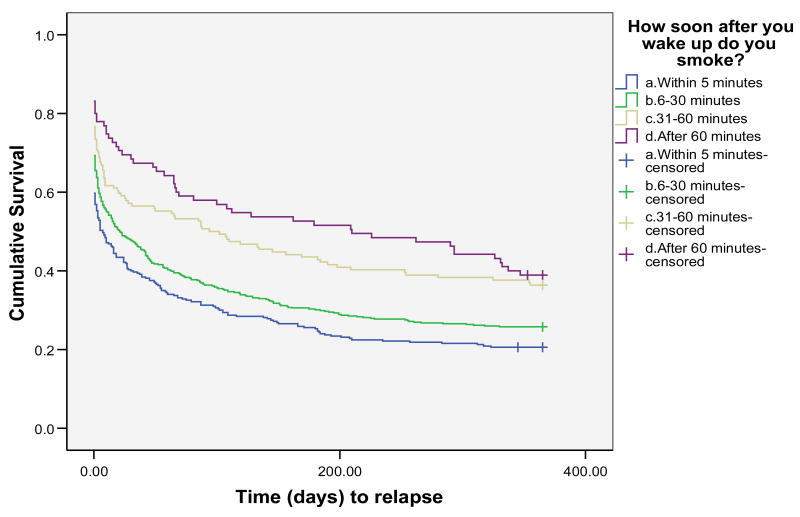
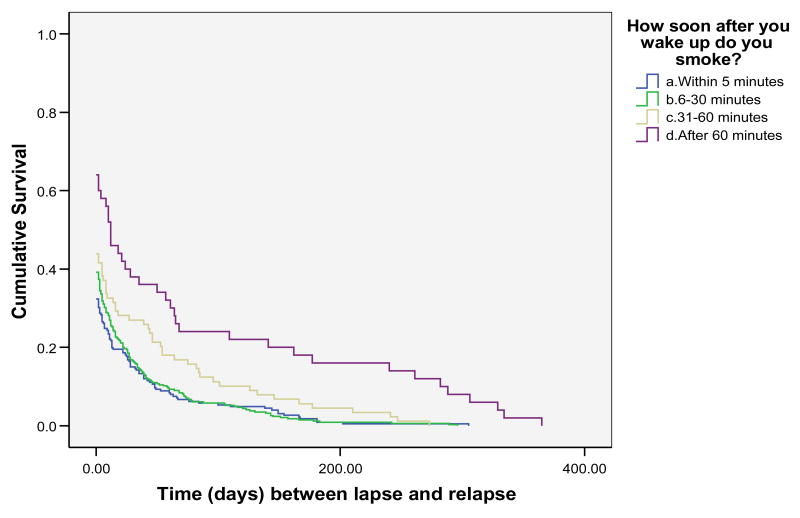
We next examined whether FTND TTFC response predicted the latency to begin to sample cigarettes (i.e., lapse), latency to return to smoking (i.e., relapse – defined as 3 consecutive days of smoking) or whether it predicted the rate at which individuals returned to daily smoking once they began to smoke (i.e., the lapse-relapse interval). These survival analyses were conducted using Wisconsin TTURC data from only two of the data sets (the Bupropion + Gum and Electronic Diary data sets) as the third data set (the Quit Line data set) did not comprise sufficiently fine-grained outcome data to permit determination of accurate survival estimates. See Figure 1 for latency to relapse survival results, Figure 2 for latency to lapse survival results, and Figure 3 for lapse-relapse latency results.
Figure 1.
Time to lapse for all 4 categories of FTND 1 response
Figure 2.
Time to relapse for all 4 categories of FTND 1 response
Figure 3.
Time between first lapse and ultimate relapse for all 4 categories of FTND 1 response
Kaplan-Meier survival analyses were conducted with data censored at 12 months or at the longest lapse-relapse interval (see Table 8). Consistent with the point-prevalence analysis, the survival analysis showed a strong relation between FTND TTFC response and relapse (Figure 1), with each response option contributing to the prediction (Log Rank = 29.02, Tarone-Ware = 33.92; p's < .01). Medians derived from the survival analyses showed that half of those smoking within 5 minutes of awakening relapsed within 7 days of the quit day; half of those smoking after 60 minutes relapsed within 210 days (see Table 8).
Table 8.
Survival analysis results from the Wisconsin Electronic Diary and Bupropion-Gum data sets using FTND TTFC to predict lapse, relapse and lapse-relapse latency
| Response Option | N | # of Events | Estimated Median Days to Event |
|---|---|---|---|
| Relapse | |||
| > 60 min | 95 | 58 | 210 |
| 31-60 min | 154 | 98 | 94 |
| 5-30 min | 501 | 372 | 21 |
| < 5 min | 320 | 254 | 7 |
| Lapse | |||
| > 60 min | 86 | 64 | 10 |
| 31-60 min | 145 | 107 | 10 |
| 5 – 30 min | 474 | 385 | 3 |
| < 5 min | 292 | 253 | 1 |
| Lapse – Relapse Latency | |||
| >60 min | 50 | 50 | 12 |
| 31-60 min | 89 | 89 | 0 |
| 5-30 min | 345 | 345 | 0 |
| < 5 min | 226 | 226 | 0 |
Note. N's vary somewhat across outcome variable categories since missing data and the nature of the dependent variable affected the number of analyzable cases. The “# of events” category reflects the number of lapse or relapse episodes.
Next, we conducted Kaplan-Meier survival analyses on the lapse data (Figure 2). This analysis again showed a strong relation between FTND TTFC and outcome (Log-Rank = 22.67, Tarone-Ware = 28.15; p's < .01). However, in this case, the relation was not linear across the response options. Those who smoked in the first 30 minutes showed markedly shorter lapse latencies than did those who smoked after 30 minutes. Coding responses dichotomously at the 30-minute mark revealed a significant survival function (p's < .01).
Table 8 shows that FTND TTFC response predicts not only the tendency to try a first post-quit cigarette, but also predicts the rate at which the person returns to daily smoking after that first cigarette (i.e., the “lapse-relapse interval”, p < .01). The results indicate that the relation with both relapse and the lapse-relapse latency is fairly strongly “dose-related.” Finally, Table 8 shows the numbers of individuals who respond to the four response options within each type of dependent variable. These data show that within this population of treatment-seeking smokers, the majority of individuals indicate that they smoke within 30 minutes of awakening; only 30% indicate that they smoke after that time period.
Relation with the WISDM
Using Wisconsin TTURC data we examined the relations between FTND TTFC and the WISDM subscales. These subscales were designed to tap relatively discrete components of dependence that may help to explain the item's predictive validity. FTND TTFC was significantly related to every WISDM subscale. However, it was related to only two of the 13 subscales at moderate to strong levels (r > .30): WISDM Tolerance (r = .66) and WISDM Automaticity (r = .36; N's = 1,479). It showed only modest relations with the following WISDM subscales (N's = 1,475-1,476, r's < .22): Affiliative Attachment, Cognitive Enhancement, Associative Processes, Negative Reinforcement, Positive Reinforcement, Social-Environmental Goads, Taste and Sensory Processes, and Weight Control. Thus, it had very modest relations with self-rated dimensions concerned with smoking for pleasure, smoking to control negative moods or distress, and smoking in response to sensory or exteroceptive cues. Tests for differences amongst zero-order correlations using Fisher's Z-transformation revealed that all of these correlations differ significantly (p's < .01) from the correlations of TTFC with Tolerance and Automaticity. Thus, TTFC is associated with smokers' ratings of the extent to which they smoke a large quantity of cigarettes, that smoking has become automatic, and that they tend to smoke constantly (c.f., Lessov et al., 2004). In this regard, it is interesting to note that these two WISDM subscales are substantially inter-related (r = .54). In addition, TTFC responses are significantly positively related to self-reported cigarettes smoked per day (r = .32; N = 1,476); but it appears that TTFC assesses more than just smoking rate since it yields more accurate predictions of relapse vulnerability than do measures of smoking rate (e.g., FTND Item 4).
Relations with withdrawal and demographics
Based on Wisconsin TTURC data, FTND TTFC was not substantially related to initial severity of the withdrawal syndrome or its trajectory during the first week post-quit (r's < .14). FTND TTFC showed, at best, modest relations with such demographic variables as age (r = .18), gender (r = .02), and education (r = -.22; all N's > 1,462).
Relation with other variables that predict cessation outcome
Another strategy for exploring the predictive validity of FTND TTFC is to identify other variables that also predict cessation outcomes and then determine the extent to which entry of FTND TTFC in the regression models reduces the predictive relations of those variables. The inference would be that to the extent that FTND TTFC and another variable shared common mechanisms of action, FTND TTFC would reduce the predictive value of the other variable when both are present in the same logistic model.
We examined the relation of variables with both 1-week and 6-month outcomes in the two data sets (the Wisconsin Electronic Diary and the Bupropion-Gum Studies) with a full array of variables. The following variables predicted 6-month outcomes in univariate logistic regression models (p's < .05): gender, race, education, age of first daily smoking, whether smoking is permitted in the home, smoking policy at work, longest period of prior abstinence after the start of smoking, level of stress at work (Prime-MD), having no one to turn to when experiencing a problem (Prime-MD), the WISDM Automaticity scale, the WISDM Tolerance scale, the NDSS Stereotypy scale, and baseline CO level assessed 1 week prior to the quit day. We then determined whether entry of the FTND TTFC resulted in substantial loss of predictive value of each of these items. Gender, race, smoking at work, longest prior abstinence, stress at work and interpersonal support continued to predict outcome after the inclusion of the FTND TTFC. Table 10 presents findings for those variables where entry of FTND TTFC resulted in a substantial reduction in magnitude of the predictive relation.
Table 10.
Results of univariate and bivariate models comprising significant predictors of 6-month smoking outcomes and the FTND TTFC from the Wisconsin Electronic Diary and Bupropion-Gum Studies.
| Variable | B | SE | Wald | P | OR | |
|---|---|---|---|---|---|---|
| Univariate | Education | -.23 | .09 | 6.18 | .01 | .79 |
| Bivariate | Education | -.14 | .10 | 2.17 | .14 | .87 |
| FTND 1 | .42 | .09 | 21.12 | .000 | 1.58 | |
| Univariate | Age/smoke | -.04 | .02 | 3.87 | .049 | .96 |
| Bivariate | Age/smoke | -.03 | .02 | 1.55 | .213 | .98 |
| FTND 1 | .44 | .09 | 23.65 | .000 | 1.55 | |
| Univariate | Home/smoke | -.34 | .16 | 4.42 | .035 | .72 |
| Bivariate | Home/smoke | -.12 | .17 | 0.50 | .48 | .89 |
| FTND 1 | .42 | .09 | 20.33 | .000 | 1.53 | |
| Univariate | WISDM-Automaticity | .14 | .05 | 8.93 | .003 | 1.15 |
| Bivariate | WISDM-Automaticity | .06 | .05 | 1.53 | .22 | 1.06 |
| FTND 1 | .41 | .10 | 18.61 | .000 | 1.51 | |
| Univariate | WISDM-Tolerance | .23 | .06 | 15.93 | .000 | 1.26 |
| Bivariate | WISDM-Tolerance | .06 | .08 | .63 | .43 | 1.06 |
| FTND 1 | .39 | .12 | 10.57 | .001 | 1.50 | |
| Univariate | NDSS-Stereotypy | .25 | .09 | 7.10 | .008 | 1.28 |
| Bivariate | NDSS-Stereotypy | .17 | .10 | 3.01 | .08 | 1.18 |
| FTND 1 | .42 | .10 | 21.53 | .000 | .98 | |
| Univariate | Baseline CO | .01 | .006 | 4.09 | .043 | 1.03 |
| Bivariate | Baseline CO | .003 | .006 | .21 | .65 | 1.00 |
| FTND 1 | .47 | .08 | 35.32 | .000 | 1.60 |
Note. “Education” = highest grade in school completed; “Age/smoke” =“ How old were you when you first started smoking daily/every day?”; “Home/smoke” = “If someone in your house wants to smoke, does he/she have to leave in order to smoke?” Relapse was coded as “1” and having a restrictive home smoking policy was also coded as 1.
The results also show that the predictive validity of FTND TTFC may be attributed to at least two factors, and perhaps more. First, it seems to capture the influence of having a restrictive smoking policy. The predictive influence of a restrictive smoking policy is captured quite efficiently by FTND TTFC: the Wald coefficient for the smoking in the home variable declines dramatically when FTND TTFC is entered into the regression model. Second, it seems to capture the impact of additional measures of particular dependence facets. In particular, it accounts for WISDM and NDSS scales that reflect frequent and “automatic smoking”. As Table 10 shows, the predictive validity of these variables declines dramatically when FTND TTFC is added to the regression models. For instance, the WISDM Automaticity subscale is highly predictive of 6-month outcomes in the univariate model with a Wald coefficient of 8.93; this value is reduced to 1.53 when FTND TTFC is entered into the model, as the logistic coefficient is essentially halved. Some sense of the meaning of the nature of the construct tapped by FTND TTFC may be gained by considering the nature of the items comprised by these two WISDM subscales along with the NDSS Stereotypy subscale (Table 11). Finally, this research revealed 17 significant predictors of 1-week or 6-month outcomes: the FTND TTFC remained a strong and robust predictor with any of these variables present in the same regression model.
Table 11.
Selected items from the WISDM Automaticity and Tolerance subscales and the NDSS Stereotypy subscale
| Scale | Selected Items |
|---|---|
| WISDM-Automaticity | I often smoke without thinking about it. |
| I smoke without deciding to. | |
| Sometimes I'm not aware that I'm smoking. | |
| WISDM-Tolerance | I can only go a couple hours between cigarettes. |
| Other smokers would consider me a heavy smoker. | |
| I usually want to smoke right after I wake up. | |
| NDSS-Stereotypy | My cigarette smoking is fairly regularly throughout the day. |
| I smoke consistently throughout the day. | |
| It's hard to estimate how many cigarettes I smoke per day because the number often changes. (oppositely keyed) |
Inspection of the predictive relations obtained with the Week 1 outcome data revealed a pattern very similar to that obtained at 6-months post-quit. While there were more significant predictors of outcome at Week 1, the predictors showing substantial overlap with FTND TTFC were essentially the same. One exception is that the WISDM Affiliative Attachment subscale predicted the 1-week outcome and its predictive validity overlapped substantially with that of FTND TTFC.
Discussion
The FTND showed impressive validity relative to other assessment instruments in terms of its ability to predict quitting success amongst a large sample of individuals enrolled in several smoking cessation trials that were conducted in different cities and involved different cessation treatments. While other nicotine dependence scales also predicted quitting success, the FTND showed the largest effect sizes of any single instrument. Moreover, the FTND yielded accurate predictions in both individuals receiving active pharmacotherapy and those receiving placebo (although some pharmacotherapies may moderate the predictive relation, cf. Fagerstrom & Schneider, 1989; Shiffman & Paton, 1999). Further analyses revealed that the first item (TTFC) showed the strongest predictive relations with quitting success both early (1 week) and late (6 months) in the follow-up period. This relation was also apparent in large nationally representative samples gathered in four countries.
Survival analyses showed that response to FTND TTFC predicted not only time to relapse, but also the latency from the quit-day for individuals to try a first cigarette (lapse latency) and the latency between a lapse and ultimate relapse (lapse-relapse latency). In general, responses showed linearity between latency to smoke in the morning and the lapse, relapse, and lapse-relapse latencies. Increases across each response category (smoking within 5 minutes, 6-30 minutes, 31-60 minutes, and after 60 minutes) tended to be associated with meaningful increases in lapse and relapse latencies. These data show that FTND TTFC reflects both a vulnerability to sample an initial cigarette and also to resume frequent use. This suggests that FTND TTFC response is associated with a vulnerability that manifests across the relapse process (cf. Shiffman et al., 1996, 1997). Thus, its validity cannot be attributed to a phase-specific element in the relapse process (e.g., discouragement or loss of self-efficacy after a lapse; see Gwaltney, Shiffman, Balabanis & Paty, 2005).
One important finding is that the FTND shared predictive validity with an item that elicited information about home smoking policy. Specifically, if an individual is not allowed to smoke in the house s/he is less likely to be smoking at follow-up. It appears that some of the predictive validity of FTND TTFC might be attributed to the fact that restrictive smoking policies may both discourage smoking early in the morning and encourage long-term abstinence. However, even with this smoking policy item in a prediction model, FTND TTFC still retained considerable predictive validity, indicating that the relation between FTND TTFC and quitting success is not merely an artifact of home smoking policy.
Concurrent validation analyses revealed that FTND TTFC was correlated fairly strongly with only two WISDM subscales (i.e., Tolerance and Automaticity), subscales that assess smoking without awareness and smoking heavily. FTND TTFC was not associated strongly with other smoking motives tapped by the WISDM. Consistent with this pattern of associations, FTND TTFC response was significantly related to self-reported cigarettes smoked per day and CO level, but was not strongly related to magnitude of the withdrawal syndrome. The lack of association with withdrawal raises doubts about the original interpretation of the FTQ, which focused on withdrawal produced by overnight deprivation (Fagerström, 1978).
FTND TTFC accounted for significant predictive validity in other variables that provide further insight. For instance, FTND TTFC response accounted statistically for the predictive validities of the following variables: education, age of first smoking, WISDM-Automaticity, WISDM-Tolerance, NDSS-Stereotypy, and baseline CO level. It is difficult to explain its association with education and age. However, its relations with the other variables suggest that the reason that FTND TTFC predicts relapse is that it taps a construct that produces a pattern of heavy, frequent smoking that generalizes across time and place (see Lessov et al., 2004). This is suggested by items such as the NDSS-Stereotypy Item, “I smoke consistently throughout the day,” and the WISDM-Tolerance Item, “I can only go a couple of hours between cigarettes.” FTND TTFC also accounted for the predictive validity of the WISDM-Automaticity scale. This scale assesses the extent to which smoking occurs without awareness or cognitive control (Curtin et al., 2006; Tiffany, 1991). Thus, even among a group of relatively heavy smokers seeking formal cessation treatment, smokers differed in the extent to which they saw their smoking as occurring outside awareness; the extent to which they did so predicted their likelihood of relapse, and FTND TTFC accounted for this relation statistically.
FTND TTFC did not account for the predictive validities of other types of variables. For instance, it did not account for the relations of stress and social support with outcome. This suggests that such items that tap an individual's psychosocial “context” may constitute somewhat independent contributions to relapse risk.
It is important to note that the validity of FTND TTFC may be due, in part, to the fact that it asks about a tangible, specific dimension of smoking that has a similar meaning across individuals (i.e., time). Smokers are asked to report a specific time at which they smoke their first cigarette in the morning. This sort of response option may be less susceptible to response style biases than are other type of options (such as rating “need” or “desire” to smoke in the morning) where thresholds for response options may differ markedly from one person to another.
In terms of theoretical significance, this research suggests that tobacco/smoking dependence, at least as manifested by relapse vulnerability, is related to a pattern of pervasive smoking, one that occurs throughout the day and that does not seem dependent upon an awareness of interoceptive or exteroceptive cueing. This is not to say that these individuals would not respond to smoking cues in the environment, but rather that their smoking is less contingent upon such cues. In fact, for these smokers cues may be so ubiquitous that their smoking may appear independent of any delimited set of cues. It is also possible that for these individuals control over smoking has shifted to internal cues of which they are unaware (Baker et al., 2004; Curtin et al., 2006).
Supporting evidence, as noted above, is the content of the questionnaires with which early morning smoking was associated (e.g., the WISDM-Automaticity and the NDSS-Priority subscales). In addition, it is noteworthy that early morning smoking was not highly related to subscales such as the WISDM-Cue Exposure/Associative Processes or the WISDM-Social Environmental Goads subscales – subscales that target smoking in response to environmental cues. In addition, such smoking was not strongly related to scales designed to reflect awareness of smoking in response to internal cues such as distress cues (e.g., WISDM-Negative Reinforcement). Findings by Lessov et al., (2004) are congruent with this conclusion. In the context of biometric twin research, these investigators found that FTND TTFC loaded most heavily on a factor that seemed to reflect sheer volume of smoking; it did not load on a factor that included withdrawal magnitude, consciously perceived quitting difficulty, or smoking despite experiencing smoking-related problems. Further evidence that suggests that highly dependent smoking is associated with a lack of contextual dependency was provided by Shiffman and Paty (2006). They recently reported that chippers, light smokers who regularly use tobacco without developing dependence, differ from other smokers in that the chippers are highly cue-dependent (Shiffman & Paty, 2006). Of course, other factors may also account for the relation of FTND TTFC with abstinence status.
This research may also have practical significance in that it suggests that a single item from the FTND can assess nicotine dependence as it is reflected in relapse vulnerability, and as it is reflected in other measures such as other WISDM and NDSS scales. The use of a single item may be important for epidemiologic or surveillance research where respondent burden is highly important. Moreover, in this research, FTND TTFC produced superior prediction of relapse than did the entire FTND questionnaire. Thus, researchers should be aware that, to the extent that they view relapse as an important endpoint, they may actually degrade their assessment of relapse vulnerability by employing the whole instrument; this is consistent with the variable inter-item correlations (Table 5). Finally, it should be noted that the present paper assessed the validity of dependence instruments against only a single criterion: quitting success. Investigators certainly would wish to consider other dependence measures to the extent that they wished to predict a broader array of dependence criteria (e.g., withdrawal; see Piper et al., 2006).
The FTND TTFC may have important clinical applications. Our data suggest that this item provides a very brief measure of relapse susceptibility. Thus, this measure could be used as a baseline screening item to target smokers beginning treatment. For instance, TTFC is already used to assign dose of nicotine lozenge therapy (Shiffman et al., 2002), and it is possible that this item could also prove useful in assigning smokers to other treatments. Finally, recent research suggests that TTFC has high heritability relative to other dependence measures (e.g., Haberstick et al., 2007; Lessov et al., 2004). Therefore, it may be well suited to serve as a phenotypic measure for genetic mapping.
Interpretive caveats
Readers should recognize that this paper, and its interpretations, rest upon self-report items. Thus, it has limited ability to shed light on such processes or phenomena as automatic information processing. In addition, it is the case that the relative validities of items and instruments may vary across different samples of smokers. For instance, West (2005) has reported findings in which other FTND items had relations with abstinence status that were as high, or higher, than the TTFC item. It is also unclear the extent to which one can generalize from the current results to instances where smoking latency data are gathered using a different response format (e.g., continuous measure of time to first cigarette). Finally, if investigators wish to use FTND TTFC as a measure of dependence, they must recognize that a meaningful portion of its predictive validity is related to its association with a secular phenomenon: a restrictive home smoking policy. Thus, in any attempt to isolate the extent to which this item assesses dependence per se, the investigators may wish to control this relation either through sample selection or through statistical means. This would be important, for instance, if investigators wished to use this item in genetics research: a portion of the variance in this item might merely reflect smoking policy rather than dependence.
Summary
The present research shows that FTND TTFC is a strong and consistent predictor of short- and long-term cessation success. Thus, this measure might be useful for both research purposes as well as for treatment planning. Because this measure is sensitive to the motivational forces that drive cessation failure, it may elucidate the nature of nicotine dependence. Construct validation efforts suggest that this item reflects smoking that is relatively heavy and noncontingent with external and internal cues.
Table 9.
Number of cigarettes smoked since last night's call by FTND TTFC response based on the Wisconsin Bupropion-Gum Study [N = 547 (pre-quit) and 534 (post-quit)].
| How soon after you wake do you smoke your first cigarette? | Time frame | Mean number of cigarettes (SD) |
|---|---|---|
| Within 5 minutes | Pre-quit | 4.43 (5.28) |
| Post-quit | 0.86 (2.24) | |
| 6-30 minutes | Pre-quit | 3.57 (3.29) |
| Post-quit | 0.52 (1.46) | |
| 31-60 minutes | Pre-quit | 2.67 (3.28) |
| Post-quit | 0.43 (1.69) | |
| After 60 minutes | Pre-quit | 3.65 (5.00) |
| Post-quit | 0.03 (0.18) |
Acknowledgments
This research was supported by a number of grants at the different participating institutions:
University of Wisconsin School of Medicine & Public Health, Center for Tobacco Research and Intervention (Supported by NIH Grants #P50-CA84724-05 and # P50-DA0197-06)
Rutgers University (Supported by NIH Grants #P50-CA84724-05 and # P50-DA0197-06)
Brown University (Supported by NIH Grant #P50-CA084719 and NIDA Grant #R01- DA016737)
University of Southern California (Supported by NIH Grant #P50-CA084735-06)
SUNY at Buffalo, School of Public Health and Health Professions (Supported by NIH Grant #P50-CA111236)
University of Minnesota (Supported by NIH Grant #P50-DA013333)
Roswell Park Cancer Institute (Supported by NIH Grant #P50-CA111236)
Yale University School of Medicine (Supported by NIH Grants # P50-DA13334, # P50-AA15632, and # K12-DA00167)
University of Pittsburgh (Supported by NIH Grant #P50-DA/CA84718)
Footnotes
This research was conducted at the University of Wisconsin, Madison
References
- Alterman AI, Gariti P, Cook TG, Cnaan A. Nicotidermal patch adherence and its correlates. Drug & Alcohol Dependence. 1999;53:159–165. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington D. C.: American Psychiatric Association; 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IA, Prescott RJ, Tjeder-Burton SM. Trnsdermal nicotine plus support in patients attending hospital with smoking-related diseases: a placebo-controlled study. Respiratory Medicine. 1996;90:47–51. doi: 10.1016/s0954-6111(96)90244-9. [DOI] [PubMed] [Google Scholar]
- Etter JF. A comparison of the content-, construct- and predictive validity of the cigarette dependence scale and the Fagerström test for nicotine dependence. Drug and Alcohol Dependence. 2005;77:259–68. doi: 10.1016/j.drugalcdep.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Fagerström KA. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerström Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–181. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Ferguson JA, Patten CA, Schroeder DR, Offord KP, Eberman KM, Hurt RD. Predictors of 6-month tobacco abstinence among 1224 cigarette smokers treated for nicotine dependence. Addictive Behaviors. 2003;28:1203–1218. doi: 10.1016/s0306-4603(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Novotny TE, Pierce JP, Giovino GA, Hatziandreu EJ, Newcomb PA, Surawicz TS, Davis RM. Methods used to quit smoking in the United States. Do cessation programs help? JAMA. 1990;263:2760–2765. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- Glynn TJ, Manley MW. How to Help Your Patients Stop Smoking: A National Cancer Institute Manual for Physicians. Bethesda, MD: US Dept of Health and Human Service, Public Health Service, National Institue of Health, National Cancer Institute; 1990. [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, Hewitt JK. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102:655–665. doi: 10.1111/j.1360-0443.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Lando H, Klesges RC, Talcott GW, Renaud EA. A study of the psychometric and predictive properties of the Fagerström Test for Nicotine Dependence in a population of young smokers. Nicotine & Tobacco Research. 1999;1:59–64. doi: 10.1080/14622299050011161. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KA. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert WS, Robinson J. Measureing the heaviness of smoking using self-reported time to first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;86:1119–1127. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression, Second Edition. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- Hughes JR. Data to estimate the similarity of tobacco research samples to intended populations. Nicotine & Tobacco Research. 2004;6:177–179. doi: 10.1080/14622200310001656993. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Giovino GA, Klevens RM, Fiore MC. Assessing the generalizability of smoking studies. Addiction. 1997;92:469–472. [PubMed] [Google Scholar]
- Hyland A, Borland R, Li Q, Yong HH, McNeill A, Fong GT, O'Connor RJ, Cummings KM. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15 3:83–94. doi: 10.1136/tc.2005.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Inaba S, Shimizu H. Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addictive Behaviors. 1999;24:155–166. doi: 10.1016/s0306-4603(98)00127-0. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans T, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HIS. Drug and Alcohol Dependence. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, Heath AC, Madden PAF. Defining nicotine dependence for genetic research: evidence from Austalian twins. Psychological Medicine. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz N, Jepson C, Patterson F, Tyndale R. Impact of CYP2A6 genotype on pretreatment smoking behavior and nicotine levels from and usage of nicotine replacement therapy. Molecular Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Califas KJ, Lento J, Wolter TD. Behavioral treatment for smokers with a history of alcoholism: predictors of successful outcome. Journal of Consulting and Clinical Psychology. 2001;69:796–801. doi: 10.1037//0022-006x.69.5.796. [DOI] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Baker TB. Review: Assessing tobacco dependence: A guide to measure evaluation and selection. Nicotine & Tobacco Research. 2006;8:339–351. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federman EB, Piasecki TM, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, et al. Controlled trial of naltrexone augmentation of nicotine replacement for smoking cessation. Archives of Internal Medicine. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Ju S. Validation of the modified Fagerström Tolerance Questionnaire with salivary cotinine among adolescents. Addictive Behaviors. 2000;25:429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine. 2002;162:1267–1276. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD. Individual differences in the context of smoking lapse episodes. Addictive Behaviors. 1997;22:797–811. doi: 10.1016/s0306-4603(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paton SM. Individual differences in smoking: gender and nicotine addiction. Nicotine & Tobacco Research. 1999 2:S153–157. doi: 10.1080/14622299050011991. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. Journal of Abnormal Psycholology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–349. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Rockville, MD: The Humana Press Inc; 1992. pp. 207–224. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Wilson VB, editors. Assessing alcohol problems: A guide for clinicians and researchers. second. Bethesda, MD: National Institute on Alcohol Abuse & Alcoholism; 2003. pp. 75–99. [Google Scholar]
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. Journal of the American Medical Association. 1994;272:1749–1756. [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- West R. Understanding nicotine and tobacco addiction (Novartis Foundation Symposium 275) Wiley; Chichester: 2005. Defining and assessing nicotine dependence in humans; pp. 36–58. [Google Scholar]
- Westman EC, Bem FM>, Simel DL, Rose JE. Smokng behavior on the first day of a quit attempt predicts long-term abstinence. Archives of Internal Medicine. 1997;157:335–340. [PubMed] [Google Scholar]