Abstract
Background
Nodes of Ranvier correspond to specialized axonal domains where voltage-gated sodium channels are highly concentrated. In the peripheral nervous system, they are covered by Schwann cells microvilli, where three homologous cytoskeletal-associated proteins, ezrin, radixin and moesin (ERM proteins) have been found, to be enriched. These glial processes are thought to play a crucial role in organizing axonal nodal domains during development. However, little is known about the molecules present in Schwann cell processes that could mediate axoglial interactions. The aim of this study is to identify by immunocytochemistry transmembrane proteins enriched in Schwann cells processes that could interact, directly or indirectly, with axonal proteins.
Results
We show that syndecan-3 (S3) and syndecan-4 (S4), two proteoglycans expressed in Schwann cells, are enriched in perinodal processes in rat sciatic nerves. S3 labeling was localized in close vicinity of sodium channels as early as post-natal day 2, and highly concentrated at nodes of Ranvier in the adult. S4 immunoreactivity accumulated at nodes later, and was also prominent in internodal regions of myelinated fibers. Both S3 and S4 were co-localized with ezrin in perinodal processes.
Conclusions
Our data identify S3 and S4 as transmembrane proteins specifically enriched in Schwann cell perinodal processes, and suggest that S3 may be involved in early axoglial interactions during development.
Background
Clustering of voltage-gated Na+ channels at nodes of Ranvier is an essential aspect of the fast saltatory propagation of action potential along myelinated axons. The nodes are devoid of myelin and flanked by paranodes where lateral loops of glial cells are tightly attached to the axon by septate-like junctions. Thus, axoglial interactions generate distinct axonal domains characterized by specific multimolecular complexes (see [1] for a review). Several proteins are highly concentrated in the nodal axolemma, including Na+ channels, comprised of an α subunit, Nav1.2, replaced by Nav1.6 later during development, and a β subunit [2,3]. Nodal Na+ channels interact with ankyrin G [4], which is associated with βIV-spectrin [5], and with two cell adhesion molecules, Nr-CAM and the 186 kDa isoform of neurofascin [6]. These nodal axonal proteins are similar to those found at axonal initial segments and are thought to form a highly interconnected multimolecular meshwork. However, in contrast to initial segments, enrichment of these proteins at nodes of Ranvier is dependent on myelinating glial cells in both central and peripheral nervous systems [7-11]. In the central nervous system where there is no direct contact between oligodendrocytes and the nodal axolemma, a diffusible factor may trigger the clustering of nodal proteins [3,8]. In contrast, in peripheral nerves, the nodal axolemma is covered by microvilli emanating from myelinating Schwann cells, and a direct contact with Schwann cells may be required for node formation [7,10,11]. However, little is known about the molecules present in Schwann cells processes that could mediate the organization of axonal nodal proteins. Ezrin, radixin and moesin (ERM proteins), three homologous proteins that are enriched in microvilli of epithelial cells, are also concentrated in Schwann cells microvilli [12,13]. They co-localize with ezrin-binding protein 50 kDa (EBP-50) [12]. Since clustering of these proteins occurs early during development at the time of the initial accumulation of ankyrin G and Na+ channels in the axon, a direct or indirect role of microvilli in the organization of nodal regions is very likely. In support of this hypothesis a recent work has demonstrated that myelinating Schwann cells emit long processes at the tip of which ERM proteins are enriched in a Rho A-dependent fashion [14]. These tips overlap with zones of ankyrin G accumulation in the axon. ERM proteins are regulated cytoplasmic cross-linkers between actin cytoskeleton and transmembrane proteins [15], and cannot mediate direct nodal axoglial contacts. Therefore it is of interest to identify transmembrane proteins enriched in Schwann cells perinodal processes that could interact with axonal proteins, either directly or indirectly.
Syndecans are a family of proteoglycans (syndecan 1–4, here abbreviated as S1–4) containing heparan sulfate and chondroitin sulfate sugar chains [16,17]. They are type I membrane proteins, with an ectodomain containing several glycosaminoglycan attachment sites, a transmembrane domain and a short C-terminal cytoplasmic tail. Their extracellular chains bind to a variety of growth factors and extracellular matrix proteins. Many cytoplasmic partners of syndecans have also been isolated, with scaffolding or signaling properties [18,19]. Here we have investigated the localization of S3, known to be expressed in Schwann cells [20], and S4, an ubiquitous protein, in rat sciatic nerve. Using specific antibodies we found that both are enriched in Schwann cells perinodal processes.
Results
Production of specific antibodies against S3 and S4
To detect the membrane-associated forms of S3 and S4 we raised antibodies against their cytoplasmic domains. As the cytoplasmic tail of each syndecan encompasses two highly conserved regions, C1 and C2, flanking a variable region (V) unique to each syndecan (Fig. 1A), sera were affinity-purified using another fusion protein encompassing only the variable part of the cytoplasmic region of the cognate protein to avoid cross-reactivity. The specificity of the purified antibodies was tested using GFP-fusion proteins encompassing the intracellular region of the various syndecans and expressed in COS-7 cells (Fig. 1B,1C,1D,1E). Both S3 and S4 antibodies were selective for their cognate antigen by immunoblotting (Fig. 1B,1D) and by immunofluorescence (Fig. 1.C, 1E).
Figure 1.
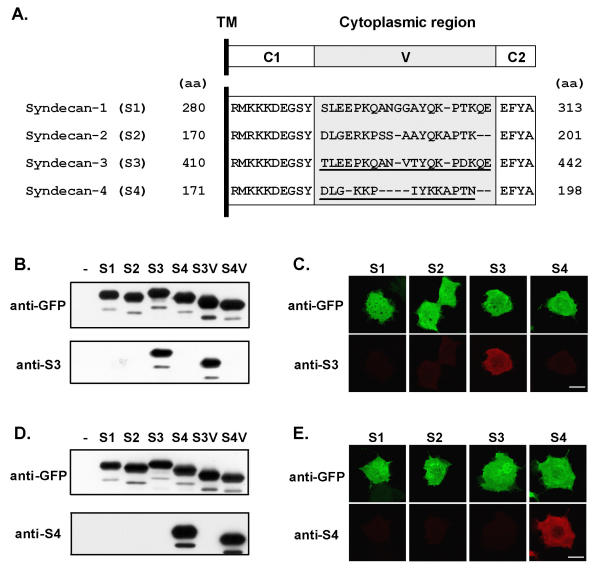
Characterization of antibodies against S3 and S4. (A) Organization in three sub-regions, C1, C2 and V (upper panel), and amino acid alignment of the cytoplasmic C-terminal regions of human S1–S4. Antibodies were raised against the entire cytoplasmic tails of S3 or S4, and purified by affinity on peptides encompassing only the variable (V) regions (underlined peptide sequences). Dashes represent gaps in the sequence introduced to optimize alignment. The position of the residues in the entire sequence of the proteins is indicated (aa). TM, transmembrane region. (B-E) Specificity of S3 and S4 antibodies was tested by immunoblotting (B, D) and immunocytochemistry (C, E) using COS-7 cells transfected with GFP-fusion proteins expressing either the intracellular regions of S1 to S4 (B-E, S1–S4) or only the variable regions of S3 and S4 (B and D, S3V and S4V). Antibodies directed against GFP (anti-GFP) were used to verify the expression of the proteins. COS-7 cells transfected with the vector alone were used as mock control in immunoblotting experiments (-). Anti-S3 and anti-S4 antibodies recognized specifically GFP-fusion proteins encompassing S3 and S4 intracellular region, respectively. Scale bars: 20 μm.
Distribution of S3 in sciatic nerve
S3 is the most abundant syndecan in the central and peripheral nervous systems, with a peak of expression in the first postnatal week [21]. In the adult central nervous system it is present along axons [22]. S3 is also expressed in Schwann cells [20] in which it allows adhesion to α4 Type V collagen [23]. In sections of neonatal rat sciatic nerves, S3 was reported to be present in membranes of Schwann cells and/or neurons [20], but its localization was not investigated at later stages. We studied the distribution of S3 immunoreactivity in sections of adult rat sciatic nerve, in parallel with that of Na+ channels (Nav), and shaker-type K+ channels (Kv1.1) enriched in juxtaparanodal regions [24]. S3 labeling was concentrated in narrow bands corresponding to nodal gaps (Fig. 2A). This localization was almost identical to that of Nav (Fig. 2B). However, in sections perpendicular to the nerve axis, the ring of S3 immunoreactivity was wider than the ring of Nav and appeared to surround it (Fig. 2C). Thus, the two proteins were not co-localized in the axon, indicating the presence of S3 in the Schwann cells perinodal processes. In teased fibers from rat sciatic nerve, a similar distribution was observed (Fig. 2D). In this preparation S3 labeling was clearly located around the nodal axon. In sciatic nerve sections as well as in teased fibers, all nodes that were identifiable by Nav immunoreactivity were stained with anti-S3 antibodies.
Figure 2.
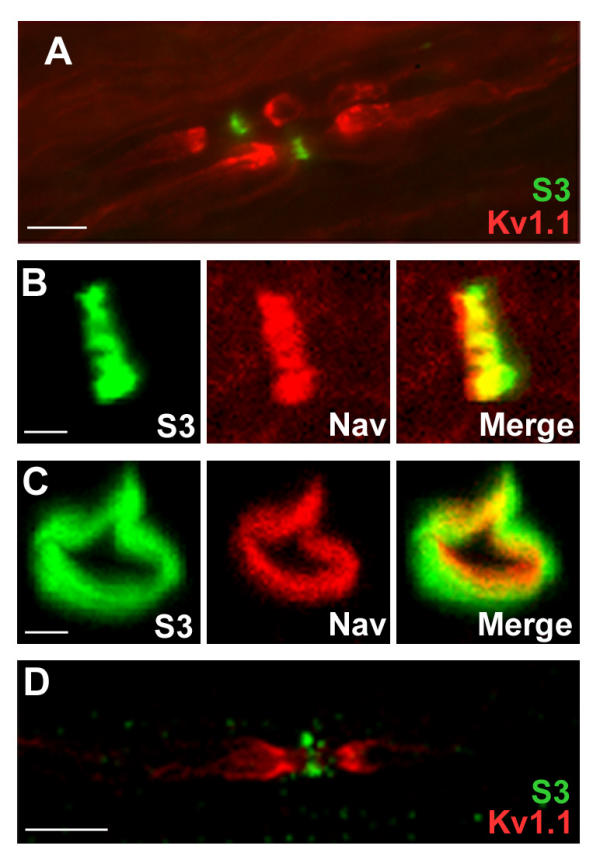
Localization of S3 in nodal regions of the PNS. (A-C) Sections through rat sciatic nerve at P30 were labeled with antibodies to S3 (green) and K+ channels (Kv1.1, A, red) or Na+ channels (Nav, B-C, red). Superposition of the two labels is shown (merge). Note that on sections perpendicular to nerve axis, S3 immunoreactivity is peripheral to Nav labeling. (D) Teased fiber from rat sciatic nerve stained for S3 (green) and Kv1.1 (red) showing that S3 immunoreactivity is on both sides of the node. B-D: confocal stacks. Scale bars: 10 μm (A, D), 1 μm (B, C).
Distribution of S4 in sciatic nerve
S4 is ubiquitously expressed and cooperates with integrins to form focal adhesions [18,19]. However, its localization in nerves is not known. In adult rat sciatic nerve, S4 antibodies labeled several structures (Fig. 3). A diffuse and irregular labeling was observed along myelinated fibers at the level of internodes, whereas paranodal regions were consistently immunonegative (Fig. 3A, 3B arrowheads). Although S4 labeling often appeared more pronounced in the vicinity of paranodal regions, suggesting a possible enrichment at juxtaparanodes, it did not co-localize with Kv1.1 (Fig. 3B). S4 immunoreactivity was also concentrated at nodes, identified by Nav co-staining (Fig. 3A). Higher magnification confocal study in sections parallel (Fig. 3C) or perpendicular (Fig. 3D) to the nerve axis demonstrated that nodal S4 immunofluorescence was located peripherally to Na+ channels, indicating its presence in the Schwann cells perinodal processes and not in the axon. Although S4 nodal labeling was less conspicuous than that of S3, due to the immunoreactivity of myelinated fibers, careful examination of the sections showed that all nodes, identified by Nav staining, were also labeled by S4 antibodies.
Figure 3.
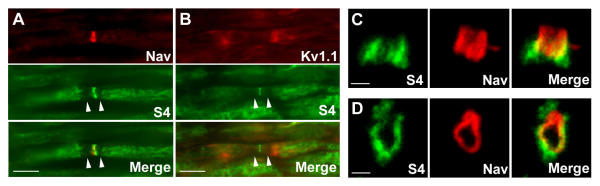
Localization of S4 in nodal regions of the PNS. Sections through rat sciatic nerve at p30 were labeled with antibodies to S4 (A-D, green) and Na+ (Nav, A, C-D, red) or K+ (Kv1.1, B, red) channels. Superposition of the labels is shown (merge). The paranodal regions are not labeled with antibodies against S4 (A, B, arrowheads). On sections perpendicular to nerve axis (D), S4 immunoreactivity is peripheral to Nav labeling. C, D: confocal stacks. Scale bars: 10 μm (A, B), 1 μm (C, D).
Colocalization of S3 and S4 with ezrin
We compared the distribution of S3 and S4 to that of ezrin in adult rat sciatic nerves. As previously described, ezrin immunoreactivity was concentrated in perinodal processes (Fig. 4A), the position of which was easily identified by double labeling with antibodies to paranodin/Caspr, a marker of paranodal regions [25]. Co-immunostaining revealed a complete colocalization of ezrin and S3 (Fig. 4B) or S4 (Fig. 4C). These results strongly indicate that S3 and S4 are co-localized with ezrin in the microvilli of Schwann cells.
Figure 4.
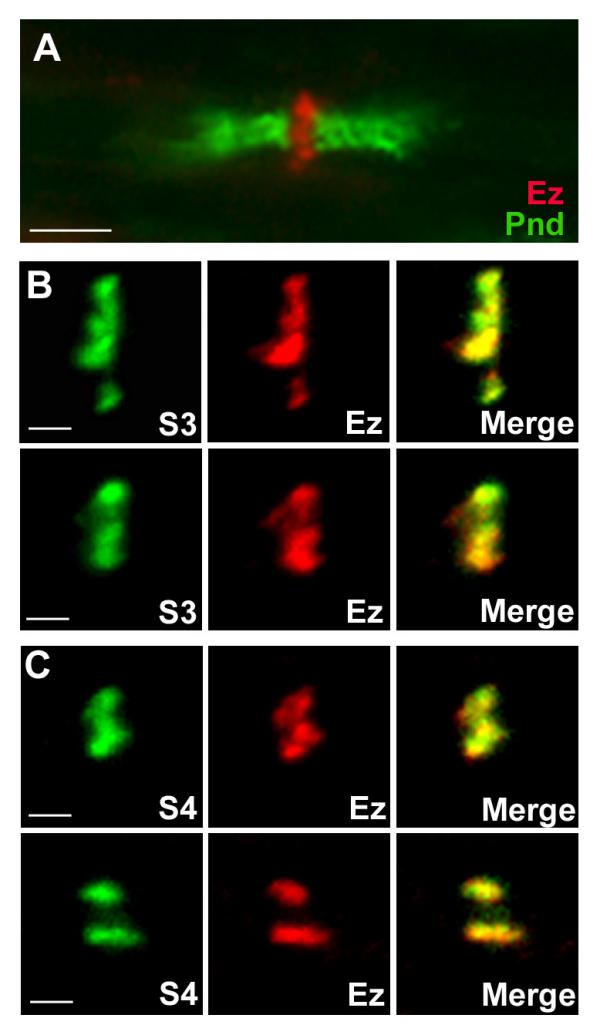
S3 and S4 co-localize with ezrin in nodal regions of PNS. (A) Sections through rat sciatic nerve at p30 were labeled with antibodies to ezrin (Ez, red) and to paranodin/Caspr (Pnd, green), a marker of paranodal regions. (B, C) Sections were double labeled with antibodies to S3 (B, green) or S4 (C, green) and antibodies to ezrin (Ez, red). Superposition of syndecan and ezrin labelings is shown (merge). Two different nodes are shown in each case. B, C: confocal stacks. Scale bars: 4 μm (A), 1 μm (B, C).
Distribution of S3 and S4 in sciatic nerve during development
Aggregates of nodal proteins start to appear early during myelination of peripheral nerves [11]. We examined the distribution of S3 and S4 with respect to the localization of Nav during the development of sciatic nerve in rat pups, from post-natal days 2 to 21 (P2 to P21, Fig. 5). At P2, Nav clusters were readily detectable (Fig. 5A.a, arrows). In the same sections, spots of concentrated S3 immunoreactivity were clearly distinguishable from the labeling of the rest of the fibers (Fig. 5A.b, arrows). However, S3 and Nav labeling did not exactly overlap, but appeared to be in very close contact with each other (Fig. 5A.a-c, insets). At P6, as the number of presumptive nodes increased, S3 and Nav immunoreactivities were always closely associated (Fig. 5A.d-f). This pattern remained similar at later stages, as nodal aggregates enlarged and acquired their mature form (Fig. 5A.g-l). When pairs of heminodes were observed [11], both were stained for S3 and Nav (Fig. 5A.g-i, arrowheads). As development proceeded, the immunoreactivity along nerve fibers decreased, and at p21 S3 staining appeared virtually restricted to the nodes of Ranvier (Fig. 5A.j-l), as in the adult (Fig. 2).
Figure 5.
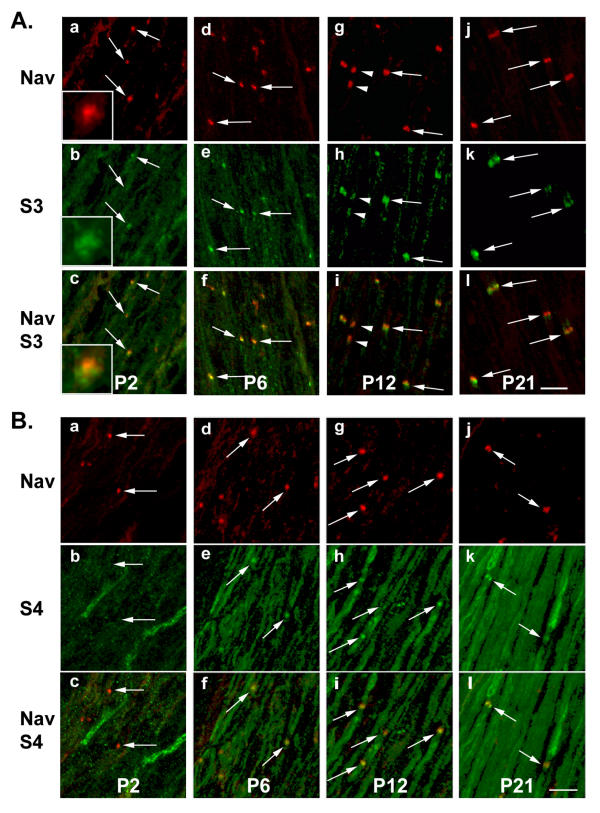
Developmental profile of S3 and S4 in rat sciatic nerve. Sections through rat sciatic nerve at P2, P6, P12, and P21 were labeled with antibodies to S3 (A, green) or S4 (B, green) and Na+ channels (Nav, A, B, red). Superposition of the two labels is indicated by arrows. At P2, S3 and Nav labelings appear to be in very close contact with each other (A.a-c, insets). For S3, a pair of heminodes is indicated by arrowheads (A.g-I). A, B: confocal stacks. Scale bars: 10 μm.
The developmental pattern of S4 immunoreactivity was also examined in rat sciatic nerves. At P2 although some S4 labeling was visible, with a few cells more intensely stained, no overlap of labeling with Nav was observed and there was no indication of the accumulation of S4 at presumptive nodes (Fig. 5Ba-c). Some nodal labeling was visible at P6 (Fig. 5B.d-f), and became more frequent at P12 and P21 (Fig. 5B.g-l). A general increase of S4 immunoreactivity in internodal regions of myelinated fibers occurred during development (Fig. 5B), leading to a pattern similar to that observed in adult rats (Fig. 3).
Discussion
Our results identify two proteoglycans, S3 and S4, as novel components of nodal regions in rat sciatic nerve. There are similarities between the distributions of the two proteins. Both S3 and S4 were found concentrated in Schwann cells perinodal processes, co-localized with ezrin, surrounding the sodium channel clusters. S3 and S4 are therefore likely to be both localized in Schwann cells microvilli. There were also marked differences between the distributions of the two proteins. S3 was detected in the vicinity of sodium channels aggregates very early in development, at a time when ezrin is also enriched in Schwann cells processes [12,14], whereas accumulation of S4 near sodium channels occurred progressively during the first postnatal weeks. In adult nerves S3 was highly concentrated at nodes as compared with any other cellular structures in the sciatic nerve. In contrast, S4 was also detected in the internodal region of myelinated fibers. These results show that at least two proteoglycans of the syndecan family are transmembrane components of Schwann cells microvilli. Since these molecules are known to have a number of intracellular and extracellular partners, it is likely that they play similar and perhaps complementary roles in the structural organization of nodal regions, as well as in signal transduction in glial processes.
Syndecans are thought to play a role in the regulation of cytoskeleton organization (for a recent review see [26]). S2 can regulate the actin cytoskeleton in different cell types, and S4 is present in focal adhesions of a number of cell lines [18]. S1 ectopically expressed in primary Schwann cells transiently co-localizes with actin filaments during cell spreading [27]. In the same transfected cells, S1 crossed-linked with anti-S1 antibodies co-localizes with actin filaments [28]. This localization is dependent on a specific region of the cytoplasmic region of the protein [29]. Several studies have suggested that signaling through the core protein of syndecans could regulate cytoskeleton organization through their clustering, binding to cytoplasmic proteins, and regulation of intracellular protein phosphorylation (for review see [26]). In this context, the ERM proteins may function as adaptors that couple syndecans to cytoskeletal proteins since S2 has been shown to interact with ezrin through a motif conserved in the others syndecans [30,31]. All these observations raise the interesting possibility that both S3 and S4 might provide plasma membrane anchors for ERM proteins in Schwann cells perinodal processes [12-14], and therefore be indirectly linked to actin microfilaments that are also enriched in microvilli [32]. In addition, the conserved C-terminal motif of syndecans can interact with PDZ (PSD-95 / discs large / ZO-1) domain-containing scaffold proteins syntenin [33], CASK/LIN [34,35], and synectin [36]. These interactions may coordinate clustering of receptors and also connection to the actin cytoskeleton [18,19]. It is not known whether syntenin, synectin or CASK/LIN are enriched in nodes of Ranvier, but it seems likely that these or other PDZ-containing proteins could be involved in the organization of perinodal glial processes.
S3 heparan sulphate chains bind to fibroblast growth factor-2 (FGF-2) [37] and heparin-binding growth-associated molecule (HB-GAM) also known as pleiotrophin or midkine [38]. FGF-2 and its high affinity receptor [39], as well as HB-GAM [40], are expressed by Schwann cells. In neuroblastoma cells S3 can activate neurite outgrowth in response to HB-GAM through a cortactin/src kinase-mediated pathway [41]. This raises the possibility that S3 might be involved in the formation and growth of Schwann cells nodal processes [14]. On the other hand, S4 binds to fibronectin and drives the formation of focal adhesions [42], while interaction of S4 with tenascin-C decreases adhesion [43]. A similar role of S4 could be important in the nodal area where loose axoglial contacts contrast with the tight septate-like junctions of neighbouring paranodes. S4 plays also a role in signaling through its cytoplasmic tail that binds and activates the catalytic domain of PKCα in the presence of phosphatidyl-inositol-4,5-bisphosphate [44,45]. Moreover, S4 regulates signaling by Rho family GTPases and focal adhesion kinase [46], and is able to bind to α-actinin [47]. All these interactions that have been described in the context of focal adhesions, may also have functional relevance for the organization of cortical cytoskeleton in Schwann cells microvilli.
Whether S3 and S4 could interact with axonal membrane proteins and contribute to the organization of the nodal region remains to be determined. Although S3 and S4 null mutant mice display relatively mild phenotypes [48-50], suggesting some redundancy between proteoglycans, their peripheral nerves have not been studied. It will be interesting to examine carefully the axoglial interactions and the molecular organization at nodes of Ranvier in these mice, as well as in double mutant mice.
Conclusions
In summary our findings show the enrichment of two syndecans in the perinodal processes of Schwann cells. These proteoglycans are receptors for extracellular proteins and growth factors. Syndecans are able to organize scaffolding proteins and activate signaling pathways involved in the reorganization of actin cytoskeleton, through tyrosine phosphorylation and activation of Rho family GTPases. Thus, they may be pivotal in the organization of Schwann cells growing tips and microvilli that seem to play an important role in the early interaction with nodal axonal proteins [14]. Since S3 accumulates earlier than S4, it is a candidate for being involved in the earliest steps of nodes of Ranvier formation.
Methods
Antibodies and constructs
Monoclonal antibodies sources were as follows: voltage-gated Na+ channel α subunit (PAN Nav, clone K58/35) [51] and ezrin (clone 3C12) [52], Sigma; Kv1.1 (clone K20/78) [53], Upstate Biotechnology; green fluorescent protein (GFP), Roche. Polyclonal antiserum against paranodin/Caspr (L-51) was described previously [25]. Alexa Fluor 488- and 594-conjugated goat anti-rabbit and anti-mouse antibodies were from Molecular Probes (Leiden, Netherlands). HRP-conjugated goat anti-mouse and anti-rabbit antibodies were from Amersham. Antibodies against S3 and S4 were generated by immunizing rabbits with the intracellular region of the human proteins (S3: residues 410–442; S4: residues 171–198, Fig. 1) fused to glutathione-S-transferase (GST), and affinity-purified on GST-fusion proteins encompassing only the variable region of the respective syndecans (S3: residues 420–439; S4: residues 181–194). Constructs for GST- and GFP-fusion proteins were generated by RT-PCR, verified by sequencing, and expressed using pGEX (Pharmacia) and pEGFP vectors (Clontech), respectively. For S1 and S2, the sequences fused to GFP encompassed residues 280–313 and 170–201 of rat S1 and human S2, respectively.
Cell culture, immunoblotting and immunocytochemistry
COS-7 cells transfection and immunoblotting were done as described [54]. For immunocytochemistry, cells were fixed for 20 min in 4% PFA/PBS, permeabilized with 0.2% Triton X-100 in PBS for 10 min, and incubated sequentially with primary and secondary antibodies for 30 min at room temperature. Immunostaining of cryostat sections (10 μm) of sciatic nerve tissues was performed essentially as previously described [9]. Sections were fixed for 20 min in methanol/acetone (50/50 vol/vol) at -20°C (for labeling with Nav antibodies) or in 4% PFA/PBS at room temperature (for labeling with Kv1.1, ezrin and paranodin antibodies), pre-incubated for 1 hour at room temperature in 0.2% porcine skin gelatin and 0.25 % Triton X-100 in PBS (PGT buffer), before incubation with primary antibodies (diluted in PGT) overnight at 4°C. After washing with PBS, coverlips were incubated for 2 hours at room temperature with secondary antibodies (diluted in PGT), washed again with PBS, and mounted in Vectashield. Images were acquired using a Leica epifluorescence microscope equipped with a CCD camera or a Leica SP laser Scanning microscope. Teased fibers were prepared from sciatic nerve as described [55]. Briefly, twenty-one days-old rats were perfused with 2% PFA in phosphate buffer (PB) at 4°C. Nerves were dissected out, post-fixed with 2% PFA in PB for 30 minutes at 4°C, and washed 1 hour in PB, before teasing and spreading on slides. Immunostaining was performed as for cryostat sections, except that slides were blocked and incubated with antibodies in PB, 0.2% porcine skin gelatin, 0.25 % Triton X-100 and 10% goat serum. Antibodies dilutions were: anti-S3 (1:300); anti-S4 (1:100); anti-Nav (1:100); anti-Kv1.1 (1:200); anti-paranodin (1:1000); anti-ezrin (1:400); anti-GFP (1:200); secondary antibodies (1:400).
Abbreviations
S: syndecan
ERM: ezrin, radixin, moesin
Nav: voltage-gated sodium channel α subunit
Kv: shaker-type K+ channels
GST: glutathione-S-transferase
GFP: green fluorescent protein
PDZ: PSD-95 / discs large / ZO-1
Authors' contributions
LG conceived of the study, produced, purified and characterized the antibodies, performed image analysis; and participated in the redaction of the manuscript. MC performed immunocytochemistry experiments on sciatic nerves. ND carried out immunocytochemistry on teased fibers. MCB participated in the purification of the antibodies. JAG participated in the design and coordination of the study, and drafted the manuscript. All authors read and approved the manuscript.
Acknowledgments
Acknowledgements
This work was supported in part by grants of Association pour la Recherche contre le Cancer, Fondation Schlumberger pour l'Enseignement et la Recherche, Fondation pour la Recherche Médicale, and Fondation NRJ. We thank Theano Eirinopolou for her help with the confocal microscope.
Contributor Information
Laurence Goutebroze, Email: goutebro@fer-a-moulin.inserm.fr.
Michèle Carnaud, Email: mcarnaud@fer-a-moulin.inserm.fr.
Natalia Denisenko, Email: nehrbass@fer-a-moulin.inserm.fr.
Marie-Claude Boutterin, Email: boutteri@fer-a-moulin.inserm.fr.
Jean-Antoine Girault, Email: girault@fer-a-moulin.inserm.fr.
References
- Girault JA, Peles E. Development of nodes of Ranvier. Curr Opin Neurobiol. 2002;12:476–485. doi: 10.1016/S0959-4388(02)00370-7. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol. 1999;28:303–318. doi: 10.1023/A:1007005528505. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Zanazzi G, Levinson SR, Salzer JL. Clustering of neuronal sodium channels requires contact with myelinating Schwann cells. J Neurocytol. 1999;28:295–301. doi: 10.1023/A:1007053411667. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Mathis C, Denisenko-Nehrbass N, Girault JA, Borrelli E. Essential role of oligodendrocytes in the formation and maintenance of central nervous system nodal regions. Development. 2001;128:4881–4890. doi: 10.1242/dev.128.23.4881. [DOI] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci. 1995;15:492–503. doi: 10.1523/JNEUROSCI.15-01-00492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Novakovic SD, Levinson SR, Schachner M, Shrager P. The clustering of axonal sodium channels during development of the peripheral nervous system. J Neurosci. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM) – positive Schwann cell processes. Proc Natl Acad Sci USA. 2001;98:1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Xu T, Crino P, Arroyo EJ, Gutmann DH. Ezrin, radixin, and moesin are components of Schwann cell microvilli. J Neurosci Res. 2001;65:150–164. doi: 10.1002/jnr.1138.abs. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Walker BJ, Lambert S. Local ERM activation and dynamic growth cones at Schwann cell tips implicated in efficient formation of nodes of Ranvier. J Cell Biol. 2003;162:489–498. doi: 10.1083/jcb.200303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecans: synergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189–192. doi: 10.1016/S0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13:578–583. doi: 10.1016/S0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Bass MD, Humphries MJ. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem J. 2002;368:1–15. doi: 10.1042/BJ20021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Evans DM, Stahl RC, Asundi VK, Conner KJ, Garbes P, Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992;117:191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Conner K, Asundi VK, O'Mahony DJ, Stahl RC, Showalter L, Cizmeci-Smith G, Hartman J, Rothblum LI. cDNA cloning, genomic organization, and in vivo expression of rat N-syndecan. J Biol Chem. 1997;272:2873–2879. doi: 10.1074/jbc.272.2.1180. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Sheng M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J Neurosci. 1999;19:7415–7425. doi: 10.1523/JNEUROSCI.19-17-07415.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman R, Stahl RC, Rothblum K, Chernousov MA, Carey DJ. Schwann cell adhesion to a novel heparan sulfate binding site in the N-terminal domain of alpha 4 type V collagen is mediated by syndecan-3. J Biol Chem. 2002;277:7619–7625. doi: 10.1074/jbc.M111311200. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Amos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003;22:25–33. doi: 10.1016/S0945-053X(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Carey DJ, Stahl RC, Cizmeci-Smith G, Asundi VK. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994;124:161–170. doi: 10.1083/jcb.124.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Stahl RC, Tucker B, Bendt KA, Cizmeci-Smith G. Aggregation-induced association of syndecan-1 with microfilaments mediated by the cytoplasmic domain. Exp Cell Res. 1994;214:12–21. doi: 10.1006/excr.1994.1228. [DOI] [PubMed] [Google Scholar]
- Carey DJ, Bendt KA, Stahl RC. The cytoplasmic domain of syndecan-1 is required for cytoskeleton association but not detergent insolubility. J Biol Chem. 1996;271:15253–15260. doi: 10.1074/jbc.271.50.32276. [DOI] [PubMed] [Google Scholar]
- Granes F, Urena JM, Rocamora N, Vilaro S. Ezrin links syndecan-2 to the cytoskeleton. J Cell Sci. 2000;113:1267–1276. doi: 10.1242/jcs.113.7.1267. [DOI] [PubMed] [Google Scholar]
- Granes F, Berndt C, Roy C, Mangeat P, Reina M, Vilaro S. Identification of a novel Ezrin-binding site in syndecan-2 cytoplasmic domain. FEBS Lett. 2003;547:212–216. doi: 10.1016/S0014-5793(03)00712-9. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Wong A, O'Connell M, Griffin JW. Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J Neurocytol. 1989;18:47–60. doi: 10.1007/BF01188423. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AR, Wood DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li M, Chen W, Simons M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol. 2000;184:373–379. doi: 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Carey DJ. N-syndecan (syndecan 3) from neonatal rat brain binds basic fibroblast growth factor. J Biol Chem. 1993;268:16810–16814. [PubMed] [Google Scholar]
- Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3) J Biol Chem. 1994;269:12999–13004. [PubMed] [Google Scholar]
- Grothe C, Nikkhah G. The role of basic fibroblast growth factor in peripheral nerve regeneration. Anat Embryol (Berl) 2001;204:171–177. doi: 10.1007/s004290100205. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ. Cellular distribution of the new growth factor pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat Embryol (Berl) 1992;186:387–406. doi: 10.1007/BF00185989. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J Biol Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- Oh ES, Woods A, Lim ST, Theibert AW, Couchman JR. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J Biol Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DK, Tumova S, Couchman JR, Woods A. Syndecan-4 associates with alpha-actinin. J Biol Chem. 2003;278:7617–7623. doi: 10.1074/jbc.M207123200. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Pavlov I, Voikar V, Lauri SE, Hienola A, Riekki R, Lakso M, Taira T, Rauvala H. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol Cell Neurosci. 2002;21:158–172. doi: 10.1006/mcne.2002.1167. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Tsuzuki S, Nakamura E, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J Biol Chem. 2000;275:5249–5252. doi: 10.1074/jbc.275.8.5249. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Schrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohling T, Turunen O, Jaaskelainen J, Carpen O, Sainio M, Wahlstrom T, Vaheri A, Haltia M. Ezrin expression in stromal cells of capillary hemangioblastoma. Am J Pathol. 1996;148:367–373. [PMC free article] [PubMed] [Google Scholar]
- Bekere-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel α- and β-subunit polypeptides. Neuropharmacology. 1996;35:851–865. doi: 10.1016/0028-3908(96)00128-1. [DOI] [PubMed] [Google Scholar]
- Toutant M, Studler JM, Burgaya F, Costa A, Ezan P, Gelman M, Girault JA. Molecular characterization of FAK neuronal isoforms. Biochem J. 2000;348:119–128. doi: 10.1042/0264-6021:3480119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Arroyo EJ, Awatramani R, Xu T, Baron P, Vallat JM, Balsamo J, Lilien J, Scarlato G, Kamholz J, et al. Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol Cell Neurosci. 2001;18:606–618. doi: 10.1006/mcne.2001.1041. [DOI] [PubMed] [Google Scholar]


