Abstract
Sequestration of malaria-infected erythrocytes in the peripheral circulation has been associated with the virulence of Plasmodium falciparum. Defining the adhesive phenotypes of infected erythrocytes may therefore help us to understand how severe disease is caused and how to prevent or treat it. We have previously shown that malaria-infected erythrocytes may form apparent autoagglutinates of infected erythrocytes. Here we show that such autoagglutination of a laboratory line of P. falciparum is mediated by platelets and that the formation of clumps of infected erythrocytes and platelets requires expression of the platelet surface glycoprotein CD36. Platelet-dependent clumping is a distinct adhesive phenotype, expressed by some but not all CD36-binding parasite lines, and is common in field isolates of P. falciparum. Finally, we have established that platelet-mediated clumping is strongly associated with severe malaria. Precise definition of the molecular basis of this intriguing adhesive phenotype may help to elucidate the complex pathophysiology of malaria.
The diversity of adhesive phenotypes expressed at the surface of infected erythrocytes has led to considerable interest in the identification of those particular phenotypes associated with severe disease. Infected erythrocytes may adhere to numerous host ligands that include thrombospondin (TSP), platelet GPIV (CD36), intercellular adhesion molecule (ICAM-1 or CD54), platelet endothelial cell adhesion molecule 1 (CD31), P-selectin (CD62P), vascular cell adhesion molecule (CD106), chrondroitin sulfate A, and hyaluronic acid (1–10). Infected erythrocytes, in some parasite lines and isolates, may rosette uninfected erythrocytes, using complement receptor 1 (CD35) on uninfected erythrocytes (11, 12). The diversity of host ligands used by parasites has suggested that the cytoadherence phenotype and the syndrome of clinical infection may be related. The adhesion of infected erythrocytes to ICAM-1 has been associated with symptomatic infection in clinical studies and with cerebral malaria in postmortem studies (13–15). Furthermore, adhesion of infected erythrocytes to chondroitin 4-sulfate and hyaluronic acid has been associated with placental malaria (8, 9). We have described an adhesive phenotype where infected erythrocytes formed apparent autoagglutinates in nonimmune serum (16). In a field study we have shown that the autoagglutinating phenotype was associated with severe malaria (17). We have now studied this phenotype and show that platelet-mediated clumping of infected erythrocytes to form apparent autoagglutinates is a common adhesive phenotype and is associated with severe disease.
Materials and Methods
Cultivation of Plasmodium falciparum-Infected Erythrocytes.
Laboratory clones of P. falciparum were cultured as previously described (16, 18). Parasite clones ITO/C10, ITO/C24, ITO/A4, and ITO/R29 were clones of the ITO4 line (16). The parasite line Malayan Camp and the nonadherent parasite line T9/96 were originally isolated in Thailand. The Palo Alto line (PAR+) is as described (19). All cultures were free of mycoplasma. Materials were from Sigma unless otherwise stated.
Preparation of Platelet-Rich Plasma (PRP), Platelet-Poor Plasma (PPP), and Platelet Suspensions.
PRP was isolated from whole blood (from donors who had not been exposed to malaria) by centrifugation (250 × g, 10 min). Platelets were separated from PRP by further centrifugation at 800 × g for 15 min and the PPP was stored at 4°C. Platelets were resuspended in PBS and stored at 4°C. The concentration of platelets was measured by using a cell counter (Coulter). Platelets from three patients with Glanzman's thrombasthenia, two patients with Bernard–Soulier syndrome, and two patients (of Afro-American origin) with platelet CD36 deficiency or Naka(−) were isolated as described (20). Control platelets (with normal expression of all surface markers including CD36) were obtained at the same time by using the same isolation and storage protocols.
Electron Microscopy.
Briefly, cells were fixed with 2.5% gluataraldehyde/0.1 M cacodylate buffer, pH 7.2. Cells were postfixed in osmium tetroxide, dehydrated, and embedded in epoxy resin. Thin sections were stained with uranyl acetate and lead citrate before examination in a Joel 1200EX transmission electron microscope. For scanning electron microscopy, cells were placed on poly-l-lysine-coated coverslips, dehydrated in acetone, and critical-point dried. The samples were then coated with gold before examination in a Phillips 505 scanning electron microscope.
Clumping and Rosetting Assays.
Autoagglutination or clumping and rosetting frequencies were assessed when the parasites had grown to the stage of pigmented trophozoites. The autoagglutination or clumping frequency was assessed after rotation of parasite cultures in 5% hematocrit in the presence of 10% PPP or 10% PRP containing a final concentration of approximately 1 × 107 platelets per ml and acridine orange (20 μg ml−1). Samples were taken at 15, 30, 60, and 120 min to permit counting of infected erythrocytes in clumps of no greater than 30 cells in size. To assess the rosetting frequency, a sample of parasite culture at 2% hematocrit was stained with acridine orange (20 μg ml−1) and viewed by using a fluorescence microscope (Zeiss III RS).
A clump was comprised of three or more infected erythrocytes and the frequency of the clumping phenotype in a line or isolate was measured as the number of infected cells in clumps in 1,000 infected cells counted in duplicate assays. Rosettes were defined as an infected cell binding two or more uninfected cells, and the frequency of the rosetting phenotype in a line or isolate was measured as the number of infected cells in rosettes in 500 infected erythrocytes in duplicate assays. In the studies of field isolates, clumping assays were performed by using 10% PPP or PRP or hyperimmune sera.
Binding of Parasitized Erythrocytes to Purified Proteins.
The adhesive phenotype of parasitized erythrocytes was determined as previously described (15, 16). Here, 2 μl of a 50 μg ml−1 solution of TSP (GIBCO/BRL), CD36 (21), ICAM-1 (a gift from Alister Craig, University of Liverpool, U.K.), CD31 (22), hyaluronic acid (2 mg ml−1), and chrondroitin sulfate A (2 mg ml−1) were adsorbed onto bacteriological plastic plates. Adherent parasitized erythrocytes were counted by light microscopy, and the number of cells bound per mm2 was corrected to 2% hematocrit and 5% parasitemia.
Inhibition of Clumping of ITO/C10 and Field Isolates by Monoclonal Antibodies.
Platelets (1 × 107 ml−1) were incubated with an anti-CD36 mAb (clone SMφ, Serotec, Raleigh, NC) or an IgM isotype control mAb at 37°C for 1 h. Platelets were then used in clumping assays as previously described.
Detection of Platelet Surface Glycoproteins.
Expression of platelet surface glycoproteins was determined by indirect immunofluorescence and flow cytometry. mAbs against CD36 (clone SMφ), CD31 (clone B-B38), glycoprotein (GP) Ia/IIa (clone P16), GPIIb/IIIa (clone 85/661), CD42b (clone AN51), αvβ3 integrin (clone LM609), P-selectin (clone WAPS12.2), TSP-1 (TSP-B7), HLA class I (clone W6/32), and FITC-conjugated rabbit anti-mouse Ig-Fab2 (Dako) were used to stain the glycoproteins by using a flow cytometer (Coulter).
Autoagglutination or Clumping in Field Parasites.
We undertook a field study in Kilifi District Hospital, Kenya during January–February 1998 and July–August 1999 to evaluate the significance of platelet-dependent autoagglutination or clumping in field isolates. We studied consecutive cases of severe and mild malaria. The severe and mild malaria cases were defined as previously described (17, 23). Patients had fever, parasitemia > 2,000 μl−1, and no illnesses other than malaria on clinical examination and laboratory investigation. All patients of severe malaria were admitted to a high-dependency ward. Children with mild malaria were able to take oral medication and were treated as outpatients. Severe malaria patients were in a coma (Blantyre coma score of <2 at least 1 h after a fit), or anemic (Hb < 5 g dl−1), or hypoglycemic (blood glucose < 2.2 mM), or showed respiratory distress, or were prostrated (unable to take oral fluids). Blood samples were collected into acid-citrate-dextrose (1/6 vol/vol), spun through Lymphoprep (Nycomed, Oslo), washed twice in supplemented RPMI medium 1640, and then cultured as described above. Parasites were grown for at least 16 h and up to 36 h. The maturity of cultured trophozoites was assessed with Giesma stained films, and assays were performed with mature trophozoites. Platelet-mediated clumping and rosetting assays were performed as described above.
Results
Autoagglutination or Clumping of Infected Erythrocytes Is Mediated by Platelets.
The apparent autoagglutinates of infected erythrocytes in nonimmune sera that we had originally observed in laboratory clones and in wild isolates could have formed by homotypic adhesion of infected cells or through bridging cells and/or molecules. We therefore used scanning and transmission electron microscopy to examine the structure of autoagglutinates or clumps. We were intrigued to find that in some cases platelets were interposed between several of the infected erythrocytes (data not shown). These findings suggested that the apparent autoagglutination of malaria- infected erythrocytes might be, at least in part, because of clumping of infected erythrocytes and platelets. To test the hypothesis, we performed autoagglutination or clumping with infected erythrocytes from our panel of laboratory parasite clones (16) with PRP and with PPP.
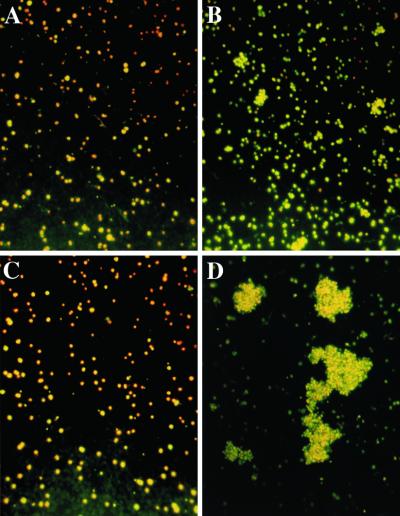
We had previously observed that the formation of autoagglutinates or clumps of infected erythrocytes in nonimmune serum was a property of the laboratory clone ITO/C10 but not of numerous other sibling clones from the same family tree (16). The ITO/C24 clone, which showed very weak autoagglutination or clumping in nonimmune serum, formed only a few aggregates of infected erythrocytes both in PPP and in PRP (Fig. 1 A and B, and quantitative data in Fig. 3). By contrast, the strongly autoagglutinating or clumping clone ITO/C10 made only a few small aggregates of infected erythrocytes in PPP but numerous, large aggregates in the presence of platelets (Fig. 1 C and D and Fig. 3). Electron micrographs confirmed that these autoagglutinates or clumps were also composed of infected erythrocytes and platelets (Fig. 2). Here, the plasma membrane of platelets was juxtaposed with the membrane of infected erythrocytes where electron-dense knobs lay under the surface of the erythrocyte membrane (Inset in Fig. 2C). We concluded that autoagglutinates or clumps of infected erythrocytes of the ITO/C10 clone that we had previously observed in nonimmune serum were aggregates of infected erythrocytes and residual platelets or platelet membranes carried over in the erythrocyte suspension and/or serum used for routine parasite culture.
Figure 1.
Clumping of infected erythrocytes in laboratory P. falciparum parasite clones. Clumps formed by ITO/C24 clone in PPP (A) and in PRP (B). Clumps formed by ITO/C10 clone in PPP (C) and in PRP (D).
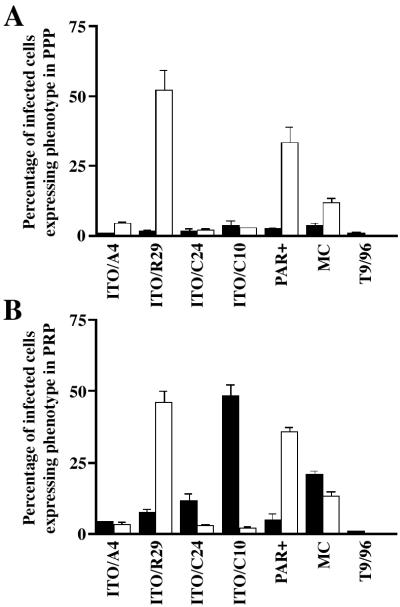
Figure 3.
Expression of clumping and rosetting phenotypes of P. falciparum clones and lines (ITO/A4, ITO/R29, ITO/C24, ITO/C10, Palo Alto, Malayan Camp, and T9/96) in PPP (A) and in PRP (B) containing approximately 1 × 107 platelets per ml. Black bars and white bars represent clumping and rosetting frequencies, respectively, of the corresponding clones.
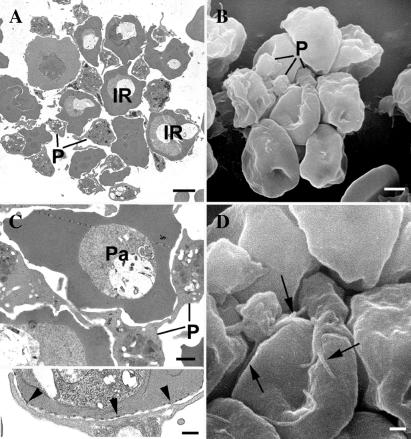
Figure 2.
Transmission and scanning electron micrographs (TEMs and SEMs) of platelet-mediated clumping. (A) TEM section of a clump formed by parasite clone ITO/C10 in PRP shows several infected erythrocytes (denoted by letters IR) aggregated with interposed platelets. An SEM (B) at the same magnification shows several platelets (P) attached to the external surface of infected erythrocytes. (Bar represents 2 μm.) (C) A closer look at one of the clumps shows platelets (P) adherent to infected erythrocytes and acting as bridging cells between two parasitized erythrocytes (Pa). (Bar represents 1 μm.) At higher magnification (C, Inset) close apposition between platelets and ITO/C10 infected erythrocytes is seen at the electron-dense knob structures (indicated by the arrowheads). (Bar represents 0.5 μm.) (D) An SEM of an autoagglutinate in PRP showing close association between platelets and infected erythrocytes (denoted by arrows). (Bar represents 1 μm.)
Platelet-Mediated Clumping Is a Distinct Phenotype in Laboratory Lines of P. falciparum.
To distinguish platelet-mediated clumping from other adhesive phenotypes that allow the formation of multicellular aggregates, we first explored whether rosetting and platelet-dependent clumping of infected erythrocytes were distinct phenotypes. The laboratory clone ITO/R29 and the unrelated Palo Alto (PAR+) line formed rosettes in both PRP and PPP whereas clone ITO/C10 formed the morphologically distinct clumps in PRP but not in PPP (Fig. 3). The parasite line Malayan Camp formed rosettes in PPP and formed both rosettes and distinct clumps in PRP (Fig. 3). Sibling clones of ITO/C10, namely ITO/A4 and ITO/C24, and the unrelated nonadherent line T9/96 showed neither autoagglutination nor rosetting in PRP and PPP (Fig. 3). Thus, platelet-mediated clumping was a distinct and specific phenotype expressed by the ITO/C10 clone.
Malaria-infected erythrocytes adhere to various host receptors, and therefore we considered whether the clumping lines ITO/C10 and Malayan Camp bound to a distinct subset of these receptors. These clones bound to CD36 and TSP-1 but not to CD31, ICAM-1, chrondroitin sulfate A, or hyaluronic acid. Thus there was no pattern of adhesion to purified receptors uniquely associated with the clumping clones (data not shown). It therefore seemed that the platelet-mediated clumping of the clone ITO/C10 is mediated either by different host ligands or by different epitopes on those receptors already described.
Presence of the Platelet Glycoprotein CD36 Is Required for Clumping.
We took advantage of the well characterized inherited abnormalities of expression of specific platelet surface glycoproteins to identify the platelet receptor or receptors required for clumping. We used platelets from patients deficient in the expression of GPIIb/IIIa (CD41/CD61, Glanzmann's thrombobasthenia), GPIb/IX (CD42a/CD42b, Bernard–Soulier syndrome) or GPIV [CD36, Naka(−) phenotype] in clumping assays (24).
Clumps of infected erythrocytes were formed by using platelets from two individuals that did not express GPIIb/IIIa or GPIb/IX. However, clumps were not formed with platelets from two individuals with the Naka(−) phenotype that did not express CD36 on the surface of platelets (data not shown). These platelets demonstrated normal expression of CD31, GPIIb/IIIa, GPIa/IIa, and GPIb/IX (data not shown). Furthermore, infected erythrocytes from the ITO/C10 clone did not bind to Naka(−) platelets adherent to plastic, but did bind to platelets deficient in GPIIb/IIIa or GPIb/IX (data not shown).
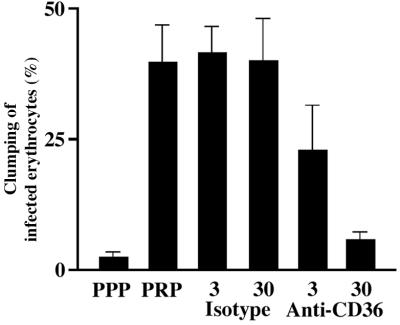
We confirmed the requirement for the expression of CD36 on platelets for the formation of platelet-mediated clumps by using an anti-CD36 antibody (clone SMφ) in clumping assays. Neither this IgM mAb to CD36 (clone SMφ) nor an isotype control antibody caused platelet aggregation at the concentrations used in these assays (data not shown). There was a dose-dependent inhibition of clumping of ITO/C10 by this anti-CD36 mAb (Fig. 4). We concluded that expression of CD36 on platelets is necessary for platelet-mediated clumping of infected erythrocytes, although these data do not exclude that other as yet undefined host receptors are also required for the expression of this phenotype.
Figure 4.
Inhibition of the clumping phenotype expressed by parasite clone ITO/C10 by using anti-CD36 mAb. Isotype indicates a control antibody. The numbers represent the concentration of the respective antibody used (3 μg ml−1 and 30 μg ml−1). The error bars represent standard error of mean from three independent experiments.
Platelet-Mediated Clumping of Field Isolates of P. falciparum.
We have previously shown that the apparent autoagglutination of infected erythrocytes in nonimmune serum is common in field isolates and is associated with severe malaria (17). However, we performed that study before we were aware of the role of platelets in the formation of clumps of infected erythrocytes. We therefore wished to determine whether the clumping of infected erythrocytes in PPP and that in PRP were independent phenomena, and whether platelet-mediated clumping was associated with severe disease. We therefore investigated these phenotypes in parasite isolates from children with severe and mild malaria.
Compared to children with mild malaria, those children who suffered from severe malaria were significantly younger (median 19 vs. 49 months), had higher admission parasitemias (median 242,800 μl−1 vs. 27,760 μl−1), and had lower hemoglobin concentrations (median 6.5 g/dl vs. 9.0 g/dl, P < 0.001 for all comparisons, Mann–Whitney U test).
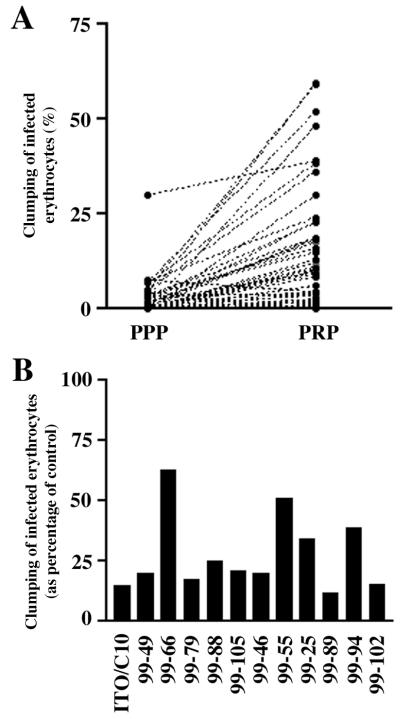
We grew P. falciparum isolates from 57 children with severe malaria and 64 children with mild malaria. Addition of platelets had a dramatic effect on the ability of these parasite isolates to form clumps. A total 104 of 121 P. falciparum isolates (86.0%) tested formed clumps in PRP. In only 6 of these 104 samples was clumping in PPP greater than clumping in PRP. Thus, in 98 of 104 isolates, the presence of platelets enhanced clumping of infected erythrocytes, indicating the formation of platelet-mediated clumps (Fig. 5A and data not shown). Furthermore, the frequency of infected erythrocytes forming clumps in PRP (mean 13.0% and median 7.4%) was significantly greater than the frequency of forming clumps of infected erythrocytes in PPP (mean 2.9% and median 0.0% of infected erythrocytes; P < 0.001, Mann–Whitney U test). We therefore concluded that most of the apparent autoagglutination observed in field isolates was indeed platelet-mediated clumping.
Figure 5.
Platelet-mediated clumping among P. falciparum field isolates. (A) Clumping in parasite isolates from children with malaria in PPP and in PRP. These data sets represent 50 consecutive parasite isolates studied in 1999. (B) Inhibition of clumping in field parasite isolates by anti-CD36 mAb. The black bars represent the clumping frequencies of individual isolates when platelets were preincubated with anti-CD36 mAb compared with when platelets were preincubated with an isotype control antibody. The degree of inhibition of clumping in the laboratory clone ITO/C10 and in 11 consecutive field isolates that grew satisfactorily from frozen samples is shown.
We determined the requirement of CD36 for clumping in all those samples where frozen duplicate samples were available and grew satisfactorily. Clumping assays with PRP were performed in these 11 samples. Here, the anti-CD36 mAb (clone SMφ) reduced clumping in PRP on average by 71.0% (range 36.7%–87.2%, median 80.0%) compared with 83.2% for the laboratory clone ITO/C10 (Fig. 5B).
Finally, we studied the association of platelet-mediated clumping with severe malaria. We derived the percentage of infected erythrocytes expressing the platelet-mediated clumping phenotype in each sample by subtracting the percentage of infected erythrocytes forming clumps in PPP from the percentage of infected erythrocytes forming clumps in PRP. The frequency of the platelet-mediated clumping phenotype was higher in isolates from children with severe malaria (median 8.1%) than in children with mild malaria (median 3.6%, P < 0.01, Mann–Whitney U test; Table 1). We also found that a rosetting phenotype was more frequently observed in isolates from severe malaria patients than in isolates from mild malaria (Table 1). However, agglutination of infected erythrocytes in serum from adults living in endemic areas, that is immune-agglutination, was not associated with severe disease (P = 0.11, Mann–Whitney U test). Furthermore, there was no correlation between rosetting and platelet-mediated clumping (r = 0.10, P = 0.30, Spearman's rank test).
Table 1.
Association of parasite phenotypes with disease severity
| Parasite phenotype | Expression, %
|
P (severe vs. mild) | |
|---|---|---|---|
| Severe disease | Mild disease | ||
| Clumping in PRP | 10.6 (27.5–3.6) | 4.3 (12.9–0.6) | 0.004* |
| Platelet-mediated clumping | 8.1 (19.6–2.5) | 3.6 (10.8–0.0) | 0.01* |
| 0.035** | |||
| Rosetting | 6.5 (34.9–2.0) | 4.1 (10.8–0.9) | 0.02* |
| 0.054** | |||
The median of the percentage of infected erythrocytes expressing the respective phenotype is given, followed in parentheses by the 75th to 25th percentiles. Comparisons were made by using Mann–Whitney U test (*) and by multinomial regression (**) (using spss Version 9.0 for Windows 95).
To determine if the parasite phenotypes were associated with severe malaria independently of age and admission parasitemia, we performed a multinomial logistic regression. We classified children with severe malaria into the most severely ill group, suffering from three or four of the syndromes of severe malaria (cerebral malaria, severe malarial anemia, respiratory distress, or hypoglycemia), the less severely ill group suffering from one or two of these syndromes, and those with mild disease. In this analysis platelet-dependent clumping (P = 0.035), age (P < 0.001), and admission parasitemia (P = 0.044) were independently associated with severe disease, and the association of rosetting with severe disease was just short of statistical significance (P = 0.054). The interactions between clumping and the other variables were not significant. The odds ratio for the association of an isolate with a clumping frequency of 10% of infected erythrocytes with the severe malaria was 2.19 (95% confidence interval 1.18–4.07). Finally, there was no significant association of platelet-mediated clumping with any particular syndrome of severe disease (data not shown).
Discussion
Here we show that the platelets mediate autoagglutination or clumping of P. falciparum-infected erythrocytes. Perhaps it is now appropriate to use the term clumping instead of autoagglutination to distinguish platelet-mediated clumping of infected erythrocytes from agglutinates of infected erythrocytes formed by antibodies or homotypic adhesion. Platelet-mediated clumping is a distinct, stable, and common adhesive property of P. falciparum. Furthermore, we have established that in the majority of field isolates in Kenya, formation of clumps of infected erythrocytes is mediated by platelets, and this specific phenotype is associated with severe malaria.
The formation of agglutinates or clumps of malaria-infected erythrocytes has been noted by many investigators but has not been studied systematically. In the older literature, Kniseley et al. (25) described agglutinates or clumps of infected erythrocytes in the circulation of monkeys infected with Plasmodium knowlesi. Fremount and Miller (26) subsequently showed by electron microscopy that infected erythrocytes in this animal model of malaria were directly apposed to form the agglutinates. Since then, several reports have described microagglutinates composed of infected and uninfected erythrocytes formed during the culture of wild isolates of P. falciparum and lines adapted to growth in primates (27–30). Previously, we had identified the phenotype of apparent autoagglutination in nonimmune serum in a laboratory clone and in field isolates (16, 17). However, until now, the cellular and molecular characteristics of this form of agglutination or clumping of infected erythrocytes had not been determined.
Here, we have shown the dramatic enhancement of clumping of infected erythrocytes by the addition of PRP (Fig. 1 D and E, Fig. 2, and Fig. 5A). Adherence of infected erythrocytes to platelets in a flow chamber (31, 32) or in culture (33, 34) has been reported, but platelet-mediated clumping has not been described as a distinct adhesive phenotype. Platelet-mediated clumping is not confined to laboratory parasite clones but also accounts for the majority of apparent autoagglutination observed among the field isolates in Kenya (Fig. 5A). We have also observed that platelet-mediated clumping by ITO/C10 clone and by the majority of field isolates tested was significantly inhibited by antibodies to CD36 (Fig. 4 and 5B). Taken together these data provide compelling evidence that platelet-mediated clumping represents the major mechanism of the apparent autoagglutination that has been seen in nonimmune serum.
These findings do not exclude other mechanisms leading to the formation of agglutinates of infected erythrocytes. Indeed, in some isolates, we did find agglutinates of parasitized erythrocytes formed independently of platelets (Fig. 5A and Table 1). Some of these agglutinates may represent the microagglutinates of infected erythrocytes seen by others (26–28). These platelet-independent agglutinates may simply be an extension of rosetting or further distinct adhesive types. Our definition of platelet-mediated clumping may allow future studies to characterize these phenotypes.
The molecular interactions that permit some but not all parasite clones and lines to form platelet-mediated clumps remain puzzling. Certainly, the formation of clumps by the ITO/C10 clone and by wild isolates requires the expression of CD36 on platelets. However, most laboratory lines and field isolates do bind to CD36 (15, 16, 35, 36) but do not form clumps (for example see ITO/A4 in Fig. 3 A and B). Furthermore, there were no characteristics linking the binding phenotype of the clumping clones to already identified receptors that were uniquely associated with this phenotype.
There are several possible explanations for this apparent paradox. First, clumping and nonclumping lines may adhere to distinct regions of CD36. Although the region defined by amino acids 139–184 on CD36 has been implicated as the principle domain for the binding of parasite-infected erythrocytes in several studies, other regions on CD36 have also been shown to support binding of parasitized erythrocytes (37–40). Definition of the relationship of clumping phenotype to particular binding sites on CD36 would be valuable. Second, it is possible that clumping clones could bind to other unidentified receptors on the platelet surface. However, if such alternative receptors exist they cannot support binding of infected cells to platelets lacking CD36. Finally, it is possible that small differences in the binding of parasites to CD36 on platelets may alter the expression of other receptors through cellular activation or increase the affinity and/or avidity of the interaction between infected erythrocytes and platelets. At present we cannot distinguish among these alternatives.
Platelet-mediated clumping is common not only in field isolates but also associated with severe malaria. These results are consistent with our previous finding that the apparent autoagglutination of infected erythrocytes in nonimmune serum was associated with severe disease (17), and our observations from this study that platelet-mediated clumping represents the major mechanism for the apparent agglutination of infected erythrocytes in nonimmune serum. Clumping assays in PRP and PPP now represent a more sensitive and specific test for this phenotype.
The association of the platelet-mediated clumping phenotype with severe disease cannot establish a causal relationship between this phenotype and pathogenesis. However, rosettes of uninfected and infected erythrocytes may increase vascular resistance (41), and we have previously suggested that clumps of infected erythrocytes may be directly associated with severe disease by causing local disturbance of blood flow and/or other important physiological functions (17). The impressive size of the robust platelet-dependent clumps in vitro does at the very least support the plausibility of this hypothesis.
The association of platelet-mediated clumping with severe disease is interesting in the light of emerging evidence suggesting that platelets may have a role in the pathology of malaria. In a model of rodent malaria, platelets sequester in the cerebral vessels and may mediate neurovascular injury (42). Indeed, platelet sequestration and agglutinates of infected erythrocytes and platelets have been recently described in the cerebral vessels of children who have died with severe malaria.¶ Furthermore, the platelets were significantly colocalized with parasite pigment (G. Grau, personal communication). These data suggest that more powerful studies to examine the association of clumping with specific syndromes of severe malaria may help to unravel the role of this phenotype in the pathogenesis of severe malaria.
We have established that platelet-mediated clumping of infected erythrocytes is a common phenotype expressed by P. falciparum isolates. The molecular interactions that allow clumps to form have not been completely defined, but in a wide range of isolates clump formation does require the platelet glycoprotein CD36. More precise description of the molecular mechanism of platelet-mediated clumping may eventually lead to strategies to ameliorate the morbidity and the mortality of falciparum malaria.
Acknowledgments
We thank Mr. R. Abel for supplying platelets from patients with Bernard–Soulier Syndrome, Dr. B. Curtis for platelets from those with Naka(−) phenotype and Glanzmann's thrombasthenia, and Mr. D. Allen for providing us with antiplatelet antibodies. We also thank Prof. C. Newbold, Dr. S. Kyes, Dr. P. Bull, and Dr. B. Elford for helpful comments, Mr. R. Roberts-Gant and Dr. K. Micklem for superb technical help in making the figures, and Dr. S. Booker for expert statistical advice. This work is published with the permission of the Director of the Kenyan Medical Research Institute (KEMRI) and supported by The Wellcome Trust and the National Blood Service, U.K.
Abbreviations
- TSP
thrombospondin
- ICAM
intercellular adhesion molecule
- PRP
platelet-rich plasma
- PPP
platelet-poor plasma
- GP
glycoprotein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Grau, G. E., Mackenzie, C. D., Carr, R., Redard, M., Pizzolato, G. P., Moulin, P., Cataldo, C., Liomba, N. G., Molyneux, M. E. & Taylor, T. E., Proceedings of the 48th Meeting of the American Society of Tropical Medical and Hygiene, Nov. 11–Dec. 2, 1999, Washington, DC, No. 780, 475.
References
- 1.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Nature (London) 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 2.Oquendo P, Hundt E, Lawler J, Seed B. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 3.Ockenhouse C F, Tandon N N, Magowan C, Jamieson G A, Chulay J D. Science. 1989;243:1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- 4.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 6.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 7.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried M, Duffy P E. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 10.Beeson J G, Rogerson S J, Cooke B M, Reeder J C, Chai W, Lawson A M, Molyneux M E, Brown G V. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udomsangpetch R, Wahlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe J A, Moulds J M, Newbold C I, Miller L H. Nature (London) 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 13.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 14.Turner G D, Morrison H, Jones M, Davis T M, Looareesuwan S, Buley I D, Gatter K C, Newbold C I, Pukritayakamee S, Nagachinta B, et al. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 15.Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 16.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Nature (London) 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts D J, Pain A, Kai O, Kortok M, Marsh K. Lancet. 2000;355:1427–1428. doi: 10.1016/S0140-6736(00)02143-7. [DOI] [PubMed] [Google Scholar]
- 18.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 19.Helmby H, Cavelier L, Pettersson U, Wahlgren M. Infect Immun. 1993;61:284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis B R, Aster R H. Transfusion. 1996;36:331–334. doi: 10.1046/j.1537-2995.1996.36496226147.x. [DOI] [PubMed] [Google Scholar]
- 21.Tandon N N, Lipsky R H, Burgess W H, Jamieson G A. J Biol Chem. 1989;264:7570–7575. [PubMed] [Google Scholar]
- 22.Fawcett J, Buckley C, Holness C L, Bird I N, Spragg J H, Saunders J, Harris A, Simmons D L. J Cell Biol. 1995;128:1229–1241. doi: 10.1083/jcb.128.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 24.Bithell T C. In: Wintrobe's Clinical Hematology. 9th Ed. Lee G R, Foerster J, Athens J W, Lukens J N, editors. Vol. 2. Philadelphia: Lea & Febiger; 1993. pp. 1397–1421. [Google Scholar]
- 25.Kniseley M H, Stratman-Thomas W K, Eliot T S. Anat Rec. 1941;79:90. [Google Scholar]
- 26.Fremount H N, Miller L H. Am J Trop Med Hyg. 1975;24:1–8. [PubMed] [Google Scholar]
- 27.Carlson J, Helmby H, Hill A V, Brewster D, Greenwood B M, Wahlgren M. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 28.Rowe A, Obeiro J, Newbold C I, Marsh K. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fandeur T, Le Scanf C, Bonnemains B, Slomianny C, Mercereau-Puijalon O. J Exp Med. 1995;181:283–295. doi: 10.1084/jem.181.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez V, Treutiger C J, Nash G B, Wahlgren M. Infect Immun. 1998;66:2969–2975. doi: 10.1128/iai.66.6.2969-2975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke B M, Morris-Jones S, Greenwood B M, Nash G B. Am J Trop Med Hyg. 1995;53:29–35. [PubMed] [Google Scholar]
- 32.Cooke B M, Nash G B. Exp Parasitol. 1995;80:116–123. doi: 10.1006/expr.1995.1013. [DOI] [PubMed] [Google Scholar]
- 33.Wahlgren M, Abrams J S, Fernandez V, Bejarano M T, Azuma M, Torii M, Aikawa M, Howard R J. Scand J Immunol. 1995;42:626–636. doi: 10.1111/j.1365-3083.1995.tb03705.x. [DOI] [PubMed] [Google Scholar]
- 34.Polack B, Peyron F, Zakiuddin I S, Kolodie L, Ambroise-Thomas P. C R Acad Sci. Series III. 1990;310:577–582. [PubMed] [Google Scholar]
- 35.Rogerson S J, Tembenu R, Dobano C, Plitt S, Taylor T E, Molyneux M E. Am J Trop Med Hyg. 1999;61:467–472. doi: 10.4269/ajtmh.1999.61.467. [DOI] [PubMed] [Google Scholar]
- 36.Ockenhouse C F, Ho M, Tandon N N, Van Seventer G A, Shaw S, White N J, Jamieson G A, Chulay J D, Webster H K. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 37.Ockenhouse C F, Tandon N N, Jamieson G A, Greenwalt D E. Infect Immun. 1993;61:2229–2232. doi: 10.1128/iai.61.5.2229-2232.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daviet L, Buckland R, Puente-Navazo M D, McGregor J L. Biochem J. 1995;305:221–224. doi: 10.1042/bj3050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asch A S, Liu I, Briccetti F M, Barnwell J W, Kwakye-Berko F, Dokun A, Goldberger J, Pernambuco M. Science. 1993;262:1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]
- 40.Baruch D I, Ma X C, Pasloske B, Howard R J, Miller L H. Blood. 1999;94:2121–2127. [PubMed] [Google Scholar]
- 41.Kaul D K, Roth E F, Nagel R L, Howard R J, Handunnetti S M. Blood. 1991;78:812–819. [PubMed] [Google Scholar]
- 42.Grau G E, Tachini-Cottier F, Vestin C, Milon G, Lou J, Piguet P, Juillard P. Eur Cytokine Netw. 1993;4:415–419. [PubMed] [Google Scholar]