Abstract
Nearly 30% of patients with mesial temporal lobe epilepsy (TLE) are resistant to treatment with anti-epileptic drugs. Neural stem cell (NSC) grafting into the hippocampus could offer an alternative therapy to hippocampal resection in these patients. As TLE is associated with reduced numbers of inhibitory GABA-ergic interneurons and astrocytes expressing the anticonvulsant glial-derived neurotrophic factor (GDNF) in the hippocampus, we tested the hypothesis that grafting of NSCs that are capable of adding new GABA-ergic interneurons and GDNF-expressing astrocytes into the epileptic hippocampus restrains spontaneous recurrent motor seizures (SRMS) in chronic TLE. We grafted NSCs expanded in vitro from embryonic medial ganglionic eminence (MGE) into hippocampi of adult rats exhibiting chronic TLE with cognitive impairments. NSC grafting reduced frequencies of SRMS by 43% and stage-V seizures by 90%. The duration of individual SRMS and the total time spent in seizures were reduced respectively by 51% and 74%. Grafting did not improve the cognitive function however. Graft-derived cells (equivalent to ~28% of injected cells) were observed in various layers of the epileptic hippocampus where they differentiated into NeuN+ neurons (13%), S-100β+ astrocytes (57%), and NG2+ oligodendrocyte-progenitors (3%). Furthermore, among graft-derived cells, 10% expressed GABA and 50% expressed GDNF. Additionally, NSC grafting restored GDNF in a vast majority of the hippocampal astrocytes but had no effect on neurogenesis. Thus, MGE-NSC therapy is efficacious for diminishing SRMS in chronic TLE. Addition of new GABA-ergic neurons and GDNF+ cells, and restoration of GDNF in the hippocampal astrocytes may underlie the therapeutic effect of MGE-NSC grafts.
Keywords: neural stem cell therapy, chronic epilepsy, glial-derived neurotrophic factor, hippocampal neurogenesis, medial ganglionic eminence, learning and memory, spontaneous seizures
Introduction
Epilepsy affects >50 million people worldwide [1]. Temporal lobe epilepsy (TLE), typified by complex partial seizures and hippocampal neurodegeneration, is seen in ~30% of epileptic patients [2]. Seizures in TLE are associated with memory impairments [3–4] and reduced hippocampal neurogenesis [5–7]. Nearly 35% of patients with TLE exhibit seizures that cannot be controlled by anti-epileptic drugs [8]. Although surgical resection of the hippocampus gives better seizure control, this option is associated with cognitive impairments [9], loss of viable tissue during resection and the possibility of continuing dependance on anticonvulsant drugs after resection [10]. Thus, alternative treatment modalities that are efficient for controlling SRMS in chronic TLE are needed.
As the occurrence of seizures in TLE is thought to be linked partially to reduced numbers of hippocampal GABA-ergic interneurons [11–12] and loss of functional inhibition [13–14], the idea of restraining spontaneous recurrent motor seizures (SRMS) via grafting of cells that release GABA has received attention [15]. Indeed, grafting of fetal GABA-ergic cells and immortalized GABA-producing cells reduces seizures in epilepsy models [15–18]. Nevertheless, clinical application of these cell types is difficult because of the non-availability of adequate amounts of cells, ethical issues concerning fetal cells [19] and the inability of GABA+ fetal/immortalized cells to have more than transient effects even with sustained GABA-ergic expression in some epilepsy models [15, 20–21]. In this context, cell types that are capable of providing an unlimited source of donor cells, giving rise to significant numbers of GABA-ergic neurons and secreting anticonvulsant proteins such as the glial derived neurotrophic factor (GDNF; 22) appear useful. Grafting of such cells might engender a sustained anticonvulsant effect over prolonged periods of time through both strengthening of the GABA-ergic circuitry and anticonvulsant actions of GDNF secreted by them.
Neural stem cells (NSCs) from the embryonic medial ganglionic eminence (MGE) appear ideal as donor cells for grafting in epilepsy, as they can be expanded in culture and have the ability to give rise to GABA-ergic interneurons. Moreover, because MGE is the region in the embryonic brain that generates most of the hippocampal GABA-ergic neurons [23–24], MGE-NSC derived GABA-ergic neurons might incorporate better into the epileptogenic hippocampal circuitry. Additionally, as NSC-derived cells express multiple neurotrophic factors including GDNF [25], NSC grafting could lead to both additions of new cells that synthesize GDNF and restorative effects on cell types in the epileptic host hippocampus. Therefore, we examined whether grafting of NSCs that are capable of adding new GABA-ergic interneurons and GDNF-expressing cells into the epileptic hippocampus would restrain SRMS in chronic TLE. We bilaterally grafted NSCs expanded in vitro from the embryonic MGE into hippocampi of rats exhibiting chronic TLE with learning and memory impairments and measured: (i) changes in the frequency and intensity of SRMS; (ii) learning and memory function; (iii) survival and differentiation of graft-derived cells; (iv) addition of GABA-ergic neurons and GDNF+ cells from grafts; and (v) effects of grafting on the number of GDNF+ astrocytes and neurogenesis in the hippocampus. This investigation represents the first study which quantified the efficacy of intrahippocampal NSC grafting for restraining spontaneous seizures in a model of chronic TLE.
Material and Methods
Animals and induction of status epilepticus
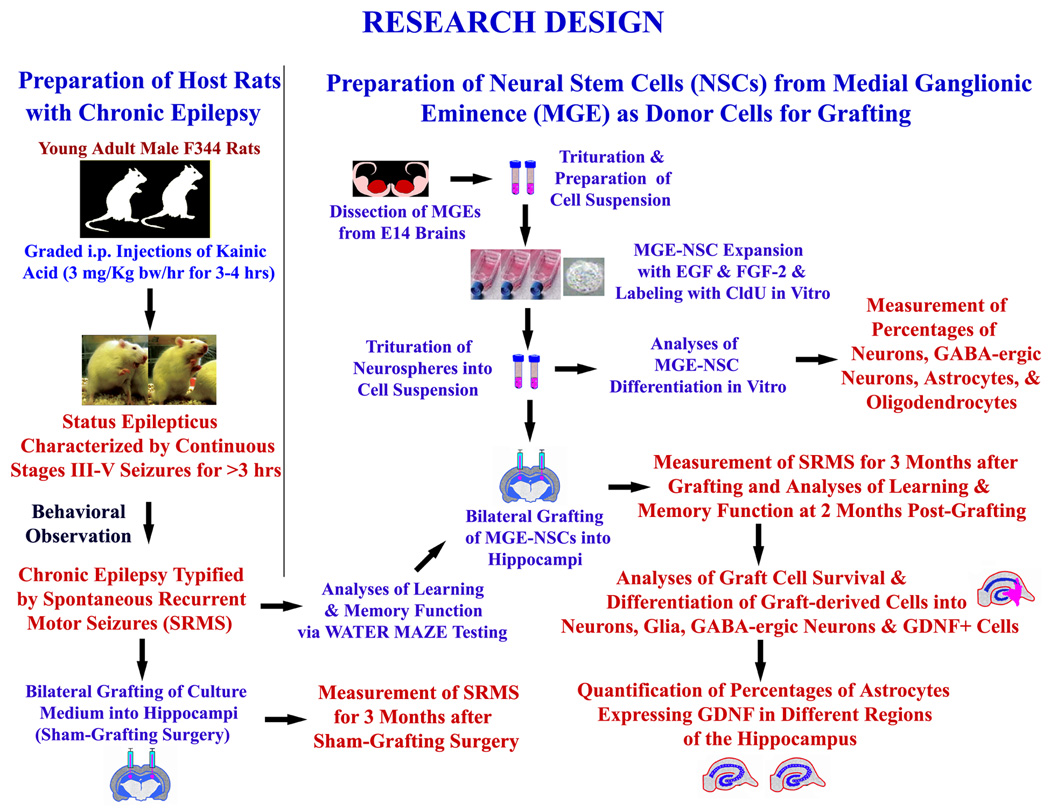
A flowchart summary of the entire experimental design is shown in Figure 1. Young adult (5-months old) Fischer 344 rats were treated with graded intraperitoneal injections of kainic acid (KA; 3.0 mg/kg/h) to induce status epilepticus (SE), as detailed in our previous reports [26–27]. The motor seizures during SE were scored according to the modified Racine scale [28] and the procedure is described in the supplemental data section.
Figure 1.
Experimental design illustrating the creation of host rats with chronic epilepsy for grafting and sham-grafting surgery (on the left side), and the preparation of fresh neural stem cells (NSCs) expanded in vitro from embryonic medial ganglionic eminence (MGE) as donor cells (upper half on right). The various measurements performed after MGE-NSC grafting include frequency, duration and severity of spontaneous seizures, learning and memory function, graft cell survival and differentiation, and effects of grafts on expression of GDNF in hippocampal astrocytes (lower half on right).
Selection of chronically epileptic rats for grafting studies
While all rats that undergo SE develop chronic epilepsy characterized by SRMS, the extent of SRMS varies considerably between animals [26,27]. Therefore, a group of age-matched chronically epileptic animals exhibiting a similar extent of SRMS (n=11) were chosen from a larger pool of animals that were chronically epileptic for prolonged periods (see supplemental section for details). This was accomplished via measuring the average frequencies of all SRMS and stage-V seizures, the average duration of individual SRMS, and the total time spent in seizures over four weeks through direct observation. The observation and scoring was done in two 4-hr sessions per week for 4 consecutive weeks prior to their selection for the grafting study.
Analyses of learning and memory function in chronically epileptic rats prior to grafting
All chronically epileptic animals chosen for grafting (n=11) were next subjected to water maze testing for ascertaining the spatial learning and memory function prior to grafting. For comparison, age-matched naïve control rats (n=6) were also included for water maze testing. The rats were trained to find the platform submerged in the water using spatial cues in eleven learning sessions (4 trials/session) followed by a probe test at 24 hrs after the last training session [29]. Rats were released from different locations in the four quadrants to ensure that hippocampal dependent learning is measured [30]. A full description of the water maze testing employed is available in the supplemental data section.
Harvesting, expansion, and labeling of MGE-NSCs in vitro
Embryonic day 14 (E14) fetuses were removed from deeply anesthetized timed pregnant F344 rats by cesarean section and collected in a culture medium [27]. The methods used for the expansion of NSCs as neurospheres and the labeling of neurosphere cells with chlorodeoxyuridine (CldU; an analog of 5’-bromodoxyuridine) are described in the supplemental data section. The neurospheres were collected, mechanically dissociated and the cell suspension was sieved through a filter with a pore size of 40 µm. The cells were washed 3–4 times in a fresh culture medium through centrifugation, viability assessed through a trypan blue exclusion test, and the concentration of viable cells was adjusted to a density of 80,000 cells/µl in a differentiation medium comprising Neurobasal, B-27 with retinoic acid and L-glutamine. Only cell suspensions that exhibited ≥75% viability were used for grafting. The CldU labeling index in each cell suspension was determined by CldU immunostaining after an overnight incubation of dissociated cells in poly-L-lysine-coated 35 mm culture dishes containing the differentiation medium described above. Only cell suspensions that exhibited ≥91% CldU labeling index were used in grafting studies. As cells were grafted shortly after adding the differentiation medium, NSCs were not given time to differentiate before transplantation.
Analyses of the differentiation potential of MGE-NSCs in vitro
The MGE-NSCs, prepared using methods that are described above for grafting, were plated onto the poly-L-lysine-coated 35 mm culture dishes containing the differentiation medium (as above) and incubated for 4–8 days at 37°C with 5% CO2 [31]. The cultures were fixed with 2% paraformaldehyde and processed for different single/dual immunofluorescence assays for identifying: (i) neurons expressing beta-III tubulin (TuJ-1); (ii) astrocytes expressing glial fibrillary acidic protein (GFAP); (iii) immature oligodendrocytes expressing the protein O1; and (iv) inhibitory interneurons expressing GABA, using methods described in our earlier reports [31–32].
Grafting of MGE-NSCs into hippocampi of chronically epileptic rats
Two months after the water maze testing, chronically epileptic rats (n=9) were grafted with MGE-NSCs. Out of the 11 chronically epileptic rats tested for water maze, two rats were excluded due to a self-mutilation injury, as per the animal protocol guidelines. Eight grafts were placed into hippocampi of each rat (i.e. 4 grafts/hippocampus) using methods detailed in our earlier reports [27,32–33] and in the supplemental data section.
Measurement of post-grafting SRMS and analyses
Changes in the extent of SRMS were measured for 3 months after grafting. This comprised measurement of the average frequencies of all SRMS and stage V seizures, the average duration of individual SRMS, and the total time spent in seizures over 3 months through direct observation. The observation and scoring was done in two 4-hr sessions per week for 3 months (i.e. 96 hrs of observation over 12 weeks). Out of the 9 rats that received grafting, one rat died within 24 hrs after the grafting due to post-operative complications and two rats died of unknown cause at 1–2 months after grafting. Cumulative pre-grafting seizure scores were then compared with post-grafting seizure scores for every month using repeated measures ANOVA for the 6 animals that survived the entire experimental period.
Analyses of learning and memory function after grafting
Two months after grafting, all surviving grafted animals (n=6) underwent a second water maze test. The methods and analyses are identical to the first water maze test and are described in the supplemental data section.
Analyses of the effects of sham-grafting surgery
To determine whether grafting surgery itself has any effects on SRMS, we analyzed changes in the frequency and duration of SRMS in an additional group of chronically epileptic rats exhibiting a similar extent of SRMS (n=6) with sham-grafting surgery involving injections of the culture medium. Pre sham-grafting seizure scores were then compared with post sham-grafting seizure scores for every month using repeated measures ANOVA.
Analyses of the yield and differentiation of graft-derived cells
All surviving grafted rats (n=6) were perfused with 4% paraformaldehyde, brain tissues were collected and 30 µm thick sections were cut coronally through the hippocampus using a cryostat and collected serially [26,27]. Every 10th section was processed for CldU immunostaining using anti CldU from Serotech and the BrdU immunostaining protocol described in our previous report [34]. Transplants in five hippocampi (from 5 different grafted animals) were analyzed for the yield of graft-derived cells. Cells positive for CldU were counted through the entire anterior-posterior extent of the hippocampus using the optical fractionator counting method in StereoInvestigator system (Microbrightfield Inc.). The stereological counting procedure used is detailed in our earlier reports [25,27,34]. Since four transplants (each comprising 80,000 live cells) were placed in each hippocampus, analyses of the yield of graft-derived cells from five hippocampi are equivalent to characterization of the yield from 20 transplants. The overall yield of graft-derived cells in each hippocampus was expressed as the percentage of injected cells (i.e. 320,000 live cells).
Differentiation of graft-derived cells into NeuN+ mature neurons, S-100β+ mature astrocytes, NG2+ oligodendrocyte progenitors, GABA+ inhibitory interneurons, and cells expressing GDNF were determined from five distinct transplants from five animals. For this, we performed dual immunofluorescence assays for CldU & NeuN, CldU & S-100β, CldU & NG2, CldU & GABA, CldU & GDNF and confocal microscopic analyses of individual dual labeled cells using 1-µm thick optical Z-stacks, as described in our previous report [25]. The primary antibodies used were anti CldU from Serotech, anti NeuN, anti NG2, anti S-100β and anti GDNF from Chemicon, and anti GABA from Sigma. The secondary antibodies used were anti rat IgG or anti mouse IgG conjugated to Alexa Fluor 488 or 594, biotinylated anti rat or anti mouse IgG, streptavidin Texas Red and streptavidin fluorescein from Molecular Probes. Separate sets of 50–100 CldU+ cells from each of the five transplants were examined for each of the five neural cell markers using Z-section analyses in a confocal microscope (LSM-510, Zeiss) for obtaining percentages of NeuN+ neurons, S-100β+ astrocytes, NG2+ oligodendrocyte progenitors, GABA+ inhibitory interneurons, and cells expressing GDNF among graft-derived cells.
Analyses of the effects of MGE-NSC grafts on GDNF+ astrocytes in the epileptic hippocampus
As chronic epilepsy is associated with decreased numbers of hippocampal astrocytes expressing GDNF and GDNF has anticonvulsant properties, we examined whether MGE-NSC grafting improves the extent of hippocampal astrocytes expressing GDNF. We quantified and compared the percentages of astrocytes expressing GDNF in three different groups of rats: age-matched naïve controls (n=4), rats with chronic epilepsy alone (n=4) and chronically epileptic rats that received MGE-NSC grafts (n=4). For this, we performed dual immunofluorescence assay for S-100β and GDNF (using primary antibodies from Chemicon) and analyzed the fractions of S-100β+ astrocytes expressing GDNF in the dentate gyrus, and the CA1 & CA3 subfields of the hippocampus using Z-section analyses in a confocal microscope (LSM-510, Zeiss). In every animal belonging to the three groups, an average of 204 S-100β+ cells in the hippocampus were examined for GDNF expression using Z-section analyses in a confocal microscope (i.e. ~816 S-100β+ cells per each of the three groups).
Analyses of the effects of MGE-NSC grafts on hippocampal neurogenesis
As chronic epilepsy is associated with substantially declined hippocampal neurogenesis [5–6], we examined whether MGE-NSC grafting improves the status of hippocampal neurogenesis using immunostaining of serial sections (every 15th) through the grafted hippocampus for doublecortin (a marker of newly born immature neurons [35]). The protocols used for DCX immunostaining has been described in our previous reports [5,25,35]. We quantified the total number of DCX+ neurons in the dentate subgranular zone and the granule cell layer of chronically epileptic rats that received MGE-NSC grafts (n=5) using stereological methods and compared these numbers with age-matched naïve control rats (n=5) and rats with chronic epilepsy alone (n=5).
Results
MGE-NSCs are multipotent and have ability to give rise to GABA-ergic neurons in culture
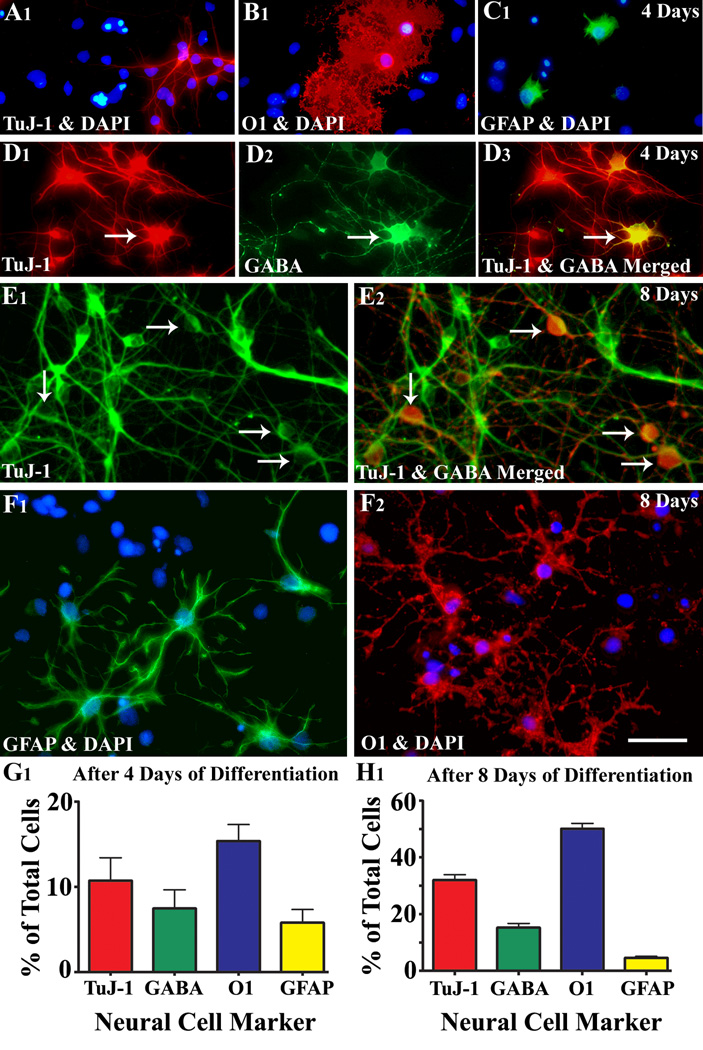
Differentiation of MGE-NSCs in culture revealed the ability of these cells to give rise to all three CNS phenotypes: neurons, oligodendrocytes and astrocytes (Fig. 2 [A1–C1, E1–F2]). Dual labeling analyses with TuJ-1 and GABA antibodies revealed that many neurons derived from MGE-NSCs express GABA (Fig. 2 [D1–E2]). After 4 days of incubation in the differentiation medium, 11% of MGE-NSCs differentiated into TuJ-1+ neurons, 15% into O1+ oligodendrocytes and 5.8% into GFAP+ astrocytes (Fig. 2 [G1]). Furthermore, 70% of TuJ-1+ neurons expressed GABA (Fig. 2 [G1]). After 8 days of incubation, most cells had differentiated (Tuj-1, 32%; GABA, 15%; O1, 50%; and GFAP, 5%; Fig.2 [H1]).
Figure 2.
Differentiation of medial ganglionic eminence-neural stem cells (MGE-NSCs) after their dissociation from CldU-labeled neurospheres and incubation in the differentiation medium for 4 days (A1–D3) or eight days (E1–F2). Differentiation of fractions of MGE-NSCs into TuJ-1+ neurons (A1, E1), O1+ oligodendrocytes (B1, F2), and GFAP+ astrocytes (C1, F1) could be seen at both time-points. Furthermore, fractions of MGE-NSCs also differentiate into GABA-ergic neurons (arrows in D1–D3 and E1–E2). Scale bar: 50 µm. The bar charts (G1, H1) illustrate percentages of MGE-NSCs that exhibit differentiation into TuJ-1+ neurons, GABA+ neurons, O1+ oligodendrocytes and GFAP+ astrocytes after incubation in the differentiation medium for 4 days (G1) or 8 days (H1). Note that the expression of GABA among TuJ-1+ neuronal population is ~70% after 4 days and ~50% after 8 days of incubation in the differentiation medium.
MGE-NSC grafting into hippocampi of chronically epileptic rats eases SRMS
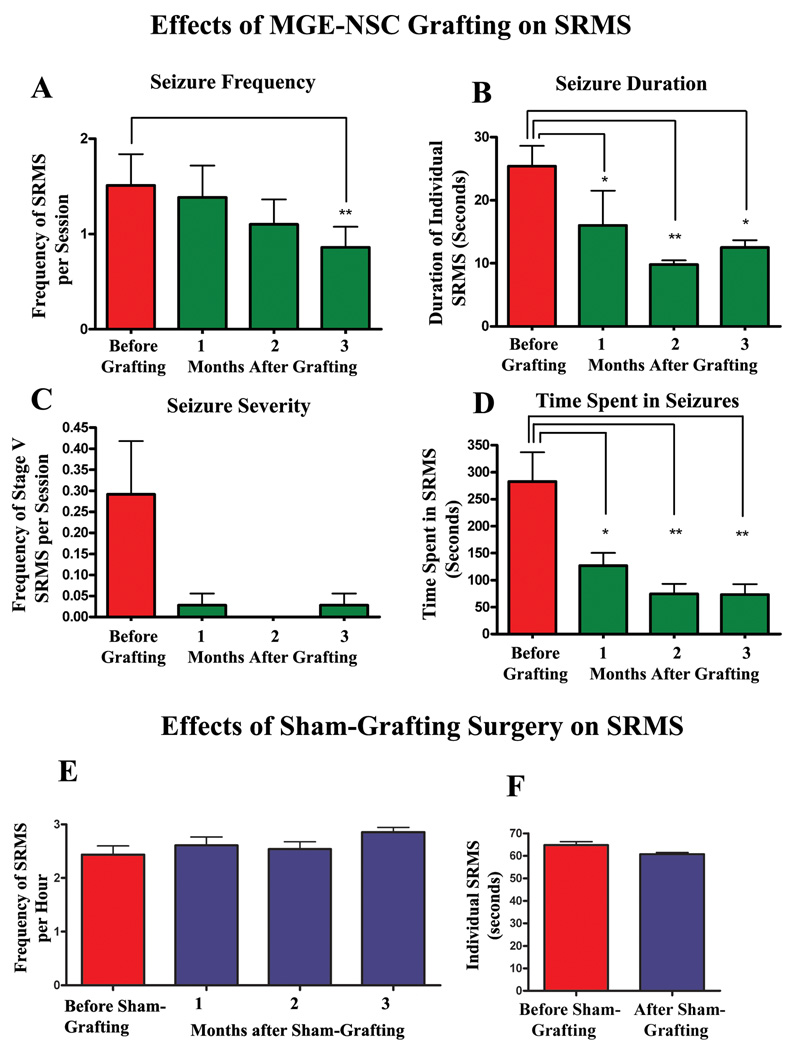
Transplantation of MGE-NSCs considerably reduced SRMS frequency, duration and severity over the observed time-frame of 3 months. In comparison to the pre-transplantation score, SRMS frequency steadily declined each month and was significantly reduced during the third month (43% reduction, p<0.01; Fig. 3[A]). The duration of individual SRMS underwent a decline in the first month and exhibited 51% decline in the 3rd month after grafting (p<0.05; Fig. 3 [B]). The overall severity of SRMS, as measured by the frequency of stage-V seizures, exhibited 90% decline in the third month after grafting (Fig. 3 [C]). Furthermore, no stage-V seizures were recorded during the second month after transplantation (Fig. 3 [C]). The total time spent in seizures per month (for the observation period of 32 hrs) displayed 74% decrease during the 3rd month after grafting (p<0.01; Fig. 3 [D]). Thus, MGE-NSC grafting resulted in considerable reductions in all parameters of SRMS.
Figure 3.
Long-term effects of MGE-NSC grafting (A–D) on spontaneous recurrent motor seizures (SRMS) in chronically epileptic rats. The Y-axis in bar charts A and C denotes the average numbers of seizures per session (4-hr block) of observation. Note that MGE-NSC grafting considerably decreases the seizure frequency (A), the duration of individual seizures (B), the severity of seizures (C) and the total time spent in seizures (D). * = p < 0.05; ** = p <0.01. The bar charts in E and F show seizure frequency and seizure duration in chronically epileptic animals that received sham-grafting surgery. Sham-grafting surgery does not alter the seizure frequency or the seizure duration in chronically epileptic rats.
Sham-grafting surgery in chronically epileptic rats has no effect on SRMS
Comparison of pre- and post-sham grafting seizure scores in this group revealed that the transplantation surgery itself has no effect on major parameters of chronic epilepsy, as animals that underwent sham-grafting surgery did not show decreases in either seizure frequency or duration of individual SRMS (Fig. 3 [E–F]).
MGE-NSC grafting does not improve learning and memory function in chronically epileptic rats
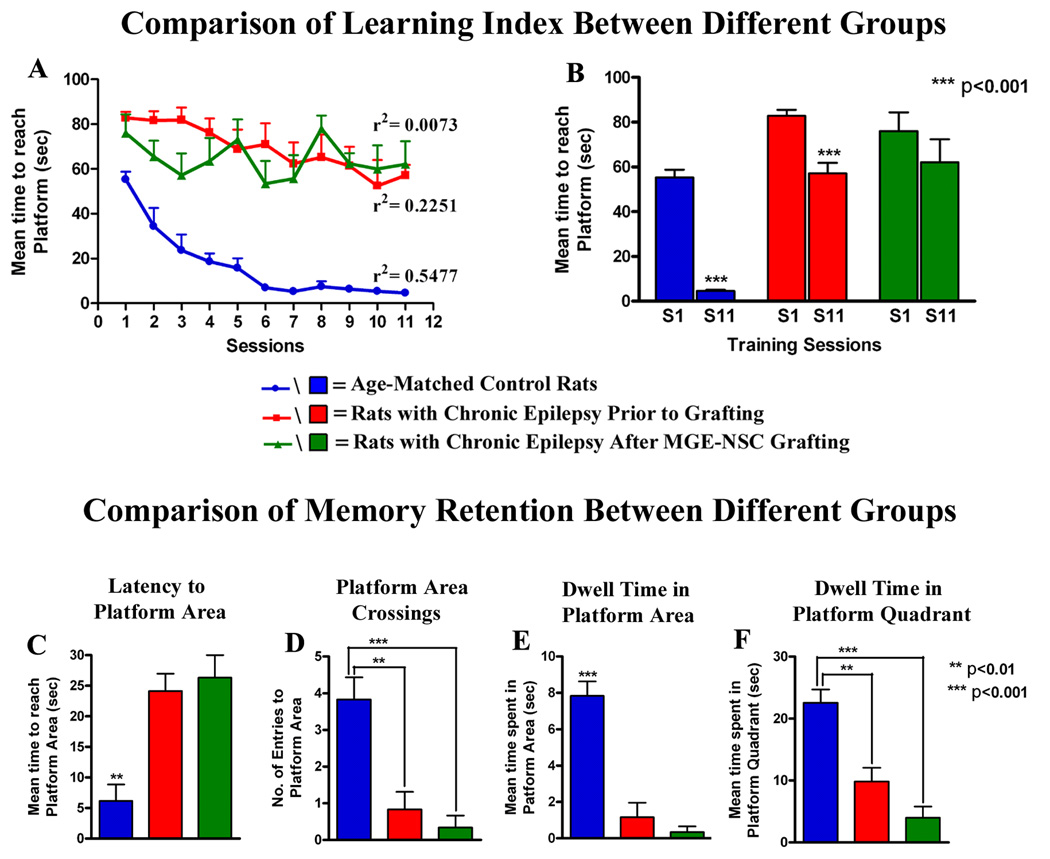
Analyses of chronically epileptic rats prior to grafting with water maze testing revealed the existence of impairments in the hippocampal-dependent learning and memory function, in comparison to age-matched control rats (Fig. 4 [A, B]). While chronically epileptic rats exhibited some ability for learning over eleven sessions as indicated by linear regression analysis and significant decrease in latency to reach the hidden platform between the first and eleventh sessions, their overall performance was much inferior to control animals (Fig. 4 [A, B]). Furthermore, the probe test conducted at 24 hrs after the last training session revealed impairments in memory encoding and retrieval ability. This was evidenced by much greater latencies to reach the platform area, and considerable reductions in platform area crossings, dwell times in the platform area and the platform quadrant, in comparison to control animals (Fig. 4 [C–F]). In the second water maze test performed at 2-months post-grafting, rats exhibited erratic learning, as evidenced by no linear regression in latencies to reach the hidden platform and no significant decrease in latency to reach the hidden platform between the first and eleventh sessions (Fig. 4 [A, B]). Overall, their post-transplantation learning scores were inferior to their pre-transplantation learning scores. The probe test revealed inability for memory retention in these rats, as also observed prior to grafting. The overall probe test scores were statistically similar to their pre-transplantation scores (Fig. 4 [C–E]). Thus, MGE-NSC grafting did not improve the hippocampal-dependent learning and memory function in chronically epileptic rats.
Figure 4.
Comparison of data pertaining to learning and memory function using water maze test between age-matched controls and chronically epileptic rats both prior to and after MGE-NSC grafting. Age-matched controls (blue lines/bars) exhibit excellent spatial learning ability with progressive decreases in the latency to reach the platform over 11 sessions (A) and a dramatic reduction in the latency to reach the platform between the first and last sessions of learning (B). Prior to grafting, chronically epileptic rats though impaired in comparison to control rats show some ability for learning (red lines/bars in A, B), which however worsens after grafting (green lines/bars in A, B). Figures C–F compare results of probe (memory retention) test performed at 24 hrs after the last learning session in the three groups. Note that control rats score well for all parameters of memory retention (latency to reach the platform area, platform area crossings, dwell time in platform area, and dwell time in the platform quadrant). In contrast, chronically epileptic rats exhibit inability for memory retention both prior to and after MGE-NSC grafting, suggesting that MGE-NSC grafting does not alleviate learning and memory impairments prevailing in chronic epilepsy.
Transplanted MGE-NSCs survive grafting into the chronically epileptic hippocampus
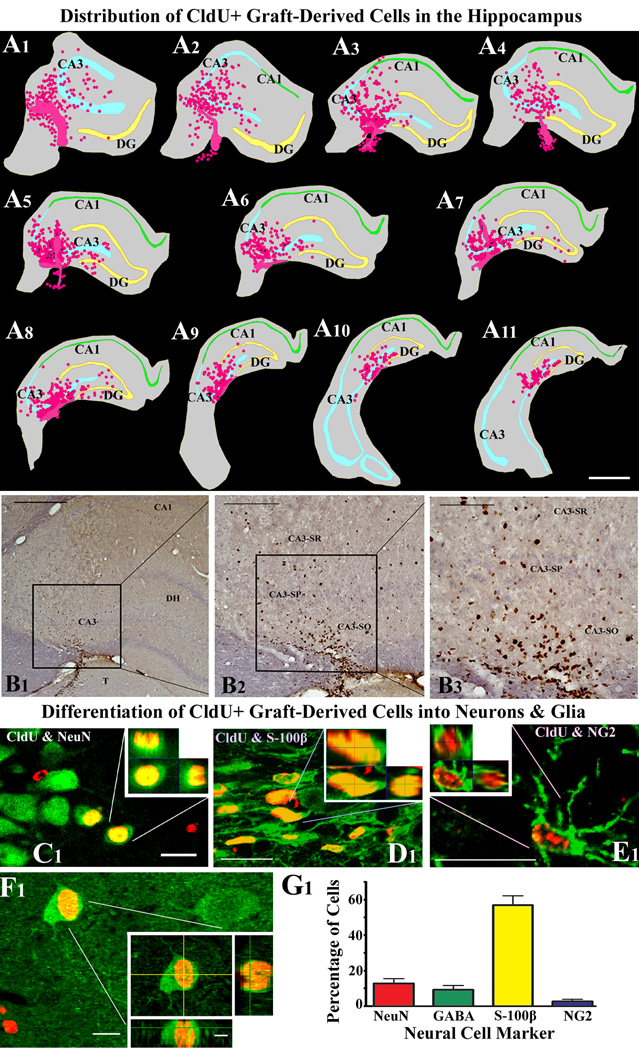
Analyses of brain tissues with CldU immunostaining revealed the presence of graft-derived cells in hippocampi after 3 months of grafting. While most graft cores were located in or attached to the CA3 region, a minority of transplant cores partially extended ventrally into the adjoining thalamus (Fig. 5 [A1–A11). However, graft-derived cells migrated mostly into different strata (strata oriens, pyramidale and radiatum) of the CA3 region and the lateral ends of the CA1 and DG regions (Fig. 5 [A1–A11, B1–B3]). Some migrated graft-derived cells could be seen in the medial regions of the dentate gyrus too (Fig. 5 [A7–A8]). Graft-derived cells were only occasionally observed in the dentate subgranular zone and granule cell layer (SGZ-GCL). Quantification of the total numbers of graft-derived cells (i.e. CldU+ cells) demonstrated a yield that is equivalent to 28.0 ± 9.2% of injected cells. Since 320,000 live cells with a CldU-labeling index of 91% were initially transplanted per hippocampus, 28% yield means that ~81,536 new cells were added to each hippocampus with MGE-NSC grafting performed in this study.
Figure 5.
Distribution and differentiation of cells derived from MGE-NSC grafts in the chronically epileptic hippocampus after 3-months of grafting. The figures A1–A11 illustrate the location of transplants and transplant-derived cells (shown in pink color based on Chlorodeoxyuridine+ [CldU+]) immunoreactivity) with respect to hippocampal cell layers and subfields in a chronically epileptic rat. These tracings, performed using the Neurolucida software (Microbrightfield Inc), represent every tenth 30-µm thick section through a chronically epileptic hippocampus that received four MGE-NSC grafts. Note that grafts and graft-derived cells were mostly located in the CA3 subfield and lateral ends of the CA1 subfield and the dentate gyrus. The core of transplants partially projected ventrally into the thalamus only at certain levels. The figures B1–B3 illustrate the distribution of CldU+ graft-derived cells in the host hippocampus. B2 is a magnified view of a region from B1 and B3 is an enlarged view of a region from B2. Both B2 and B3 show engraftment of graft-derived cells into strata oriens (SO), pyramidale (SP) and radiatum (SR) of the CA3 subfield. Scale bar, A1–A11, 1000 µm; B1, 500µm; B2, 200 µm; B3, 100 µm. Figures C1–F1 illustrates differentiation of MGE-NSC graft-derived CldU+ cells into NeuN+ mature neurons (C1), S-100β+ mature astrocytes (D1), NG2+ oligodendrocyte precursors (E1) and GABA+ neurons (F1), visualized through dual immunolabeling for CldU (red) and markers of neurons/glia (green) and Z-section analyses in a confocal microscope. Scale bar, C1–E1, 20 µm; F1, 10 µm; orthogonal inset of F1, 5 µm. The bar chart in G1 depicts the percentages of graft-derived cells that differentiate into NeuN+ neurons, GABA+ neurons, S-100β+ astrocytes and NG2+ oligodendrocyte precursors.
Grafted MGE-NSCs give rise to neurons, astrocytes, oligodendrocyte progenitors and GABA+ neurons
Grafted MGE-NSCs differentiated into NeuN+ neurons, GABA+ interneurons, astrocytes and oligodendrocyte progenitors (Fig. 5 [C1–F1]). Among graft-derived cells, 13% differentiated into NeuN+ mature neurons, 57% into S-100β+ mature astrocytes and 3% into NG2+ oligodendrocyte progenitor cells (Fig. 5 [G1]). Moreover, 10% of graft-derived cells differentiated into GABA-ergic neurons (Fig. 5 [G1]), indicating that a vast majority of neurons derived from grafts were GABA+. Extrapolation of the yield of graft-derived cells with percentages of different cell types suggests that MGE-NSC grafting in this study resulted in addition of ~10,600 new neurons, ~46,476 new astrocytes, ~2,446 new oligodendrocyte progenitors and ~8,154 new GABA-ergic neurons into each hippocampus of chronically epileptic rats.
Transplanted MGE-NSCs give rise to substantial numbers of new GDNF+ cells
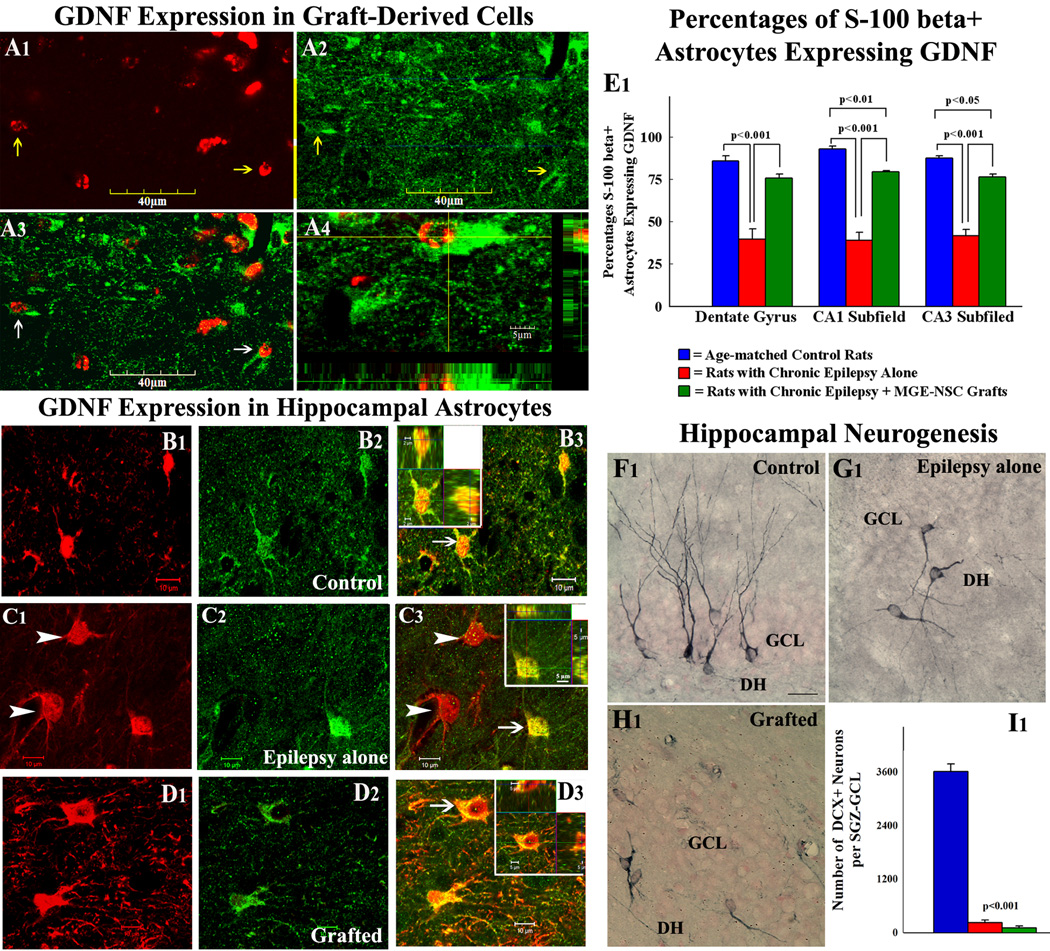
A fraction of cells derived from MGE-NSC grafts expressed GDNF, which morphologically appeared to be astrocytes (Fig. 6 [A1–A4]). Immunopositivity for GDNF was also found in graft-derived cells that migrated into different layers of the hippocampus. Quantification using CldU and GDNF dual immunofluorescence and confocal microscopic analyses revealed that ~50% (Mean ± S.E.M. = 50.2 ± 5.6) of graft-derived cells differentiated into cells expressing GDNF. Extrapolation of the yield of graft-derived cells with percentages of graft-derived cells expressing GDNF suggested that MGE-NSC grafting resulted in addition of ~40,768 new GDNF+ cells into each hippocampus of epileptic rats.
Figure 6.
Expression of glial-derived neurotrophic factor (GDNF) in cells derived from MGE-NSC grafts (A1–A4), and MGE-NSC grafting mediated changes in GDNF expression of host hippocampal astrocytes (B1–E1) and hippocampal neurogenesis (F1–I1). Arrows in figures A1–A3 show examples of graft-derived CldU+ cells (red) that express GDNF (green). A4 shows orthogonal views of a GDNF+ graft derived cell (with CldU+ red nucleus and GDNF+ green cytoplasm). Scale bar, A1–A3 = 40µm; A4 = 5µm. Figures B1–D3 show confocal microscopic images of S-100β+ hippocampal astrocytes (red) that exhibit GDNF expression (green) in an age-matched control rat (B1–B3), a rat with chronic epilepsy alone (C1–C3) and a chronically epileptic rat that received MGE-NSC grafts (D1–D3). The insets in B3, C3, and D3 show orthogonal views of cells indicated by arrows. Note that these cells are positive for both S-100β and GDNF. Arrowheads in C1 and C3 denote S-100β+ astrocytes that lack GDNF expression in the epilepsy alone group. Scale bar, B1–D3 = 10 µm. The bar chart in E1 shows percentages of S-100β+ astrocytes expressing GDNF in the dentate gyrus, and CA1 & CA3 subfields of the hippocampus in different groups. Note that GDNF expression in astrocytes declines substantially with chronic epilepsy in all regions of the hippocampus but recovers dramatically after MGE-NSC grafting. Figures F1–H1 illustrates examples of newly born (doublecortin+) neurons in the dentate gyrus in the three groups. Newly born neurons in the rat with chronic epilepsy alone (G1) and the chronically epileptic rat that received MGE-NSC grafts (H1) exhibit abnormal polarity and basal dendrites, in comparison to normal orientation of dendrites in newly born neurons of the age-matched control rat (F1). DH, dentate hilus; GCL, granule cell layer. Scale bar, F1–H1 = 25µm. The bar chart in I1 compares numbers of newly born neurons between the three groups. Note that rats with chronic epilepsy alone (red) and chronically epileptic rats that received MGE-NSC grafts (green) exhibit greatly declined hippocampal neurogenesis compared to age-matched control rats (blue).
MGE-NSC grafting enhances the fraction of GDNF+ astrocytes in the chronically epileptic hippocampus
We investigated whether seizure-suppressing effects of MGE-NSC grafting are linked to increased GDNF expression in the host hippocampus, as ~50% of graft-derived cells expressed GDNF and previous studies have suggested that GDNF has anticonvulsant properties [22,36]. Dual immunofluorescence for GDNF and S-100β in age-matched control animals revealed that virtually all GDNF+ cells in the hippocampus are S-100β+, suggesting that GDNF is mostly found in the mature astrocytes (Fig. 6 [B1–B3). Quantification of cells that express GDNF among S-100β+ astrocytes revealed GDNF expression in ≥86% of astrocytes in the dentate gyrus, and CA1 and CA3 subfields (Fig. 6 [E1]). Similar analyses in rats with chronic epilepsy alone revealed substantial loss of GDNF expression in the S-100β+ astrocyte population (Fig. 6 [C1–C3], which was evidenced by GDNF expression in ≤42% of astrocytes in the dentate gyrus, and CA1 and CA3 subfields (p<0.001 in comparison to control animals; Fig. 6 [E1]). In contrast, rats with chronic epilepsy that received MGE-NSC grafts exhibited GDNF expression in ≥76% of S-100β+ astrocytes in the dentate gyrus, and CA1 and CA3 subfields (p<0.001 in comparison to rats with chronic epilepsy alone; Fig. 6 [E1]). Thus, MGE-NSC grafting into the chronically epileptic hippocampus restores GDNF expression in S-100β+ astrocytes in different regions of the hippocampus. When compared to GDNF expression in the S-100β+ astrocyte population of age-matched control animals, this restoration appeared complete for the dentate gyrus (p>0.05) but partial for the CA1 and CA3 subfields (p<0.05 to p<0.01; Fig. 6 [E1]).
MGE-NSC grafting has no effect on neurogenesis in the chronically epileptic hippocampus
We investigated the effects of MGE-NSC grafting on the status of hippocampal neurogenesis because of the likelihood that decreased hippocampal neurogenesis observed in chronic epilepsy partly underlies learning and memory impairments [5,6,37]. In comparison to an apparently normal distribution of newly born (DCX+) neurons in the age-matched control hippocampus (Fig. 6 [F1]), both epileptic groups (i.e. rats with chronic epilepsy alone and rats with chronic epilepsy plus MGE-NSC grafts) rarely showed DCX+ neurons along the antero-posterior extent of the hippocampus (Fig. 6 [G1, H1]). Furthermore, DCX+ neurons in both epileptic groups exhibited abnormal polarity with basal dendrites extending into the dentate hilus (Fig. 6 [G1, H1]), in comparison to the characteristic growth of apical dendrites into the molecular layer observed in control rats (Fig. 6 [F1]). Quantification of numbers of DCX+ neurons in the dentate SGZ-GCL of different groups revealed no effects of MGE-NSC grafting on hippocampal neurogenesis in chronically epileptic animals. In comparison to age-matched control animals, the overall decrease in hippocampal neurogenesis was 93% in rats with chronic epilepsy alone and 97% in chronically epileptic rats that received MGE-NSC grafts (p<0.001; Fig. 6 [I1]). Thus, MGE-NSC grafting did not improve hippocampal neurogenesis in chronically epileptic rats.
Discussion
This study provides the first evidence that MGE-NSC grafting into the hippocampus is an effective approach for suppressing SRMS in chronic TLE. Animals that were chronically epileptic for prolonged periods exhibited considerable reductions in seizure frequency, duration and severity, and the amount of time spent in seizures at 1–3 months after grafting of MGE-NSCs. Furthermore, MGE-NSC grafting caused addition of ~8,154 GABA-ergic neurons and ~40,768 GDNF+ cells into each hippocampus of epileptic animals. Moreover, MGE-NSC grafting restored GDNF expression in a vast majority of hippocampal astrocytes. Considering the inhibitory function of GABA-ergic neurons in synaptic transmission and antiepileptic properties of GDNF, it is likely that addition of new GABA-ergic neurons and GDNF+ cells, and restoration of GDNF in host hippocampal astrocytes underlie seizure suppression mediated by MGE-NSC grafting. Additionally, despite the restraint on SRMS, MGE-NSC grafting did not improve cognitive function in epileptic animals, which might be attributable at least partially to the failure of grafts to normalize the waned neurogenesis in the chronically epileptic hippocampus for prolonged periods.
Significance of the animal prototype and the choice of donor NSCs
Studies on NSC grafts in epilepsy models conducted so far employed grafting as a pre-treatment strategy for preventing chronic epilepsy development after acute seizures [38–45]. While such studies are needed for unraveling anti-epileptogenic effects of NSC grafts, they are not germane to treating people afflicted with chronic TLE. As patients with drug-resistant epilepsy are the most likely candidates for NSC grafting therapy among the epilepsy patient population as an alternative to hippocampal resection, we chose rats that were epileptic for prolonged periods. As seizures in TLE mainly originate from the hippocampus and are associated with cognitive impairments and reduced hippocampal neurogenesis [3–7], we tested the effects of bilateral grafting of NSCs into hippocampi of rats that were not only chronically epileptic but also had cognitive impairments. The choice of MGE-NSCs as donor cells in this study was based on several properties of MGE cells. As MGE is the source of all hippocampal GABA-ergic interneurons in the developing brain, we presumed that MGE-NSCs exhibit a high propensity for generating hippocampal-specific GABA-ergic interneurons after grafting. Furthermore, GABA-ergic interneurons derived from primary MGE cells have the ability for functional integration and increasing the extent of inhibition when grafted into the normal postnatal brain [23].
Efficacy of MGE-NSC grafts for treating spontaneous seizures in chronic TLE
This study provides several novel results regarding the efficacy of MGE-NSC grafts for treating chronic TLE. First, cells derived from MGE-NSC grafts exhibited ability for long-term survival and engraftment in the chronically epileptic hippocampus, as evidenced by 28% yield of injected cells after 3-months of grafting. The overall yield of graft-derived cells is better than the ~10–20% yield of fetal MGE cells observed following grafting into the brain of normal/Kv1.1 mutant mice [16,23] and ~1% yield reported for SVZ-NSC grafts in a mouse model of epilepsy [46]. Second, most (~73%) graft-derived cells could be accounted by their differentiation into mature NeuN+ neurons, GABA+ neurons, S-100β+ mature astrocytes or NG2+ oligodendrocyte precursors. Third, MGE-NSC grafts did not form tumors unlike the inclination of NSCs derived from embryonic stem cells for tumor formation [44]. More importantly, MGE-NSC grafting reduced the overall SRMS frequency by 43%, severity by 90%, and duration by 74%. These anti-seizure effects appeared to be mediated by MGE-NSC grafts because sham-grafting surgery did not alter the frequency or intensity of SRMS in chronically epileptic rats.
Potential mechanisms underlying the seizure-suppression mediated by MGE-NSC grafts
Our results suggest two potential mechanisms: addition of new GABA-ergic neurons, and addition of new GDNF+ cells with restoration of GDNF expression in hippocampal astrocytes. With regard to GABA-ergic neurons, MGE-NSC grafting resulted in addition of ~8,154 GABA-ergic neurons into each hippocampus. This addition is substantial, considering the extent of GABA-ergic interneuron loss in chronic TLE [11,12,47,48]. In light of the previous findings that grafting of cells that simply secrete GABA induces transient anti-seizure effects [15,18,20,49,50], one can surmise that the anti-seizure effect is a consequence of increase in GABA concentration mediated by GABA-ergic neurons derived from grafts resulting in an increased threshold for the occurrence of SRMS. Nevertheless, since the anti-seizure effect of grafts progressively improved over the post-grafting survival period and substantial numbers of graft-derived GABA-ergic neurons could be recovered after 3-months of grafting, inhibitory synaptic integration of graft-derived GABA-ergic neurons on hippocampal principal excitatory neurons cannot be ruled out. Indeed, previous studies on primary MGE cell grafts have demonstrated that axons of graft-derived GABA-ergic neurons integrate with the host brain circuitry and increase the level of inhibition [16,23]. Thus, seizure-suppression in this study is likely mediated at least partially by the incorporation of graft-derived GABA-ergic neurons into the inhibitory hippocampal circuitry. Electrophysiological and electron-microscopic studies are needed in future to confirm this possibility however.
Pertaining to GDNF+ cells, MGE-NSC grafting resulted in addition of ~40,768 new GDNF+ cells into each hippocampus of epileptic animals. Furthermore, MGE-NSC grafting induced GDNF expression in a vast majority (≥76%) of hippocampal astrocytes. Because this level of expression is closer to that of age-matched controls (GDNF in ≥86% of astrocytes) and substantially higher than that observed in animals with chronic epilepsy alone (GDNF in ≤42% of astrocytes), the restoration of GDNF levels after MGE-NSC grafting is apparent. Addition of new GDNF+ cells and restoration of GDNF expression in hippocampal astrocytes have functional implications because of the anti-epileptic effects of GDNF. These include seizure-suppressing effects with rAAV-GDNF when administered to the hippocampus either prior to or after kindling or during the self-sustained phase of SE, and suppression of generalized seizures with implantation of encapsulated cells that are genetically modified to produce GDNF into the hippocampus of kindled rats [22,36]. Thus, seizure suppression after MGE-NSC grafting is likely also linked to addition of new GDNF-synthesizing cells as well as restoration of GDNF expression in hippocampal astrocytes.
Cognitive function in chronically epileptic rats after MGE-NSC grafting
Transplantation of MGE-NSC grafts did not reverse the hippocampal-dependent spatial learning and memory deficits in chronically epileptic rats. Bearing in mind the importance of neurogenesis for the hippocampal-dependent learning and memory function [51–54], the lack of improvement might be attributable at least partially to the inability of grafts to induce hippocampal neurogenesis that almost shuts down in chronic epilepsy [5–6, 37]. Indeed, no effects of MGE-NSC grafting were observed on neurogenesis in this study when examined after 3-months of grafting. Lack of graft-mediated effects on neurogenesis likely reflects insufficient engrafting of graft-derived NSCs into the dentate SGZ [25]. This is because most graft-derived cells differentiated into neurons and glia and migration of graft-derived cells into the dentate SGZ-GCL was minimal. While our study might have missed the possible flourish of neurogenesis occurring early after grafting (due to analyses at only a long-term time-point after grafting), the results nevertheless point out that MGE-NSC grafting does not normalize neurogenesis for prolonged periods. Another reason for lack of improvement in cognition is that virtually all neurons derived from MGE-NSC grafts differentiated into GABA-ergic neurons rather than giving rise to CA1/CA3 pyramidal neurons to replenish the decline in CA1 and CA3 pyramidal cell layers. Thus, grafts did not appear to repair the disrupted hippocampal circuitry in terms of replacing the connectivity of lost CA1 and CA3 pyramidal neurons. Rather, grafts have likely reduced the excitatory activity of surviving principal neurons via increased inhibition mediated by the addition of new GABA-ergic neurons and GDNF+ cells, and restoration of GDNF in hippocampal astrocytes. Restoration of cognitive pathways might require grafting of NSCs that are capable of engrafting substantially into the dentate SGZ and/or cells that have the ability to differentiate into hippocampal pyramidal neurons and repair the disrupted circuitry [12,33,55].
Conclusion and future directions
Our results demonstrate that MGE-NSC grafting into the hippocampus has great promise for treating chronic TLE, particularly for seizure control. However, several issues need to be resolved prior to its clinical application. These include finding human NSCs that are comparable to rat MGE-NSCs, testing the ability of human NSC or embryonic stem cell-derived GABA-ergic neurons [56] for long-term survival, synaptic integration and seizure suppression in chronically epileptic prototypes, and development of strategies that both increase the yield of NSC graft-derived GABA-ergic neurons and promote the engraftment of graft-derived NSCs into the dentate SGZ to restore hippocampal neurogenesis, and analyses of transplants at multiple time points to account for depreciation of graft-derived cells with time.
Supplementary Material
Acknowledgement
Supported by grants from the National Institute of Neurological Disorders and Stroke (RO1 NS054780 to A.K.S) and Department of Veterans Affairs (VA Merit Award to A.K.S.). Ben Waldau was partially supported by the 2007 Congress of Neurological Surgeons (CNS) Translational/Basic Science Resident Research fellowship. We thank M.S. Rao and M. Acharya for their contribution to raising some of the chronically epileptic rats used in this study and B. Shuai for outstanding technical assistance.
Footnotes
Author contributions:
BW: Design, collection, assembly, analyses and interpretation of data, manuscript writing
BH: Design, collection, assembly, analyses and interpretation of data, final approval of manuscript
RK: Collection, assembly and analyses of data, final approval of manuscript
AKS: Conception, design, collection, assembly, analyses and interpretation of data, manuscript writing, and financial support
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Strine TW, Kobau R, Chapman DP, et al. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46:1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher PD, Sperber EF, Moshe SL. Hippocampal sclerosis revisited. Brain Dev. 1998;20:563–573. doi: 10.1016/s0387-7604(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 3.Astur RS, Taylor LB, Mamelak AN, et al. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 4.Detour J, Schroeder H, Desor D, et al. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46:499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]
- 5.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirttila TJ, Manninen A, Jutila L, et al. Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann Neurol. 2005;58:211–223. doi: 10.1002/ana.20545. [DOI] [PubMed] [Google Scholar]
- 8.Engel J. Epilepsy in the world today: medical point of view. Epilepsia. 2002;43 Suppl 6:12–13. doi: 10.1046/j.1528-1157.43.s.6.6.x. [DOI] [PubMed] [Google Scholar]
- 9.Helmstaedter C. Temporal lobe resection--does the prospect of seizure freedom outweigh the cognitive risks? Nat Clin Pract Neurol. 2008;4:66–67. doi: 10.1038/ncpneuro0657. [DOI] [PubMed] [Google Scholar]
- 10.Shamim S, Wiggs E, Heiss J, et al. Temporal lobectomy: resection volume, neuropsychological effects, and seizure outcome. Epilepsy Behav. 2009;16:311–314. doi: 10.1016/j.yebeh.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lanerolle NC, Kim JH, Robbins RJ, et al. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 12.Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci. 2000;20:8788–8801. doi: 10.1523/JNEUROSCI.20-23-08788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd KG, Bossi L, Morselli PL, et al. Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol. 1986;44:1033–1044. [PubMed] [Google Scholar]
- 14.Cornish SM, Wheal HV. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28:563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- 15.Loscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy--promising avenues or blind alleys? Trends Neurosci. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Baraban SC, Southwell DG, Estrada RC, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008;212:468–481. doi: 10.1016/j.expneurol.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson K. Transplantation of GABA-producing cells for seizure control in models of temporal lobe epilepsy. Neurotherapeutics. 2009;6:284–294. doi: 10.1016/j.nurt.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner DA, Shetty AK. Clinical prospects for neural grafting therapy for hippocampal lesions and epilepsy. Neurosurgery. 2003;52:632–644. doi: 10.1227/01.neu.0000047825.91205.e6. [DOI] [PubMed] [Google Scholar]
- 20.Gernert M, Thompson KW, Loscher W, et al. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol. 2002;176:183–192. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- 21.Löscher W, Ebert U, Lehmann H, et al. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Kanter-Schlifke I, Georgievska B, Kirik D, et al. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther. 2007;15:1106–1113. doi: 10.1038/sj.mt.6300148. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Dolado M, Calcagnotto ME, Karkar KM, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pleasure SJ, Anderson S, Hevner R, et al. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 25.Hattiangady B, Shuai B, Cai J, et al. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 26.Rao MS, Hattiangady B, Reddy DS, et al. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- 27.Rao MS, Hattiangady B, Rai KS, et al. Strategies for promoting anti-seizure effects of hippocampal fetal cells grafted into the hippocampus of rats exhibiting chronic temporal lobe epilepsy. Neurobiol Dis. 2007;27:117–132. doi: 10.1016/j.nbd.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty AK. Progenitor cells from the CA3 region of the embryonic day 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004;14:595–614. doi: 10.1002/hipo.10206. [DOI] [PubMed] [Google Scholar]
- 32.Shetty AK, Rao MS, Hattiangady B. Behavior of hippocampal stem/progenitor cells following grafting into the injured aged hippocampus. J Neurosci Res. 2008;86:3062–3074. doi: 10.1002/jnr.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattiangady B, Rao MS, Zaman V, et al. Incorporation of embryonic CA3 cell grafts into the adult hippocampus at 4-months after injury: effects of combined neurotrophic supplementation and caspase inhibition. Neuroscience. 2006;139:1369–1383. doi: 10.1016/j.neuroscience.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 36.Kanter-Schlifke I, Fjord-Larsen L, Kusk P, et al. GDNF released from encapsulated cells suppresses seizure activity in the epileptic hippocampus. Exp Neurol. 2009;216:413–419. doi: 10.1016/j.expneurol.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49 Suppl 5:26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuruba R, Hattiangady B, Shuai B, et al. Effects of grafting of hippocampal stem/progenitor cells shorly after status epilepticus on the development of chronic epilepsy. Cell Transplant. 2009;18:221–221. [Google Scholar]
- 39.Richardson RM, Barbaro NM, Alvarez-Buylla A, et al. Developing cell transplantation for temporal lobe epilepsy. Neurosurg Focus. 2008;24:E17. doi: 10.3171/FOC/2008/24/3-4/E16. [DOI] [PubMed] [Google Scholar]
- 40.Maisano X, Carpentino J, Becker S, et al. Embryonic stem cell-derived neural precursor grafts for treatment of temporal lobe epilepsy. Neurotherapeutics. 2009;6:263–277. doi: 10.1016/j.nurt.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty AK, Hattiangady B. Concise review: prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu K, Kim M, Jung KH, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023:213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Steinbeck JA, Lusardi T, et al. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 44.Carpentino JE, Hartman NW, Grabel LB, et al. Region-specific differentiation of embryonic stem cell-derived neural progenitor transplants into the adult mouse hippocampus following seizures. J Neurosci Res. 2008;86:512–524. doi: 10.1002/jnr.21514. [DOI] [PubMed] [Google Scholar]
- 45.Jing M, Shingo T, Yasuhara T, et al. The combined therapy of intrahippocampal transplantation of adult neural stem cells and intraventricular erythropoietin-infusion ameliorates spontaneous recurrent seizures by suppression of abnormal mossy fiber sprouting. Brain Res. 2009 doi: 10.1016/j.brainres.2009.07.079. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Raedt R, Van Dycke A, Waeytens A, et al. Unconditioned adult-derived neurosphere cells mainly differentiate towards astrocytes upon transplantation in sclerotic rat hippocampus. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.08.009. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47.Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- 48.Shetty AK, Hattiangady B, Rao MS. Vulnerability of Hippocampal GABA-ergic Interneurons to Kainate Induced Excitotoxic Injury During Old Age. J Cell Mol Med. 2009;13:2408–2423. doi: 10.1111/j.1582-4934.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson K, Anantharam V, Behrstock S, et al. Conditionally immortalized cell lines, engineered to produce and release GABA, modulate the development of behavioral seizures. Exp Neurol. 2000;161:481–489. doi: 10.1006/exnr.1999.7305. [DOI] [PubMed] [Google Scholar]
- 50.Castillo CG, Mendoza-Trejo S, Aguilar MB, et al. Intranigral transplants of a GABAergic cell line produce long-term alleviation of established motor seizures. Behav Brain Res. 2008;193:17–27. doi: 10.1016/j.bbr.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupret D, Revest JM, Koehl M, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 53.Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jessberger S, Clark RE, Broadbent NJ, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shetty AK, Zaman V, Turner DA. Pattern of long-distance projections from fetal hippocampal field CA3 and CA1 cell grafts in lesioned CA3 of adult hippocampus follows intrinsic character of respective donor cells. Neuroscience. 2000;99:243–255. doi: 10.1016/s0306-4522(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 56.Maroof AM, Brown K, Shi SH, et al. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J Neurosci. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.