Abstract
Cardiovascular disease continues to be the leading cause of death, suggesting that new therapies are needed to treat the progression of heart failure post-myocardial infarction. As cardiac tissue has a limited ability to regenerate itself, experimental biomaterial therapies have focused on the replacement of necrotic cardiomyocytes and repair of the damaged extracellular matrix. While acellular and cellular cardiac patches are applied surgically to the epicardial surface of the heart, injectable materials offer the prospective advantage of minimally invasive delivery directly into the myocardium to either replace the damaged extracellular matrix or to act as a scaffold for cell delivery. Cardiac-specific decellularized matrices offer the further advantage of being biomimetic of the native biochemical and structural matrix composition, as well as the potential to be autologous therapies. This review will focus on the requirements of an ideal scaffold for catheter-based delivery as well as highlight the promise of decellularized matrices as injectable materials for cardiac repair.
Keywords: Injectable, Minimally Invasive, Heart Failure, Therapy, Biomaterials, Decellularized
Introduction
The progressive pathological changes post-myocardial infarction (MI) include an initial inflammatory response [1], loss of cardiomyocytes [2, 3], and degradation of the left ventricular (LV) extracellular matrix (ECM) by matrix metalloproteases [4, 5], which lead to wall thinning, infarct expansion [6], scar tissue formation, and eventual LV dilatation and decrease in cardiac function [7]. This process of negative LV remodeling post-MI is thought to independently contribute to heart failure (HF) [8]. Currently, the only successful treatments for end-stage HF post-MI are total heart transplantation and the use of an LV assist device. As coronary heart disease continues to be the leading cause of death in the USA and the Western world [9], it is clear that new therapies are needed.
To replace the necrotic cardiomyocytes and damaged ECM, cardiac tissue engineering approaches have become a major research focus for therapy post-MI. These approaches include biomaterial scaffolds in combination with cells, as well as biomaterials alone that are intended to recruit cells into the damaged region. Initially, cellular therapy, or cellular cardiomyoplasty, involved the injection of cells in saline or media without any type of scaffold. Although this technique has shown promise in preclinical and clinical trials [10–14], the lack of an appropriate extracellular environment for cellular adhesion has limited cell retention, survival, and integration into the host tissue within the damaged infarct region [15, 16]. More recently, biomaterial scaffolds have been explored for use as cellular and acellular cardiac patches [17–22] and also as injectable materials [23–27], often referred to as in situ gelling materials [28]. In addition to cellular cardiac patches, used to increase cell transplant survival [17–19], acellular cardiac patches have been developed from a variety of materials to provide structural support for the damaged ventricle and/or encourage cellular recruitment into the material [20–22]. While both have been somewhat effective at preserving cardiac function in animal models, implantation of these patch materials involves an invasive open chest procedure, such as sternotomy or thoracotomy. Furthermore, patch materials are sutured to the epicardial surface of the heart, limiting the region of therapeutic benefit. In contrast, injectable materials offer a unique solution of replacing the damaged myocardial ECM and/or delivering cells directly to the infarcted region while offering the potential for minimally invasive delivery.
Injectable Scaffold Requirements
As a variety of injectable materials have been explored for cardiac tissue engineering and the prevention of HF, it is important to consider the factors necessary for an ideal injectable scaffold for the heart. The ideal injectable material would be one that mimics the native cardiac extracellular milieu and meets the clinical need of minimally invasive catheter delivery. From a tissue engineering perspective, it is important that a material be biocompatible and provide the appropriate cell–matrix interactions to allow for cell adhesion proliferation and/or maturation [29–31]. The native extracellular environment provides cells with a complex combination of proteins and polysaccharides. Thus, while scaffolds have been functionalized with simple peptides such as RGD to promote adhesion [32], it is known that more complex, combinatorial signals promote significant changes in cell behavior [33, 34], indicating that the biochemical composition of a scaffold is critical to instruct cells. Furthermore, material properties such as degradation products play an important role. It is essential that the degradation products be non-toxic and that the degradation rate allows for new tissue in-growth. Beyond biochemical composition, structural properties, such as porosity, are considerations that effect degradation and cellular infiltration [35, 36]. Pores should be interconnected with a diameter >10 μm to allow for cellular and vascular infiltration as well as diffusion of nutrients [30]. Biochemical composition and porosity that allows for cellular infiltration should thus allow for vascular cell infiltration and revascularization of the ischemic region. The angiogenic potential of a scaffold is important for most tissue engineering applications [29, 30, 37], but is particularly critical in the ischemic region of the heart post-MI as microvascular dysfunction is a known predictor of LV dilation [38–40].
For a material to be compatible with clinically relevant catheter delivery, allowing for a minimally invasive procedure, additional design requirements become important. Initial human cellular transplantation studies involved direct epicardial injection while a patient received an open chest procedure, such as cardiac artery bypass graft or the implantation of a LV assist device [41, 42]. However, recent advances in catheter technology allow for percutaneous transcoronary or transendocardial delivery [10–14, 24], eliminating the need for an invasive surgical procedure. Thus, materials designed as injectable scaffolds should meet design requirements for coronary or endocardial catheter delivery. The material must have the proper gelation properties and kinetics to remain liquid within the catheter while allowing the formation of a solid gel within the myocardial tissue. Materials currently being tested in small animal models, via direct epicardial injection [23, 25–27, 43, 44], would likely have difficulty translating to catheter delivery due to gelation properties or other delivery constraints. For example, a quick-gelling material may clog the catheter, preventing injectability. Additionally, some materials are multi-component, requiring a double-barreled injector for delivery [27, 43–45], which is not compatible with current catheter technology.
Synthetic Materials
Synthetic materials for tissue engineering offer the advantage of tunability, allowing the design of a material with the appropriate porosity, mechanical stability, and degradation properties [30]. Recently, variations of poly(N-isopropylacrylamide) (PNIPAAM) and poly(ethylene glycol) (PEG) have been developed and evaluated as injectable therapies. PNIPAAM, a synthetic hydrogel, is advantageous because it undergoes a phase transition just below physiologic temperature, causing it to form a gel at 37°C, yet remains a liquid at room temperature. Variations of PNIPAAM, including biodegradable dextran (Dex) grafted poly(E-caprolactone)-2-hydroxylethyl methacrylate (PCL-HEMA)/PNIPAAm (Dex-PCL-HEMA/PNIPAAm) [46, 47] and copolymerization of NIPAAm, acrylic acid (AAc), and hydroxyethyl methacrylate-poly(trimethylene carbonate) (HEMAPTMC), to create poly(NIPAAm-co-AAc-co-HEMAPTMC) [48] have shown preserved or improved cardiac function in small animal infarct models. Additionally, PEG, a common bioinert material, has been explored as a non-degradable option. However, injection of PEG-vinyl sulfone immediately following permanent ligation in a rat model showed no difference in long-term functional benefit as compared to saline injection [49]. A recent study in our lab involving the injection of PEG suggests that the injection of a bioinert, non-degradable material is insufficient to reduce negative LV remodeling and prevent the progression of HF despite an increase in infarct wall thickness (data unpublished). Thus, while synthetic materials offer the benefit of tunability, they lack inherent bioactivity, important for cellular adhesion, proliferation, and growth, which may play a key role in cardiac regeneration.
Naturally Derived or Inspired Materials
The primary focus for injectable materials has been on naturally derived or inspired materials to improve cell survival or preserve cardiac function. The advantage of natural materials is that the components are recognized by the body and are easily degraded into safe by-products [30]. The first materials explored as injectable scaffolds include collagen and fibrin glue, both commonly used tissue engineering materials. Collagen, a helical structure with a defined pattern of amino acids that can be easily recognized by the body, is the main protein component of most extracellular environments and is thus a common material choice for tissue engineering scaffolds [50]. Commercially available collagen products have been used as injectable scaffolds for cardiac tissue engineering [26, 51, 52], yet advances with collagen have slowed since the initially reported studies. Fibrin, another commonly used fibrillar protein, is involved in the coagulation cascade, in wound healing responses [53], and in promoting angiogenesis [54, 55]. Fibrin glue is a commercially available two-component product consisting of the fibrin precursor, fibrinogen, and the activating enzyme, thrombin, that self-assemble upon contact to create a fibrin gel. Fibrin glue was the first material used to demonstrate that an injectable biomaterial can improve cell survival [43] as well as induce neovascularization and preserve cardiac function with or without cells [27, 43, 51, 56]. However, fibrin glue requires a double-barreled injection system that is not currently compatible with catheter delivery, unless the catheter is flushed with saline between injections. This method is likely not clinically feasible, as multiple flushes of material into the bloodstream would occur. Both collagen and fibrin are single proteins that, although common for tissue engineering applications, do not mimic the native ECM, which is a tissue-specific network of proteins and polysaccharides.
To create a more complex extracellular environment, Matrigel is a commonly used material [16]. Matrigel refers to the purified matrix derived from Engelbreth–Holm–Swarm mouse sarcoma cells and includes a complex mixture of laminin, collagen IV, entactin, heparan sulfate proteoglycans, and growth factors. Another attractive characteristic is that Matrigel undergoes a phase transition to create a gel at physiologic temperature. However, being that Matrigel is derived from a mouse sarcoma line, it is not tissue-specific and presents the risk of tumor induction [57, 58], which will likely limit its clinical translation. Additionally, Matrigel may not be conducive to allowing neovascularization, as recent studies present varied results [51, 59]. In one study, a lack of cellular infiltration, including a lack of vascular cell infiltration, into injected Matrigel was reported [59].
Chitosan and alginate are two non-mammalian polysaccharide materials that have been explored for cardiac tissue engineering. Chitosan, derived from the structural component of crustacean shells, is a polysaccharide with tunable chemistry that allows for the control of degradation properties [60]. The ability to undergo temperature-phase transition at physiologic temperature allows chitosan to gel in situ upon injection into the myocardium with or without cells [23]. Mouse embryonic stem cells delivered into an ischemic border zone showed improved retention and engraftment when injected with chitosan, in addition to improved function and neovascular formation [23]. Potential barriers to using chitosan include the lack of solubility in neutral solutions and inconsistency in cellular attachment [50]. Alginate, a polysaccharide derived from brown seaweed, has the unique ability to undergo a phase transition, utilizing the calcium present in the myocardial tissue, upon injection into the LV free wall [24]. The use of alginate has shown positive results in both acute and chronic rat MI models [25] and additionally has been delivered in combination with growth factors [61] or modification with RGD [32]. This past year, alginate was shown to improve function in a porcine model upon minimally invasive transcoronary catheter delivery [24]. Despite the recent success, known limitations associated with alginate include poor cell adhesion and infiltration, as the hydrophilic nature of alginate leads to the prevention of protein adsorption and mammalian cell interaction [50]. Furthermore, as chitosan and alginate are both derived from non-mammalian sources, they likely do not provide the appropriate extracellular microenvironment.
As naturally inspired materials, self-assembling peptides, RAD16-I and RAD16-II, which are able to form nanofibrous gels at physiologic pH have also been explored. Initial in vivo studies using the RAD16-II self-assembling peptides alone showed promising results in healthy myocardium, including the infiltration of endothelial cells and cardiomyocytes, as well as differentiation of human embryonic stem cells (hESCs) to cardiomyocyte (CM) upon injection [59]. However, upon injection in MI models, success has only been achieved with the addition of tethered growth factors, with variable results depending on the growth factor and the cell type. It has been shown that incorporation of insulin-like growth factor-1 (IGF-1) with self-assembling peptides aids in CM survival [62], improves survival and proliferation of cardiac progenitor cells (CPC) [63], and improves cardiac function upon delivery with CM or CPC cells [62, 63]. Self-assembling peptides injected with platelet derived growth factor (PDGF) showed improved fractional shortening, although they did not improve function when injected alone [64, 65]. Furthermore, nanofibers delivered with a protease-resistant stromal cell derived factor-1 (SDF-1) were shown to recruit endogenous stem cells and improve cardiac function [66]. However, both self-assembling peptide sequences, with or without PDGF, failed to improve skeletal myoblast survival and cardiac function in a rat model [67]. These varied data suggest the potential importance of an injected biomaterial to create the appropriate microenvironment for the cell type being delivered or recruited.
Several groups have explored combination materials, or incorporated additional components, such as growth factors to create a more complex microenvironment, to aid in cell survival, and/or preserve cardiac function. Combination materials include a collagen–Matrigel combination [68], a fibrin–alginate biocomposite [45], and a PEGylated fibrin biomatrix [42]. While these combination materials provide a more complex scaffold, they do not properly mimic the native cardiac ECM.
Decellularized Materials
In native healthy tissue, the extracellular environment is a complex composition of proteins and proteoglycans that guides cellular attachment, survival, migration, proliferation, and differentiation [30, 31, 69–72]. Thus, as described, cardiac tissue engineering strategies have focused on the development of injectable scaffolds to replace the native ECM, with the hope of providing cells the proper environment to develop [15]. It follows that the best replacement of the complex milieu is the native ECM itself. Therefore, recent focus has been placed on the utilization of decellularized ECM for a variety of medical applications, including cardiac repair.
Decellularization involves the physical, chemical, or enzymatic removal of an organ or tissue’s cellular content, thus leaving only the ECM. Although it may alter the chemical and structural composition of the ECM, decellularization is beneficial as it allows for the removal of cellular antigens, which could induce a foreign body reaction, inflammation, and potential transplant rejection [73]. The removal of cellular antigens and the fact that ECM proteins are fairly well conserved among species [74] allow xenogeneic decellularized materials to be well tolerated [75]. In fact, numerous decellularized ECMs, such as small intestine submucosa (SIS), pericardium, skin, and heart valve from both bovine and porcine sources are FDA-approved and used clinically [75].
For cardiac applications, decellularized urinary bladder matrix has been used as an epicardial cardiac patch material, showing cellular infiltration into the patch and functional benefits [21, 22, 76]. However, the use of a non-cardiac-specific decellularized ECM patch material resulted in undesirable cartilage tissue formation within the myocardium [22], potentially a result of non-tissue-specific cell–ECM interactions. In addition, patch materials are limited to the epicardial surface of the heart and thus do not provide the advantages of minimally invasive delivery and treatment within the infarct region, as injectable materials offer.
For these reasons, the use of decellularized matrices as injectable therapies for cardiac repair has gained recent focus. Decellularized SIS has been processed to be a powder and injected, as an emulsion, directly into rat myocardium following an ischemic–reperfusion event [79]. Results showed cellular recruitment into the injection and without intermediate flushing of the catheter. However, it has been shown that while the general ECM components of each tissue are similar, each individual tissue does contain its own unique combination of proteins and proteoglycans [30, 70]. Thus, cardiac-specific decellularized tissue would be the appropriate choice to replace the damaged myocardial ECM. It was recently shown that intact rat and porcine hearts can be decellularized via perfusion with detergents, utilizing the vasculature to access, lyse, and remove all of the cells [77, 78], thus opening up the possibility of using decellularized myocardial ECM for cardiac repair.
Decellularized ventricular and pericardial ECM are two attractive options recently developed for cardiac tissue engineering. Our lab has shown that decellularized porcine ventricular tissue, as well as porcine and human pericardial tissue, can be processed to create a solubilized liquid with the ability to gel via self-assembly at physiologic temperature both in vitro and in vivo upon injection into myocardial tissue [80, 81]. Briefly, ventricular or pericardial tissue was harvested and decellularized using sodium dodecyl sulfate detergents (Figs. 1 and 2). The decellularized matrix was then lyophilized and milled to create a fine powder (Fig. 3a, b). This powder can be solubilized using enzymatic digestion to create a liquid matrix for catheter delivery (Fig. 3c).
Fig. 1.
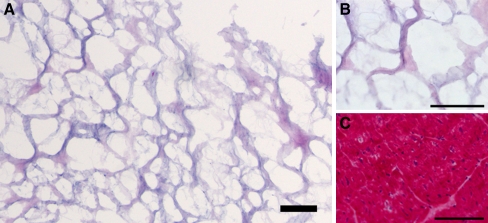
Decellularized ventricular ECM and non-decellularized porcine ventricular tissue (hematoxylin and eosin-stained sections). a, b Intact decellularized ventricular ECM prior to processing. c Intact non-decellularized ventricular tissue. Scale bars, 100 μm. Note the absence of cells in the decellularized ECM. Reprinted with permission from [80]
Fig. 2.
Decellularized and non-decellularized human and porcine pericardial tissue (hematoxylin and eosin-stained sections). a, b Human; c, d Porcine; a, c Non-decellularized pericardial tissue; b, d Decellularized pericardial ECM. Scale bars, 500 μm. Note the absence of cells in the decellularized ECM. Reprinted with permission from [81]
Fig. 3.
Decellularization process of ventricular extracellular matrix. a Decellularized, lyophilized (dried) ECM. b Milled powder. c Solubilized myocardial matrix in a 1-mL syringe, prepared for injection
As it is critical that a material for cardiac therapy mimic the native ECM to provide the appropriate microenvironment to facilitate cell–matrix interactions, the decellularized ventricular ECM biochemical composition was characterized and shown to retain a complexity of proteins, peptides, and glycosaminoglycans [80]. The complex solubilized myocardial matrix was shown to reassemble in vitro at physiologic pH and temperature into a nanofibrous and porous structure. Initial in vivo feasibility testing showed gelation within healthy rat myocardial tissue upon direct epicardial injection, with pore size of ~30 μm and a structure resembling that of the intact decellularized tissue, prior to processing (Fig. 4a). In addition, the myocardial matrix was shown to promote the migration of human coronary artery endothelial cells and rat aortic smooth muscle cells in vitro as well as promote the infiltration of vascular cells and the formation of arterioles in vivo [80]. The myocardial matrix has been shown to preserve LV volume and ejection fraction in a rat infarct model (data unpublished). To assess clinical feasibility, the matrix has further been tested in a porcine model via transendocardial catheter delivery (data unpublished). Briefly, multiple injections were made throughout the LV free wall and septal wall, as guided by an electromechanical voltage (NOGA) map. The resistance of each injection was rated and did not increase with time, indicating that the material did not gel within the catheter. Thus, the feasibility of the material to meet the criteria for transendocardial delivery has been demonstrated. The presented myocardial matrix thus provides a cardiac-specific matrix that meets clinical design criteria and is a promising option for translation.
Fig. 4.
In vivo gelation of injectable myocardial and pericardial matrix scaffolds (hematoxylin and eosin-stained sections). a Arrow indicates area of injected myocardial matrix, and the inset is intact decellularized ventricular ECM prior to processing. Scale bar, 100 μm. Note the similar structure of the self-assembled matrix to the decellularized ECM. b Arrow indicates area of injected human pericardial matrix. Scale bar, 500 μm. c Arrow indicates area of injected porcine pericardial matrix. Scale bar, 500 μm. Reprinted with permission from [80, 81]
Our lab has also tested the initial feasibility of a decellularized pericardial gel which has the potential to be an autologous therapy [81]. While the pericardium provides some structural support for the heart, it is considered non-essential for survival and is routinely cut or removed from patients during surgery, without adverse effects [82]. Thus, a portion of the pericardium can be removed and processed to be an autologous treatment, as has been done for valve replacement and left ventricular repair [83, 84]. Pericardial matrix gels have been created from porcine pericardium, as well as human pericardium, following a similar preparation as the myocardial matrix [81]. Human pericardium was obtained from patients undergoing cardiothoracic surgery, who would be the likely targets of this autologous therapy. Initial in vivo results demonstrate that both human and porcine pericardial matrices are able to gel in vivo (Fig. 4b, c) and promote the infiltration of vascular cells, including arteriole formation. Although pericardial matrix is not an exact ECM match for ventricular myocardium, the pericardium is thought to influence myocardial contraction and epicardial vessel properties [82] and offers the additional benefit of providing a potentially autologous treatment option.
Conclusions
A variety of materials are being explored to prevent the progression of HF post-MI, including synthetic and naturally derived or inspired materials. Keeping in mind the outlined requirements of an injectable scaffold, decellularized matrices show great promise for clinical translation. Synthetic materials, while able to be modified or allow for angiogenesis through their porous structure [85], do not offer the bioactivity to replace the damaged ECM and allow for the appropriate cell–matrix interactions. Many of the recently explored naturally derived and inspired materials offer the benefit of bioactivity, although have not been designed specifically for cardiac repair. Fibrin and collagen are single proteins, each lacking the complexity of a native ECM. Matrigel, while more complex, will likely not progress to the clinic as it is derived from a mouse sarcoma line. Furthermore, fibrin, collagen, and Matrigel would potentially gel prematurely during catheter delivery due to their rapid gelation kinetics. Although chitosan and alginate may be suited for catheter delivery, both are from non-mammalian sources and thus do not offer a mimic of native ECM.
Decellularized materials offer the advantage of being cardiac-specific and potentially autologous injectable therapies. The myocardial and pericardial matrices have shown initial feasibility within healthy rat myocardium via in vivo gelation and vascular cell infiltration. In addition, gelation of myocardial matrix occurs with the proper kinetics to allow for transendocardial catheter delivery, as assessed in a porcine model. Being that the myocardial matrix and pericardial matrix have similar gelation kinetics, we anticipate that the pericardial matrix will also be compatible with catheter injection. Catheter compatibility is critical because while many materials have shown preserved or improved function upon injection with or without cells in small animal models [23–27], most will likely not translate to catheter delivery. In addition, the ventricular and pericardial decellularized matrices retain complex biochemical ECM cues, providing an extracellular milieu that cannot currently be mimicked by other materials, and thus offer promising solutions as injectable therapies to replace the degraded ECM for the treatment of MI and HF.
Acknowledgments
Unpublished data from the Christman lab was supported in part by the NIH Director's New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004309-01.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovascular Research. 2002;55(2):329–340. doi: 10.1016/S0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 2.Weisman HF, Bush DE, Mannisi JA, Weisfeldt ML, Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988;78(1):186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, et al. Apoptosis in myocytes in end-stage heart failure. The New England Journal of Medicine. 1996;335(16):1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi SC, Campbell SE, Reddy HK, Tjahja E, Voelker DJ. Matrix metalloproteinase activity expression in infarcted, noninfarcted and dilated cardiomyopathic human hearts. Molecular and Cellular Biochemistry. 1996;155(1):13–21. doi: 10.1007/BF00714328. [DOI] [PubMed] [Google Scholar]
- 6.Hutchins GM, Bulkley BH. Infarct expansion versus extension: Two different complications of acute myocardial infarction. The American Journal of Cardiology. 1978;41(7):1127–1132. doi: 10.1016/0002-9149(78)90869-X. [DOI] [PubMed] [Google Scholar]
- 7.Jeremy RW, Hackworthy RA, Bautovich G, Hutton BF, Harris PJ. Infarct artery perfusion and changes in left ventricular volume in the month after acute myocardial infarction. Journal of the American College of Cardiology. 1987;9(5):989–995. doi: 10.1016/S0735-1097(87)80298-X. [DOI] [PubMed] [Google Scholar]
- 8.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100(9):999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 10.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107(18):2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 11.Dib N, Diethrich EB, Campbell A, Goodwin N, Robinson B, Gilbert J, et al. Endoventricular transplantation of allogenic skeletal myoblasts in a porcine model of myocardial infarction. Journal of Endovascular Therapy. 2002;9(3):313–319. doi: 10.1583/1545-1550(2002)009<0313:ETOASM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Dib N, Campbell A, Jacoby DB, Zawadzka A, Ratliff J, Miedzybrocki BM, et al. Safety and feasibility of percutaneous autologous skeletal myoblast transplantation in the coil-infarcted swine myocardium. Journal of Pharmacological and Toxicological Methods. 2006;54(1):71–77. doi: 10.1016/j.vascn.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. Journal of the American College of Cardiology. 2003;42(12):2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. The New England Journal of Medicine. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circulation Research. 2005;97(1):8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 17.Kellar RS, Shepherd BR, Larson DF, Naughton GK, Williams SK. Cardiac patch constructed from human fibroblasts attenuates reduction in cardiac function after acute infarct. Tissue Engineering. 2005;11(11–12):1678–1687. doi: 10.1089/ten.2005.11.1678. [DOI] [PubMed] [Google Scholar]
- 18.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102(19 Suppl 3):III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann WH, Didie M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106(12 Suppl 1):I151–I157. [PubMed] [Google Scholar]
- 20.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. Journal of the American College of Cardiology. 2007;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NAF, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112(suppl I):I-135–I-143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 22.Badylak SF, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. The Heart Surgery Forum. 2002;6(2):E20–E26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 23.Lu WN, Lu SH, Wang HB, Li DX, Duan CM, Liu ZQ, et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Engineering. Part A. 2009;15:1437–1447. doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 24.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. Journal of the American College of Cardiology. 2009;54(11):1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 26.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: A novel approach to preserve cardiac function after myocardial infarction. Journal of the American College of Cardiology. 2005;46(4):714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 27.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Engineering. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 28.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. Journal of the American College of Cardiology. 2006;48(5):907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: A review. Journal of Tissue Engineering and Regenerative Medicine. 2007;1(5):327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 30.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 31.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacology & Therapeutics. 2005;105(2):151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30(5):751–756. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Brafman DA, Shah KD, Fellner T, Chien S, Willert K. Defining long-term maintenance conditions of human embryonic stem cells with arrayed cellular microenvironment technology. Stem Cells and Development. 2009;18:1141–1154. doi: 10.1089/scd.2008.0410. [DOI] [PubMed] [Google Scholar]
- 34.Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells and Development. 2008;17(1):29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 35.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Advanced Drug Delivery Reviews. 2007;59(4–5):263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. Journal of Materials Chemistry. 2004;14(13):1915–1923. doi: 10.1039/b401791a. [DOI] [Google Scholar]
- 37.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. Journal of Biomaterials Science, Polymer Edition. 2004;15(6):701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 38.Sakuma T, Hayashi Y, Sumii K, Imazu M, Yamakido M. Prediction of short- and intermediate-term prognoses of patients with acute myocardial infarction using myocardial contrast echocardiography one day after recanalization. Journal of the American College of Cardiology. 1998;32(4):890–897. doi: 10.1016/S0735-1097(98)00342-8. [DOI] [PubMed] [Google Scholar]
- 39.Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109(9):1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 40.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93(2):223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 41.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: Four-year follow-up. Circulation. 2005;112(12):1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Engineering. Part A. 2008;14(6):1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 43.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. Journal of the American College of Cardiology. 2004;44(3):654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 44.Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. The Journal of Thoracic and Cardiovascular Surgery. 2004;128(4):571–578. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee R, Zavadzkas JA, Saunders SM, McLean JE, Jeffords LB, Beck C, et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. The Annals of Thoracic Surgery. 2008;86(4):1268–1276. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, Wu DQ, Jiang XJ, Zhang XZ, Li XY, Zhang JF, et al. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. European Journal of Heart Failure. 2009;11(1):14–19. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 47.Li XY, Wang T, Jiang XJ, Lin T, Wu DQ, Zhang XZ, et al. Injectable hydrogel helps bone marrow-derived mononuclear cells restore infarcted myocardium. Cardiology. 2010;115(3):194–199. doi: 10.1159/000281840. [DOI] [PubMed] [Google Scholar]
- 48.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30(26):4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. Journal of Cardiac Failure. 2009;15(7):629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical Reviews. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 51.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Engineering. 2005;11(11–12):1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 52.Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, et al. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. Journal of the American College of Cardiology. 2003;41(11):1964–1971. doi: 10.1016/S0735-1097(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 53.Sierra DH. Fibrin sealant adhesive systems: A review of their chemistry, material properties and clinical applications. Journal of Biomaterials Applications. 1993;7(4):309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 54.Naito M, Stirk CM, Smith EB, Thompson WD. Smooth muscle cell outgrowth stimulated by fibrin degradation products. The potential role of fibrin fragment E in restenosis and atherogenesis. Thrombosis Research. 2000;98(2):165–174. doi: 10.1016/S0049-3848(99)00202-9. [DOI] [PubMed] [Google Scholar]
- 55.Thompson WD, Smith EB, Stirk CM, Marshall FI, Stout AJ, Kocchar A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. The Journal of Pathology. 1992;168(1):47–53. doi: 10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 56.Chekanov V, Akhtar M, Tchekanov G, Dangas G, Shehzad MZ, Tio F, et al. Transplantation of autologous endothelial cells induces angiogenesis. Pacing and Clinical Electrophysiology. 2003;26(1 Pt 2):496–499. doi: 10.1046/j.1460-9592.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 57.Albini A, Melchiori A, Garofalo A, Noonan DM, Basolo F, Taraboletti G, et al. Matrigel promotes retinoblastoma cell growth in vitro and in vivo. International Journal of Cancer. 1992;52(2):234–240. doi: 10.1002/ijc.2910520214. [DOI] [PubMed] [Google Scholar]
- 58.Yue W, Brodie A. MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors. The Journal of Steroid Biochemistry and Molecular Biology. 1993;44(4–6):671–673. doi: 10.1016/0960-0760(93)90278-5. [DOI] [PubMed] [Google Scholar]
- 59.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111(4):442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–2349. doi: 10.1016/S0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 61.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovascular Research. 2007;75(1):178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padin-Iruegas MEMD, Misao YMD, Davis MEP, Segers VFMMDP, Esposito GP, Tokunou TMDP, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120(10):876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. The Journal of Clinical Investigation. 2006;116(1):237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsieh PC, MacGillivray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114(7):637–644. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 66.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116(15):1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 67.Dubois G, Segers VF, Bellamy V, Sabbah L, Peyrard S, Bruneval P, et al. Self-assembling peptide nanofibers and skeletal myoblast transplantation in infarcted myocardium. Journal of Biomedical Materials Research. Part B: Applied Biomaterials. 2008;87(1):222–228. doi: 10.1002/jbm.b.31099. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Zhang H, Wang H, Wei Y, Hu S. Artificial matrix helps neonatal cardiomyocytes restore injured myocardium in rats. Artificial Organs. 2006;30(2):86–93. doi: 10.1111/j.1525-1594.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 69.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28(25):3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 70.Uriel S, Labay E, Francis-Sedlak M, Moya ML, Weichselbaum RR, Ervin N, et al. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Engineering. Part C Methods. 2008;15:309–321. doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macfelda K, Kapeller B, Wilbacher I, Losert UM. Behavior of cardiomyocytes and skeletal muscle cells on different extracellular matrix components—Relevance for cardiac tissue engineering. Artificial Organs. 2007;31(1):4–12. doi: 10.1111/j.1525-1594.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 72.Brown L. Cardiac extracellular matrix: A dynamic entity. American Journal of Physiology. Heart and Circulatory Physiology. 2005;289(3):H973–H974. doi: 10.1152/ajpheart.00443.2005. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF, Prockop DJ. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution . Biochemistry. 1983;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- 75.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112(suppl I):I-144–I-149. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 77.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Natural Medicines. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 78.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Engineering. Part C. Methods. 2010;16:525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao ZQ, Puskas JD, Xu D, Wang NP, Mosunjac M, Guyton RA, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. Journal of the American College of Cardiology. 2010;55(12):1250–1261. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 80.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: A potentially autologous scaffold for cardiac tissue engineering. Tissue Engineering. Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuster V. Hurst's the heart. 10. New York: McGraw-Hill Medical Publishing Division; 2001. [Google Scholar]
- 83.David TE, Feindel CM, Ropchan GV. Reconstruction of the left ventricle with autologous pericardium. The Journal of Thoracic and Cardiovascular Surgery. 1987;94(5):710–714. [PubMed] [Google Scholar]
- 84.Duran CM, Gometza B, Kumar N, Gallo R, Martin-Duran R. Aortic valve replacement with freehand autologous pericardium. The Journal of Thoracic and Cardiovascular Surgery. 1995;110(2):511–516. doi: 10.1016/S0022-5223(95)70248-2. [DOI] [PubMed] [Google Scholar]
- 85.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Engineering. 2007;13(9):2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]