Abstract
Properdin, a positive regulator of the alternative pathway (AP) of complement is important in innate immune defenses against invasive Neisserial infections. Recently, commercially-available unfractionated properdin was shown to bind to certain biological surfaces, including Neisseria gonorrhoeae, which facilitated C3 deposition. Unfractionated properdin contains aggregates or high-order oligomers, in addition to its physiological ‘native’ (dimeric, trimeric and tetrameric) forms. We examined the role of properdin in AP activation on diverse strains of N. meningitidis and N. gonorrhoeae specifically using native versus unfractionated properdin. C3 deposition on Neisseria decreased markedly when properdin function was blocked using an anti-properdin mAb or when properdin was depleted from serum. Maximal AP-mediated C3 deposition on Neisseriae even at high (80%) serum concentrations required properdin. Consistent with prior observations, pre-incubation of bacteria with unfractionated properdin followed by the addition of properdin-depleted serum resulted in higher C3 deposition than when bacteria were incubated with properdin-depleted serum alone. Unexpectedly, none of ten Neisserial strains tested bound native properdin. Consistent with its inability to bind to Neisseriae, preincubating bacteria with native properdin followed by the addition of properdin-depleted serum did not cause detectable increases in C3 deposition. However, reconstituting properdin-depleted serum with native properdin a priori enhanced C3 deposition on all strains of Neisseria tested. In conclusion, the physiological forms of properdin do not bind directly to either N. meningitidis or N. gonorrhoeae but play a crucial role in augmenting AP-dependent C3 deposition on the bacteria through the ‘conventional’ mechanism of stabilizing AP C3 convertases.
Keywords: Neisseria meningitidis, Neisseria gonorrhoeae, complement, properdin, C3, alternative pathway
INTRODUCTION
Properdin is the only known positive regulator of the complement system. Each properdin monomer is a 53 kDa molecule. In plasma, properdin exists as cyclic dimers (P2), trimers (P3), and tetramers (P4) formed by head-to-tail association of monomers (1–3). The AP C3 convertase, C3b, Bb, has a half life of only 1.5 min. Properdin carries out the important function of binding to and stabilizing the C3b, Bb complex, thereby increasing its half-life 5–10 fold (4).
When properdin was first discovered more than 50 years ago, it was thought to be an initiator of the AP (5). This original theory was later changed in favor of the more widely accepted role of properdin, that of stabilizing the AP C3 convertase. Recent studies have showed that properdin can bind directly to AP activator surfaces such as zymosan and rabbit erythrocytes (6) and serve to initiate the AP by forming a platform for assembly of AP C3 convertases (6, 7). Of note, Spitzer et al reported that properdin bound to a strain of N. gonorrhoeae and a ‘rough’ LPS mutant of E. coli K12, and that bacteria-bound properdin was capable of enhancing C3 deposition on these bacteria following the addition of properdin-deficient serum (6). These data have important implications because properdin deficiency in humans is associated with an increased incidence of invasive infections with N. meningitidis (8–20). The finding that properdin binds to N. gonorrhoeae and activates complement has been extrapolated to N. meningitidis (21, 22). This newly proposed (or perhaps more appropriately, rediscovered) mechanism provided an attractive theory to explain why properdin-deficient individuals are exclusively predisposed to meningococcal disease (21, 22).
We questioned the ability of Neisseriae to bind to properdin under physiological conditions for the following reasons. First, both N. meningitidis and N. gonorrhoeae have evolved several intricate mechanisms to evade killing by human complement (23–34); binding of properdin by bacteria would place these pathogens at a distinct disadvantage for survival in their human host. Second, the study of properdin binding to N. gonorrhoeae used commercially available properdin that has undergone freeze-thawing and contains high-order oligomers, or aggregates of properdin (2, 35). These aggregates are also called ‘activated’ properdin or Pn (35). Unlike the physiological forms of properdin, ‘activated’ properdin can promote complement activation and consumption when added to serum (35). In addition, aggregates are also likely to bind with higher avidity, or perhaps non-specifically, to surfaces that native forms of properdin may not.
In this study, we have evaluated the role of native properdin in activating the AP on N. meningitidis and N. gonorrhoeae and have contrasted this with unfractionated properdin. These studies provide important mechanistic insights into the role of properdin in complement activation on the pathogenic Neisseriae.
MATERIALS AND METHODS
Bacterial strains
One representative strain from each of the five major meningococcal serogroups that cause disease worldwide (A, B, C, W-135 and Y) and their isogenic unencapsulated mutants were used in this study. With rare exceptions (36, 37), almost all meningococci isolated from the blood or cerebrospinal fluid are encapsulated (38). Isogenic unencapsulated mutants were also studied because strains carried in the nasopharynx often do not express capsules (39) and further, invasive is olates must down-regulate capsule production while traversing epithelial barriers (40, 41). To minimize regulation of the AP by sialylation of lipooligosaccharide (LOS) (reviewed in (32)), the LOS sialyltransferase gene (lst) of serogroups B, C, W-135 and Y strains was abrogated by insertional inactivation (lst::KanR) as described previously (42). Serogroup A N. meningitidis do not synthesize 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA), the donor molecule for LOS sialylation, and therefore can sialylate its LOS only when CMP-NANA is added to growth media. Five strains of N. gonorrhoeae that differed in their PorB type, sensitivity to the bactericidal action of normal human serum and the clinical syndrome they caused were used in this study. In addition, LOS mutations of one of the strains, F62, was used to examine the role of LOS truncation on interaction of this strain with properdin. Insertional inactivation of LOS glycosyl transferase lgtE and lgtF of F62 using methods described previously (43) yielded isogenic mutant strains that expressed truncated LOS molecules (lgtE mutant, Glc → HepI; lgtF mutant, HepI unsubstituted). Sialylation of F62 gonococcal lipooligosaccharide (LOS) was achieved by adding CMP-NANA to the growth media to a final concentration of 1 μg/ml. All Neisserial strains were routinely cultured on chocolate agar plates at 37°C in the presence of 5% CO2. Strains and their relevant characteristics are listed in Table I.
Table I.
Bacterial strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| N. meningitidis | ||
| A2594 | A:4:P1.9:ST-5; Germany, 1991; encapsulated; LOS not sialylated | (25) |
| A2594 mynB | Insertional inactivation of mynB (mynB:: CmR) of A2594; unencapsulated, LOS not sialylated | (25) |
| H44/76 lst | Insertional inactivation of lst of H44/76 (B:15:P1.7,16: ST-32; Norway, 1976); (lst::KmR); encapsulated, LOS not sialylated | This study |
| H44/76 siaD lst | Insertional inactivation of siaD of H44/76 lst (siaD::CmR); unencapsulated, LOS not sialylated | (25) |
| C2120 lst | Insertional inactivation of lst of strain C2120 (C:NT:P1.5,2:ST-11; Germany, 1997) (siaD::CmR); encapsulated, LOS not sialylated | (42) |
| C2120 siaD lst | Insertional inactivation of siaD of strain C2120 lst (siaD::CmR); unencapsulated, LOS not sialylated | (25) |
| W171 lst | Insertional inactivation of lst of W171 (W135:NT:P1.10:ST-11) (lst::KmR); encapsulated, LOS not sialylated | This study |
| W171 siaD lst | Insertional inactivation of siaD of W171 lst (siaD::CmR); unencapsulated, LOS not sialylated | (25) |
| Y2220 lst | Insertional inactivation of lst of Y2220 (Y:21:P1.15:ST-172) (lst::KmR); encapsulated, LOS not sialylated | This study |
| Y2220 siaD lst | Insertional inactivation of lst of Y2220 siaD (lst::KmR); unencapsulated, LOS not sialylated | (25) |
| N. gonorrhoeae | ||
| F62 | PorB.1B, serum sensitive | (53) |
| 24-1 | PorB.1B, serum sensitive | (54) |
| 398079 | PorB.1B, serum sensitive | (55) |
| WG | PorB.1B, serum resistant (DGIA isolate) | (56) |
| 15253 | PorB.1A, serum resistant (DGI isolate) | (48) |
| F62 lgtE | F62 lgtE::kan; Glc→HepI | This study |
| F62 lgtF | F62 lgtF::spc; HepI unsubstituted | This study |
Disseminated gonococcal infection
Sera, C3, antibodies, and zymosan
Properdin-depleted serum (P-depleted serum) was purchased from Quidel (Catalog no. A512). Serum depleted of C3 by immunoaffinity chromatography was purchased from Complement Technology, Inc (Cat. No. A314). C3 was purified from human plasma by PEG precipitation and DEAE Sephacel chromatography as described previously (44). Two anti-properdin mAbs were purchased from Quidel (Cat. Nos. A233 and A235). MAb A233 blocks properdin function while mAb A235 binds to properdin but does not block the function of properdin. C3 deposition on bacteria and zymosan was detected by anti-human C3c conjugated to FITC from Biodesign (now Meridian Life Science, Inc.). Sera obtained fresh from 10 normal adults (normal human serum; NHS) were pooled and stored at −80°C until used. All sera contained 10 mM EGTA and 10 mM Mg2+ to selectively activate the AP.
Purification and fractionation of properdin
Properdin was purified from normal human plasma as described (45) and stored at −80°C until further fractionated. Alternatively, purified human properdin was obtained either from Complement Technology, Inc. or from Quidel Corporation. Commercially available properdin or properdin that was purified in the laboratory and stored at −80°C is referred to in this study as unfractionated properdin. Pure, frozen properdin was thawed and the physiological (P2-P4) forms and the aggregated Pn forms were separated by cation exchange chromatography, followed by size exclusion chromatography (2). Briefly, thawed properdin was separated using a 1 mL Mono S cation exchange column and the recovered oligomers further separated by gel filtration on Phenomenex Bio Sep-Sec-S4000 column. The properdin sample, in PBS, was loaded onto the 600 × 7.8 mm molecular sieve column and eluted at a flow rate of 0.5 ml/min. Fractionated properdin was stored at 4°C and was used in experiments within 2 weeks to minimize reaggregation of the properdin that can occur with prolonged storage (2).
Properdin binding and C3 deposition by flow cytometry
Briefly, 108 bacteria were harvested from a 12–14 hour culture on a chocolate agar plate and suspended in Hanks’ balanced salt solution (HBSS) containing 0.15 mM CaCl2 and 1 mM MgCl2 (HBSS++). Zymosan was also suspended in HBSS++ at a concentration of 3 × 108 particles/ml. Bacteria or zymosan were washed once with HBSS++ and 5 × 107 bacteria or zymosan particles were incubated for 30 min at 37°C with either: properdin (10 μg/ml); NHS-Mg/EGTA;C3-depleted serum-Mg/EGTA; properdin-depleted serum-Mg/EGTA or C3-depleted serum-Mg/EGTA reconstituted with purified C3 (1 mg/ml), each used in a final reaction volume of 100 μl; the final concentration of sera in these experiments was 20%. Properdin that bound to bacteria or zymosan was detected using anti-human properdin mAb (Quidel Cat. No. A235) at a dilution of 1:100, followed by anti-mouse IgG conjugated to Alexafluor 647 (1:400). Data were collected either on a LSR II flow cytometer (Becton Dickinson [Franklin Lakes, NJ]) or a FACSCalibur instrument (Becton Dickinson) and analyzed using the FlowJo analysis software program (Version 7.2.4, TreeStar Inc. [Ashland, OR]).
The functional importance of properdin in mediating AP-dependent C3 deposition (includes both the C3b and iC3b fragments) on Neisseria was assessed by two methods: i) the function of properdin was blocked with an anti-properdin mAb and ii) C3 deposited on bacteria by properdin-depleted serum was compared with C3 deposited by properdin-depleted serum reconstituted with purified properdin. The concentration of properdin in reconstituted sera was 10 μg/ml. All reaction mixtures contained 10 mM Mg2+ and 10 mM EGTA to inhibit classical and lectin pathway activation (both dependent on Ca2+) and restrict complement activation to the Mg2+-dependent AP. In the first method, properdin function in serum was blocked by adding anti-properdin mAb (Quidel cat. No. A233) to Mg/EGTA-NHS to a final concentration of 50 μg/ml. Total C3 deposition on bacteria was detected following incubation of bacteria with Mg/EGTA-NHS (20% v/v) containing the anti-properdin mAb in a final volume of 100 μl. Bacteria incubated with Mg/EGTA-NHS (no anti-properdin mAb added) served as a control. Reaction mixtures were incubated for 30 min at 37°C and total C3 (C3b plus iC3b) binding to bacteria was detected by flow cytometry using anti-C3c FITC as described previously (26, 28). In the second method, C3 deposition on bacteria incubated with properdin-depleted serum was compared with C3 deposition using properdin-depleted serum that was reconstituted either with unfractionated properdin or with properdin fractions (described above), each to a concentration of 10 μg/ml.
To determine whether preincubation of bacteria with properdin would enhance C3 deposition, bacteria were incubated with purified unfractionated properdin or properdin fractions (10 μg/ml in a final volume of 100 μl) for 20 min at 37°C, washed once to remove unbound properdin, followed by the addition of P-depleted serum to a final concentration 20% (v/v)). As a comparator, we measured C3 deposition on bacteria that were incubated with properdin-depleted serum reconstituted with the corresponding properdin form to a final concentration of 10 μg/ml; this reconstituted serum was added to the bacteria to achieve a final serum concentration of 20% (v/v) in the reaction mixture. BaselineC3 deposition independent of properdin function was measured by incubating bacteria with properdin-depleted serum. Total C3 (C3b plus iC3b) deposited on bacteria was detected using anti-human C3c conjugated to FITC (1:100 dilution in HBSS++/1% BSA) as described previously (26, 28).
RESULTS
Binding of unfractionated purified properdin to Neisseriae
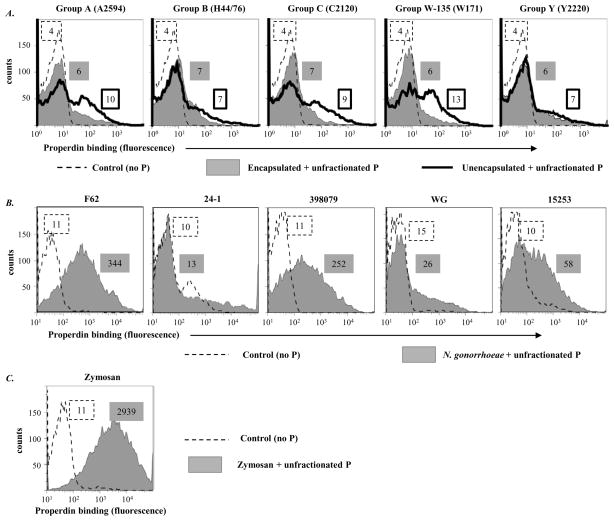
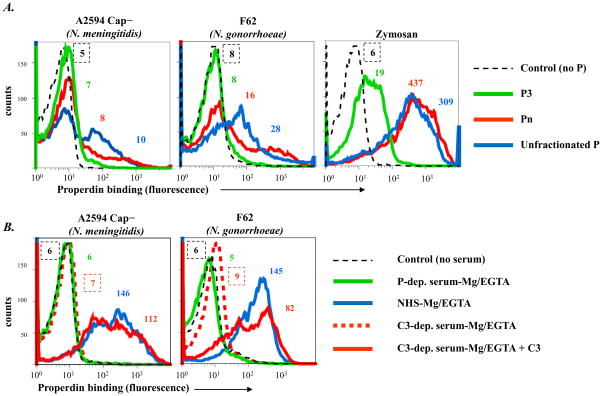
We quantified the binding of unfractionated purified properdin to five meningococcal strains and their isogenic unencapsulated mutants and also to five strains of N. gonorrhoeae. Zymosan has been shown previously to bind to purified properdin directly by flow cytometry (6) and was used as a positive control. Only minimal binding of unfractionated purified properdin to unencapsulated meningococcal subpopulations of serogroups A, C and W-135 strains was seen relative to controls; capsule expression further decreased properdin binding to these serogroups (Figure 1A). Gonococcal strains bound varying amounts of purified properdin; the highest levels of properdin binding were observed with N. gonorrhoeae F62, while minimal binding was seen with strains WG and 24-1 (Figure 1B). No correlation was apparent between the ability of a gonococcal strain to resist complement-dependent killing (Table 1) and the amount of properdin binding (Figure 1B). Furthermore, sialylation of the LOS of N. gonorrhoeae F62 by growth in media containing CMP-NANA did not affect properdin binding (data not shown). As expected, zymosan bound unfractionated properdin well (Figure 1C).
Figure 1.
Binding of properdin to Neisseriae following incubation with commercially available unfractionated purified human properdin (10 μg/ml). A. Five encapsulated strains of N. meningitidis (one representative strain from each of the five major serogroups – A, B, C, W-135 and Y) and their isogenic unencapsulated mutants. Bacteria-bound properdin was detected by flow cytometry using an anti-properdin mAb (Quidel; Cat. No. A235) followed by anti-mouse IgG conjugated to Alexafluor 647. In all graphs, the x-axis represents fluorescence on a log10 scale and the y-axis the number of events. Controls, where properdin was omitted from the reaction mixture, are shown by the broken line histograms. Numbers adjacent to the histograms represent median fluorescence intensities (MFIs) of P binding; numbers in the box with the dashed border represents fluorescence of the control bacteria, numbers in the grey shaded box binding to encapsulated bacteria and the box with a solid black outline binding to unencapsulated meningococci. B. properdin binding to five strains of N. gonorrhoeae and C. properdin binding to zymosan. One experiment of two reproducibly repeated experiments is shown for each of the histograms.
Properdin plays a critical role in AP-mediated C3 deposition on meningococci and gonococci
The functional importance of properdin in mediating AP-dependent C3 deposition on N. meningitidis and N. gonorrhoeae was assessed by two methods: i) use of an anti-properdin mAb to block the function of properdin present in serum and ii) use of properdin-depleted serum.
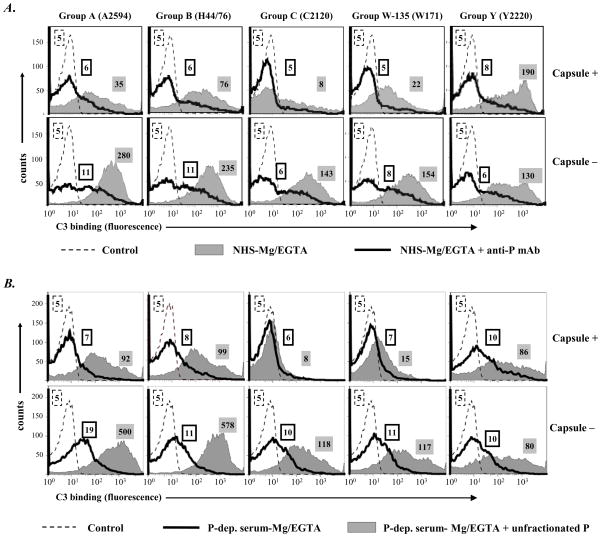
Meningococcal and gonococcal strains described in Figure 1 were used in the following experiments. First, the function of properdin in AP-mediated C3 deposition on Neisseriae was evaluated by using a mAb that blocks properdin function (50 μg mAb A233/ml serum). Shown in Figure 2A, C3 deposition on both encapsulated (upper panel, all except serogroup C) and unencapsulated (lower panel, all) meningococcal strains decreased markedly when properdin function was blocked with the anti-properdin mAb. These data point to a central role for properdin in enhancing AP activation on meningococci. This observation was confirmed by using properdin-depleted Mg/EGTA-treated human serum, shown in Figure 2B, where minimal deposition of C3 occurred as a result of marked diminution of AP activation in properdin-depleted serum; reconstitution of depleted serum with physiologic concentrations of unfractionated properdin increased C3 binding to all strains except encapsulated serogroup C and W-135 isolates. These studies were performed with unfractionated properdin to simulate prior observations made by Spitzer et al (6).
Figure 2.
Properdin augments AP-mediated C3 deposition on N. meningitidis. A. Five encapsulated strains of N. meningitidis (upper panel) and their isogenic unencapsulated mutants (lower panel) were incubated with NHS-Mg/EGTA (20% (v/v)) either in the absence (grey shaded histograms) or in the presence (histograms depicted by solid lines) of an anti-properdin mAb that blocks properdin function (Quidel; Cat. No. A233). Total C3 deposited on the bacterial surface was measured by flow cytometry using sheep polyclonal anti-human C3c conjugated to FITC. B. C3 deposition was assessed on the ten strains of N. meningitidis up on incubation with properdin (P-) depleted serum-Mg/EGTA (20% (v/v)) (histograms depicted by solid lines), or with P-depleted serum-Mg/EGTA reconstituted with purified unfractionated properdin to a concentration of 10 μg/ml (grey shaded histograms). Detection of C3 was performed as indicated above. Axes are as described in Figure 1. Controls (no serum added to bacteria) are shown by the broken lines. Controls with heat-inactivated serum show nearly identical tracings and have been omitted for simplicity. Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of C3 binding. One representative experiment of two separately performed and reproducibly repeated experiments is shown for each of the histograms.
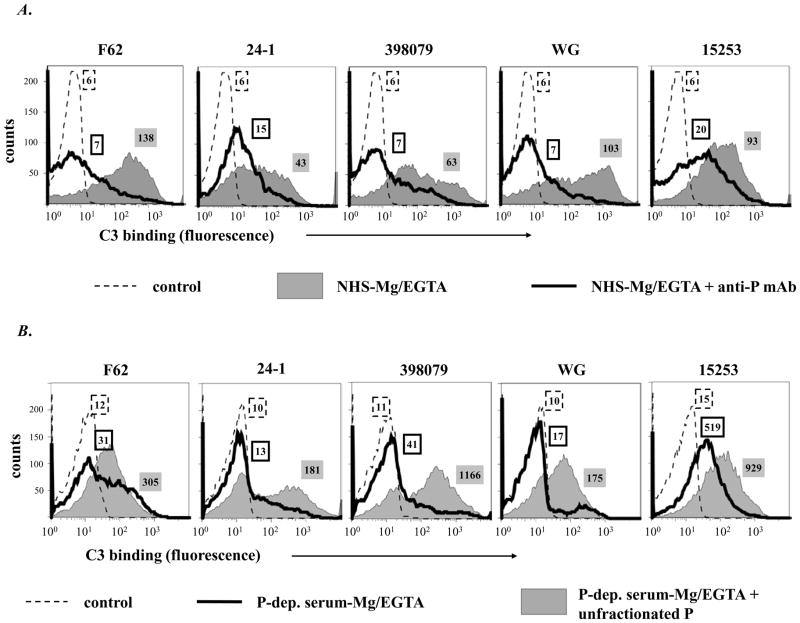
Similarly, the importance of properdin in promoting AP activation on N. gonorrhoeae was demonstrated. A 4- to 10-fold decrease in C3 binding (fluorescence) to all 5 strains occurred when properdin function was blocked with anti-properdin mAb (Figure 3A). Incubation of N. gonorrhoeae in properdin-depleted Mg/EGTA-NHS resulted in small amounts of C3 deposited; addition of properdin to Mg/EGTA-NHS augmented C3 deposition on all strains, minimally to strain15253 (Figure 3B). N. gonorrhoeae strain F62 was chosen for further studies because it bound the greatest amount of C3.
Figure 3.
Properdin augments AP-mediated C3 deposition on N. gonorrhoeae. A. C3 deposition on 5 strains of N. gonorrhoeae incubated with NHS-Mg/EGTA (20% (v/v) alone (grey shaded histograms) or with added anti-P mAb (Quidel mAb A233) that blocks properdin function (histograms depicted by solid lines). B. C3 deposition on gonococcal strains after incubation with P-depleted serum-Mg/EGTA (20% (v/v)) (histograms depicted by solid lines) or with P-depleted serum-Mg/EGTA reconstituted with purified unfractionated P (grey shaded histograms). Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of C3 binding. Detection of C3 fragments was performed as in Fig. 1A. One representative experiment of two separately performed and reproducibly repeated experiments is shown for each of the histograms..
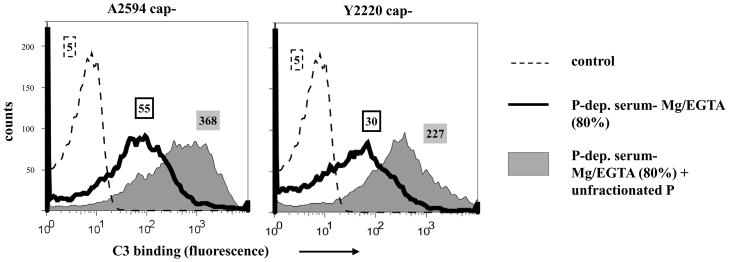
Because activity of the AP is concentration dependent (46), we tested whether higher serum concentrations would permit optimal AP activation in the absence of properdin. Shown in Figure 4, increasing the concentration of properdin-deficient Mg/EGTA-NHS to 80% resulted in no increase in the amount of C3 deposition at 30 min on two meningococcal strains tested, compared to similar experiments where20% serum was used (Figure 2B).
Figure 4.
Properdin (P) mediated deposition of C3 on Neisseriae at 80% serum concentration. Total C3 deposition on the unencapsulated derivatives of strains A2594 and Y2220 incubated either with P-depleted serum-Mg/EGTA (final concentration 80% (v/v); solid black histograms) or P-depleted serum-Mg/EGTA(final concentration 80% (v/v)) reconstituted with purified unfractionated properdin (10 μg/ml; shaded grey histograms). Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of C3 binding. Detection of C3 fragments was performed as in Fig. 1A. One representative experiment of two separately performed and reproducibly repeated experiments is shown for each of the histograms.
The presence of physiologic concentrations of properdin to serum greatly enhanced C3 binding (Figure 2B and 4). These results show that properdin is required to boost AP activation on meningococci even in the presence of high complement concentrations (as would be encountered by bacteria in the bloodstream).
Preincubation of Neisseriae with unfractionated properdin is less efficient in depositing C3 than serum reconstituted with properdin
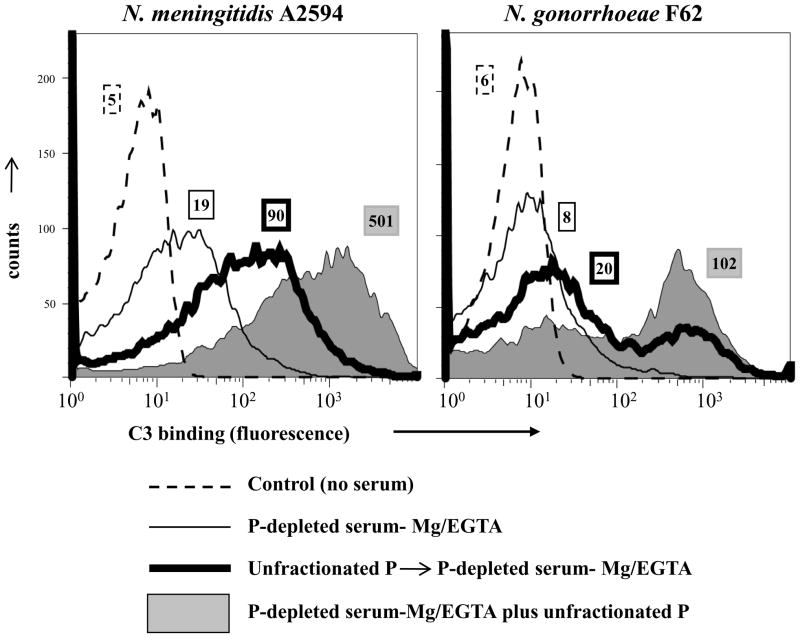
N. gonorrhoeae incubated with unfractionated properdin were reported to show enhanced C3 deposition following addition of properdin-deficient serum compared with diminished C3 deposition on bacteria that were incubated with properdin-deficient serum alone (6). While those data suggested a role for bacteria-bound properdin in fixing C3 on gonococci, the relative balance between properdin bound to bacteria directly versus properdin bound indirectly to bacteria via C3b, Bb, which is necessary to maximally deposit C3, was not determined. To address this, we compared C3 deposition on Neisseriae that were: i) first incubated with unfractionated purified properdin followed by the addition of properdin-depleted serum or ii) incubated with properdin-depleted serum that was reconstituted with unfractionated properdin a priori. The unencapsulated mutant of A2594 and gonococcal strain F62 were chosen because these strains bound the highest amounts of unfractionated properdin. Bacteria either were incubated with unfractionated purified properdin (10 μg/ml in a final volume of 100 μl), washed once to remove unbound properdin, followed by the addition of properdin depleted serum (final concentration 20% (v/v)) or were incubated in 20% properdin depleted serum with unfractionated properdin added to achieve a final serum properdin concentration of 10 μg/ml. The concentration of properdin in the reaction mixture where bacteria were preincubated with properdin (10 μg/ml) was 5-fold higher than the final concentration of properdin in the reaction mixture that contained 20% reconstituted serum ([properdin] 2 μg/ml). Shown in Figure 5, bacteria preincubated with unfractionated properdin followed by the addition of properdin-depleted serum bound less C3 than bacteria that were incubated with properdin-sufficient serum.
Figure 5.
Preincubation of Neisseriae with unfractionated properdin (P) enhances C3 deposition. Unencapsulated mutant of meningococcal strain A2594 and N. gonorrhoeae strain F62 were incubated either with P-depleted serum-Mg/EGTA (thin solid lines) or preincubated with unfractionated P (10 μg/ml). Organisms were washed then P-depleted serum-Mg/EGTA (labeled ‘Unfractionated P→P-depleted serum-Mg/EGTA’) was added (thick black lines) or organisms were incubated with P-depleted serum-Mg/EGTA reconstituted with unfractionated P (shaded grey histograms). Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of C3 binding. Detection of C3 fragments was performed as in Fig. 1A. One representative experiment of two separately performed and reproducibly repeated experiments is shown for each of the histograms.
Neisseriae do not bind to native properdin
Unfractionated properdin contains high order oligomers of properdin formed as a result of freeze-thawing (35). Properdin in serum contains only dimers, trimers and tetramers (native properdin) (2, 35). We hypothesized that an artificial increase in avidity caused by high order oligomerization and aggregation could result in binding of properdin to surfaces that otherwise do not bind the native forms of properdin. To explore this possibility on Neisseriae, properdin was fractionated by size exclusion chromatography and properdin dimers, trimers and tetramers (called P2, P3 and P4, respectively) were tested for direct binding (in the absence of C3 convertases) to Neisseriae by flow cytometry. Properdin that eluted in the void volume (higher order oligomers, or Pn) and commercially available unfractionated pure properdin were used as controls.
Compared to unfractionated properdin there was barely any detectable binding of native properdin to any meningococcal or gonococcal strains tested. Representative examples with N. meningitidis strain A2594 (unencapsulated mutant) and N. gonorrhoeae strain F62, the strains that bound the highest amounts of unfractionated properdin, are shown in Figure 6A (left and middle graphs). No binding was seen to P2, P3 or P4 fractions;(P3 data only are shown for simplicity). In contrast, P2, P3 and P4 all bound to a similar extent to zymosan (data with P3 shown in Figure 6A, right graph). High amounts of Pn and unfractionated properdin bound to zymosan.
Figure 6.
Binding of fractionated properdin (P) to Neisseriae. A. P was fractionated into dimers (P2), trimers (P3), tetramers (P4) and higher order oligomers (Pn; properdin in the void volume of a size-exclusion chromatograph). Binding of each of these fractions to N. meningitidis A2594 (unencapsulated) and N. gonorrhoeae F62 was measured by flow cytometry. Binding of P2, P3 and P4 yielded nearly identical results; results with the P3 fraction (only) are shown for simplicity (solid green line). Binding of Pn is shown by the red line; binding of commercially available unfractionated P by the blue line. B. Serum P binds to Neisseriae in the presence of the alternative pathway of complement. P binding to unencapsulated N. meningitidis A2594 and N. gonorrhoeae F62 following incubation with C3-depleted serum-Mg/EGTA (broken red histograms), P-depleted serum-Mg/EGTA(green histograms), C3-depleted serum-Mg/EGTA reconstituted with purified C3 (solid red histograms), or normal human serum-Mg/EGTA (blue histograms). All sera contained 10 mM Mg2+ and 10 mM EGTA and the final concentration of sera in all reaction mixtures was 20%. Axes are as described in Figure 1. Controls (no added serum) are shown by the black broken lines. Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of P binding. One representative experiment of two separately performed and reproducibly repeated experiments is shown for each of the histograms.
It was previously reported that binding of unfractionated properdin to E. coli K12 or Salmonella typhimurium strains increased with LPS truncation (loss of the O-antigen, which contain repeating saccharide structures (6, 47)). While unfractionated properdin did not bind to wild-type enterobacterial strains that expressed the O-antigen, mutants that expressed only the core oligosaccharide, or that lacked part or all of the core oligosaccharide, bound well to unfractionated properdin (6). Neisseria lack O-antigens; all Neisserial strains examined in this study, except gonococcal strain 15253, express at least 4–6 hexose residues extending outward from HepI of the LOS core; the HepI of 15253 is substituted with a lactose residue (48). To rule out the possibility that LOS hexose extensions present in, for example, wild-type strain F62 may have blocked binding of native properdin, we examined the binding of properdin fractions to the LOS truncated lgtE(Glc → HepI) and lgtF(Hep1 unsubstituted) mutants of F62. No binding of any of the native forms of properdin to these mutants was observed (data not shown). As expected, unfractionated properdin bound well to the mutants with truncated LOS (not shown).
To determine whether serum components other than C3 convertases could affect binding (either negatively or positively) of properdin to Neisseriae, we measured properdin binding to bacteria in the presence of C3-depleted serum. Properdin-depleted serum served as a negative control and C3-depleted serum reconstituted with C3 and NHS served as positive controls; all sera contained Mg/EGTA to permit selective activation of the AP. No properdin binding was measured on bacteria that were incubated with C3-depleted serum (Figure 6B, broken red histograms) and simulated controls with properdin-depleted serum (green histograms). Properdin binding was observed in sera that contained both properdin and active C3 (NHS and reconstituted C3-depleted serum, depicted by solid red and blue histograms, respectively).
Preincubation of Neisseria with native forms of properdin does not result in C3 deposition; native properdin augments C3 deposition by stabilizing C3 convertases
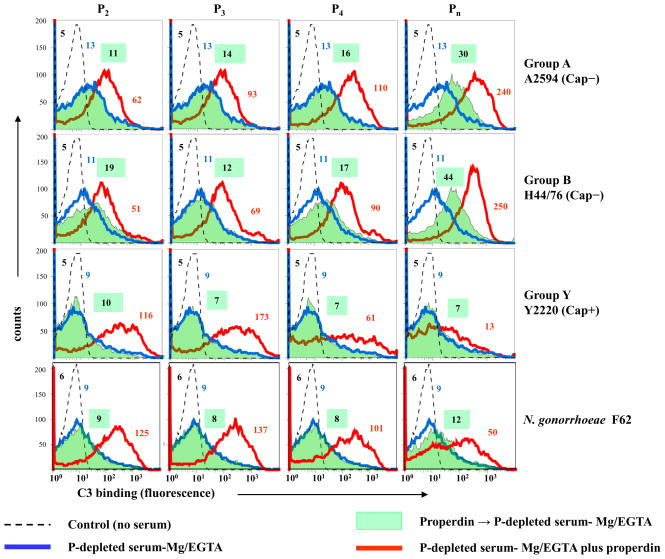
We assessed a downstream functional consequence of the interaction of native properdin with Neisseria, which distinct from unfractionated properdin, had not resulted in direct binding of properdin to bacteria. We used native oligomers of properdin (2, 35) to confirm its ‘conventional’ role in promoting C3 deposition on bacteria that result from secondary (or indirect) binding of properdin to bacteria via C3b, Bb to stabilize AP C3 convertases. Unencapsulated derivatives of strains A2594 and H44/76, encapsulated serogroup Y strain 2220, and gonococcal strain F62 were preincubated with either P2, P3, P4 or Pn followed by the addition of Mg-EGTA treated properdin-depleted serum. Controls included bacteria plus properdin-depleted serum that was reconstituted with each of the properdin fractions separately. Total C3 deposited on bacteria was measured by flow cytometry. Shown in Figure 7, preincubation of bacteria with P2, P3 or P4 did not enhance C3 binding to any of the strains (shaded green histograms) compared to properdin-deficient serum alone (blue histograms). Pre-incubation of meningococcal strains A2594 and H44/76 (both unencapsulated), but not Y2220 (encapsulated) or F62, with Pn resulted in enhanced C3 binding to bacteria (shaded green histograms, right column) compared to incubation with properdin-depleted serum alone (solid blue histograms). In contrast, reconstitution of properdin-depleted serum with each of the native fractions resulted in an increase in C3 deposition on all strains (solid red lines).
Figure 7.
Preincubation with native properdin (P) does not enhance C3 deposition on Neisseriae. Unencapsulated derivatives of serogroup A strain 2594 and serogroup B strain H44/76, encapsulated serogroup Y strain 2220 and gonococcal strain F62 were pre-incubated either with properdin dimers (P2), trimers (P3), tetramers (P4) or higher-order oligomers (Pn) each to a final concentration of 10 μg/ml and washed. P-depleted serum-Mg/EGTA was then added to a final concentration of 20%. C3 deposition on bacteria was measured by flow cytometry (shaded green histograms). Bacteria incubated only with P-depleted serum-Mg/EGTA (final concentration 20%) are shown by the blue histograms. C3 deposition was also measured on bacteria that were incubated with P--depleted serum-Mg/EGTA that had been reconstituted with each of the purified properdin (P2, P3, P4 or higher-order oligomer [Pn]) fractions (final concentration of each P fraction was 10 μg/ml; shown by the red histograms). Axes are as described in Figure 1. Controls (serum lacking from the reaction mixture) are indicated by the broken lines. Numbers adjacent to histograms represent the median fluorescence intensities (MFIs) of P binding. One representative of two separately performed and reproducibly repeated experiments is shown for each of the histograms.
Collectively, these data indicate that the primary mechanism of native properdin-mediated augmentation of C3 deposition on Neisseria involves stabilization of C3 convertases. C3 deposition seen when bacteria are preincubated with unfractionated properdin is likely mediated by the high-order oligomers in the preparations and may not reflect physiological conditions in vivo.
DISCUSSION
Antibody-dependent bactericidal activity is important for protection against meningococcal infection (49, 50). The AP plays an important role in amplifying C3 deposition on the bacterial surface. C3 activation represents the convergence of the classical, lectin and APs. The subsequent activation of the terminal complement components can lead to C5b-9 insertion into the membrane of gram-negative pathogens, resulting in complement-dependent killing. Deficiencies of the terminal complements (C5 through C9) and AP components such as factor D and properdin predispose individuals to invasive meningococcal infections (11, 20, 51). Properdin deficiency is rare, but individuals with properdin deficiency are predisposed to severe invasive meningococcal infections, often with a higher mortality than normal individuals (11, 20, 51). Both N. meningitidis and N. gonorrhoeae have evolved several intricate mechanisms to evade complement. The previously reported ability of N. gonorrhoeae to bind to properdin and activate complement (6) would provide a distinct disadvantage to the bacteria in vivo.
Two important observations have emerged from this study. First, properdin is critical for optimal AP-dependent C3 deposition on the pathogenic Neisseriae and second, native properdin does not bind directly to any of the strains of N. meningitidis or N. gonorrhoeae tested and does not initiate AP activation when preincubated with Neisseriae. Together, these results strongly suggest that properdin acts to enhance AP activation on Neisseriae through the “conventional” mechanism – i.e., by stabilizing AP C3 convertases. These studies emphasize the importance of using native properdin for functional assays. The only forms of properdin reported in serum are dimers, trimers and tetramers (P2, P3 and P4, respectively) that are present in the ratio of 26:54:20 (2, 35). Higher order oligomers (aggregates of properdin) that form when properdin is freeze-thawed (as seen in commercial preparations), or with prolonged storage of native properdin, can promote fluid phase complement activation and consumption when added to serum (2, 35). Furthermore, a recent study shows that higher order oligomers can bind non-specifically to live cell surfaces where they promote complement activation (45).
There was a wide variation among strains in their ability to bind to C3 (Figures 2 and 3), which could reflect differences in the ability of strains to activate the AP and/or availability of targets for C3 on the bacterial surface. An important observation was that expression of groups A, B, C and W-135, but not group Y, capsules all resulted in less AP activation (shaded graphs in the upper panels of Figures 2A and 2B) as evidenced by less C3 deposition compared to their isogenic unencapsulated mutants (grey shaded histograms in the lower panels of Figures 2A and 2B). The mechanism of AP suppression by select meningococcal capsular polysaccharides is currently the subject of a separate investigation.
The importance of properdin in promoting AP activation on Neisseriae was shown using a mAb against properdin that blocked its function and resulted in a marked decrease in C3 deposition on all meningococci and gonococci tested. These findings were confirmed by an independent method where C3 deposition on Neisseriae was enhanced when properdin-depleted serum was reconstituted either with native or unfractionated properdin.
It is noteworthy that preincubating N. gonorrhoeae stra in F62 with the unfractionated commercial properdin preparation followed by the addition of properdin-depleted serum (Figure 5, right graph, “Unfractionated P → P-depleted serum-Mg/EGTA”) resulted in increased levels of C3 deposition compared to preincubation of strain F62 with Pn (void volume eluate of a molecular sieve column), shown in Figure 7 (shaded green histogram, lower right graph). This may be explained by lower amounts of Pn binding to F62 relative to unfractionated properdin (Figure 6A). High order oligomers present in commercial properdin preparations may have also been retained by the molecular sieve column. Potentially, these retained aggregates in Pn may have influenced binding to strain F62 and consequent C3 deposition that simulated C3 binding brought on by unfractionated commercial properdin. This may have also influenced higher C3 binding by unencapsulated Group A and B N. meningitidis by the Pn preparation, which enhanced C3 deposition when added either before P-depleted serum-Mg/EGTA (Figure 7, shaded green histogram in the two upper graphs in the Pn column) or together with P-depleted serum-Mg/EGTA (Figure 7, red histograms in the same graphs).
It is clear that certain complement activator surfaces such as zymosan bind to purified native properdin (Fig. 6A). Other complement activator surfaces such as rabbit erythrocytes have also been reported to bind to commercially available unfractionated properdin (6), although a recent study shows that the native properdin forms do not (45). Studies that define ligands or functions of properdin using unfractionated properdin that may contain aggregates need to be interpreted with caution. In addition, other molecules such as serum amyloid P component have been reported to interfere with the ability of properdin to bind to surfaces (52) and may limit the ability of properdin to initiate complement activation in the context of serum.
In conclusion, our results emphasize the importance of using native forms of properdin to analyze the biological and functional roles of this molecule. The ‘conventional’ mechanism of properdin function, which is to bind to and stabilize AP C3 convertases, remains the principal mechanism of function on the surface of Neisseria. The lack of this essential mechanism may explain why properdin-deficient individuals are more susceptible to meningococcal infections.
Acknowledgments
We thank Dr. Ulrich Vogel (Universität Würzburg, Germany) for providing meningococcal strains and mutants used in this study. We thank Dr. Daniel Stein for providing the plasmid to make F62 lgtF, Dr. Asesh Banerjee for the plasmid to make F62 lgtE, and Mrs. Staci Snyder and Connie Elliot for their excellent technical assistance.
Abbreviations used in this paper
- AP
alternative pathway
- NHS
normal human serum
- LOS
lipooligosaccharide
- lgt
lipooligosaccharide glycosyl transferase
- Hep
heptose
- Glc
glucose
- CMP-NANA
5′-cytidinemonophospho-N-acetylneuraminic acid
- HBSS
Hank’s Balanced Salt Solution
Footnotes
This work was supported by National Institutes of Health grants AI054544 (S.R.), AI32725 and AI084048 (P.A.R), and DK-35081 (M.K.P.), and American Heart Association National Scientist Development Grant 0735101N (V.P.F.).
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, hold the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI(online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version-derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
The authors declare the following disclosure: M.K.P. is an officer of and has a financial interest in Complement Technology, Inc., a supplier of complement reagents.
References
- 1.Nolan KF, Kaluz S, Higgins JM, Goundis D, Reid KB. Characterization of the human properdin gene. Biochem J. 1992;287(Pt 1):291–297. doi: 10.1042/bj2870291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol. 1989;142:202–207. [PubMed] [Google Scholar]
- 3.Smith CA, Pangburn MK, Vogel CW, Muller-Eberhard HJ. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. JBiol Chem. 1984;259:4582–4588. [PubMed] [Google Scholar]
- 4.Fearon DT, Austen KF. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 7.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 8.Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–926. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- 9.Fijen CA, Kuijper EJ, Hannema AJ, Sjoholm AG, van Putten JP. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups [see comments] Lancet. 1989;2:585–588. doi: 10.1016/s0140-6736(89)90712-5. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen HE, Koch C, Magnussen P, Lind I. Complement deficiencies in selected groups of patients with meningococcal disease. Scand J Infect Dis. 1989;21:389–396. doi: 10.3109/00365548909167442. [DOI] [PubMed] [Google Scholar]
- 11.Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984;63:243–273. [PubMed] [Google Scholar]
- 12.Sjoholm AG, Braconier JH, Soderstrom C. Properdin deficiency in a family with fulminant meningococcal infections. Clin Exp Immunol. 1982;50:291–297. [PMC free article] [PubMed] [Google Scholar]
- 13.Braconier JH, Sjoholm AG, Soderstrom C. Fulminant meningococcal infections in a family with inherited deficiency of properdin. Scand J Infect Dis. 1983;15:339–345. doi: 10.3109/inf.1983.15.issue-4.04. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen HE, Koch C. Congenital properdin deficiency and meningococcal infection. Clin Immunol Immunopathol. 1987;44:134–139. doi: 10.1016/0090-1229(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 15.Genel F, Atlihan F, Gulez N, Sjoholm AG, Skattum L, Truedsson L. Properdin deficiency in a boy with fulminant meningococcal septic shock. Acta Paediatr. 2006;95:1498–1500. doi: 10.1080/08035250600603008. [DOI] [PubMed] [Google Scholar]
- 16.Cunliffe NA, Snowden N, Dunbar EM, Haeney MR. Recurrent meningococcal septicaemia and properdin deficiency. J Infect. 1995;31:67–68. doi: 10.1016/s0163-4453(95)91550-8. [DOI] [PubMed] [Google Scholar]
- 17.Spath PJ, Sjoholm AG, Fredrikson GN, Misiano G, Scherz R, Schaad UB, Uhring-Lambert B, Hauptmann G, Westberg J, Uhlen M, Wadelius C, Truedsson L. Properdin deficiency in a large Swiss family: identification of a stop codon in the properdin gene, and association of meningococcal disease with lack of the IgG2 allotype marker G2m(n) Clin Exp Immunol. 1999;118:278–284. doi: 10.1046/j.1365-2249.1999.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlesinger M, Mashal U, Levy J, Fishelson Z. Hereditary properdin deficiency in three families of Tunisian Jews. Acta Paediatr. 1993;82:744–747. doi: 10.1111/j.1651-2227.1993.tb12550.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlesinger M, Nave Z, Levy Y, Slater PE, Fishelson Z. Prevalence of hereditary properdin, C7 and C8 deficiencies in patients with meningococcal infections. Clin Exp Immunol. 1990;81:423–427. doi: 10.1111/j.1365-2249.1990.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hourcade DE. Properdin and complement activation: a fresh perspective. Curr Drug Targets. 2008;9:158–164. doi: 10.2174/138945008783502458. [DOI] [PubMed] [Google Scholar]
- 23.Jarva H, Ram S, Vogel U, Blom AM, Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174:6299–6307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis GA, Vedros NA. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. Factor H Binding and Function in Sialylated Pathogenic Neisseriae is Influenced by Gonococcal, but Not Meningococcal, Porin. J Immunol. 2007;178:4489–4497. doi: 10.4049/jimmunol.178.7.4489. [DOI] [PubMed] [Google Scholar]
- 26.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons NJ, Patel PV, Tan EL, Andrade JRC, Nairn CA, Goldner M, Cole JA, Smith H. Cytidine 5′-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human red blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog. 1988;5:303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- 28.Ram S, Cullinane M, Blom A, Gulati S, McQuillen D, Monks B, O’Connell C, Boden R, Elkins C, Pangburn M, Dahlback B, Rice PA. Binding of C4b-binding Protein to Porin: A molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–296. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 32.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15:233–240. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009 doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel U, Frosch M. Mechanisms of neisserial serum resistance. Mol Microbiol. 1999;32:1133–1139. doi: 10.1046/j.1365-2958.1999.01469.x. [DOI] [PubMed] [Google Scholar]
- 35.Farries TC, Finch JT, Lachmann PJ, Harrison RA. Resolution and analysis of ‘native’ and ‘activated’ properdin. Biochem J. 1987;243:507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Findlow H, Vogel U, Mueller JE, Curry A, Njanpop-Lafourcade BM, Claus H, Gray SJ, Yaro S, Traore Y, Sangare L, Nicolas P, Gessner BD, Borrow R. Three cases of invasive meningococcal disease caused by a capsule null locus strain circulating among healthy carriers in Burkina Faso. J Infect Dis. 2007;195:1071–1077. doi: 10.1086/512084. [DOI] [PubMed] [Google Scholar]
- 37.Hoang LM, Thomas E, Tyler S, Pollard AJ, Stephens G, Gustafson L, McNabb A, Pocock I, Tsang R, Tan R. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis. 2005;40:e38–42. doi: 10.1086/427875. [DOI] [PubMed] [Google Scholar]
- 38.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B71–77. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claus H, Maiden MC, Maag R, Frosch M, Vogel U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–1819. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- 40.Deghmane AE, Giorgini D, Larribe M, Alonso JM, Taha MK. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol Microbiol. 2002;43:1555–1564. doi: 10.1046/j.1365-2958.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- 41.Hammerschmidt S, Muller A, Sillmann H, Muhlenhoff M, Borrow R, Fox A, van Putten J, Zollinger WD, Gerardy-Schahn R, Frosch M. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 42.Vogel U, Claus H, Heinze G, Frosch M. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect Immun. 1999;67:954–957. doi: 10.1128/iai.67.2.954-957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ram S, Ngampasutadol J, Cox AD, Blom AM, Lewis LA, St Michael F, Stupak J, Gulati S, Rice PA. Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect Immun. 2007 doi: 10.1128/IAI.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Rear LD, Ross GD. Isolation and purification of C3 from human plasma. Curr Protoc Immunol. 2001;Chapter 13(Unit 13):13. doi: 10.1002/0471142735.im1303s14. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira VP, Cortes C, Pangburn MK. Native Polymeric Forms of Properdin Selectively Bind to Targets and Promote Activation of the Alternative Pathway of Complement. Immunobiology. 2010 doi: 10.1016/j.imbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber RD, Morrison DC, Podack ER, Muller-Eberhard HJ. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979;149:870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinrichs DE, Yethon JA, Amor PA, Whitfield C. The assembly system for the outer core portion of R1-and R4-type lipopolysaccharides of Escherichia coli. The R1 core-specific beta-glucosyltransferase provides a novel attachment site for O-polysaccharides. J Biol Chem. 1998;273:29497–29505. doi: 10.1074/jbc.273.45.29497. [DOI] [PubMed] [Google Scholar]
- 48.Yamasaki R, Kerwood DE, Schneider H, Quinn KP, Griffiss JM, Mandrell RE. The structure of lipooligosaccharide produced by Neisseria gonorrhoeae, strain 15253, isolated from a patient with disseminated infection: evidence for a new glycosylation pathway of gonococcal lipooligosaccharide. J Biol Chem. 1994;269:30345–30351. [PubMed] [Google Scholar]
- 49.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell L, Hourcade D. Inhibition of properdin-directed complement activation by serum amyloid P component. Mol Immunol. 2008;45:4103. [Google Scholar]
- 53.Schneider H, Griffiss JM, Williams GD, Pier GB. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982;128:13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- 54.Densen P, Gulati S, Rice PA. Specificity of antibodies against Neisseria gonorrhoeae that stimulate neutrophil chemot axis. J Clin Invest. 1987;80:78–87. doi: 10.1172/JCI113067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulati S, Cox A, Lewis LA, Michael FS, Li J, Boden R, Ram S, Rice PA. Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci. Infect Immun. 2005;73:7390–7397. doi: 10.1128/IAI.73.11.7390-7397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice PA, Kasper DL. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate: roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982;70:157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]