Abstract
Although comprising a minority of the transplant population, acute liver failure (ALF) patients represent some of the most challenging cases in terms of the level and complexity of care required. An ALF patient requires much more than a single skilled intensivist, gastroenterologist, or surgeon. Successful care of the ALF patient begins with early diagnosis and triage to the appropriate level of care where a multitude of specialties are required to work together to maximize the chance of recovery and/or extend the window of opportunity for transplant.
Keywords: Acute liver failure, liver transplant, hepatic encephalopathy, hepatitis, acetaminophen
Acute liver failure (ALF) represents a small percentage of liver transplants performed annually in the United States but is comprised of a particularly ill cohort of individuals with extremely high short-term morbidity and mortality. The management of these patients requires a focused multidisciplinary team approach. The selection of individuals who are viable to survive transplant, while requiring transplant to survive, represents a challenge in clinical practice.
Definitions and Etiologies
ALF typically presents in the absence of any previously known liver disease with clinical and laboratory evidence of significant liver injury that leads to impaired hepatic function. ALF has also been called fulminant hepatic failure or acute hepatic failure, with the basic definition of the onset of hepatic encephalopathy (HE) within 8 weeks of the patient becoming jaundiced. Certain researchers propose further subdivision of ALF into hyperacute (0–7 days), acute (8–28 days), and subacute (29 days to <26 weeks), though these classifications have not been widely embraced.1,2
Drug-induced hepatic failure, which is commonly divided into acetaminophen- and nonacetaminophen-related etiologies, occurs at higher rates in Western countries compared to Asian and African countries, where viral hepatitis is a more common cause of ALT. According to the United States ALF Group Registry statistics from 1998 to 2008, the most common etiologies of ALF were acetaminophen (46%), followed by indeterminate causes (14%), other drugs (12%), hepatitis B (7.7%), and autoimmune causes (5.9%).3 Less common causes included ischemia, Wilson disease, Budd-Chiari syndrome, and pregnancy.
Although European countries have similar statistics, viral hepatitis (mainly hepatitis B and A) is the predominant cause of ALF worldwide. Drug-induced hepatitis is much less common in developing nations, though antituberculosis therapy warrants special mention as the most common cause of drug-induced ALF in South Asia.4 ALF secondary to hepatitis B is also on the rise in Europe and the United States due to immigration, with some researchers attributing 5–10% of new ALF cases to hepatitis B infection.5 Although only 1% of patients who develop acute hepatitis B progress to ALF, the rate approaches 20% in cases of hepatitis D virus co-infection.6 Older patients and those with hepatitis C virus infection also have higher rates of ALF in acute hepatitis B infection.7
The majority of acute hepatitis A infections do not result in ALF, and many young patients with acute hepatitis A are anicteric and asymptomatic. Older patients with acute hepatitis A develop ALF approximately 0.2–0.4% of the time and accounted for 2.6% of cases within the ALF registry in the United States.
Regardless of the etiology, ALF is considered an uncommon condition, with 2,000–2,300 cases per year in the United States.8 In 2009, diagnoses of acute hepatic necrosis accounted for 4.2% (243/5,748) of all adult liver transplants performed in the United States.9 Although the high mortality rate seen with ALF has improved with advances in liver transplantation and intensive care unit (ICU) management, it still reaches 60–80%, far worse than most 1-year survival rates (80–90%) for liver transplant due to chronic liver disease.10–12 Early recognition and treatment, as well as consideration of liver transplant, are the major factors for improving survival rates.
Diagnosis
The initial clinical presentation of ALF is often non-specific and easily misdiagnosed, as it often occurs in otherwise healthy individuals without a history of chronic liver disease. Malaise, fatigue, and nausea usually appear first, followed by jaundice. Mental status changes usually appear after the onset of jaundice, with early onset changes associated with a higher risk of cerebral edema and intracranial hypertension (ICH).13 The subset of ALF patients with a delayed presentation of jaundice and more gradual onset of hepatic insufficiency has lower rates of cerebral edema. These subfulminant hepatic failure patients were traditionally thought to have a worse overall prognosis, though recent guidelines emphasize that the etiology of ALF is more important than the length of the illness.2
History-taking is essential to narrow diagnostic possibilities, with exposures to medications and viral infections topping the list of questions to ask patients and their families. The use of herbal or over-the-counter supplements should be specifically investigated, as patients often do not consider these substances to be medications. Supplements such as hydroxycut, used for bodybuilding, have been well established as potential hepatotoxins, whereas many others such as green tea have been implicated in case reports.14,15 Mushroom ingestion should also be specifically addressed, as ingestion of Amanita phalloides may produce ALF.16 Sexual contacts, tattoos, travel, alcohol use, and recreational drug use should also be examined.
Laboratory confirmation of ALF is fairly straightforward. A prolonged prothrombin time of approximately 4–6 seconds or more (international normalized ratio [INR] of greater than 1.5) with any degree of encephalopathy substantiates the diagnosis of ALF and necessitates hospital admission. Other early laboratory tests that should be obtained include a complete blood count, complete metabolic panel with serum chemistries and liver-associated enzymes, arterial blood gases, and lactate. A serum acetaminophen level is important to obtain, though early therapy with n-acetylcysteine (NAC) may be beneficial even in nonacetaminophen ALF.17 The complete list of laboratory tests for the initial evaluation of ALF is shown in Table 1.
Table 1.
Laboratory Tests to Perform in the Initial Evaluation of Acute Liver Failure
| Type of Tests | Specific Laboratory Tests |
|---|---|
| Serum chemistries |
|
| Hepatic panel |
|
| Hematology |
|
| Arterial blood |
|
| Toxicology |
|
| viral hepatitis serologies |
|
| Autoimmune markers |
|
| Urine |
|
| Other |
|
- Ab=
antibody;
- Ag=
antigen;
- ALT=
alanine aminotransaminase;
- AST=
aspartate aminotransferase;
- HAV=
hepatitis A virus;
- HCV=
hepatitis C virus;
- HEV=
hepatitis E virus;
- HSV=
herpes simplex virus;
- Ig=
immunoglobulin;
- INR=
international normalized ratio;
- PCR=
polymerase chain reaction;
- PT=
prothrombin time;
- PTT=
partial thromboplastin time;
- VZV=
varicella zoster virus.
Numerous criteria and scoring systems based upon laboratory values and clinical findings have been developed to risk-stratify patients with ALF. A system that is currently widely used comes from King's College in London and divides patients into acetaminophen versus nonacetaminophen ALF. This scoring system (Table 2) is generally quite accurate in predicting poor prognosis and, along with clinical judgment, is useful for ensuring timely transfer to a liver transplant center.
Table 2.
King's College Criteria for Poor Prognosis in ALF
| Acetaminophen-induced ALF | Nonacetaminophen-induced ALF |
|---|---|
| Arterial pH <7.30 after fluid resuscitation | Prothrombin time >100 sec (INR >6.5) |
Or all of the following:
|
Or any 3 of the following:
|
- ALF=
acute liver failure;
- INR=
international normalized ratio.
Treatment
Treatment of ALF should begin even before its etiology is confirmed, particularly in cases of toxicity. Although the number of liver-directed therapies in ALF is relatively limited, it is of paramount importance to recognize and administer the correct therapy. The well-established nomogram for acetaminophen level and time from exposure is useful to predict the chance of injury in a single acute ingestion, but it is important to note that this nomogram is not helpful in subacute acetaminophen toxicity or when acetaminophen is ingested in combination with alcohol. As NAC may also be helpful in nonacetaminophen-associated ALF, it is reasonable to administer it as initial therapy, either orally or via 72-hour infusion.18 Side effects, which are usually mild, include nausea, vomiting, and, on rare occasions, bronchospasm or urticaria that responds to discontinuation, antihistamines, or epinephrine (for bronchospasm).
Mushroom poisoning also warrants special mention, as specific antidotes should be administered at an early stage, if possible. Penicillin G and milk thistle (silibinin) are thought to be helpful. Milk thistle dosing is somewhat problematic in the United States, as it is not available as a licensed drug, but rather as a supplement. The usual recommended dose is 30–40 mg/kg/day intravenously or orally, for an average of 3–4 days.19 The standard dose of penicillin G for this indication is 300,000–1,000,000 units/kg/day.20
Treatment of viral hepatitis–induced ALF has also benefited from the development of specific antiretrovirals. In hepatitis B–induced ALF, antiviral treatment with new-generation nucleoside or nucleotide analog therapy should be considered, based upon early studies that showed that lamivudine was beneficial and potentially prevented the need for liver transplant when introduced at an early phase.21 It is important to emphasize that the overwhelming majority of acute hepatitis B cases do not require antiviral therapy, as most patients recover on their own, but in the setting of ALF, antiviral therapy is indicated, as evidenced by encephalopathy or coagulopathy.
Hepatitis D co-infection may occur in conjunction with acute hepatitis B and is a risk factor for the development of ALF. Although there is no specific therapy for hepatitis D infection, diagnosis is important, as the disease follows a characteristic biphasic pattern in which initial recovery is followed by clinical deterioration. Hepatitis E also has no specific antiviral therapy but can cause ALF on rare occasions, particularly in pregnant women. Herpes simplex virus–related ALF requires early recognition and immediate medical treatment with intravenous acyclovir.22 This relatively rare cause of ALF can occur in pregnant women and immunosuppressed patients but has also been described in healthy individuals.
Another relatively uncommon but important cause of ALF is Wilson disease, which accounts for 1–3% of ALF cases in the United States annually. The typical fulminant Wilson disease patient is young, with an abrupt onset of hemolytic anemia, hyperbilirubinemia (direct and indirect), renal failure, and low serum alkaline phosphatase and uric acid levels.2 Although serum ceruloplasmin is usually low, it may be normal in up to 15% of cases; thus, 24-hour urinary copper as well as hepatic copper (via liver biopsy) are more sensitive diagnostic markers. Treatment with penicillamine is not recommended in the setting of significant hepatic dysfunction; rather, hemofiltration via albumin dialysis or plasma exchange is recommended. Some researchers suggest that early treatment with penicillamine prior to onset of encephalopathy may obviate the need for liver transplant.23 However, most cases of fulminant Wilson disease are thought to require transplant for patient survival, though this may be a function of the patient presenting late in their disease secondary to difficulty in diagnosis.
Autoimmune hepatitis is another cause of ALF that is important to recognize. Autoantibodies may be absent, and liver biopsy is often required to obtain a definitive diagnosis. A small but significant subset of these patients will respond to treatment with steroids, whereas others will require liver transplantation.
Pregnancy-related ALF is usually related to acute fatty liver of pregnancy or Hemolysis, Elevated Liver Enzymes, Low Platelets syndrome. Jaundice, coagulopathy, low platelets, and hypoglycemia are notable findings.2These alterations resolve with delivery in the majority of patients, with a relative minority eventually requiring liver transplantation. As certain viral infections are more common among pregnant women, it is important not to overlook alternative or coexisting diagnoses in a jaundiced pregnant patient.
Acute Budd-Chiari syndrome is defined by acute hepatic vein thrombosis and can present with ALF. Although the first-line treatment of Budd-Chiari syndrome is venous decompression via transjugular intrahepatic portosystemic shunts or a similar procedure, some patients present with hepatic dysfunction severe enough to warrant liver transplantation. One important caveat is to evaluate for the underlying cause of the thrombotic event and determine whether it is compatible with liver transplantation. For example, hypercoagulable states associated with malignancy would preclude liver transplantation, whereas other hypercoagulable states might be effectively managed with postoperative anticoagulation.
Intensive Care Unit Management of the Acute Liver Failure Patient
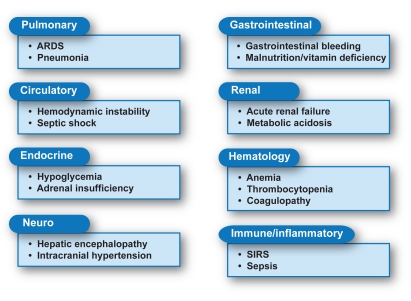
Effective ICU management of ALF patients requires an experienced multidisciplinary team (Figure 1). The first step in this process is the decision to admit the patient to the ICU. The presence and degree of HE is important for making this decision. Patients with stage I HE experience changes in behavior, with minimal changes in their level of consciousness, and may be initially managed in a step-down or ward setting. On the other hand, patients with drowsiness, asterixis, and any degree of disorientation (stage II HE) are at a high risk of decompensation and warrant ICU admission. Patients with more severe neurologic impairment (stages III –Iv HE) require intubation and mechanical ventilation.
Figure 1.
Acute liver failure involvement of multiple organ systems.
- ARDS=
- adult respiratory distress syndrome;
- SIRS=
- systemic inflammatory response syndrome.
The pathophysiology of HE is not completely understood. An association has been described between arterial ammonia levels, ICH, and the risk of cerebral herniation.24Ammonia-lowering therapy with lactulose has been the mainstay of treatment for HE, but oral administration is often problematic in the setting of altered mental status. Once mechanically ventilated, a nasogastric tube may be used to administer the frequent doses of this medication, though there is a lack of randomized controlled trials showing that lactulose directly improves outcomes.25 Appropriately formulated rectal lactulose may also be used in the absence of a nasogastric tube. Rifaximin (Xifaxan, Salix), an oral antibiotic that has been used in both the treatment and prevention of HE in chronic liver disease, has not been evaluated for its efficacy in ALF and, therefore, cannot be recommended as monotherapy for HE. However, given this nonabsorbable antibiotic's efficacy and safety, it would appear reasonable to administer rifaximin in combination with lactulose for severe cases of HE at a dose of 400 mg 3 times daily.26 With lactulose therapy, the potential side effect of colonic distention should be taken into consideration, particularly among patients who are being prepared for liver transplant surgery.
Cerebral edema and ICH are the most dramatic complications of ALF and can lead to death from cerebral herniation. Higher grades of encephalopathy correlate with a higher risk of ICH, and 20–25% of ALF deaths have been attributed to ICH historically, though more recent data show significantly fewer deaths (5–13%) directly resulting from ICH.3,27,28 ICH may be inferred from computed tomography scan or directly measured via intracranial pressure (ICP) measurements, but it is important to initiate treatment early in high-risk patients with elevated arterial ammonia levels (>150– 200 μmol/L) or grades III–Iv HE.3 Recommended management includes intubation, sedation, head-of-bed elevation to at least 30 degrees, and efforts to minimize interventions and stimuli that result in increased ICP. Prophylactic dosing of antiseizure medications has not been shown to improve outcomes.29
Neurologic monitoring includes transcranial Doppler, continuous electroencephalography, and jugular venous oxygen saturation (SvJO2). The need for ICP monitoring and the decision of when to place an ICP monitor is a matter of debate, as there are no randomized controlled trials evaluating these questions. The placement of ICP monitoring devices can cause significant intracranial bleeding, though this risk declines once the device is in place.30 Goal ICP is less than 20 mmHg, with a cerebral perfusion pressure (CPP) of greater than 50–60 mmHg (CPP=mean arterial pressure – ICP).2Intravenous mannitol at a dose of 1 g/kg body weight (dose range 0.25–2 g/kg) is a first-line therapy that may be used. Hypertonic saline given in serial intravenous boluses to reach a serum sodium level of 145–155 mmol/L has also been shown to reduce ICP.31 Hyperventilation to reduce PaCO2 to 25–30 mmHg has been used as a shortterm solution to decrease cerebral blood flow and lower ICP. Moderate hypothermia (32–34 degrees Celsius) or indomethacin may also be used to lower ICP when other approaches have failed.32
Infection has consistently remained the number one cause of death in ALF patients, and treatment approaches vary considerably among transplant centers. Daily surveillance of blood, urine, and sputum cultures has been shown to be useful and can direct therapy. Prophylactic antibiotics have been shown to reduce infection rates, though their use has not been directly linked to improved outcomes. The addition of selective gut decontamination to prophylactic antibiotics does not appear to improve efficacy.33 Fungal infections such as Candida albicans can be found in up to one third of patients with ALF and are associated with high mortality.34 A reasonable approach adopted by several centers, including our own, is to obtain pan cultures of patients daily and to consider empiric antibiotics and antifungals in high-risk individuals with severe HE, renal failure, or mechanical ventilation.
The circulatory disturbance in ALF is characterized by decreased systemic vascular resistance and elevated cardiac output, a pattern similar to that seen in septic shock. Management consists of intravenous fluid resuscitation and vasopressors guided by invasive hemodynamic monitoring. Adrenal insufficiency is commonly seen in ALF, and the presence of persistent hypotension despite volume resuscitation and vasopressors should lead to an assessment of adrenal function with a cosyntropin stimulation test.35 Empiric treatment for adrenal insufficiency with intravenous steroids while awaiting the results of the adrenal insufficiency tests is both prudent and indicated, if clinical suspicion is high.
Mechanical ventilation is often required in the setting of volume resuscitation or significant HE. Acute respiratory distress syndrome is commonly seen in ALF patients, and protective ventilation strategies designed to minimize lung injury are important.31
Acute kidney injury (AKI) is common in ALF, particularly in cases of acetaminophen toxicity. volume depletion, direct nephrotoxicity of medications, acute tubular necrosis, and hepatorenal syndrome are contributing mechanisms.36 Renal failure often coexists with a variety of metabolic derangements, including lactic acidosis, hyponatremia, hypophosphatemia (particularly in acetaminophen toxicity), and, often, profound hypoglycemia requiring continuous glucose infusion.2 In the setting of AKI, nephrotoxins such as intravenous contrast agents, aminoglycosides, and nonsteroidal anti-inflammatory drugs should be avoided. volume resuscitation is also an important initial therapy with a preference for a combination of isotonic crystalloid and colloid solutions.
The decision of when to begin renal replacement therapy (RRT) has been a subject of debate. There is agreement that when an acute indication for RRT exists, continuous RRT, rather than intermittent hemodialysis, is preferred to minimize cardiovascular disturbances and increased ICPs. In the absence of an acute indication such as refractory acidosis, hyperkalemia, or volume overload, some researchers advocate early RRT in the setting of decreased urine output or rising creatinine in an attempt to prevent cardiovascular instability, reduce levels of circulating ammonia, and, in some cases, induce a relative hypothermia.31 Preliminary studies suggest a potential mortality benefit, but large randomized controlled trials are lacking.37
Bleeding in the setting of ALF is another important consideration, though, in practice, significant bleeding is seen only in 5% of ALF cases.38 The risk of bleeding is increased in the setting of AKI and ICH. Patients with ALF often have markedly elevated prothrombin times or INRs, as well as thrombocytopenia, though these measures do not always correlate to increased rates of bleeding. Gastrointestinal prophylaxis with either a proton pump inhibitor or histamine receptor 2 antagonist is standard at most centers. Correction of coagulopathy with fresh frozen plasma or thrombocytopenia with platelet transfusion is not indicated unless an invasive procedure is planned. Commonly cited targets that allow placement of a central venous catheter or ICP monitor include an INR of no more than 1.5 and a platelet count of at least 50,000 cells/microliter; however, these targets are somewhat arbitrary and are not supported by robust evidence. Preliminary data suggest that recombinant factor vIIa may be an effective and rapid method to correct coagulopathy for procedures, yet the safety of this approach has not been established.39Similarly, the use of a general strategy to minimize blood product use in the absence of overt bleeding or an invasive procedure appears prudent, particularly given the known complications of transfusion and the risk of thromboembolism associated with recombinant factor vIIa.
Nutrition is another consideration in ALF patients, with electrolyte and vitamin replacement being important, as well as early initiation of enteral nutrition. Protein restriction is not indicated, and 1 g/kg per day of protein is recommended. Specific amino acid formulations (branched chain) appear to be no more effective than standard supplements.2 Parenteral nutrition may be needed if enteral nutrition is contraindicated.
Bridge to Transplant
In selected ALF patients who have failed medical therapy but for whom a liver transplant is not available, other interventions have been envisioned to avoid further decompensation that would preclude liver transplant. Treatments at this point reside in the experimental domain, with case studies reporting success but no ideal approach. Hepatectomy with portocaval shunt has been described as a successful bridge to transplant in the setting of severe hemodynamic instability attributable in part to the “toxic” failing liver.40,41 Artificial liver support systems have also been investigated, though no single system has proven to be safe and effective at performing the liver's many functions of detoxification and synthesis. These charcoal (or similar) devices often worsen coagulopathy and thrombocytopenia, and newer devices using tissue culture hepatocytes have failed to show a mortality benefit in early studies.42,43 One artificial liver support system that is based upon the selective removal of albumin bound toxins from blood is the molecular adsorbent recirculating system, whose effect on survival with and without transplantation may be beneficial.44
Liver Transplant
Despite aggressive medical management, many patients with ALF deteriorate to a point where transplantation remains their only option for survival. The decision to list a patient with ALF for transplant is difficult, though most physicians agree that there is a window of opportunity where the patient clearly requires transplantation and is well enough to survive the operation. Usually, this window comes after initial stabilization and before sepsis and multisystem organ failure. Relative contraindications for liver transplantation include sustained cerebral hypo-perfusion with cerebral perfusion pressures of less than 40 mmHg for more than 2 hours, the need for high-dose vasopressors, and the presence of acute respiratory distress syndrome with the need for inhaled oxygen at more than 60% FiO2 and peak end expiratory pressure of more than 12 cm H20.
Long-term outcomes following liver transplantation for ALF are worse than those in chronic liver diseases, with 1-year survival rates of 58–92% and 5-year survival rates of 61–76%.45 Good-quality whole donor organs obtained after a short waiting period are considered the key to a successful outcome. The previously mentioned King's College Criteria are relatively accurate in predicting poor outcome, whereas other scoring systems such as the Acute Physiology and Chronic Health Evaluation System, levels of factor v, and the Model for End-Stage Liver Disease score have variable sensitivity and specificity.2 The etiology of liver failure also appears to be associated with prognosis, with acetaminophen, hepatitis A, shock liver, or pregnancy-related disease being associated with significantly higher transplant-free survival rates.11 Psychosocial issues are also important to consider, particularly relative to long-term outcomes, as patients with poor social support or overdose as the cause of their ALF are difficult to manage after their initial recovery.
Summary
Although comprising a minority of the transplant population, ALF patients represent some of the most challenging cases in terms of the level and complexity of care required. Successful care of the ALF patient begins with early diagnosis and triage to the appropriate level of care where a multitude of specialties are required to work together to maximize the chance of recovery and/or extend the window of opportunity for transplant. Transplant outcomes at several large centers dedicated to ALF appear to be similar to those for chronic liver disease, but these findings have not been universally seen.
Contributor Information
Dawn McDowell Torres, Dr. McDowell Torres is a Transplant Hepatology Fellow..
Robert D. Stevens, Dr. Stevens serves as Associate Professor in the Departments of Anesthesiology/Critical Care Medicine, Neurology, Neurosurgery, and Radiology in the Division of Neurosciences Critical Care at the Johns Hopkins University School of Medicine..
Ahmet Gurakar, Dr. Gurakar serves as Associate Professor of Medicine and Medical Director of Liver Transplantation in the Division of Gastroenterology and Hepatology at the Johns Hopkins University School of Medicine in Baltimore, Maryland.
References
- 1.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 2.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 3.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Shalimar, Bhatia v, et al. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–1674. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman ML. Management of acute hepatitis B. Clin Liver Dis. 2010;14:75–91. doi: 10.1016/j.cld.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Shukla NB, Poles MA. Hepatitis B infection: co-infection with hepatitis C virus, hepatitis D virus and human immunodeficiency virus. Clin Liver Dis. 2004;8:445–460. doi: 10.1016/j.cld.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Sagnelli E, Coppola N, Pisaturo M, et al. HBv superinfection in HCv chronic carriers: a disease that is frequently severe but associated with the eradication of HCv. Hepatology. 2009;49:1090–1097. doi: 10.1002/hep.22794. [DOI] [PubMed] [Google Scholar]
- 8.Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008;14(suppl 2):S67–79. doi: 10.1002/lt.21612. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health & Human Services. OPTN: Organ Procurement and Transplantation Network [Web site] [March 23, 2010]. Available at: http://optn.transplant.hrsa.gov/latestdata/rptdata.asp.
- 10.Mas A, Rodes J. Fulminant hepatic failure. Lancet. 1997;349:1081–1085. doi: 10.1016/S0140-6736(96)08054-3. [DOI] [PubMed] [Google Scholar]
- 11.Ostapowicz G, Fontana RJ, SchiØdt Fv, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 12.Farmer DG, Anselmo DM, Ghobrial RM, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg. 2003;237:666–675. doi: 10.1097/01.SLA.0000064365.54197.9E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 14.Fong TL, Klontz KC, Canas-Coto A, et al. Hepatotoxicity due to hydroxycut: a case series. Am J Gastroenterol. 2010 Jan 26; doi: 10.1038/ajg.2010.5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gloro R, Hourman-Olivier I, Mosquet B, et al. Fulminant hepatitis during self medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol. 2005;17:1135–1137. doi: 10.1097/00042737-200510000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Ganzert M, Felgenhauer N, Zilker T. Reassessment of predictors of fatal outcome in amatoxin poisoning: some critical comments. J Hepatol. 2007;47:424–425. doi: 10.1016/j.jhep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotelo N, de los Angeles Durazo M, Gonzalez A, Dhanakotti N. Early treatment with N-acetylcysteine in children with acute liver failure secondary to hepatitis A. Ann Hepatol. 2009;8:353–358. [PubMed] [Google Scholar]
- 19.Hruby K, Csomos G, Fuhrmann M, Thaler H. Chemotherapy of Amanita phalloides poisoning with intravenous silibinin. Hum Toxicol. 1983;2:138–185. doi: 10.1177/096032718300200203. [DOI] [PubMed] [Google Scholar]
- 20.Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning--from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96:3195–3198. doi: 10.1111/j.1572-0241.2001.05283.x. [DOI] [PubMed] [Google Scholar]
- 21.Tillmann HL, Hadem J, Leifeld L, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J viral Hepatol. 2006;13:256–263. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 22.Ichai P, Roque Afonso AM, Sebagh M, et al. Herpes simplex virus-associated acute liver failure: a difficult diagnosis with a poor prognosis. Liver Transpl. 2005;11:1550–1555. doi: 10.1002/lt.20545. [DOI] [PubMed] [Google Scholar]
- 23.Durand F, Bernuau J, Giostra E, et al. Wilson's disease with severe hepatic insufficiency: beneficial effects of early administration of D-penicillamine. Gut. 2001;48:849–852. doi: 10.1136/gut.48.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalan R, Olde Damink SW, Hayes PC, Deutz NE, Lee A. Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia, and cerebral blood flow. J Hepatol. 2004;41:613–620. doi: 10.1016/j.jhep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Alba L, Hay JE, Angulo P, Lee WM. Lactulose therapy in acute liver failure. J Hepatol. 2002;36(suppl 1):33. [Google Scholar]
- 26.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 27.Barshes NR, Lee TC, Balkrishnan R, et al. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195–201. doi: 10.1097/01.tp.0000188149.90975.63. [DOI] [PubMed] [Google Scholar]
- 28.Bernal W, Cross TJ, Auzinger G, et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol. 2009;50:306–313. doi: 10.1016/j.jhep.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia v, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure – a controlled clinical trial. J Hepatol. 2004;41:89–96. doi: 10.1016/j.jhep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 30.vaquero J, Fontana RJ, Larson AM, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–1589. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 31.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464–470. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 32.Bernal W, Auzinger G, Sizer E, Wendon J. Intensive care management of acute liver failure. Semin Liver Dis. 2008;28:188–200. doi: 10.1055/s-2008-1073118. [DOI] [PubMed] [Google Scholar]
- 33.Rolando N, Wade JJ, Stangou A, et al. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg. 1996;2:8–13. doi: 10.1002/lt.500020103. [DOI] [PubMed] [Google Scholar]
- 34.Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl. 2005;11:594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 35.Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395–402. doi: 10.1053/jhep.2002.34514. [DOI] [PubMed] [Google Scholar]
- 36.Larson AM. Acute liver failure. Dis Mon. 2008;54:457–485. doi: 10.1016/j.disamonth.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Wu vC, Ko WJ, Chang HW, et al. Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg. 2007;205:266–276. doi: 10.1016/j.jamcollsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Pereira LM, Langley PG, Hayllar KM, Tredger JM, Williams R. Coagulation factor v and vII/v ratio as predictors of outcome in paracetamol-induced fulminant hepatic failure: relation to other prognosotic indicators. Gut. 1992;33:98–102. doi: 10.1136/gut.33.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shami vM, Caldwell SH, Hespenheide EE, Arseneau KO, Bickston SJ, Macik BG. Recombinant activated factor vII for coagulopathy in fulminant hepatic failure compared with conventional therapy. Liver Transpl. 2003;9:138–143. doi: 10.1053/jlts.2003.50017. [DOI] [PubMed] [Google Scholar]
- 40.Ringe B, Lübbe N, Kuse E, Frei U, Pichlmayr R. Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg. 1993;218:3–9. doi: 10.1097/00000658-199307000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferraz-Neto BH, Moraes-Junior JM, Hidalgo R, et al. Total hepatectomy and liver transplantation as a two-stage procedure for toxic liver: case reports. Transplant Proc. 2008;40:814–816. doi: 10.1016/j.transproceed.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 42.Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artifical and bioartifical support systems for acute and acute-on-chronic liver failure: a systemic review. JAMA. 2003;289:217–222. doi: 10.1001/jama.289.2.217. [DOI] [PubMed] [Google Scholar]
- 43.Akdogan M, Aladag M, Rashwan S, et al. Fulminant hepatic failure and the potential role of liver dialysis. Int J Artif Organs. 2004;27:956–961. doi: 10.1177/039139880402701108. [DOI] [PubMed] [Google Scholar]
- 44.Saliba F. The molecular adsorbent recirculating system in the intensive care unit: a rescue therapy for patients with hepatic failure. Crit Care. 2006;10:118. doi: 10.1186/cc4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan G, Taqi A, Marotta P, et al. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl. 2009;15:1696–1702. doi: 10.1002/lt.21931. [DOI] [PubMed] [Google Scholar]