Kaposi sarcoma is a low-grade vascular tumor associated with human herpesvirus-8 infection (HHV-8). The first description of this tumor dates back to 1872 and was made by Dr. Moritz Kaposi, a Hungarian dermatologist who described 5 cases of “idio-pathic multiple pigmented sarcomas of the skin.”1 In total, 4 forms of this disease have been described. As HHV-8 has been detected in all 4 forms of Kaposi sarcoma,2 these forms likely represent different manifestations of the same pathologic process.
The classical variant of Kaposi sarcoma occurs predominantly in elderly men from Eastern Europe and Mediterranean countries.3–5 This form is not associated with HIV, but it coincides with an altered immune system and malignant diseases. Clinically, this variant is distinguished by multiple red-to-purple nodules on the lower limbs. These nodules slowly grow larger and are subsequently also found in more proximal regions. The tumors are usually asymptomatic and are rarely systemically progressive. The second variant is the lymphadenopathy-associated form of Kaposi sarcoma, also called the endemic or African form. This form is very aggressive6and is often found in South Africa in young Bantu children with local or generalized lymphadenopathy.7Skin lesions are rare in this variant. The third variant is the transplant- or immunosuppression-associated form of Kaposi sarcoma. This form develops between several months to several years after organ transplantation with immunosuppressive therapy. Lesions develop on the skin, but in approximately half of the cases, they are also found in internal organs and lymph nodes.8–10 The fourth variant of Kaposi sarcoma is the AIDS-associated (epidemic) form. This form is found in approximately one fourth of all AIDS patients and is the most common AIDS-associated tumor in the United States. Kaposi sarcoma occurs in AIDS-affected homosexual men 20 times more frequently than in nonhomosexual AIDS patients with the same degree of immunodeficiency. AIDS-associated Kaposi sarcoma has no preferred locations but is widely scattered, and involvement of the lymph nodes and intestine occurs relatively early.3,11
Case Report
A 40-year-old African-American man with a history of anemia and small-bowel thickening on computed tomography scan was referred for single-balloon small-bowel enteroscopy. His past medical history was suggestive of asthma and depression, and his medications included an albuterol inhaler as needed. The patient had a history of unprotected sexual contact but was currently living with his girlfriend of many years. He denied having any history of drug allergies, smoking, alcohol, or intravenous drug abuse, or blood transfusions. On examination, the patient was moderately built and nourished, with stable vitals, a weight of 183 lbs, and no evidence of skin lesions. Laboratory studies revealed a hemoglobin level of 10.5 g/dL, hematocrit of 31.9%, white blood cell count of 7,800/cmm with a differential of 55.6% neutrophils and 28.4% lymphocytes, platelet count of 470 units, and mean corpuscular volume of 82 fL. Computed tomography scan showed 3 small-bowel masses of uncertain etiology. Small-bowel enteroscopy revealed scattered umbilicated nodules with central ulceration extending from the left tonsillar area to the distal jejunum (Figures 1–4). Biopsy specimens were obtained from the small bowel (Figures 5–7) and gastric lesions (Figures 8–11).
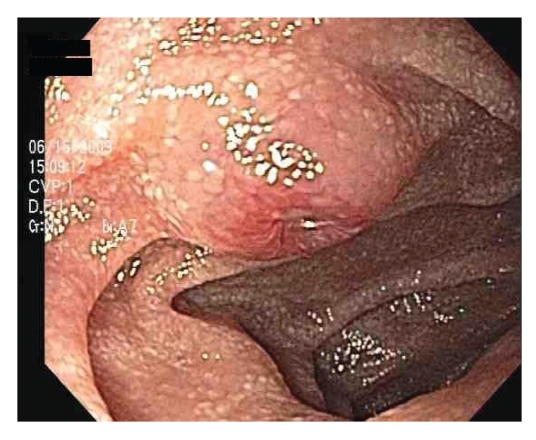
Figure 1.
Umbilicated nodule with central ulceration in the mid-jejunum.
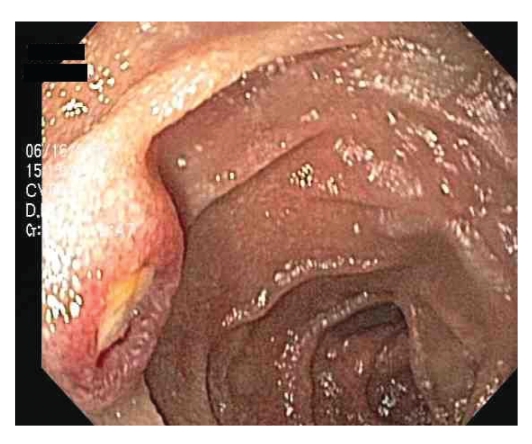
Figure 2.
Umbilicated nodule with central ulceration in the proximal jejunum.
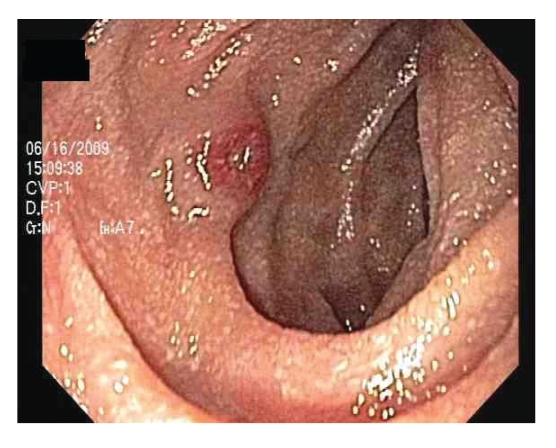
Figure 3.
Umbilicated nodule with central ulceration in the duodenum.
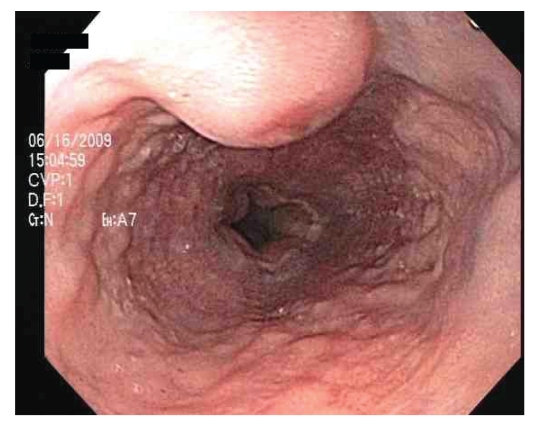
Figure 4.
Umbilicated nodule in the esophagus.
Figure 5.
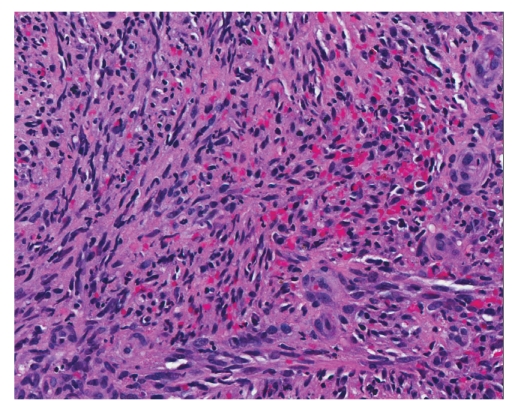
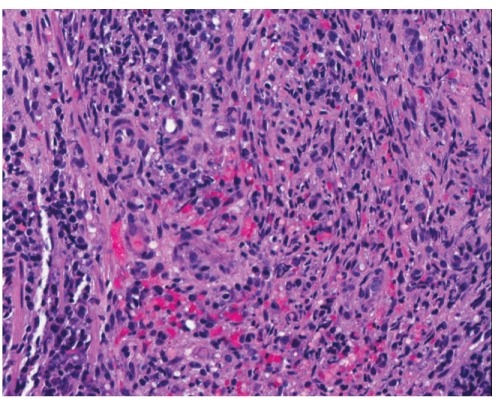
Hematoxylin and eosin stain showing the small intestine with Kaposi sarcoma (×200).
Figure 6.
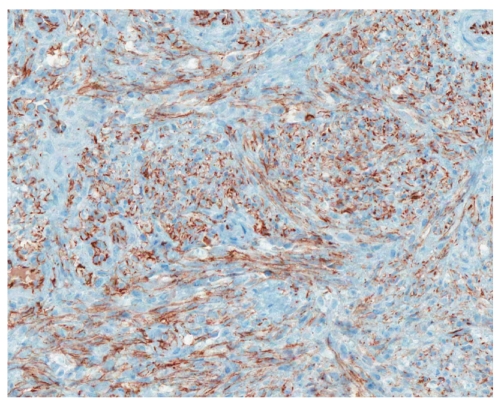
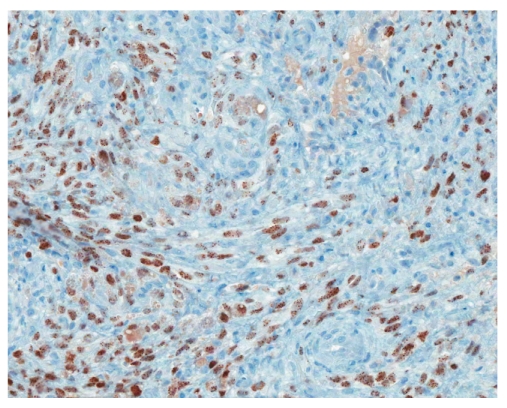
CD31 immunostain revealing the small intestine with Kaposi sarcoma (×200).
Figure 7.
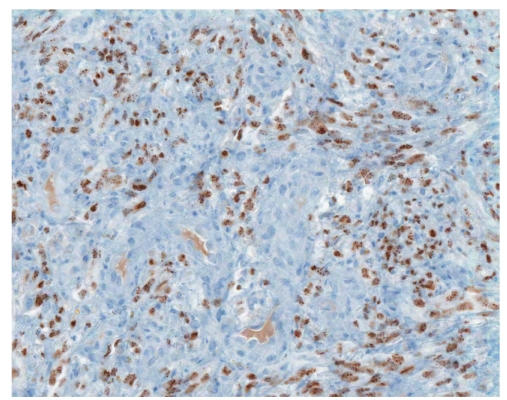
Human herpes virus-8 immunostain showing the small intestine with Kaposi sarcoma (×200).
Figure 8.
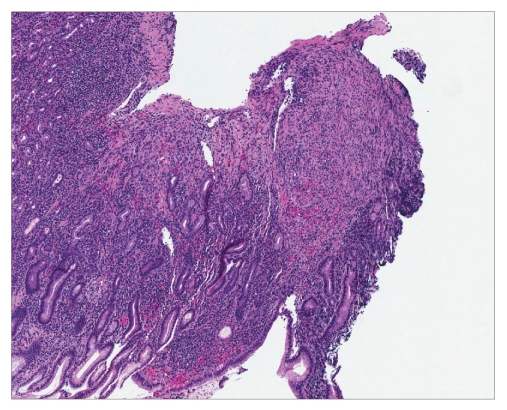
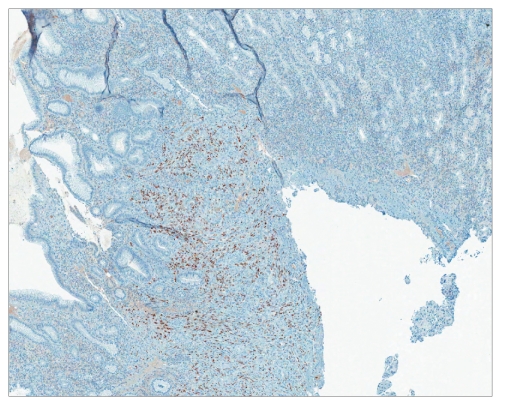
Hematoxylin and eosin stain revealing stomach mucosa with Kaposi sarcoma (×40).
Figure 9.
Hematoxylin and eosin stain showing stomach mucosa with Kaposi sarcoma (×200).
Figure 10.
Human herpes virus-8 immunostain revealing stomach mucosa with Kaposi sarcoma (×40).
Figure 11.
Human herpes virus-8 immunostain showing stomach mucosa with Kaposi sarcoma (×200).
Histology is similar in each form of Kaposi sarcoma, with submucosal vascular spindle-shaped cells, and does not allow for distinguishing the 4 forms. In our patient, the biopsy specimens revealed whorls of spindle-shaped cells and neovascularization with small-vessel proliferation suggestive of Kaposi sarcoma. Immunostains for HHV-8 and CD31 were positive, supporting the above diagnosis. The patient tested positive for HIV, with a CD4 count of less than 50 cells per cubic millimeter of blood. The patient received a referral for an infectious diseases consultation for the initiation of highly active antiretroviral treatment (HAART).
Discussion
Kaposi sarcoma is the most common gastrointestinal malignancy in AIDS (seen in approximately 40% of patients) and is often asymptomatic.1 The presentation of Kaposi sarcoma led to the establishment of an AIDS diagnosis in our patient. A greater-than-50% incidence of Kaposi sarcoma of the gastrointestinal tract has been seen in AIDS patients with cutaneous Kaposi sarcoma.
Although gastrointestinal Kaposi sarcoma is usually asymptomatic, hemorrhages from the oral cavity, esophagus, stomach, and large bowel have been reported in this disease.12,13 Some patients present with abdominal pain, weight loss, nausea, vomiting, malabsorption, or diarrhea.2Further complications of gastrointestinal Kaposi sarcoma can be perforation14 or obstruction15 of the bowel.
One case of HIV-related Kaposi sarcoma of the appendix and acute appendicitis has been described in the literature.16 As a differential diagnosis of Kaposi sarcoma, non-Hodgkin lymphomas frequently involve the gut in AIDS patients.15 Furthermore, tumors of the gut with spindle-shaped cells such as leiomyomas, rhabdomyosarcomas, high-grade pleomorphic sarcomas, or gastrointestinal stromal tumors have to be considered in the differential diagnosis. The primary diagnosis of Kaposi sarcoma in the stomach or small or large intestine should be considered in elderly men from Eastern Europe, Mediterranean and Arabian regions, and, naturally, in immunosuppressed and AIDS patients with corresponding lesions. The diagnosis of Kaposi sarcoma with a negative HIV test and positive test for HHV-8 should lead to the consideration of other causes such as iatrogenic or tumor-related immunosuppression (lymphoproliferative disorders).
The origin of the proliferating spindle cells in Kaposi sarcoma is uncertain, though these cells are currently believed to be derived from lymphatic endothelium.17Immunohistochemistry shows expression of CD34, CD31, and D2-40.18,19 Another lymphatic endothelial cell marker (hyaluronan receptor LYVE-1), expressed by endothelial cells of normal lymphatic vessels but not blood vessels, is positive in angiosarcomas and Kaposi sarcomas.20 A monoclonal antibody (FHI-1) against the carboxyl terminal end of the FLI-1 protein can be reliably applied in the differential diagnosis of tumors of endothelial differentiation. All rhabdomyosarcomas, desmo-plastic small round cell tumors, high-grade pleomorphic sarcomas, and colonic adenocarcinomas are negative for FLI-1.21 Therefore, FLI-1 can help in the differential diagnosis of nonvascular tumors such as gastrointestinal stromal tumors. Infection with HHV-8 is necessary for the development of Kaposi sarcoma in HIV patients, and, at present, it is considered the definitive cause of Kaposi sarcoma. Over 95% of Kaposi sarcoma lesions, regardless of their source or clinical subtype, have been found to be infected with HHV-8.22 The long-lasting expression of HHV-8 latency genes is important for Kaposi sarcoma spindle-cell progression,23 and the lesional spindle cells in our patient's biopsies confirmed HHV-8 infection using immunohistochemistry staining.
Overall, the visceral involvement of the Kaposi sarcoma is usually associated with poor prognosis.24 Treatment is usually palliative and aimed primarily at improving symptoms and preventing progression. Options may include antiretroviral medications, radiation therapy, chemotherapy, or combination therapy.3 Depending upon the severity of HIV and the disease burden of Kaposi sarcoma, HAART could be first-line therapy. Antiretrovirals may help decrease the proportion of new lesions, promote regression of existing lesions, and improve survival with or without chemotherapy.3 Systemic chemotherapy is usually reserved for cases with widespread disease. Due to favorable response rates and toxicity profiles, liposomal anthracyclines (eg, doxorubicin) have become first-line systemic agents for treatment of disseminated Kaposi sarcoma.4
In conclusion, we suggest that Kaposi sarcoma be included within the differential diagnosis of small-bowel nodules in otherwise asymptomatic patients. Although the era of HAART has significantly decreased the incidence of Kaposi sarcoma and its gastrointestinal manifestations, a high index of suspicion in susceptible populations may increase the likelihood of early diagnosis and aid management of this aggressive disease.
References
- 1.Kaposi M. Idiopatisches multiples pigmentsarkom der haut. Arch Dermatol Syph. 1872;3:265–273. [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Biggar RJ, Horm J, Fraumeni JF, Jr, Greene MH, Goedert JJ. Incidence of Kaposi's sarcoma and mycosis fungoides in the United States including Puerto Rico, 1973-1981. J Natl Cancer Inst. 1984;73:89–94. [PubMed] [Google Scholar]
- 4.Laor Y, Schwartz RA. Epidemiologic aspects of American Kaposi's sarcoma. J Surg Oncol. 1979;12:299–303. doi: 10.1002/jso.2930120403. [DOI] [PubMed] [Google Scholar]
- 5.Ross RK, Casagrande JT, Dworsky RL, Levine A, Mack T. Kaposi's sarcoma in Los Angeles, California. J Natl Cancer Inst. 1985;75:1011–1015. [PubMed] [Google Scholar]
- 6.Dezube BJ, Pantanowitz L, Aboulafa DM. Management of AIDS-related Kaposi sarcoma: advances in target discovery and treatment. AIDS Read. 2004;14:236–238. 243-244, 251-253. [PubMed] [Google Scholar]
- 7.Friedman SL, Wright TL, Altman DF. Gastrointestinal Kaposi's sarcoma in patients with acquired immune deficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89:102–108. doi: 10.1016/0016-5085(85)90750-4. [DOI] [PubMed] [Google Scholar]
- 8.Farge D, Lebbe C, Marjanovic Z, et al. Human herpes virus-8 and other risk factors for Kaposi's sarcoma in kidney transplant recipients. Groupe Cooperatif de Transplantation d'Ile de France (GCIF) Transplantation. 1999;67:1236–1242. doi: 10.1097/00007890-199905150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Margolius L, Stein M, Spencer D, Bezwoda WR. Kaposi's sarcoma in renal transplant recipients. Experience at Johannesburg Hospital, 1966–1989. S Afr Med J. 1994;84:16–17. [PubMed] [Google Scholar]
- 10.Shepherd FA, Maher E, Cardella C, et al. Treatment of Kaposi's sarcoma after solid organ transplantation. J Clin Oncol. 1997;15:2371–2377. doi: 10.1200/JCO.1997.15.6.2371. [DOI] [PubMed] [Google Scholar]
- 11.Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 12.Lin CH, Hsu CW, Chiang YJ, Ng KF, Chiu CT. Esophageal and gastric Kaposi's sarcomas presenting as upper gastrointestinal bleeding. Chang Gung Med J. 2002;25:329–333. [PubMed] [Google Scholar]
- 13.Neville CR, Peddada AV, Smith D, Kagan AR, Frost DB, Sadoff L. Massive gastrointestinal hemorrhage from AIDS-related Kaposi's sarcoma confined to the small bowel managed with radiation. Med Pediatr Oncol. 1996;26:135–138. doi: 10.1002/(SICI)1096-911X(199602)26:2<135::AID-MPO12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida EM, Chan NH, Chan-Yan C, Baird RM. Perforation of the jejunum secondary to AIDS-related gastrointestinal Kaposi's sarcoma. Can J Gastroenterol. 1997;11:38–40. doi: 10.1155/1997/425861. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL. Kaposi's sarcoma and lymphoma of the gut in AIDS. Baillieres Clin Gastroenterol. 1990;4:455–475. doi: 10.1016/0950-3528(90)90012-6. [DOI] [PubMed] [Google Scholar]
- 16.Zebrowska G, Walsh NM. Human immunodeficiency virus-related Kaposi's sarcoma of the appendix and acute appendicitis. Report of a case and review of the literature. Arch Pathol Lab Med. 1991;115:1157–1160. [PubMed] [Google Scholar]
- 17.Hong YK, Foreman K, Shin JW, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 18.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 19.Kaiserling E. Immunohistochemical identification of lymph vessels with D2-40 in diagnostic pathology [in German] Pathologe. 2004;25:362–374. doi: 10.1007/s00292-004-0693-6. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Edwards JR, Espinosa O, Banerji S, Jackson DG, Athanasou NA. Expression of a lymphatic endothelial cell marker in benign and malignant vascular tumors. Hum Pathol. 2004;35:857–861. doi: 10.1016/j.humpath.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol. 2004;17:547–552. doi: 10.1038/modpathol.3800065. [DOI] [PubMed] [Google Scholar]
- 22.Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 23.Kahl P, Buettner R, Friedrichs N, Merkelbach-Bruse S, Wenzel J, Carl Heukamp L. Kaposi's sarcoma of the gastrointestinal tract: report of two cases and review of the literature. Pathol Res Pract. 2007;203:227–231. doi: 10.1016/j.prp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Huang WY, Pantanowitz L, Dezube BJ. Unusual sites of malignancies: CASE 3. AIDS-related Kaposi's sarcoma of the gastrointestinal tract. J Clin Oncol. 2005;23:2098–2099. doi: 10.1200/JCO.2005.01.171. [DOI] [PubMed] [Google Scholar]