Abstract
Background and Objective
Low level light (or laser) therapy (LLLT) is a rapidly growing modality used in physical therapy, chiropractic, sports medicine and increasingly in mainstream medicine. LLLT is used to increase wound healing and tissue regeneration, to relieve pain and inflammation, to prevent tissue death, to mitigate degeneration in many neurological indications. While some agreement has emerged on the best wavelengths of light and a range of acceptable dosages to be used (irradiance and fluence), there is no agreement on whether continuous wave or pulsed light is best and on what factors govern the pulse parameters to be chosen.
Study Design/Materials and Methods
The published peer-reviewed literature was reviewed between 1970 and 2010.
Results
The basic molecular and cellular mechanisms of LLLT are discussed. The type of pulsed light sources available and the parameters that govern their pulse structure are outlined. Studies that have compared continuous wave and pulsed light in both animals and patients are reviewed. Frequencies used in other pulsed modalities used in physical therapy and biomedicine are compared to those used in LLLT.
Conclusion
There is some evidence that pulsed light does have effects that are different from those of continuous wave light. However further work is needed to define these effects for different disease conditions and pulse structures.
Keywords: low level light therapy, photobiomodulation, frequency, pulse duration, duty cycle, clinical trials
INTRODUCTION
Since the introduction of low-level laser (light) therapy in 1967, over two hundred randomized, double-blinded, and placebo-controlled phase III clinical trials have been published from over a dozen countries. Whereas there is some degree of consensus as to the best wavelengths of light and acceptable dosages to be used, there is no agreement on whether continuous wave (CW) or pulsed wave (PW) light is more suitable for the various applications of LLLT. This review will raise (but not necessarily answer) several questions. How does pulsed light differ from CW on the cellular and molecular level, and how is the outcome of LLLT affected? If pulsing is more efficacious, then at what pulse parameters is the optimal outcome achieved? In particular, what is the ideal pulse repetition rate or frequency to use?
PULSE PARAMETERS AND LIGHT SOURCES
There are five parameters that could be specified for pulsed light sources. The pulse width or duration or ON time (PD) and the pulse Interval or OFF time (PI) are measured in seconds. Pulse repetition rate or frequency (F) is measured in Hz. The duty cycle (DC) is a unitless fractional number or %. The peak power and average power are measured in Watts.
Pulse duration, pulse repetition rate, and duty cycle are related by the simple equation:
Peak power is a measure of light intensity during the pulse duration, and related to the average power (measured in Watts) by:
Alternatively,
In all cases, it is necessary to specify any two out of three of: PD, F, and DC, and either the peak or average power for the pulse parameters to be fully defined.
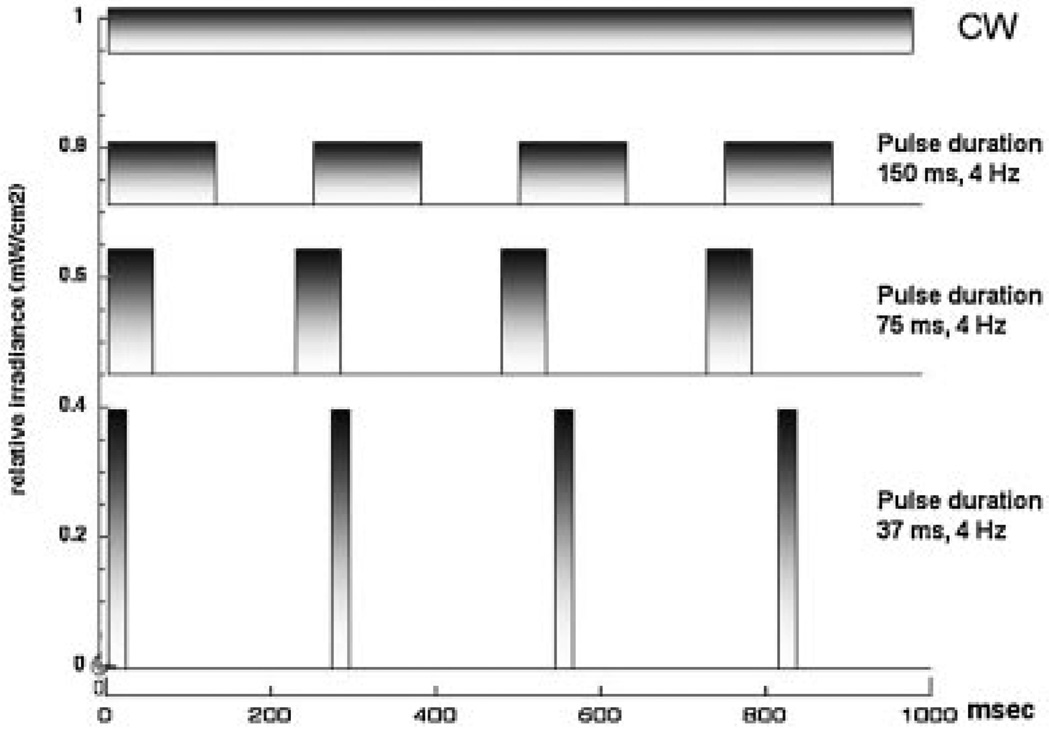
Figure 1 graphically shows the relationship between peak power and pulse duration.
Fig. 1.
Conceptual diagram comparing the structure of CW with pulsed light of various pulse durations.
TYPES OF PULSED LIGHT SOURCES
Five major types of pulsed lasers (or other light sources) are commonly utilized: (1) Q-switched, (2) Gain-switched, (3) Mode-locked, (4) Superpulsed, and (5) Chopped or gated. Each utilizes a different mechanism to generate light in a pulsed as opposed to continuous manner, and vary in terms of pulse repetition rates, energies, and durations. However the first three classes of “truly” pulsed lasers mentioned above are in general not used for LLLT; instead superpulsed or gated lasers are mainly used. The concept of super-pulsing was originally developed for the carbon dioxide laser used in high power tissue ablative procedures. The idea was that by generating relatively short pulses (µsecond) the laser media could be excited to higher levels than those normally allowed in CW mode where heat dissipation constraints limit the maximum amounts of energy that can be used to excite the lasing media. With the original carbon dioxide superpulsed lasers, the short pulses would confine the thermal energy in the tissue (by making the pulse duration less than the thermal diffusion time) reducing collateral thermal damage to normal tissue.
Another type of laser that particularly benefited from super-pulsing is the gallium-arsenide (GaAs) diode laser. This laser has a wavelength in the region of 904-nm and pulse duration usually in the range of 100–200 nanoseconds. Another semiconductor laser amenable to superpulsing is the indium-gallium-arsenide (In-Ga-As) diode laser. It emits light at a similar wavelength (904–905-nm) as the GaAs diode laser, producing very brief pulses (200 nanoseconds) of high frequencies (in the range of kilohertz). These pulses are of very high peak powers (1–50 W) and an average power of 60 mW. Theoretically, the super-pulsed GaAs and In-Ga-As lasers allow for deep penetration without the unwelcome effects of CW (such as thermal damage), as well as allowing for shorter treatment times.
The other major class of pulsed light sources used in LLLT are simply CW lasers (usually diode lasers) that have a pulsed power supply generated by a laser driver containing a pulse generator. This technology is described as “chopped” or “gated.” It is also equally feasible to use pulse generator technology to pulse LEDs or LED arrays [1].
WHY COULD PULSING BE IMPORTANT IN LLLT?
Pulsed light offers numerous potential benefits. Because there are “quench periods” (pulse OFF times) following the pulse ON times, pulsed lasers can generate less tissue heating. In instances where it is desirable to deliver light to deeper tissues increased powers are needed to provide adequate energy at the target tissue. This increased power can cause tissue heating at the surface layers and in this instance pulsed light could be very useful. Whereas CW causes an increase in temperature of the intervening and target tissues or organ, pulsed light has been shown to cause no measurable change in the temperature of the irradiated area for the same delivered energy density. Anders et al. administered pulsed light to pig craniums, and found no significant change in temperature of the scalp or skull tissue (J.J. Anders, personal communication). Ilic et al. [2] found that pulsed light (peak power densities of 750 mW/cm2) administered for 120 seconds produced no neurological or tissue damage, whereas an equal power density delivered by CW (for the same number of seconds) caused marked neurological deficits.
Aside from safety advantages, pulsed light might simply be more effective than CW. The “quench period” (pulse OFF times) reduces tissue heating, thereby allowing the use of potentially much higher peak power densities than those that could be safely used in CW. For example, when CW power densities at the skin of ≥2 W/cm2 are used, doubling the CW power density would only marginally increase the treatment depth while potentially significantly increasing the risk of thermal damage; in contrast, peak powers of ≥5 W/cm2 pulsed using appropriate ON and OFF times might produce little, or no tissue heating. The higher peak powers that can be safely used by pulsing light can overcome tissue heating problems and improve the ability of the laser to penetrate deep tissues achieving greater treatment depths.
There may be other biological reasons for the improved efficacy of pulsed light (PW) over CW. The majority of the pulsed light sources used for LLLT have frequencies in the 2.5–10,000 Hz range and pulse durations are commonly in the range of a few millisecond. This observation suggests that if there is a biological explanation of the improved effects of pulsed light it is either due to some fundamental frequency that exists in biological systems in the range of tens to hundreds of Hz, or alternatively due to some biological process that has a time scale of a few milliseconds. Two possibilities for what these biological processes could actually be occur to us. Firstly, it is known that mammalian brains have waves that have specific frequencies [3]. Electroencephalography studies have identified four distinct classes of brain waves [4,5]. Alpha waves (8–13 Hz) occur in adults who have their eyes closed or who are relaxed [6]. Beta waves (14–40 Hz) mainly occur in adults who are awake, alert or focused [7]. Delta waves (1–3 Hz) occur mainly in infants, adults in deep sleep, or adults with brain tumors [8]. Theta waves (4–7 Hz) occur mainly in children ages 2–5 years old and in adults in the twilight state between sleeping and waking or in meditation [9]. The possibility of resonance occurring between the frequency of the light pulses and the frequency of the brain waves may explain some of the results with transcranial LLLT using pulsed light.
Secondly, there are several lines of evidence that ion channels are involved in the subcellular effects of LLLT. Some channels permit the passage of ions based solely on their charge of positive (cationic) or negative (anionic) while others are selective for specific species of ion, such as sodium or potassium. These ions move through the channel pore single file nearly as quickly as the ions move through free fluid. In some ion channels, passage through the pore is governed by a “gate,” which may be opened or closed by chemical or electrical signals, temperature, or mechanical force, depending on the variety of channel. Ion channels are especially prominent components of the nervous system. Voltage-activated ion channels underlie the nerve impulse and while transmitter-activated or ligand-gated channels mediate conduction across the synapses.
There is a lot of literature on the kinetics of various classes of ion channels but in broad summary it can be claimed that the time scale or kinetics for opening and closing of ion channels is of the order of a few milliseconds. For instance Gilboa et al. [10] used pulses having a width 10 milliseconds and a period of 40 milliseconds (25 Hz). Other reports on diverse types of ion channels have given kinetics with timescales of 160 milliseconds [11], 3 milliseconds [12] and one paper giving three values of 0.1, 4 and 100 milliseconds [13]. Potassium and calcium ion channels in the mitochondria and the sarcolemma may be involved in the cellular response to LLLT [14–16].
Thirdly there is the possibility that one mechanism of action of LLLT on a cellular level is the photodissociation of nitric oxide from a protein binding site (heme or copper center) such as those found in cyctochrome c oxidase [17]. If this process occurs it is likely that the NO would rebind to the same site even in the presence of continuous light. Therefore if the light was pulsed multiple photodissociation events could occur, while in CW mode the number of dissociations may be much smaller.
PENETRATION DEPTH
The most important parameter that governs the depth of penetration of laser light into tissue is wavelength. Both the absorption and scattering coefficients of living tissues are higher at lower wavelength so near-infrared light penetrates more deeply that red and so on. It is often claimed that pulsed lasers penetrate more deeply into tissue than CW lasers with the same average power. Why exactly should this be so? Let us suppose that at a certain wavelength (for instance 810-nm) the depth of tissue at which the intensity of a laser is reduced to 10% of its value at the surface of the skin is 1-cm. Therefore if we are using a laser with a power density (irradiance) of 100 mW/cm2 at the skin, the power density remaining at 1 cm below the skin is 10 mW/cm2 and at 2-cm deep is 1 mW/cm2. Now let us suppose that a certain threshold power density (minimum number of photons per unit area per unit time) at the target tissue is necessary to have a biological effect and that this value is 10 mW/cm2. The effective penetration depth at CW may be said to be 1-cm. Now let us suppose that the laser is instead pulsed with a 10-milliseconds pulse duration at a frequency of 1 Hz (DC = 1 Hz×0.010 seconds = 0.010) and the same average power. The peak power and peak power densities are now 100 times higher (peak power = average power/DC = average power×100). With a peak power density of 10 W/cm2 at the skin, the tissue depth—at which this peak power density is attenuated to the threshold level of 10 mW/cm2—is now 3-cm rather than 1-cm in CW mode. But what we have to consider is that the laser is only on for 1% of the time so the total fluence delivered to the 3-cm depth in pulsed mode is 100 times less than that delivered to 1-cm depth in CW mode. However it would be possible to increase the illumination time by a factor of 100 to reach the supposed threshold of fluence as well as the threshold of power at the 3-cm depth. In reality the increase in effective penetration depth obtained with pulsed lasers is more modest than simple calculations might suggest. Many applications of LLLT do not require deep penetration such as tendinopathies and joint pain.
Similarly, deep penetration is often not required to alleviate joint pain. The target tissue in such cases is the synovia; with the exception of back, neck, and hip, most joints have readily accessible synovia. Bjordal et al. [19] conducted a review of literature and concluded that “superpulsed” lasers (904 nm) were not significantly more effective than CW lasers (810–830 nm); both types of laser achieved similar results, but half the energy was needed to be used for superpulsed lasers. On the other hand, deeper penetrance is needed to reach back, neck, and hip joints. If power densities greater than a few mW/cm2 are to be safely delivered to target tissues >5 cm below the skin, it appears likely that this can only be done by using pulsed lasers. It is postulated that successful LLLT treatments in such joints bring benefit not by reaching the deep target tissue but by inhibiting superficial nociceptors. In other words, they bring relief primarily by attenuating pain perception, as opposed to decreasing inflammation. Does deeper penetration via pulsed lasers offer any significant benefit over CW? It is quite possible that a relatively higher fluence is necessary to attenuate pain, whereas a lower fluence decreases inflammation. If this is indeed the case, for musculo-skeletal applications achieving higher doses at the level of the target tissue may not be ideal. Further studies must be done to confirm this hypothesis, as well as to determine if there is any real benefit to the deeper penetration attained by pulsed lasers. Muscles such as the biceps and rectus femoris are not small organs, and have quite deep target tissue. Yet, various studies have shown significant improvement with CW lasers and CW LED. It remains to be seen whether or not pulsed lasers offer any additional advantage. Similarly, depression [20] and stroke studies [21] using LLLT have demonstrated that CW LED’s and CW lasers (respectively) produce a beneficial therapeutic effect. There are reports from Anders’ laboratory that fluences as low as 0.1–0.2 J/cm2 may be optimal for cells in the brain [22]. However, further studies must be done to determine whether pulsed light, with higher peak power densities deeper into the brain tissues, might increase the effectiveness of these therapies.
STUDIES COMPARING CW AND PW
In this review thirty-three studies involving pulsed LLLT were examined. Of these studies, nine of them directly compared continuous wave (CW) with pulsed wave (PW) light, as recorded in Table 1. Six of these nine studies found PW to be more effective than CW. One study comparing CW and PW found both modes of operation to be equally effective, with no statistically significant difference between the two. Only two of the nine articles reported better results with CW than PW, although in both of these studies PW treated subjects were found to have better outcomes than placebo groups. One of the recurring limitations of the papers in this review was that like for like irradiation parameters were not used. For instance, Gigo-Benato et al. [23] found CW superior to PW in nerve regeneration, but is this because of the mode of operation (CW or PW) or because the CW laser used 808 nm and the pulsed laser used 905 nm?
TABLE 1.
Studies Comparing CW and PW
| Refs. | Subject | Condition | λ (nm) | f (Hz) | Other reported parameters | Results? |
|---|---|---|---|---|---|---|
| Kymplova et al. [24] | Humans | Wound healing | 670 | 10, 25, and 50 | Power: 20 mW; energy density: 2 J/cm2 | PW > CW |
| Brondon et al. [36] | In vitro (human HEP-2 cells) |
Increasing the penetration depth of light through melanin filters |
670 | 6, 18, 36, 100, and 600 |
Power: 10 mW; energy density: 5 J/cm2 | PW > CW |
| Lapchak et al. [26] | Rabbits | Ischemic stroke | 808 | 100 and 1,000 | Power density: 7.5 mW/cm2; ON time: 0.3 milliseconds (1,000 Hz), 2 milliseconds (100 Hz); average energy delivered to the brain: 0.9–1.2 J; duty cycle: 30% and 20% |
PW > CW |
| Lapchak and De Taboada [27] |
Rabbits | Ischemic stroke | 808 | 100 | Cortical irradiance: 7.5 mW/cm2 (CW), 37.5 mW/cm2 (PW); cortical fluence: 0.9 J/cm2 (CW), 4.5 J/cm2 (PW) |
PW > CW |
| Gigo-Benato et al. [23] | Rats | Nerve regeneration | 808 (CW), 905 (PW), 808 + 905 (CW + PW) |
10,000 | Power: 416 mW (CW), 28 W (PW); energy density: 29 J/cm2 (CW), 40 J/cm2 (PW); pulse duration: 454 seconds (CW), 200 nanoseconds (PW) |
Combined (CW + PW) > CW > PW |
| Braverman et al. [61] |
Rabbits | Wound healing | 632.8 (CW), 904 (PW) |
4,672 | Power: 10 mW (CW), 50 mW (PW); energy density: 1.65 J/cm2 (CW), 8.25 J/cm2 (PW); pulse duration: 200 nanoseconds |
CW = PW |
| Al-Watban and Zhang [28] |
Rats | Wound healing | 635 | 100, 200, 300, 400, and 500 |
Power density: 0.89 mW/cm2; energy density: 1.0 J/cm2 |
CW > PW |
| Ueda and Shimizu [37] |
In vitro (osteoblast-like cells from fetal rat calvariae) |
Bone stimulation | 830 | 1, 2, and 8 | N/A | PW > CW |
| Sushko et al. [25] | Mice | Pain | 610–670, 850–910 |
10, 600, and 8,000 | N/A | PW > CW |
Of the six studies that found PW to be more effective than CW, four of them involved the use of LLLT to cure the following pathologies in vivo: wound healing, pain, and ischemic stroke. The two remaining studies reported pulsing to be beneficial in vitro; in the first such study, PW promoted bone stimulation more so than CW. The other in vitro study comparing CW and PW found the latter mode of operation better able to penetrate through melanin filters, indicating that pulsing may be beneficial in reaching deep target tissue in dark-skinned patients.
In the wound healing study, Kymplova et al. [24] used a large sample size of women to study the effects of phototherapy on wound repair following surgical episiotomies (one of the most common surgical procedures in women). A pulsed laser emitted light (wavelength of 670 nm) at various frequencies (10, 25, and 50 Hz). The pulsed laser promoted wound repair and healing more so than the CW light source.
In the pain study, Sushko et al. [25] investigated the role of pulsed LLLT to attenuate pain in white male mice. The same wavelength of light was used as in Kymplova et al.’s study (670 nm), with the frequencies of 10, 600, and 8,000 Hz. Both modes of delivery (CW and PW) reduced the behavioral manifestations of somatic pain as compared to controls, but pulsed light (10 and 8,000 Hz in particular) was more effective.
The two studies involving pulsed LLLT and stroke were both done by Lapchak et al. [26]. Ischemic strokes were induced in rabbits, and a pulsed laser with a wavelength of 808 nm was used. In the first study, two frequencies of pulsed light were used (100 and 1,000 Hz), both of which reduced neurological deficits more so than CW. Accordingly, pulsed LLLT may play a major role in the management of stroke patients. Lapchak et al.’s second study attempted to prove the hypothesis that LLLT’s neuroprotective effect following stroke was a result of enhanced mitochondrial energy production (increased ATP synthesis) [27]. As with the previous study, LLLT was administered following stroke induction. CW radiation raised cortical ATP levels but was unable to bring them back to baseline. PW radiation, on the other hand, not only mitigated the effects of stroke on cortical ATP levels, but was able to raise cortical ATP levels to higher than those found in healthy rabbits (those in which stroke was not induced). This study provides valuable insight into one of the potential cellular and molecular mechanisms behind the enhanced neurogenesis (and improved clinical outcomes) observed in subjects receiving transcranial LLLT following stroke.
One of the nine studies reviewed found CW and PW to be equally effective in the promotion of wound healing. This study compared the effects of a CW laser (632.8 nm) and a PW laser (904 nm) on the promotion of wound healing in rabbits. Both lasers improved tensile strength during wound healing, but did not significantly improve wound-healing rates. A combined laser (CW+PW) was also tested. All three of the laser regimens improved tensile strength to a similar extent.
As mentioned earlier, there were nine studies that compared CW and PW, only two of which found CW to be more effective. These two studies involved wound healing and nerve regeneration respectively. Al-Watban and Zhang [28] study involved rats that were inflicted with aseptic wounds. The rats were divided into three groups: a control group, those irradiated with continuous wave light, and those irradiated with pulsed light at various repetition rates (100, 200, 300, 400, and 500 Hz). Of the pulse repetition rates administered, 100 Hz was the most efficacious and 500 Hz the least. Both CW and PW (635 nm) promoted wound healing, but CW was more efficacious. These results conflict with earlier studies that found pulsed light to be more beneficial in the promotion of wound healing. However, it should be noted that the difference between CW and PW treated subjects was small (a relative wound healing rate of 4.81 as compared to 4.32).
The second study that found CW to be more effective than PW involved nerve regeneration. There were three articles involving nerve regeneration, all of which found pulsed LLLT to be ineffective, as discussed in the section below entitled “Studies Involving Nerve Conduction and Regeneration.” Of these three, only Gigo-Benato et al. [23] compared CW (808 nm) and PW (905 nm). This study involved rats in which the left median nerve was completely transected and then repaired by end-to-end neurorrhaphy. The CW laser (808 nm) promoted faster nerve and muscle recovery than the pulsed laser (905 nm). However, Gigo-Benato also tested a combination of the CW and pulsed lasers, finding this to be the most effective of all. In other words, seven of the nine studies comparing CW and PW found pulsing to play a beneficial role. Only one of the nine studies found no role of PW, and even in this study the benefit of CW over PW was minimal.
STUDIES INVOLVING THE USE OF COMBINED LASERS (CW+PW)
We reviewed three studies, as recorded in Table 2, which investigated the role of a combined laser (using both CW and PW). Of these, only Gigo-Benato’s study compared the combined laser to stand alone CW or PW. This study has been discussed in the above section: the combined laser was found to be effective in stimulating nerve regeneration, more so than CW or PW alone.
TABLE 2.
Studies Involving the Use of Combined Lasers (CW + PW)
| Refs. | Subject | Condition | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|
| Gigo-Benato et al. [23] |
Rats | Nerve regeneration | 808 (CW), 905 (PW), 808 + 905 (CW + PW) |
10,000 | Power: 416 mW (CW), 28 W (PW); energy density: 29 J/cm2 (CW), 40 J/cm2 (PW); pulse duration: 454 seconds (CW), 200 nanoseconds (PW) |
Combined (CW + PW) > CW > PW |
| Branco and Naeser [62] |
Humans | Pain | 670 (CW), 904 (PW) | 73, 584, 3,500 | N/A | Combined regimen (CW + PW + TEN- TENS) effective |
| Naeser et al. [29] | Humans | Pain | 632.8 (CW), 904 (PW) | 73, 584, 3,500 | Power: 15 mW (CW), 9.4 W (PW) | Combined regimen (CW + PW + TEN- TENS) effective |
The two other studies used a combined laser (CW and PW) to administer laser acupuncture, along with Transcutaneous Electrical Nerve Stimulation (TENS), to patients with symptoms of pain. Naeser et al. [29] administered this “triple therapy” to patients suffering from carpal tunnel syndrome (CTS). Eleven patients with mild-to-moderate symptoms of CTS were selected, all of who had failed to respond to standard medical or surgical treatment regimens. Subjects were divided into two groups, one of which received sham irradiation and the other that received a combined treatment of LLLT (CW and pulsed) and TENS. As compared to controls, the treated group experienced statistically significant improvement and remained stable for 1–3 years. The results of this study are promising, and indicate a possible role of LLLT and TENS in the conservative management of CTS.
Ceccherelli et al. [30] administered laser acupuncture to patients suffering from myofascial pain. In this double-blinded placebo controlled trial, patients received either the same “triple therapy” as in the Naeser et al. study (CW, PW, and TENS) or sham irradiation, every other day over the course of 24 days. Results were encouraging, with the treatment group experiencing a significant improvement in symptoms, both immediately after the treatment regimen and at a 3-month follow up visit.
In both preceding studies, the combined regimen of CW, PW, and TENS was compared to untreated controls, and found to be effective. However, neither study compared CW and PW or administered CW, PW, or TENS individually. As such, it is difficult to determine whether standalone CW or PW would have produced similar results, or if the combined regimen (along with TENS) was necessary.
STUDIES EVALUATING THE USE OF PULSED LASERS
Of the 33 studies reviewed, 21 of them compared PW treated subjects with untreated controls, as reported in Table 3. Of these, fourteen studies found pulsed LLLT to be effective, whereas seven of them found PW treated subjects to have no benefit over untreated controls. Only one study found PW to have a worse outcome than controls. Of the fourteen studies that found pulsed LLLT to be effective, seven involved the promotion of wound healing, four involved the attenuation of pain, two involved the promotion of bone and cartilage growth respectively, and one involved the treatment of a very rare condition (hyperphagic syndrome caused by traumatic brain injury). Of the seven studies that found no benefit to pulsed light, three involved the promotion of nerve conduction, two involved the promotion of nerve regeneration, and the remaining two involved the attenuation of pain.
TABLE 3.
Studies Evaluating the Use of Pulsed Lasers
| Refs. | Subject | Condition | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|
| Schubert [63] | Humans | Wound healing | 637, 956 | 8.58, 15.6, 31.2, 78, 287, and 702 |
Irradiance: 21 W/m2, 55 W/m2; duty cycle: 80% |
PW > untreated controls |
| Rezvani et al. [35] | Pigs | Prevention of post-radiation complication of dermal necrosis |
660, 820, 880, 950 |
2.5 and 5,000 | Power: 15 mW; power density: 120 mW/cm2; energy density: 0.22, 0.54, 1.08, 2.16, 4.32, and 10.8 J/cm2; duty cycle: 80%; pulse duration: 320 milliseconds with 80 milliseconds-pause between pulses |
PW > untreated controls; 5 kHz most effective |
| Bagis et al. [64] | Rats | Nerve regeneration | 904 | 4, 8, 16, 32, 64, and 128 |
Power: 27 W; energy density: 0.005–2.5 J/cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
| Chen et al. [41] | Rats | Nerve regeneration | 904 | 5,000 and 20,000 |
Energy density: 2.23–3.88 J/cm2 (5 kHz), 8.92–15.5 J/cm2 (20 kHz) |
PW decreased (as opposed to increased) nerve regeneration as compared to sham-irradiated controls |
| Walsh et al. [38] | Humans | Nerve conduction | 820 | 9.12 and 73 | Power: 50 mW; energy density: 9.55 J/cm2; duty cycle: 80% |
PW made no statistically significant effect as compared to untreated controls |
| Bagis et al. [39] | Frogs | Nerve conduction | 904 | 4, 8, 16, 64, and 128 |
Power: 27 W; energy density: 0.005–2.5 J/cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
| Comelekoglu et al. [40] |
Frogs | Nerve conduction | 904 | 1, 4, 16, and 64 | Power: 27 W; energy density: 0.005–2.5 J/cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
| Longo et al. [31] | Rats | Wound healing | 904 | 1,500 and 3,000 |
Power: 20 W; power density: 5 mw/cm2 (1.5 kHz), 10 mw/cm2 (3 kHz); energy density: 3 J; pulse duration: 200 nanoseconds |
PW > untreated controls; only 3,000 Hz was effective in promoting wound healing |
| Korolev and Zagorskaia [32] |
Rats | Wound healing | 850–910 | 500 and 3,000 | N/A | PW > untreated controls; 500 Hz more effective |
| Vasheghani et al. [65] |
Rats | Wound healing | 890 | 80 | Power: 75 W; energy density: 0.396 J/cm2; pulse duration: 180 microseconds |
PW > untreated controls |
| Hopkins et al. [66] | Humans | Wound healing | 820 | 700 | Energy density: 8 J/cm2 | PW > untreated controls |
| Kucerova et al. [67] |
Humans | Wound healing | 670, 632.8 | 5, 292, and 9,000 |
Power: 20 mW; energy density: 1.5 J/cm2 |
PW > untreated controls |
| el Sayed and Dyson [33] |
Rats | Wound healing | 820 | 2.5, 20, 292, and 20,000 |
Power: 800 mW/cm2; energy density: 21.6 J km; pulsing duration: 360, 45, 3, and 0.045 milliseconds for 2.5, 20, 292, and 20,000 Hz respectively; duty cycle: 90% |
PW > untreated controls; 20 and 292 Hz most effective |
| Morrone et al. [68] | In vitro (chondrocytes from humans) |
Cartilage growth | 100, 300, and 500 |
Power: 1W; energy: 300 J | PW > untreated controls | |
| Ponnudurai et al. [34] |
Rats | Pain | 632.8 | 4, 60, and 200 | N/A | PW > untreated controls; 4Hz acted most rapidly but transiently; 60 Hz had delayed but longer lasting effect; 200 Hz had no effect |
| Mokhtar et al. [69] |
Humans | Pain | 660–950 | 16 and 73 | Power: 532 mW; pulse duration: 50 milliseconds with 12.5 milliseconds period between pulses; energy: 383 J; duty cycle: 80% |
PW > untreated controls (significant but very minimal hypoalgesic effect was found) |
| Miriutova et al. [70] |
Humans | Pain | 850–910 | N/A | N/A | PW > untreated controls |
| Craig et al. [42] | Humans | Pain | 660–950 | 2.5, 5, and 20 | Total average radiant exposure: 31.7 J/cm2 |
PW made no statistically significant effect as compared to untreated controls |
| de Bie et al. [43] | Humans | Pain | 904 | 500 and 5,000 | Power: 25 W; energy density: 0.5 and 5 J/cm2; pulse duration: 200 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
| Ceccherelli et al. [30] |
Humans | Pain | N/A | N/A | N/A | PW > untreated controls |
| Read et al. [71] | Human | Brain damage (causing hyperphagia and pica) |
950 | 2.5 and 10 | Power: 25 mW | PW > untreated control; significant but transient reduction of symptoms |
Studies Comparing Various Pulse Repetition Rates
If pulsed LLLT is effective (or ineffective), then what pulse repetition rates are to be used (or avoided)? Ten of the 33 articles reviewed tested and compared various repetition rates, as reported in Table 4. Four of these studies involved the use of pulsed LLLT to promote wound healing. Longo et al. [31] used the pulse repetition rates of 1,500 and 3,000 Hz, and found only the latter setting to promote wound healing. Korolev et al. [32] similarly used two pulse repetition rates, 500 and 3,000 Hz. In this case, both were found to be effective but 500 Hz was more so. Al-Watban and Zhang [28] compared five different pulse repetition rates (100, 200, 300, 400 and 500 Hz), finding 100 Hz to be the most effective and 500 Hz the least. el Sayed and Dyson [33] compared four different pulse repetition rates (2.5, 20, 292, and 20,000 Hz), and found only the two middle values (20 and 292 Hz) beneficial. The more effective pulse repetition rates in these four studies were very disparate, including 20, 100, 292, 500, and 3,000 Hz (a range of 20–3,000 Hz).
TABLE 4.
Studies Comparing Various Pulse Repetition Rates
| Refs. | Subject | Condition | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|
| Rezvani et al. [35] |
Pigs | Prevention of post-radiation complication of dermal necrosis |
660, 820, 880, 950 |
2.5 and 5,000 | Power: 15 mW; power density: 120 mW/cm2; energy density: 0.22, 0.54, 1.08, 2.16, 4.32, and 10.8 J/cm2; duty cycle: 80%; pulse duration: 320 milliseconds with 80 milliseconds-pause between pulses |
PW > untreated controls; 5 kHz most effective |
| Brondon et al. [36] |
In vitro (human HEP-2 cells) |
Increasing the penetration depth of light through melanin filters |
670 | 6, 18, 36, 100, and 600 |
Power: 10 mW; energy density: 5 J/cm2 | PW > CW; 100 and 600 Hz most effective |
| Lapchak et al. [26] |
Rabbits | Ischemic stroke | 808 | 100 and 1,000 | Power density: 7.5 mW/cm2; ON time: 0.3 milliseconds (1,000 Hz), 2 milliseconds (100 Hz); average energy delivered to the brain: 0.9–1.2 J; duty cycle: 30% and 20% |
PW > CW; both 1 kHz and 100 Hz had similar effect with no significant difference between the two |
| Longo et al. [31] |
Rats | Wound healing | 904 | 1,500 and 3,000 | Power: 20 W; power density: 5 mw/cm2 (1.5 kHz), 10 mw/cm2 (3 kHz); energy density: 3 J; pulse duration: 200 nanoseconds |
PW > untreated controls; only 3,000 Hz was effective in promoting wound healing |
| Korolev and Zagorskaia [32] |
Rats | Wound healing | 850–910 | 500 and 3,000 | N/A | PW > untreated controls; 500 Hz more effective |
| Al-Watban and Zhang [28] |
Rats | Wound healing | 635 | 100, 200, 300, 400, and 500 |
Power density: 0.89 mW/cm2; energy density: 1.0 J/cm2 |
CW > PW; of the pulsed frequencies used, 100 Hz was most effective (but less so than CW) |
| el Sayed and Dyson [33] |
Rats | Wound healing | 820 | 2.5, 20, 292, and 20,000 |
Power: 800 mW/cm2; Energy density: 21.6 J km; pulsing duration: 360, 45, 3, and 0.045 milliseconds for 2.5, 20, 292, and 20,000 Hz respectively; duty cycle: 90% |
PW > untreated controls; 20 and 292 Hz most effective |
| Ueda and Shimizu [37] |
In vitro (osteoblast-like cells from fetal rat calvariae) |
Bone stimulation | 830 | 1, 2, and 8 Hz | N/A | PW > CW; 2 Hz most effective |
| Ponnudurai et al. [34] |
Rats | Pain | 632.8 | 4, 60, and 200 | N/A | PW > untreated controls; 4 Hz acted most rapidly but transiently; 60 Hz had delayed but longer lasting effect; 200 Hz had no effect |
| Sushko et al. [25] |
Mice | Pain | 610–670, 850–910 |
10, 600, and 8,000 |
N/A | PW > CW; 10 and 8,000 Hz most effective |
Two studies compared the role of various pulse repetition rates in the attenuation of pain. Ponnudurai et al. [34] used laser photobiostimulation to decrease pain levels in rats, and investigated the effect of using various pulsing frequencies (4, 60, and 200 Hz). The rat tail-flick test was utilized, and tail-flick latencies were measured at five intervals between 30 minutes and 7 days following irradiation. The pulsing frequency of 4 Hz increased pain threshold rapidly but very transiently, whereas 60 Hz produced a delayed but longer lasting effect. On the other hand, 200 Hz failed to produce any hypoalgesic effect whatsoever. Sushko et al. [25] conducted a similar experiment, using mice instead of rats. The center of pain was irradiated (610–910 nm) for 10 minutes with either CW or pulsed light (10, 600, and 8,000 Hz). Both modes of delivery (CW and pulsed) reduced the behavioral manifestations of somatic pain as compared to controls, but pulsed light was more effective. In particular, 10 and 8,000 Hz produced the best effect. The more effective pulse repetition rates from these two studies (involving pain attenuation) included 4, 10, 60, and 8,000 Hz (a range of 4–8,000 Hz), and the less effective pulse repetition rates included 200 and 600 Hz.
Lapchak et al. [26] not only compared CW and PW, but also pulsed light at two different repetition rates, P1 (1,000 Hz) and P2 (100 Hz). Ischemic strokes were induced in rabbits, and the neuroprotective effects of LLLT were assessed via behavioral analysis 48 hours post-stroke. Both P1 (1,000 Hz) and P2 (100 Hz) produced a similar effect (superior to CW).
Rezvani et al. [35] studied the use of low level light therapy to prevent X-ray induced late dermal necrosis. An X-ray dose of 23.4 Gy is known to invariably cause dermal necrosis after 10–16 weeks. This dose was delivered to pigs, which were then treated with LLLT for several weeks using various wavelengths (660, 820, 880, and 950 nm) pulsed at either 2.5 or 5,000 Hz. Light pulsed at 2.5 Hz did not reduce the incidence of dermal necrosis. On the other hand, light pulsed at 5,000 Hz significantly reduced (P = 0.001) the incidence to 52% when given 6–16 weeks after irradiation.
Of the 10 articles reviewed that compared various pulse repetition rates, two of them involved in vitro experiments. Brondon et al. [36] undertook a study to determine if pulsing light would overcome the filtering effects of melanin. Melanin filters were placed in front of human HEP-2 cells, which were then irradiated for 72 hours (670 nm wavelength) with either CW or pulsed light at various repetition rates (6, 18, 36, 100, and 600 Hz). Both cell proliferation and oxidative burst activity, were increased in the group treated with pulsed light, indicating that pulsed light is indeed better able to penetrate melanin rich skin. Specifically, cell proliferation was maximal at 100 Hz at 48 and 72 hours (n = 4, P≤0.05), and oxidative burst was maximal at 600 Hz (n = 4, P≤0.05).
Ueda and Shimizu [37] studied the effects of pulsed low-level light on bone formation in vitro. Osteoblast-like cells were isolated from fetal rat calvariae; one group was not irradiated at all, another was irradiated with continuous wave light, and the third group with pulsed light at three repetition rates (1, 2, and 8 Hz). As compared to the control group, both CW and PW light resulted in increased cellular proliferation, bone nodule formation, alkaline phosphatase (ALP) gene expression, and ALP activity. Pulsed light at 2 Hz stimulated these factors the most.
Out of all 10 articles that compared various pulse repetition rates, the following pulse repetition rates were found to be beneficial: 2, 10, 20, 100, 292, 500, 600, 1,000, 3,000, 5,000, and 8,000 Hz. In this wide range of frequencies (2–8,000 Hz), no particular frequencies stood out as being particularly more or less useful than others.
STUDIED INVOLVING WOUND HEALING
Ten studies out of the 33 involved LLLT’s role in the promotion of wound healing, as recorded in Table 5. Only two of these studies compared CW and PW. Kymplova et al. [24] found pulsed LLLT to promote wound healing over CW, whereas Al-Watban and Zhang [28] found CW to be slightly more effective than PW. Both studies used light of a similar wavelength (670 vs. 635 nm), although the pulse repetition rates used by Kymplova et al. were lower (10–50 Hz vs. 100–500 Hz in Al-Watban et al.’s study). The energy densities applied were also different (2 J/cm2 vs. 1 J/cm2).
TABLE 5.
Studied Involving Wound Healing
| Refs. | Subject | CW | PW | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|---|
| Schubert [63] | Humans | X | 637, 956 | 8.58, 15.6, 31.2, 78, 287, and 702 |
Irradiance: 21 and 55 W/m2; duty cycle: 80% |
PW > untreated controls | |
| Kymplova et al. [24] | Humans | X | X | 670 | 10, 25, and 50 | Power: 20 mW; energy density: 2 J/cm2 | PW > CW |
| Longo et al. [31] | Rats | X | 904 | 1,500 and 3,000 | Power: 20 W; power density: 5 mw/cm2 (1.5 kHz), 10 mw/cm2 (3 kHz); energy density: 3 J; pulse duration: 200 nanoseconds |
PW > untreated controls; only 3,000 Hz was effective in promoting wound healing |
|
| Braverman et al. [61] | Rabbits | X | X | 632.8 (CW), 904 (PW) |
4,672 | Power: 10 mW (CW), 50 mW (PW); energy density: 1.65 J/cm2 (CW), 8.25 J/cm2 (PW); pulse duration: 200 nanoseconds |
Both CW and PW improved tensile strength, and no statistically significant difference between the two |
| Korolev and Zagorskaia [32] | Rats | X | 850–910 | 500 and 3,000 | N/A | PW > untreated controls; 500 Hz more effective |
|
| Al-Watban and Zhang [28] | Rats | X | 635 | 100, 200, 300, 400, and 500 |
Power density: 0.89 mW/cm2; energy density: 1.0 J/cm2 |
CW > PW; of the pulsed frequencies used, 100 Hz was most effective (but less so than CW) |
|
| Vasheghani et al. [65] | Rats | X | X | 890 | 80 | Power: 75 W; energy density: 0.396 J/cm2; pulse duration=180 microseconds |
PW > untreated controls |
| Hopkins et al. [66] | Humans | X | 820 | 700 | Energy density: 8 J/cm2 | PW > untreated controls | |
| Kucerova et al. [67] | Humans | X | 670, 632.8 | 5, 292, and 9,000 | Power: 20 mW; energy density: 1.5 J/cm2 |
PW > untreated controls | |
| el Sayed and Dyson [33] | Rats | X | 820 | 2.5, 20, 292, and 20,000 |
Power: 800 mW/cm; energy density: 21.6 J km; pulsing duration: 360, 45, 3, and 0.045 milliseconds for 2.5, 20, 292, and 20,000 Hz respectively; duty cycle: 90% |
PW > untreated controls; 20 and 292 Hz most effective |
Every study reviewed found pulsed LLLT effective in promoting wound healing (as compared to untreated controls), including the Al-Watban et al. study. Six of these studies used light in the wavelength range of 820–956 nm, and four in the range of 632.8–670 nm. Once again, a wide range of frequencies were used (2.5–20,000 Hz), most of which were found to promote wound healing. (Tested frequencies included 2.5, 5, 8.58, 10, 15.6, 20, 25, 31.2, 50, 78, 80, 287, 292, 500, 700, 3,000, 4,672, 9,000, and 20,000 Hz). Most of these articles also reported energy densities, usually in the range of 1–2 J/cm2.
STUDIES INVOLVING NERVE CONDUCTION AND REGENERATION
We reviewed three articles evaluating the role of pulsed LLLT in the promotion of nerve conduction, and another three involving nerve regeneration, as reported in Table 6. Unlike the studies involving wound healing where positive outcomes were reported, all six of these studies reported negative outcomes with pulsed light. Five of these studies found PW to have no statistically significant effect on outcome, whereas one of them found PW to have a deleterious effect. There was no study that directly compared CW and PW in regards to nerve conduction. Walsh et al. [38] conducted a study with 32 human volunteers to determine if pulsed LLLT would influence nerve conduction in the superficial radial nerve. Action potentials were measured pre- and post-irradiation (at 5, 10, and 15 minutes). No significant difference was appreciated between control and treatment groups, indicating that LLLT with those particular pulsing parameters and dosimetry had no specific neurophysiologic effects on nerve conduction. Bagis et al. [39] and Comelekoglu et al. [40] obtained similar negative results using frog nerves. Walsh et al. used a wavelength of 820 nm, whereas Bagis et al. used a 904 nm laser. All three studies tested pulse repetition rates within the range of 1–128 Hz.
TABLE 6.
Studies Involving Nerve Conduction and Regeneration
| Refs. | Subject | Condition | CW | PW | λ (nm) | f (Hz) | Other reported parameters |
Results |
|---|---|---|---|---|---|---|---|---|
| Gigo-Benato et al. [23] | Rats | Nerve regeneration |
X | X | 808 (CW), 905 (PW), 808 + 905 (CW + PW) |
10,000 | Power: 416 mW (CW), 28 W (PW); energy density: 29 J/cm2 (CW), 40 J/cm2 (PW); pulse duration: 454 seconds (CW), 200 nanoseconds (PW) |
Combined (CW + PW) > CW > PW |
| Bagis et al. [64] | Rats | Nerve regeneration |
X | 904 | 4. 8, 16, 32, 64, and 128 |
Power: 27 W; energy density: 0.005–2.5 J/ cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
|
| Chen et al. [41] | Rats | Nerve regeneration |
X | 904 | 5,000 and 20,000 |
Energy density: 2.23–3.88 J/cm2 (5 kHz), 8.92–15.5 J/cm2 (20 kHz) |
PW decreased (as opposed to increased) nerve regeneration as compared to sham- irradiated controls |
|
| Walsh et al. [38] | Humans | Nerve conduction |
X | 820 | 9.12 and 73 |
Power: 50 mW; energy density: 9.55 J/cm2; duty cycle: 80% |
PW made no statistically significant effect as compared to untreated controls |
|
| Bagis et al. [39] | Frogs | Nerve conduction |
X | 904 | 4, 8, 16, 64, and 128 |
Power: 27 W; energy density: 0.005–2.5 J/cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
|
| Comelekoglu et al. [40] | Frogs | Nerve conduction |
X | 904 | 1, 4, 16, and 64 |
Power: 27 W; energy density: 0.005–2.5 J/cm2; pulse duration: 220 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
Similarly, the nerve regeneration studies reviewed reported negative outcomes. Chen et al. [41] found PW to have a counterproductive effect, reducing nerve regeneration as compared to untreated controls. Only one study compared CW with PW, and found the former to be superior to the latter. However, the combined laser (CW+PW) was superior to CW alone, indicating that there might in fact be a role of pulsing in nerve regeneration.
STUDIES INVOLVING PAIN ATTENUATION
Nine of the thirty-three studies involved pulsed LLLT’s role in the attenuation of pain, as reported in Table 7. Of these, only one of them directly compared CW and PW. This study was conducted by Sushko et al. [25] and found that although both CW and PW decreased pain levels, PW was more effective. This study also determined that pulse repetition rates of 10 and 8,000 Hz were more effective than 600 Hz. Ponnudurai et al. [34] similarly compared various pulse repetition rates (4, 60, and 200 Hz). A rapid but transient analgesic effect was exhibited with 4 Hz, whereas a delayed but longer lasting effect was achieved with 60 Hz. On the other hand, 200 Hz failed to produce any analgesic effect whatsoever.
TABLE 7.
Studies Involving Pain Attenuation
| Refs. | Subject | CW | PW | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|---|
| Ponnudurai et al. [34] |
Rats | X | 632.8 | 4, 60, and 200 | N/A | PW > untreated controls; 4 Hz acted most rapidly but transiently; 60 Hz had delayed but longer lasting effect; 200 Hz had no effect |
|
| Mokhtar et al.[69] | Humans | X | 660–950 | 16 and 73 | Power: 532 mW; pulse duration: 50 milliseconds with 12.5 milliseconds period between pulses; energy: 383 J; duty cycle: 80% |
PW > untreated controls | |
| Miriutova et al.[70] | Humans | X | 850–910 | N/A | PW > untreated controls | ||
| Craig et al.[42] | Humans | X | 660–950 | 2.5, 5, and 20 | Total average radiant exposure: 31.7 J/cm2 |
PW made no statistically significant effect as compared to untreated controls |
|
| Sushko et al.[25] | Mice | X | X | 610–670, 850–910 | 10, 600, and 8,000 |
N/A | PW > CW; 10 and 8,000 Hz most effective |
| de Bie et al.[43] | Humans | X | 904 | 500 and 5,000 | Power: 25 W; energy density: 0.5 and 5 J/cm2; pulse duration: 200 nanoseconds |
PW made no statistically significant effect as compared to untreated controls |
|
| Ceccherelli et al.[30] | Humans | X | N/A | N/A | N/A | PW > untreated controls | |
| Branco and Naeser [62] |
Humans | X | X | 670 (CW), 904 (PW) | 73, 584, 3,500 | N/A | Combined regimen (CW, pulsed, and TENS) improved outcome as compared to untreated controls (CW and PW not administered individually in this study) |
| Naeser et al. [29] | Humans | X | X | 632.8 (CW), 904 (PW) |
73, 584, 3,500 | Power: 15 mW (CW), 9.4 W (PW) |
Combined regimen (CW, PW, and TENS) improved outcome as compared to untreated controls (CW and PW were not administered individually in this study) |
Two of the studies used a combined laser (CW+PW) along with TENS; both found the combined regimen to be effective. The five remaining studies compared pulsed LLLT with untreated controls. Three of these studies found pulsed LLLT to be effective, whereas two did not. Of the nine total studies on pain attenuation, seven found pulsed LLLT to be effective in its role of attenuating pain. Only two studies found no statistically significant effect. However, it should be noted that both of these involved pain of a different nature than commonly tested in pulsed LLLT studies. The first of these was by Craig et al. [42] and involved the use of pulsed LLLT to relieve the symptoms of delayed-onset muscle soreness (DOMS). DOMS refers to the feeling of pain and muscle stiffness that can result 1–3 days after intense sporting activity such as weightlifting. This pain is duller in quality than that tested in the other studies. The second study that showed no benefit to pulsed LLLT, published by de Bie et al. [43], involved the treatment of lateral ankle sprains.
STUDIES INVOLVING ISCHEMIC STROKE
Table 8 records the two studies that involved pulsed LLLT and stroke. In the first study, PW but not CW decreased neurological deficits when delivered six hours post-stroke. Two pulse repetition rates were tested (100 and 1,000 Hz) and found to be equally effective. On the other hand, both CW and PW produced no benefit if delivered 12 hours post-stroke, indicating that timely administration of LLLT is essential.
TABLE 8.
Studies Involving Stroke
| Refs. | Subject | Condition | CW | PW | λ (nm) | f (Hz) | Other reported parameters | Results |
|---|---|---|---|---|---|---|---|---|
| Lapchak et al. [26] |
Rabbits | Ischemic stroke |
X | X | 808 | 100 and 1,000 | Power density: 7.5 mW/cm2; on time: 0.3 milliseconds (1,000 Hz), 2 milliseconds (100 Hz); average energy delivered to the brain: 0.9–1.2 J; duty cycle: 30% and 20% |
PW > CW; both 1 kHz and 100 Hz had similar effect with no significant difference between the two |
| Lapchak and De Taboada [27] |
Rabbits | Ischemic stroke |
X | X | 808 | 100 | Cortical irradiance: 7.5 mW/cm2 (CW), 37.5 mW/cm2 (PW); cortical fluence: 0.9 J/cm2 (CW), 4.5 J/cm2 (PW) |
PW > CW |
The second study investigated the possible mechanisms behind the neuroprotective effect of LLLT. It was postulated that LLLT enhances mitochondrial energy production (and ATP synthesis), which allows for enhanced neurogenesis. This hypothesis was tested using the rabbit small clot embolic stroke model (RSCEM). Four groups of rabbits were used: (1) a naïve control group which was neither embolized or irradiated, (2) a placebo group which was embolized and sham irradiated, (3) an embolized group which was irradiated with CW (808 nm), and (4) an embolized group which was irradiated with pulsed light (808 nm) at two different frequencies. Forty-five percent less cortical ATP was measured in the second group (placebo) as compared to the first (naïve), confirming the hypothesis that ischemic strokes decrease cortical mitochondrial energy. All laser irradiated groups were able to mitigate this effect. CW radiation managed to raise the cortical ATP levels by 41%, whereas PW administration raised these levels by over 150%. Surprisingly, this was even higher than the cortical ATP content measured in naïve rabbits that had never suffered stroke.
OTHER APPLICATIONS OF PULSED MODALITIES IN BIOMEDICINE
Many of the modalities of treatment employed in biomedicine and physical therapy are used in pulsed format [44]. Electricity, electromagnetic fields and ultrasound are applied with particular pulse structures. It may be possible to gain some insight into the effect of pulsing structures in LLLT by a brief review of the other pulsed modalities. Transcutaneous electrical neural stimulation (TENS) is the application of pulses of electric current to the skin [45]. This application stimulates the brain and has been used for the treatment of various psychological and neurological conditions, including Parkinson’s, epilepsy, chronic pain, depression, and neuromuscular rehabilitation. Frequencies usually fall between 5 and 25 Hz, but may range from 2 to 80 Hz [46]. Deep brain stimulation (DBS) is a surgical treatment involving the implantation of a brain pacemaker, a medical device that sends electrical impulses to specific parts of the brain. DBS has the potential to provide substantial benefit to patients suffering from a variety of neurological conditions, including epilepsy, Parkinson’s disease, dystonia, Tourette’s syndrome, and depression [47]. The Food and Drug Administration (FDA) approved DBS at 130 Hz as a treatment for essential tremor in 1997, for Parkinson’s disease in 2002, and dystonia in 2003. Pulsed electromagnetic field (PEMF) therapy has been used for a wide range of conditions, including bone healing and regeneration [48], osteoporosis [49], arthritis [50] wound healing and pain [51], carpal tunnel syndrome [52], spinal cord injury [53], nerve regeneration [54], soft tissue injuries [55], and cancer [56]. Frequencies used for these conditions range from 1 Hz (“low”) to 200 Hz (“high”). Transcranial magnetic stimulation (TMS) is a noninvasive method used to excite neurons in the brain. Weak electric currents are induced by butterfly coils positioned above the head. TMS has been approved for the treatment of resistant depression in several countries and is under investigation for migraine [57], aphasia [58], and tinnitus [59]. Low-intensity pulsed ultrasound (LIPUS) utilizes a non-thermal mechanism of action, which can be used to promote bone healing by inducing the expression of growth factors and prostaglandins, which stimulate osteoblasts, chondrocytes and fibroblasts [60].
CONCLUSION
There has been remarkably little information available in the peer-reviewed literature on the rationale for using pulsed lasers or pulsed light in LLLT rather than CW. Moreover there is no consensus on the effects of different frequencies and pulse parameters on the physiology and therapeutic response of the various disease states that are often treated with laser therapy. This has allowed manufacturers to claim advantages of pulsing without hard evidence to back up their claims.
CW light is the gold standard and has been used for all LLLT applications. However, this review of the literature indicates that overall pulsed light may be superior to CW light with everything else being equal. This seemed to be particularly true for wound healing and post-stroke management. On the other hand, PW as a solo treatment may be less beneficial than CW in patients requiring nerve regeneration. This could possibly be explained by the mechanism of action LLLT that can either cause cell stimulation or cell inhibition or both stimulation and inhibition at the same time on different cell types. It is possible that stimulation in neurons is desired to promote neurogenesis following stroke (increased mitochondrial synthesis of ATP results in more energy for neurons to regenerate themselves), whereas inhibition of inflammatory cells, inhibition of immune response or inhibition of the glial scar may also occur at the same time. The logic in favor of PW is that cells may need periods of rest, without which they can no longer be stimulated further.
Considering that the biology of LLLT is known to be complex, it is likely that there may several optimal sets of pulse parameters and that these may relate to the specific wavelengths and chromophores and may well also be affected by other optical properties of tissues.
It was impossible to draw any meaningful correlations between pulse frequency and pathological condition, due to the wide-ranging and disparate data. As for other pulse parameters, these were in general poorly and inconsistently reported. It is suggested that future researchers consistently report the following parameters: wavelength, frequency, duty cycle, peak power, average power, peak power density, average power density, and energy density. Careful reporting of these values would make it easier for future reviewers to find useful patterns. Furthermore, a concerted effort should be made to use like-for-like parameters when comparing CW and pulsed lasers. This limitation notwithstanding, this review indicates that pulsing will continue to play an important role in LLLT especially for applications where deep tissue penetration is required. Nevertheless, much more research remains to be done at both basic science and clinical levels before the role of pulsing in LLLT becomes clear and completely understood.
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: R01AI050875; Contract grant sponsor: Center for Integration of Medicine and Innovative Technology; Contract grant number: DAMD17-02-2-0006; Contract grant sponsor: CDMRP Program in TBI; Contract grant number: W81XWH-09-1-0514; Contract grant sponsor: Air Force Office of Scientific Research; Contract grant number: FA9950-04-1-0079.
Footnotes
Conflict of Interest: Luis De Taboada is an employee and stockholder in Photothera, Inc. that does phototherapy for stroke. James Carroll is owner of THOR Photomedicine a company that makes phototherapy devices.
REFERENCES
- 1.Valchinov ES, Pallikarakis NE. Design and testing of low intensity laser biostimulator. Biomed Eng Online. 2005;4(1):5. doi: 10.1186/1475-925X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomed Laser Surg. 2006;24(4):458–466. doi: 10.1089/pho.2006.24.458. [DOI] [PubMed] [Google Scholar]
- 3.Freeman WJ. The wave packet: An action potential for the 21st century. J Integr Neurosci. 2(1):3–30. doi: 10.1142/s0219635203000214. [DOI] [PubMed] [Google Scholar]
- 4.Westmoreland BF, Klass DW. Unusual EEG patterns. J Clin Neurophysiol. 1990;7(2):209–228. doi: 10.1097/00004691-199004000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lopes da Silva F. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79(2):81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- 6.Kirschfeld K. The physical basis of alpha waves in the electroencephalogram and the origin of the “Berger effect”. Biol Cybern. 2005;92(3):177–185. doi: 10.1007/s00422-005-0547-1. [DOI] [PubMed] [Google Scholar]
- 7.Thuroczy G, Kubinyi G, Bodo M, Bakos J, Szabo LD. Simultaneous response of brain electrical activity (EEG) and cerebral circulation (REG) to microwave exposure in rats. Rev Environ Health. 1994;10(2):135–148. doi: 10.1515/reveh.1994.10.2.135. [DOI] [PubMed] [Google Scholar]
- 8.Picchioni D, Killgore WD, Balkin TJ, Braun AR. Positron emission tomography correlates of visually-scored electroencephalographic waveforms during non-Rapid Eye Movement sleep. Int J Neurosci. 2009;119(11):2074–2099. doi: 10.1080/00207450903139770. [DOI] [PubMed] [Google Scholar]
- 9.Baijal S, Srinivasan N. Theta activity and meditative states: Spectral changes during concentrative meditation. Cogn Process. 2010;11(1):31–38. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- 10.Gilboa G, Chen R, Brenner N. History-dependent multiple-time-scale dynamics in a single-neuron model. J Neurosci. 2005;25(28):6479–6489. doi: 10.1523/JNEUROSCI.0763-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priestley T, Kemp JA. Kinetic study of the interactions between the glutamate and glycine recognition sites on the N-methyl-d-aspartic acid receptor complex. Mol Pharmacol. 1994;46(6):1191–1196. [PubMed] [Google Scholar]
- 12.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406(6798):889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 13.Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: Implications for spike-timing dependent synaptic plasticity. J Physiol. 2004;556:337–345. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow RT, David MA, Armati PJ. 830nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root ganglion neurons: Implications for the analgesic effects of 830nm laser. J Peripher Nerv Syst. 2007;12(1):28–39. doi: 10.1111/j.1529-8027.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 15.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84(5):1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 16.Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004;80(2):366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 17.Lane N. Cell biology: Power games. Nature. 2006;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 18.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: A systematic review with meta-analysis. Photomed Laser Surg. 2010;28(1):3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]
- 19.Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, Stergioulas A, Johnson MI. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow) BMC Musculoskelet Disord. 2008;9:75. doi: 10.1186/1471-2474-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46–59. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 22.Anders JJ. The potential of light therapy for central nervous system injury and disease. Photomed Laser Surg. 2009;27(3):379–380. doi: 10.1089/pho.2009.0053. [DOI] [PubMed] [Google Scholar]
- 23.Gigo-Benato D, Geuna S, de Castro Rodrigues A, Tos P, Fornaro M, Boux E, Battiston B, Giacobini-Robecchi MG. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: A double-blind randomized study in the rat median nerve model. Lasers Med Sci. 2004;19(1):57–65. doi: 10.1007/s10103-004-0300-3. [DOI] [PubMed] [Google Scholar]
- 24.Kymplova J, Navratil L, Knizek J. Contribution of phototherapy to the treatment of episiotomies. J Clin Laser Med Surg. 2003;21(1):35–39. doi: 10.1089/10445470360516725. [DOI] [PubMed] [Google Scholar]
- 25.Sushko BS, Lymans’kyi Iu P, Huliar SO. Action of the red and infrared electromagnetic waves of light-emitting diodes on the behavioral manifestation of somatic pain. Fiziol Zh. 2007;53(3):51–60. [PubMed] [Google Scholar]
- 26.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: An extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5’-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Al-Watban FA, Zhang XY. The comparison of effects between pulsed and CW lasers on wound healing. J Clin Laser Med Surg. 2004;22(1):15–18. doi: 10.1089/104454704773660921. [DOI] [PubMed] [Google Scholar]
- 29.Naeser MA, Hahn KA, Lieberman BE, Branco KF. Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: A controlled study. Arch Phys Med Rehabil. 2002;83(7):978–988. doi: 10.1053/apmr.2002.33096. [DOI] [PubMed] [Google Scholar]
- 30.Ceccherelli F, Altafini L, Lo Castro G, Avila A, Ambrosio F, Giron GP. Diode laser in cervical myofascial pain: A double-blind study versus placebo. Clin J Pain. 1989;5(4):301–304. doi: 10.1097/00002508-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Longo L, Evangelista S, Tinacci G, Sesti AG. Effect of diodes-laser silver arsenide-aluminium (Ga-Al-As) 904nm on healing of experimental wounds. Lasers Surg Med. 1987;7(5):444–447. doi: 10.1002/lsm.1900070513. [DOI] [PubMed] [Google Scholar]
- 32.Korolev LN, Zagorskaia NZ. The effect of infrared laser radiation of different frequencies on the healing of skin wounds. Vopr Kurortol Fizioter Lech Fiz Kult. 1996;(3):8–10. [PubMed] [Google Scholar]
- 33.el Sayed SO, Dyson M. Effect of laser pulse repetition rate and pulse duration on mast cell number and degranulation. Lasers Surg Med. 1996;19(4):433–437. doi: 10.1002/(SICI)1096-9101(1996)19:4<433::AID-LSM8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 34.Ponnudurai RN, Zbuzek VK, Wu WH. Hypoalgesic effect of laser photobiostimulation shown by rat tail flick test. Acupunct Electrother Res. 1987;12(2):93–100. doi: 10.3727/036012987816358896. [DOI] [PubMed] [Google Scholar]
- 35.Rezvani M, Nissan M, Hopewell JW, van den Aardweg GJ, Robbins ME, Whitehouse EM. Prevention of X-ray-induced late dermal necrosis in the pig by treatment with multi-wavelength light. Lasers Surg Med. 1992;12(3):288–293. doi: 10.1002/lsm.1900120308. [DOI] [PubMed] [Google Scholar]
- 36.Brondon P, Stadler I, Lanzafame RJ. Pulsing influences photoradiation outcomes in cell culture. Lasers Surg Med. 2009;41(3):222–226. doi: 10.1002/lsm.20740. [DOI] [PubMed] [Google Scholar]
- 37.Ueda Y, Shimizu N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg. 2003;21(5):271–277. doi: 10.1089/104454703322564479. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DM, Baxter GD, Allen JM. Lack of effect of pulsed low-intensity infrared (820nm) laser irradiation on nerve conduction in the human superficial radial nerve. Lasers Surg Med. 2000;26(5):485–490. doi: 10.1002/1096-9101(2000)26:5<485::aid-lsm8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Bagis S, Comelekoglu U, Sahin G, Buyukakilli B, Erdogan C, Kanik A. Acute electrophysiologic effect of pulsed gallium-arsenide low energy laser irradiation on configuration of compound nerve action potential and nerve excitability. Lasers Surg Med. 2002;30(5):376–380. doi: 10.1002/lsm.10057. [DOI] [PubMed] [Google Scholar]
- 40.Comelekoglu U, Bagis S, Buyukakilli B, Sahin G, Erdogan C, Kanik A. Acute electrophysiological effect of pulsed gallium-arsenide low-energy laser irradiation on isolated frog sciatic nerve. Lasers Med Sci. 2002;17(1):62–67. doi: 10.1007/s10103-002-8268-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen YS, Hsu SF, Chiu CW, Lin JG, Chen CT, Yao CH. Effect of low-power pulsed laser on peripheral nerve regeneration in rats. Microsurgery. 2005;25(1):83–89. doi: 10.1002/micr.20079. [DOI] [PubMed] [Google Scholar]
- 42.Craig JA, Barlas P, Baxter GD, Walsh DM, Allen JM. Delayed-onset muscle soreness: Lack of effect of combined phototherapy/low-intensity laser therapy at low pulse repetition rates. J Clin Laser Med Surg. 1996;14(6):375–380. doi: 10.1089/clm.1996.14.375. [DOI] [PubMed] [Google Scholar]
- 43.de Bie RA, de Vet HC, Lenssen TF, van den Wildenberg FA, Kootstra G, Knipschild PG. Low-level laser therapy in ankle sprains: A randomized clinical trial. Arch Phys Med Rehabil. 1998;79(11):1415–1420. doi: 10.1016/s0003-9993(98)90237-4. [DOI] [PubMed] [Google Scholar]
- 44.Canapp DA. Select modalities. Clin Tech Small Anim Pract. 2007;22(4):160–165. doi: 10.1053/j.ctsap.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Cameron M, Lonergan E, Lee H. Transcutaneous electrical nerve stimulation (TENS) for dementia. Cochrane Database Syst Rev. 2003;(3) doi: 10.1002/14651858.CD004032. CD004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8(8):623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 47.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker NA, Denegar CR, Preische J. Low-intensity pulsed ultrasound and pulsed electromagnetic field in the treatment of tibial fractures: A systematic review. J Athl Train. 2007;42(4):530–535. [PMC free article] [PubMed] [Google Scholar]
- 49.Tabrah F, Hoffmeier M, Gilbert F, Jr, Batkin S, Bassett CA. Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (PEMFs) J Bone Miner Res. 1990;5(5):437–442. doi: 10.1002/jbmr.5650050504. [DOI] [PubMed] [Google Scholar]
- 50.Ay S, Evcik D. The effects of pulsed electromagnetic fields in the treatment of knee osteoarthritis: A randomized, placebo-controlled trial. Rheumatol Int. 2009;29(6):663–666. doi: 10.1007/s00296-008-0754-x. [DOI] [PubMed] [Google Scholar]
- 51.Strauch B, Herman C, Dabb R, Ignarro LJ, Pilla AA. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J. 2009;29(2):135–143. doi: 10.1016/j.asj.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Weintraub MI, Cole SP. A randomized controlled trial of the effects of a combination of static and dynamic magnetic fields on carpal tunnel syndrome. Pain Med. 2008;9(5):493–504. doi: 10.1111/j.1526-4637.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 53.Crowe MJ, Sun ZP, Battocletti JH, Macias MY, Pintar FA, Maiman DJ. Exposure to pulsed magnetic fields enhances motor recovery in cats after spinal cord injury. Spine (Phila, PA 1976) 2003;28(24):2660–2666. doi: 10.1097/01.BRS.0000099385.46102.0D. [DOI] [PubMed] [Google Scholar]
- 54.Mert T, Gunay I, Gocmen C, Kaya M, Polat S. Regenerative effects of pulsed magnetic field on injured peripheral nerves. Altern Ther Health Med. 2006;12(5):42–49. [PubMed] [Google Scholar]
- 55.Owegi R, Johnson MT. Localized pulsed magnetic fields for tendonitis therapy. Biomed Sci Instrum. 2006;42:428–433. [PubMed] [Google Scholar]
- 56.Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, DeNardo GL. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res. 2005;11(19 Pt 2):7093s–7103s. doi: 10.1158/1078-0432.CCR-1004-0016. [DOI] [PubMed] [Google Scholar]
- 57.Philip R. Holland SFCA, Schembri Carol T, Fredrick Joe P, Sunnyvale CA, Goadsby Peter J, San Francisco CA. Abstract published American Academy of Neurology 2009. ranscranial Magnetic Stimulation for the Treatment of Migraine Aura. ranscranial Magnetic Stimulation for the Treatment of Migraine Aura. [Google Scholar]
- 58.Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, Fregni F, Seekins H, Doron K, Pascual-Leone A. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25(2):181–191. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- 59.Kleinjung T, Vielsmeier V, Landgrebe M, Hajak G, Langguth B. Transcranial magnetic stimulation: A new diagnostic and therapeutic tool for tinnitus patients. Int Tinnitus J. 2008;14(2):112–118. [PubMed] [Google Scholar]
- 60.Suzuki A, Takayama T, Suzuki N, Sato M, Fukuda T, Ito K. Daily low-intensity pulsed ultrasound-mediated osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys Sin (Shanghai) 2009;41(2):108–115. doi: 10.1093/abbs/gmn012. [DOI] [PubMed] [Google Scholar]
- 61.Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989;9(1):50–58. doi: 10.1002/lsm.1900090111. [DOI] [PubMed] [Google Scholar]
- 62.Branco K, Naeser MA. Carpal tunnel syndrome: Clinical outcome after low-level laser acupuncture, microamps transcutaneous electrical nerve stimulation, and other alternative therapies–an open protocol study. J Altern Complement Med. 1999;5(1):5–26. doi: 10.1089/acm.1999.5.5. [DOI] [PubMed] [Google Scholar]
- 63.Schubert V. Effects of phototherapy on pressure ulcer healing in elderly patients after a falling trauma. A prospective, randomized, controlled study. Photodermatol Photoimmunol Photomed. 2001;17(1):32–38. doi: 10.1034/j.1600-0781.2001.017001032.x. [DOI] [PubMed] [Google Scholar]
- 64.Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C. No effect of GA-AS (904nm) laser irradiation on the intact skin of the injured rat sciatic nerve. Lasers Med Sci. 2003;18(2):83–88. doi: 10.1007/s10103-003-0258-6. [DOI] [PubMed] [Google Scholar]
- 65.Vasheghani MM, Bayat M, Dadpay M, Habibie M, Rezaei F. Low-level laser therapy using 80-Hz pulsed infrared diode laser accelerates third-degree burn healing in rat. Photomed Laser Surg. 2009;27(6):959–964. doi: 10.1089/pho.2008.2366. [DOI] [PubMed] [Google Scholar]
- 66.Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J Athl Train. 2004;39(3):223–229. [PMC free article] [PubMed] [Google Scholar]
- 67.Kucerova H, Dostalova T, Himmlova L, Bartova J, Mazanek J. Low-level laser therapy after molar extraction. J Clin Laser Med Surg. 2000;18(6):309–315. [PubMed] [Google Scholar]
- 68.Morrone G, Guzzardella GA, Tigani D, Torricelli P, Fini M, Giardino R. Biostimulation of human chondrocytes with Ga-Al-As diode laser: ‘In vitro’ research. Artif Cells Blood Substit Immobil Biotechnol. 2000;28(2):193–201. doi: 10.3109/10731190009118581. [DOI] [PubMed] [Google Scholar]
- 69.Mokhtar B, Baxter GD, Walsh DM, Bell AJ, Allen JM. Double-blind, placebo-controlled investigation of the effect of combined phototherapy/low intensity laser therapy upon experimental ischaemic pain in humans. Lasers Surg Med. 1995;17(1):74–81. doi: 10.1002/lsm.1900170109. [DOI] [PubMed] [Google Scholar]
- 70.Miriutova NF, Abdulkina NG, Luksha LV, Levitskii EF. Laser therapy and electric stimulation in rehabilitation treatment of peripheral neuropathy. Vopr Kurortol Fizioter Lech Fiz Kult. 2002;(4):25–27. [PubMed] [Google Scholar]
- 71.Read A, Beaty P, Corner J, Sommerville VilleC. Reducing naltrexone-resistant hyperphagia using laser acupuncture to increase endogenous opiates. Brain Injury. 1996;10(12):911–919. doi: 10.1080/026990596123882. [DOI] [PubMed] [Google Scholar]