Abstract
Photodynamic inactivation (PDI) has been investigated to cope with the increasing incidence of multidrug-resistant (MDR) pathogens. In Hong Kong, methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli are the two commonest MDR pathogens. Here, we studied the photodynamic inactivation (PDI) mediated by poly-L-lysine chlorin(e6) conjugate (pL-ce6) and toluidine blue O (TBO) in clinical MRSA and ESBL producing E. coli, together with their corresponding American Type Culture Collection (ATCC) strains. Both pL-ce6 and TBO mediated a light- and drug dose-dependent efficacy for the four pathogens. pL-ce6 was more effective. pL-ce6 at 8 µM, 30 Jcm−2, attained 5 log killing for ESBL-producing E. coli and E. coli (ATCC 25922); 4 log killing for MRSA, and 3 log killing for S. aureus (ATCC 25923). TBO at 80 µM, 30 Jcm−2, only exhibited 3 log killing in MRSA and 2 log killing in S. aureus (ATCC 25923). TBO (400 µM, 30 Jcm−2) induced equal killing for ESBL-producing E. coli and E. coli (ATCC 25922). Our studied MRSA isolate responded better than S. aureus (ATCC 25923). Thus, pL-ce6-mediated PDI in other MRSA isolates deserves further investigation.
Keywords: Photodynamic inactivation, Poly-L-lysine chlorin(e6) conjugate, Toluidine blue O, Methicillin-resistant Staphylococcus aureus, Extended spectrum β-lactamase (ESBL), Escherichia coli
Introduction
The relentless increase of multidrug-resistant (MDR) pathogens, together with the limited development of novel antibiotics, makes it imperative to discover new anti microbial strategies to combat MDR pathogens. Methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli are two of the commonest MDR pathogens at present causing worldwide concern.1,2 It is reported that 69.8% of all S. aureus isolates are resistant to oxacillin,3 while ESBL-producing E. coli accounts for 11% of all E. coli isolates in Hong Kong.4
Antimicrobial photodynamic inactivation (PDI) is perhaps an alternative strategy to combat these MDR pathogens. PDI employs nontoxic photosensitizers (PSs), which localize in the microbial cells and are activated by a specific wavelength of visible light.5 The excited-state PS reacts with in situ molecular oxygen and transfers its energy to generate reactive oxygen species (ROS), such as singlet oxygen and superoxide anion. These ROS can cause damage to cell walls, proteins, nucleic acids, and membrane lipids, eventually causing cell death.6,7
Toluidine blue O (TBO), a phenothiazinium salt, is a moderately effective cationic PS causing damage to the bacterial cell membrane.8 Poly-L-lysine chlorin(e6) conjugate (pL-ce6) is a highly effective polycationic PS designed to bind and penetrate multiple classes of microbial cells.9 It consists of tetrapyrrole chlorin(e6) covalently bonded to poly-l-lysine chains and is photobactericidal against both Gram-positive and Gram-negative bacteria, as well as fungi, by destroying the bacterial/fungal membrane structure.10,11 The main purpose of this study was to explore the PDI effect of pL-ce6 and TBO on ATCC (American Type Culture Collection) S. aureus and E. coli, and clinical isolates of MRSA and ESBL-producing E. coli.
Materials and methods
Bacterial strains used in this study were S. aureus (ATCC 25923), E. coli (ATCC 25922), a clinical isolate of MRSA, and a clinical isolate of ESBL-producing E. coli. All clinical isolates were collected from the University Clinic, Hong Kong Polytechnic University. The MRSA was multiresistant to the following antibiotics: oxacillin, clindamycin, cloxacillin, erythromycin, and ofloxacin. The ESBL-producing E. coli was multiresistant to the following antibiotics: ampicillin, cefuroxime IV, cefuroxime axetil, gentamicin, ofloxacin, and ampicillin/sulbactam.
Toluidine blue O (TBO) was purchased from Sigma (St. Louis, MO, USA). A 1-mM TBO stock solution was prepared by dissolving TBO in sterile distilled water. The poly-l-lysine chlorin(e6) conjugate (pL-ce6) was prepared according to previously described methods.12 It had an average of six chlorin(e6) molecules conjugated to a polymer with an average length of 164 lysine residues, and the stock solution was 3.3 mM ce6 equivalent. Both stock solutions were stored at 4°C in the dark and diluted with phosphate-buffered saline (PBS) to give working solutions of the PS.
The bacterial strains were grown exponentially to a cell density of 1 × 108 cells/ml at 37°C in nutrient broth (Oxoid, Basingstoke, England). Aliquots of bacterial suspensions were sensitized with a range of concentrations of PSs for 30 min. The PS-loaded cells were centrifuged, washed twice with sterile PBS, and resuspended in nutrient broth. Aliquots of 150 µl treated cells were placed in a 96-well microtiter plate and irradiated with red light (10–30 Jcm−2) emitted from a 400-W quartz-halogen lamp equipped with a heat isolation filter and a long-pass filter (600 nm).13 Then 100 µl was withdrawn and serially diluted in PBS; 10 µl from each dilution mixture was spread on nutrient agar plates in triplicate as described.14 The plates were incubated at 37°C overnight. The numbers of colonies was enumerated to determine the survival fractions. Light-alone controls (no-PS) and dark controls (PS-treated cell suspension without light) were included.
Results
Values for results are presented as means and SEs of five independent studies. Statistics were analyzed by two-tailed unpaired Student’s t-test. The significance level was set at P < 0.05.
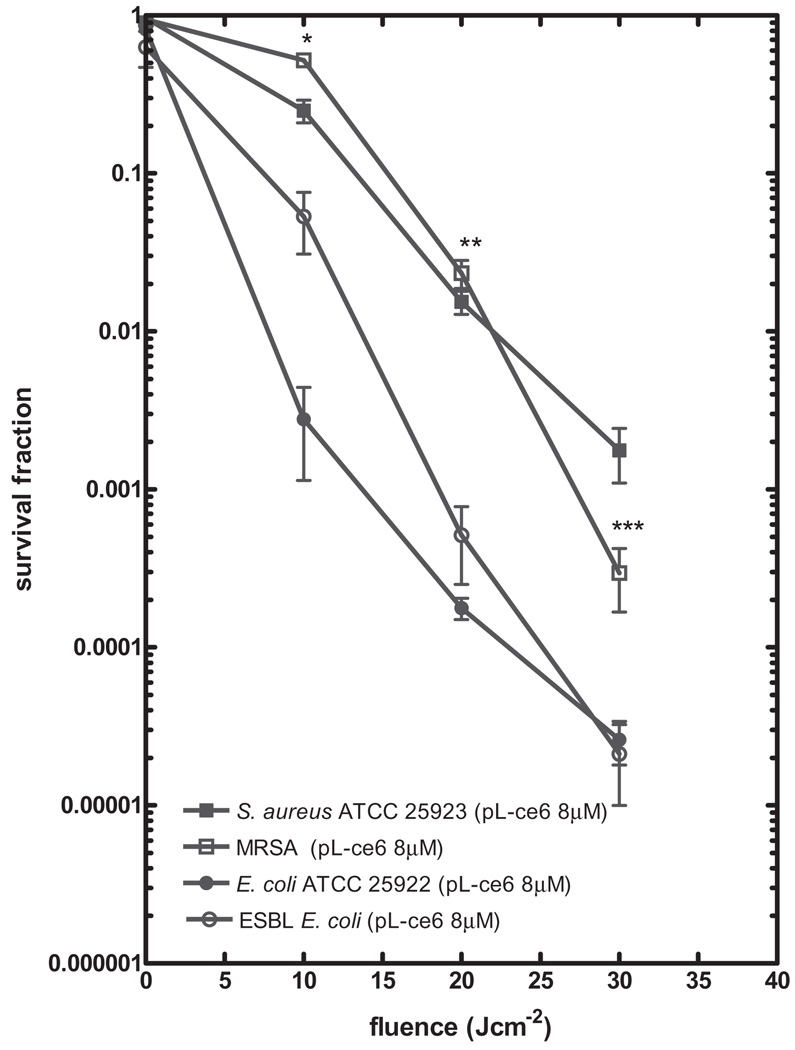
For photodynamic inactivation (PDI) mediated by pL-ce6, concentrations ranging from 4 to 8 µM with light doses from 10 to 30 Jcm−2 were employed; 4 log killing was obtained for MRSA treated with 8-µM pL-ce6 at 30 Jcm−2. However, the S. aureus (ATCC 25923) only showed 2.8 log killing (Fig. 1). For pL-ce6-mediated PDI against the E. coli strains, the degrees of killing observed for ESBL-producing E. coli were identical to those observed for E. coli (ATCC 25922) at every PS dose and at every light dose. Overall, the effectiveness of PDI against E. coli was significantly greater than that observed against S. aureus; 8 µM pL-ce6 and 30 Jcm−2 gave almost 5 log killing of E. coli strains compared to 2.5–3.5 log killing of S. aureus strains (P < 0.005).
Fig. 1.
Photodynamic inactivation mediated by poly-L-lysine chlorin(e6) conjugate (pL-ce6) in Staphylococcus aureus American Type Culture Collection (ATCC) 25923, methicillin-resistant S. aureus (MRSA), Escherichia coli ATCC 25922, and extended-spectrum β-lactamase (ESBL)-producing E. coli; 1 × 108 cells/ml were sensitized with 8 µM pL-ce6 and then exposed to 0–30 Jcm−2 light doses. The values shown are means of at least five independent experiments, and bars indicate SEs. *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed unpaired Student’s t-test comparing the ATCC strain and clinical resistant strain
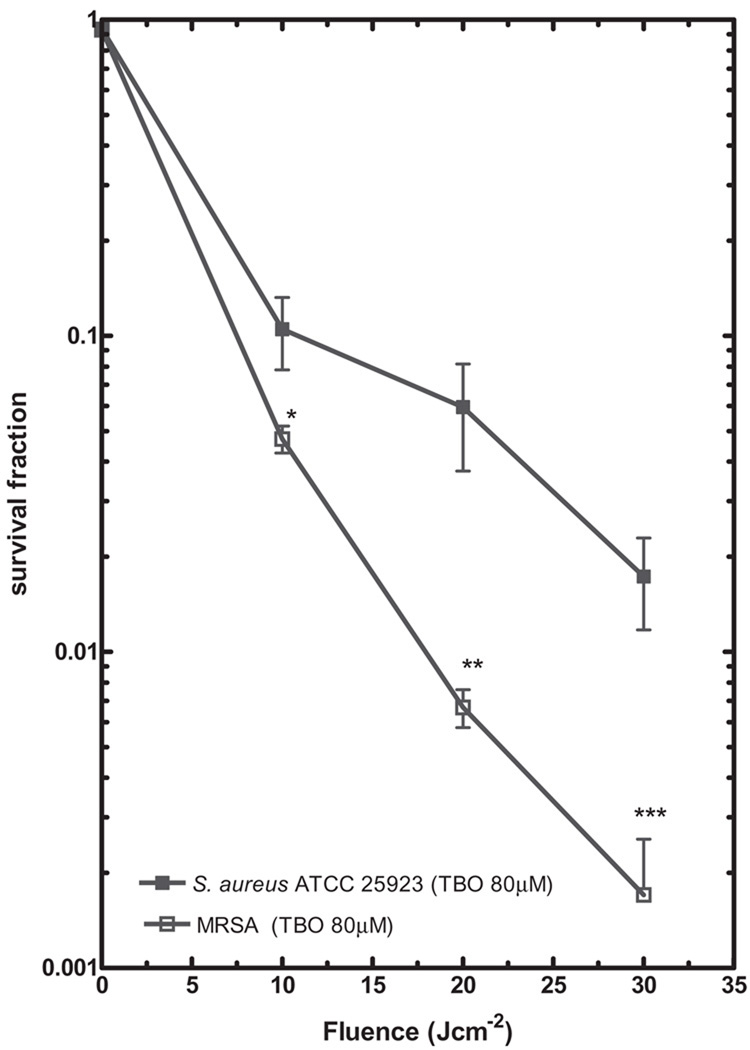
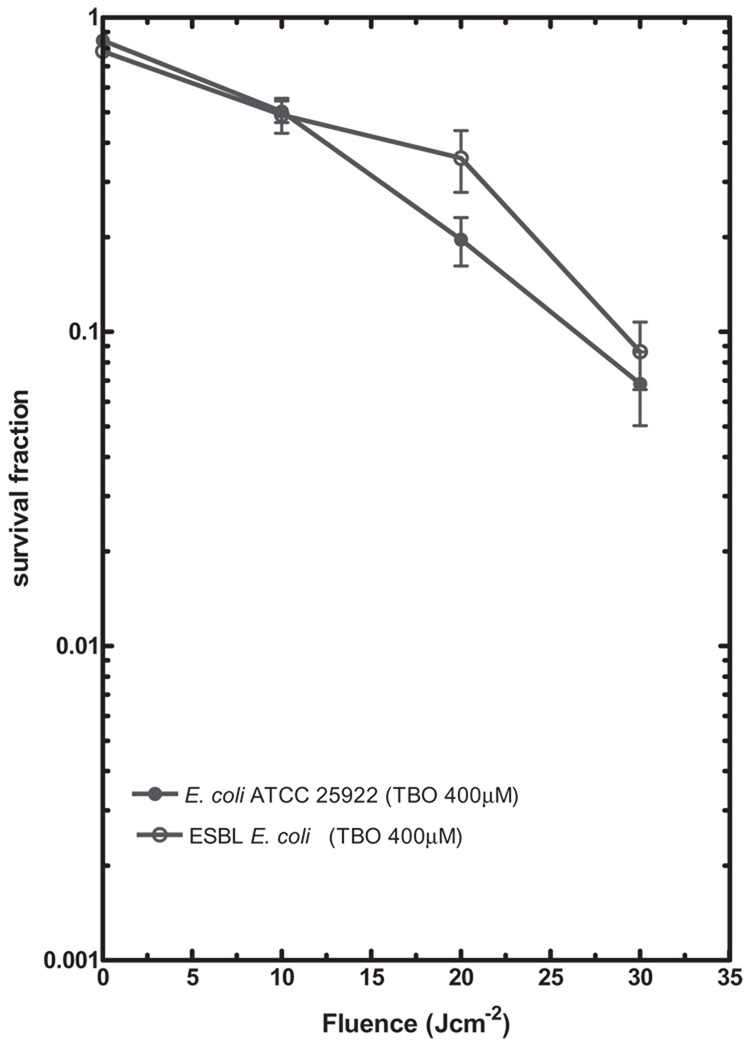
Figure 2 shows the results of PDI mediated by TBO in both S. aureus strains. Various concentrations of TBO (40, 60, and 80 µM) and different light doses (0–30 Jcm−2) were applied to clinical MRSA and S. aureus (ATCC 25923). Under the same TBO concentration and light dose, the clinical MRSA isolate was consistently killed more when compared with S. aureus (ATCC 25923). With 80 µM TBO at 30 Jcm−2, 3 log killing was obtained for the MRSA and 2 log killing was obtained for S. aureus (ATCC 25923). In contrast, when 80 µM TBO and 30 Jcm−2 of light were delivered to E. coli strains, there was no loss of viability (data not shown) and therefore higher concentrations of TBO (100–400 µM) were employed for the PDI of E. coli. Figure 3 shows the PDI mediated by 400 µM TBO towards clinical ESBL-producing E. coli and E. coli (ATCC 25922) with light doses of 10–30 Jcm−2. Both strains showed modest but identical degrees of killing; 95% killing at 400 µM TBO and 30 Jcm−2. Even further increasing the TBO concentration to 1 mM gave no further killing of either strain (data not shown).
Fig. 2.
Photodynamic inactivation mediated by toluidine blue O (TBO) in S. aureus ATCC 25923 and MRSA; 1 × 108 cells/ml of S. aureus and MRSA were sensitized with 80 µM TBO and then exposed to 0–30 Jcm−2 light doses. The values shown are means of at least five independent experiments, and bars indicate SEs. *P < 0.05; **P < 0.01; ***P < 0.001 by two-tailed unpaired Student’s t-test, comparing the ATCC strain and clinical resistant strain
Fig. 3.
Photodynamic inactivation mediated by TBO in E. coli ATCC 25922 and ESBL-producing E. coli; 1 × 108 cells/ml of E. coli ATCC 25922 and ESBL-producing E. coli were sensitized with 400 µM TBO and then exposed to 0–30 Jcm−2 light doses. The values shown are means of at least five independent experiments, and bars indicate SEs
Discussion
This study has shown, for the first time, that this isolated MRSA strain is more sensitive to PDI-mediated killing than S. aureus (ATCC 25923), using two different cationic PSs. This study also contains the first report comparing the PDI-mediated killing of ESBL E. coli with that of E. coli (ATCC 25922) and demonstrating that both phenotypes are equally killed. There have been several previous literature reports comparing the PDI of MRSA with that of S. aureus. Maisch et al.15 used XF porphyrins as antimicrobial PSs and found identical killing of MRSA (and methicillin-resistant S. epidermidis to that found with the wild-type strains). Wainwright et al.16 found that PDI with phenothiazinium dyes killed MRSA somewhat less efficiently than wild-type S. aureus strains. Embleton et al.17 employed a phage delivery system to carry out PDI with the PS, Sn-ce6, and again found that MRSA was somewhat less susceptible compared to wild-type S. aureus strains. How can we explain our observation of the increased susceptibility of MRSA, compared to S. aureus (ATCC 25923), to PDI mediated by pL-ce6 and TBO? A possible reason may be due to differences in their cell-wall structure. The presence of altered penicillin-binding protein (PBP 2′a) encoded by the mecA gene in MRSA exerts decreased affinity for methicillin and other β-lactams. PBP 2′a plays an important role in catalyzing the transpeptidational activity which is crucial for the cross-linkage of peptidoglycan.18 This may mean that MRSA actually has a more permeable cell wall than the S. aureus naïve strain. The altered architectural structure in MRSA may therefore favor the penetration of pL-ce6 and TBO across the cell wall, resulting in the higher killing of MRSA upon illumination. In order to test for this hypothesis, the PDI effect of pL-ce6 and TBO against other clinical MRSA isolates that exhibit different antibiotic patterns from this study will be further included in order to verify whether MRSA was more susceptible to pL-ce6 and TBO mediated PDI.
Comparing the PDI effects of pL-ce6 and TBO towards all the bacteria, pL-ce6 was a much more potent PS than TBO. pL-ce6 showed a higher phototoxic effect toward the Gram-negative E. coli compared to the Gram-positive S. aureus. The reverse situation was found with TBO, where S. aureus was more susceptible compared to E. coli. The relative susceptibility order of the different species (Gram-positive and Gram-negative) in response to the TBO and pL-ce6-mediated PDI was consistent with that reported by Demidova and Hamblin.12 It is known that the double lipid bilayer cell envelope structure of Gram-negative E. coli is more complicated than that of Gram-positive S. aureus. In addition, the presence of strongly negatively charged lipopolysaccharides (LPS) in the outer membrane provides the Gram-negative bacteria with a very effective permeability barrier to exclude hydrophobic and large hydrophilic solutes. As a result, anionic and neutral PSs do not bind well to the Gram-negative outer membrane, due to absence of electrostatic charge attraction, and furthermore, they cannot penetrate the permeability barrier and are therefore unable to inactivate these bacteria.5,6,19 However, positively charged PS or cationic PS-conjugates bind to the negatively charged bacterial surface and displace the naturally occurring divalent cations (Ca2+ and Mg2+) that anchor the LPS in place. This displacement weakens the outer membrane structure, creating “cracks” in the permeability barrier, which allows the PS to penetrate to the inner plasma membrane that is thought to be the crucial site of photodamage.20 For the Gram-positive bacteria, there is a relatively porous peptidoglycan layer surrounding the cytoplasmic membrane. They possess a slightly negatively charged surface and are also sensitive to cationic PS, although anionic and neutral PSs can also be used to eradicate Gram-positive bacteria.5,6,19
The susceptibility differences observed between E. coli and S. aureus and pL-ce6 and TBO may be due to their different chemical properties, as indicated by Demidova and Hamblin et al.12 Although both pL-ce6 and TBO are cationic, pL-ce6 possesses a relatively larger molecular weight and the molecule has a greater number of potentially positively charged groups than TBO. The large molecular weight of pL-ce6 may not facilitate its ready penetration into the cell wall of the Gram-positive S. aureus leading to photodestruction. In the Gram-negative E. coli, the polycationic nature of the pL-ce6 conjugate gives higher binding affinity to LPS and is better at displacing the divalent cations referred to above, thus causing “self-promoted uptake”.5,20,21
In summary, in our study, the selected MDR isolates were more susceptible to PDI-mediated killing than their ATCC reference strains. The PDI efficacies of pL-ce6 and TBO were not affected by the antibiotic resistance mechanism that presented in MRSA and ESBL-producing E. coli. These data reinforce suggestions that PDI may be an alternative treatment for localized infections caused by these two MDR pathogens.
Acknowledgments
This work was supported by Competitive Earmarked Research Grant (B-Q874) from Hong Kong Research Grant Council. Dr. M.R. Hamblin was supported by a grant from the US NIAID (R01AI050875).
Contributor Information
Hi M Tang, Department of Health Technology and Informatics, Medical Laboratory Science Section, The Hong Kong Polytechnic University, Hung Hom, Hong Kong SAR.
Michael R. Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA Department of Dermatology, Harvard Medical School, Boston, MA, USA; Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA.
Christine M.N. Yow, Department of Health Technology and Informatics, Medical Laboratory Science Section, The Hong Kong Polytechnic University, Hung Hom, Hong Kong SAR, Tel. +852-3400-8575; Fax +852-2362-4365, htcyow@inet.polyu.edu.hk
References
- 1.Bootsma MC, Diekmann O, Bonten MJ. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci USA. 2006;103:5620–5625. doi: 10.1073/pnas.0510077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellou H. Multidrug resistance in Gram-negative bacteria that produce extended-spectrum beta-lactamases (ESBLs) Clin Microbiol Infect. 2005;11 Suppl 4:1–16. doi: 10.1111/j.1469-0691.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 3.Bell JM, Turnidge JD. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY antimicrobial surveillance program, 1998–1999. Antimicrob Agents Chemother. 2002;46:879–881. doi: 10.1128/AAC.46.3.879-881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho PL, Tsang DN, Que TL, Ho M, Yuen KY. Comparison of screening methods for detection of extended-spectrum beta-lactamases and their prevalence among Escherichia coli and Klebsiella species in Hong Kong. APMIS. 2000;108:237–240. doi: 10.1034/j.1600-0463.2000.d01-50.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisch T, Szeimies RM, Jori G, Abels C. Antibacterial photodynamic therapy in dermatology. Photochem Photobiol Sci. 2004;3:907–917. doi: 10.1039/b407622b. [DOI] [PubMed] [Google Scholar]
- 7.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 8.Usacheva MN, Teichert MC, Biel MA. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J Photochem Photobiol B. 2003;71:87–98. doi: 10.1016/j.jphotobiol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–1726. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 12.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yow CM, Mak NK, Szeto S, Chen JY, Lee YL, Cheung NH, et al. Photocytotoxic and DNA damaging effect of temoporfin (mTHPC) and merocyanine 540 (MC540) on nasopharyngeal carcinoma cell. Toxicol Lett. 2000;115:53–61. doi: 10.1016/s0378-4274(00)00174-0. [DOI] [PubMed] [Google Scholar]
- 14.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 15.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 17.Embleton ML, Nair SP, Heywood W, Menon DC, Cookson BD, Wilson M. Development of a novel targeting system for lethal photosensitization of antibiotic-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:3690–3696. doi: 10.1128/AAC.49.9.3690-3696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger-Bachi B, Rohrer S. Factors influencing methicillin resistance in staphylococci. Arch Microbiol. 2002;178:165–171. doi: 10.1007/s00203-002-0436-0. [DOI] [PubMed] [Google Scholar]
- 19.Jori G, Brown SB. Photosensitized inactivation of microorganisms. Photochem Photobiol Sci. 2004;3:403–405. doi: 10.1039/b311904c. [DOI] [PubMed] [Google Scholar]
- 20.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock RE, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988;7:713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]