Abstract
Vascular endothelial growth factor (VEGF-A or VEGF) is a potent growth factor for the development of retinal and choroidal vasculatures. To define the temporal requirement of the retinal pigmented epithelium (RPE)-derived VEGF in choroidal vascular development, we generated conditional VEGF knockout mice using an inducible Cre/lox system. The loss of the RPE-derived VEGF was confirmed with immunoblotting and immunohistochemistry. Retinal function and structure were assessed with electroretinography and histology, respectively. Choroidal vascular density was analyzed with computer-assisted semi-quantitative assay using fluorescently labeled choroidal flat-mounts. Induction of RPE-specific VEGF disruption at embryonic day 10 (E10) or E13 for two days caused regulatable decreases in choroidal vascular density, photoreceptor function, and photoreceptor outer nuclear layer thickness. The loss of the RPE-produced VEGF after E15 did not cause detectable defects in choroidal vasculatures and photoreceptor function and morphology. These results suggest that the RPE-derived VEGF plays a critical role in choroidal vascular development during organogenesis before embryonic day 15.
Keywords: choroid, RPE, VEGF, inducible, Cre/lox
INTRODUCTION
Vascular endothelial growth factor (VEGF-A or VEGF) is a major growth factor for embryogenesis, vasculogenesis, and angiogenesis (Shibuya 2001, Ferrara et al. 2003). VEGF is a potent endothelial mitogen that regulates proliferation, migration, and tube formation during the development of new blood vessels (for review, see (Saint-Geniez & D'Amore 2004)). Loss of a single VEGF allele prevents angiogenic growth of new blood vessels and leads to embryonic lethality in mice between embryonic day 11 to 12 (Carmeliet et al. 1996, Ferrara et al. 1996). In the eye VEGF is an essential growth factor for the development of retinal neurons (Yang & Cepko 1996), retinal vasculature (Stalmans et al. 2002, Haigh et al. 2003), and choroidal vasculature (Marneros et al. 2005), through VEGF receptor-mediated signaling pathways (for review, see (Penn et al. 2008)). VEGF is also a major pathogenic factor for a number of ocular vascular diseases, including diabetic retinopathy (DR) and age-related macular degeneration (AMD), leading causes of blindness that are associated with abnormal function of blood-retina barriers.
Retinal pigment epithelium (RPE), Müller cells, endothelial cells, astrocytes, and ganglion cells are major VEGF-producing cells in the retina (Stone et al. 1995, Aiello et al. 1995, Pierce et al. 1995, Miller et al. 1997, Stone et al. 1996). VEGF produced by some retinal cells may be beneficial to the integrity of the retina (Blaauwgeers et al. 1999, Nishijima et al. 2007, Kilic et al. 2006, Saint-Geniez et al. 2008). RPE-derived VEGF was speculated to play a significant role in physiological fenestration and retinal maintenance (Blaauwgeers et al. 1999). The role of RPE-derived VEGF was investigated using transgenic models previously. While these studies demonstrated its post-developmental function (Schwesinger et al. 2001, Oshima et al. 2004), they are not designed for dissecting the role of the RPE-derived VEGF in retinal development. Disruption of the RPE-derived VEGF in mice at embryonic day 10 (E10) caused dramatic defects, including absence of choriocapillaris, occurrence of microphthalmia, and complete loss of visual function (Marneros et al. 2005). To establish a mouse model for defining the temporal requirement of the RPE-derived VEGF in choroidal vascular development and dissecting its post-developmental function, we used tetracycline-inducible gene expression technology and Cre/lox system (for review see (Gossen et al. 1994, Le & Sauer 2000)) and generated mice with inducible VEGF disruption in the RPE at various time. This report described our investigation on the temporal role of the RPE-derived VEGF in choroidal vascular development.
MATERIALS AND METHODS
Generation of conditional VEGF knockout mice and animal treatment
All experiments with animals strictly follow the guidelines established by the ARVO statement for the “Use of Animals in Ophthalmic and Vision Research” and were approved by the Institutional Animal Care and Use Committees of the University of Oklahoma Health Sciences Center, the Dean A. McGee Eye Institute, and the Oklahoma Medical Research Foundation. The conditional VEGF knockout (KO) mice were generated by mating the inducible RPE-specific Cre mice with existing floxed VEGF mice (Gerber et al. 1999, Zhu et al. 2010). To minimize the influence of genetic background in our study, hemizygous (HEMI) and wild-type (WT) littermates were used as controls throughout this study. Induction of VEGF disruption in mice was achieved by feeding pregnant females with doxycycline (dox, 2 mg/ml) in 5% sucrose.
Genotyping and RT-PCR analysis
Genotyping of wild-type (WT, cre−Vegfff) mice and hemizygous (HEMI, cre−Vegff+) and homozygous (cre+Vegfff) VEGF KO mice was performed using primer pairs a (5'-CCT GGC CCT CAA GTA CAC CTT-3') and b (5'-TCC GTA CGA CGC ATT TCT AG-3') to detect a 100 bp product for the WT allele and a 150 bp product for the floxed VEGF allele. PCR diagnosis for cre was performed according to a previously described procedure (Le & Sauer 2000) using primers c (5’- AGG TGT AGA GAA GGC ACT TAG C -3’) and d (5’- CTA ATC GCC ATC TTC CAG CAG G -3’) to detect a 411-bp product. PCR diagnosis of the Cre-activatable lacZ reporter gene in R26R mice was performed with primers e (5'-GAG TTG CGT GAC TAC CTA CGG-3') and f (5'-GGC TTC ATC CAC CAC ATA CAG G-3') to detect a 495-bp product.
RT-PCR analysis of Cre-activatable lacZ expression to detect a 495-bp product was performed according to a previous procedure (Le et al. 2003), using primers e and f (described above). Primers g (5'-GAC GAG GCG CAG AGC AAG AGA GG-3') and h (5'-CTC TTT GAT GTC ACG CAC GAT TTC-3') were used to detect a 450-bp β-actin product as internal control.
β-Galactosidase assay, immunoblotting, and immunostaining
For localization of Cre function in the retina, double transgenic mice derived from transgenic Cre mice and Cre-activatable reporter mice (R26R) (Soriano 1999) were used and β-galactosidase assay was carried out as described previously (Le et al. 2006). For immunoblotting of VEGF, a polyclonal anti-VEGF antibody (1:500 dilution, Santa Cruz Biotechnologies, Santa Cruz, CA) was used. The peroxidase-linked anti-rabbit IgG and mouse IgG secondary antibodies (1:5,000 dilution, Amersham Biosciences, Buckinghamshire, England) were used. The images were detected by chemiluminescence using a Super Signal West Dura Extended Duration Substrate (PIERCE, Rockford, IL) and a Kodak Molecular Image Station 4000R and were analyzed with KODAK Molecular Imaging Software V.4.0.0.
For immunostaining of choroidal vessels with anti-CD31 antibody, eyes were fixed with 4% paraformaldehyde in PBS for 1 h and were then dissected to remove the sclera, lens, and retina. The remaining RPE/choroid was blocked in 10% horse serum for 1 h and incubated with a rat anti-mouse antibody against CD31 (1:100 dilution, BD Pharmingen, San Diego, CA) in 1%Triton-PBS at 4°C overnight. The RPE/choroid was incubated with Alexa Fluor 488–conjugated goat anti–rat IgG secondary antibody (Molecular Probes, Eugene, OR) in 1%Triton-PBS for 2 h, flat-mounted, and examined with fluorescent microscopy or confocal microscopy. Retinal vessels were stained with fluorescently labeled isolectin B4 (Vector, Burlingame, CA), as described previously (Bai et al. 2009). Immunostaining of VEGF was performed according to an established condition (Bai et al. 2009) using a polyclonal anti-VEGF antibody (1:100 dilution, Santa Cruz Biotechnologies) and Alexa Fluor 488–conjugated goat anti–rabbit IgG secondary antibody (Molecular Probes). For nuclear staining, 4, 6 diamidino-2-phenylindole (DAPI) and mounting media were used according to manufacturer’s instruction (Vector).
Quantification of RPE and retinal vessel density
RPE density was quantified by using the average number of RPE nuclei from 8 images of 730 pixels × 550 pixels. For analysis of retinal vessel density, isolectin B4 stained images (8 images of 100 pixels × 100 pixels) were used to calculate the mean microvessel intersections, as described previously (Browning et al. 1997).
Quantification of choroidal vascular density
To quantify the choroidal vascular density, the anti-CD31 antibody stained choroid images, generated with fluorescent or confocal microscopy, were analyzed with Adobe Photoshop (7.0) Software. For each image, appropriate thresholds were set in such a way that fluorescent images were converted to black-white images, as illustrated in Figure 2B–C. Black-white images were then be used to calculate the percentage of choroidal vessels based on the area of staining, rather than fluorescent intensity. The average percentage in each area was plotted and expressed as the mean ± SEM. Differences were assessed with Student’s t-test. P < 0.05 was considered significant.
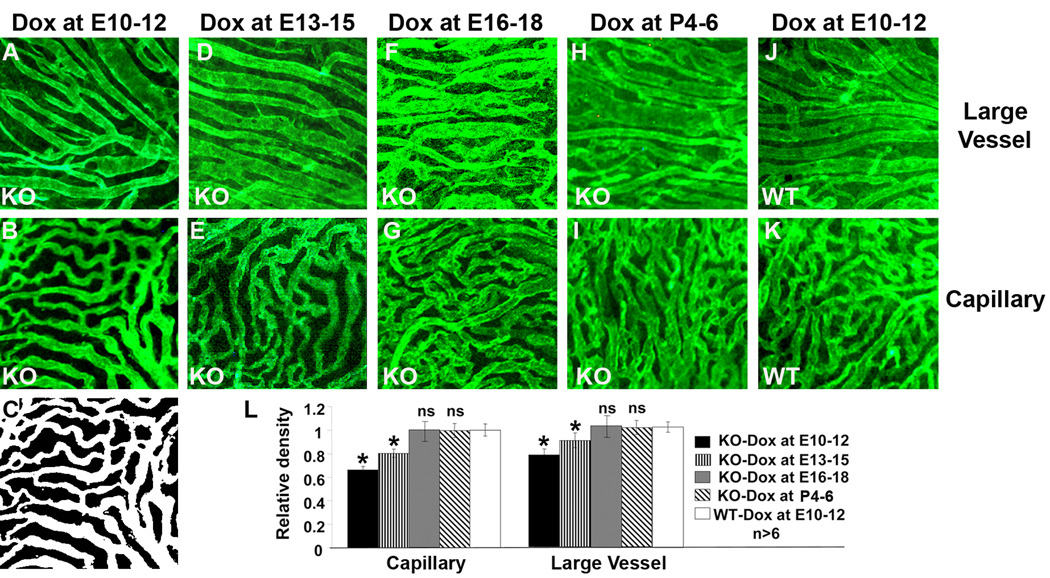
Figure 2.
Temporal requirement of the RPE-derived VEGF in choroidal vascular development. A–K: representative images of anti-CD31 antibody-stained large choroidal vessels and capillaries from P21 conditional VEGF KO mice and WT mice after dox induction at E10-12, E13-15, E16-18, or P4–P6. C: black-white image converted from B, which was used to calculate the area of vessels. M: quantification of choroidal vascular density. *: p<0.05 vs WT in t-test. ns: not significant. Inducible disruption of the RPE-derived VEGF at various times resulted in regulatable changes in choroidal vascular density and the RPE-derived VEGF was required for the development of choroidal vasculature before embryonic day 15.
Retinal function and morphology
Scotopic ERG was examined with a UTAS-E 3000 ERG system (LKC technologies, Inc., Gaithersburg, MD), according to the conditions described previously (Le et al. 2004, Zheng et al. 2006). For retinal morphology, the mice were euthanized and the eyes were dissected and fixed at room temperature for 48 h in Perfix (4% paraformaldehyde, 20% isopropanol, 2% trichloroacetic acid, 2% zinc chloride). The fixed eyes were then embedded in paraffin and sectioned in 5-µm thickness. The sections were stained with hematoxylin and eosin (H & E) and were observed under a light microscopy. Outer nuclear layer thickness (ONL) was represented by average thickness in superior and inferior at 0.5 mm from optic nerve head, as described previously (Bai et al. 2009).
Transmission electron microscopy
Mouse eyes were fixed with 1% glutaraldehyde and 4% paraformaldehyde in PBS, postfixed with 1% osmium tetroxide and 0.8% potassium ferricyanide in 0.1 M cacodylate, pH 7.4, en bloc stained with uranyl acetate, dehydrated and embedding in epoxy resin. Ultrathin sections were then cut and stained with uranyl acetate and examined with a Hitachi H-7600 Transmission Electron Microscope.
RESULTS
Generation of conditional VEGF knockout mice
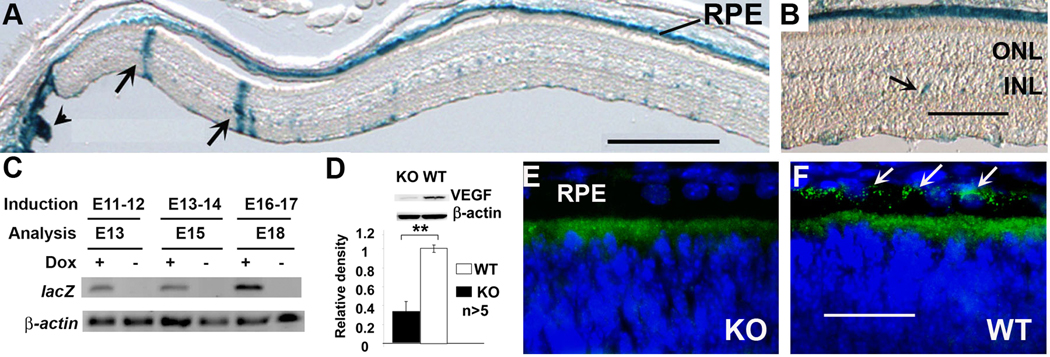
During the generation of VMD2 promoter controlled tetracycline-inducible Cre mice (Le et al. 2008), we obtained several transgenic founders. The retinal expression pattern of these transgenic mouse lines were characterized by using a Cre-activatable β-galactosidase reporter mouse line R26R (Soriano 1999, Zhu et al. 2010). One of them, named VMD2-RPE-Cre-2 (VRC2), demonstrated strongest Cre activity in the RPE, as judged by Cre-activated β-galactosidase activity (Figure 1A–B). Expression of Cre-activated lacZ was detected one day after dox induction at E11-12, E13-14, or E16-17 (Figure 1C), suggesting that Cre-mediated recombination could induced at various time after E10. Therefore, this VRC2 mouse line is suitable for disruption of VEGF in the RPE at any time after E10. The VRC2 mice were mated with mouse carrying a floxed VEGF allele to generate conditional VEGF KO mice (Gerber et al. 1999). Immunoblotting (IB) of RPE extracts demonstrated a significant reduction (67.7%) of the RPE-derived VEGF at P5 in conditional VEGF KO mice with dox induction at E16-18 (Figure 1D), which was similar to that with dox induction at E10-12 (data not shown). This result was confirmed with immunohistochemistry (IHC) (Figure 1E–F).
Figure 1.
Generation of conditional VEGF KO mice. A–B: localization of Cre expression in retinal sections of VRC2/R26R mice. Arrows in A: expression in Müller cells. Arrow head: expression outside the retina. Arrows in B: expression in unidentified cells in inner nuclear layer (INL). ONL: outer nuclear layer. Scale bars in A and B equal to 250 µm and 100 µm, respectively. C: RT-PCR analysis of Cre activated lacZ expression (30 cycles) in the retina of VRC2/R26R mice one day after dox induction at E11-12, E13-14, or E16-17. β-Actin (20 cycles) served as an internal control. D–F: Analysis of VEGF expression in P5 conditional VEGF knockout mice with immunoblotting and immunohistochemistry after dox induction at E16-18. Scale bar in F equals to 40 µm. **: p<0.001 vs WT in t-test. RPE-derived VEGF was expressed near the basolateral side of the RPE (towards choroid, arrows in F) in WT mice and was disrupted in the conditional VEGF KO mice.
Choroidal vascular density
To determine the temporal requirement of the RPE-derived VEGF in choroidal development, we recently established a semi-quantitative method to analyze choroidal vascular density. In this procedure, mouse choroidal vessels were immunostained with an anti-CD31 antibody and were detected by fluorescently labeled secondary antibody. Confocal or fluorescent microscopic images were than converted into black-white images, as shown in Figure 2B–C. The area of the black-white images, not the intensity of fluorescent signals, was then used to represent choroidal vascular density. Using this method, we examined the choroidal vascular density at postnatal day 21 (P21) in conditional VEGF KO mice after dox induction at various times. Compared with WT controls, dox induction at E10-12 resulted in significant reduction in choroidal vascular density of both choroiocapillaris (31.4%) and large choroidal vessels (near the sclera, 21.0%) in P21 conditional VEGF KO mice (Figure 2A–C, L). Dox induction at E13-15 caused a less dramatic reduction in the density of both choriocapillaris (19.8%) and large choroidal vessels (9.3%) in P21 conditional VEGF KO mice (Figure 2D–E, L). Dox induction at E16-18 or P4-6 did not have any apparent effect on the density of both choriocapillaris and large choroidal vessels in P21 conditional VEGF KO mice (Figure 2F–I, L). These results suggest that inducible RPE-specific VEGF disruption caused a regulatable choroidal vascular density in mice. While choroidal vascular development required the RPE-derived VEGF at E10, its dependence on RPE-derived VEGF reduced gradually until E16 when it is no longer needed for this process.
Photoreceptor function and morphology
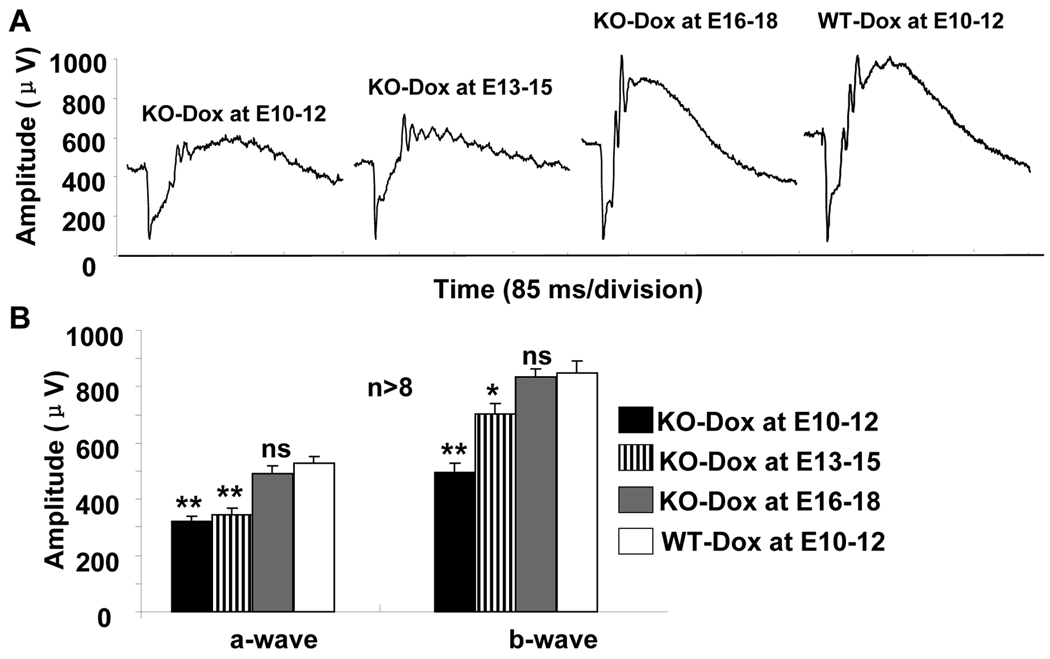
To determine if the reduction of choroidal vascular density resulted in any change in the retina, we examined photoreceptor function in conditional VEGF KO mice with dox induction at various times. Compared with WT controls, dox induction at E10-12 caused a significant loss of scotopic ERG a-wave (38.9%) and b-wave (41.1%) amplitudes in P21 conditional VEGF KO mice (Figure 3). Dox induction at E13-15 resulted in 34.3% and 17% reduction of scotopic ERG a-wave and b-wave amplitudes in P21 conditional VEGF KO mice (Figure 3). There was no significant change in photoreceptor function in P21 conditional VEGF KO mice if dox induction occurred after E15 (Figure 3). These results indicate that disruption of the RPE-derived VEGF at different times caused regulatable changes in rod photoreceptor function.
Figure 3.
Photoreceptor function in P21 conditional VEGF KO mice after dox induction at various time for two days. A: Representative scotopic ERG of the conditional VEGF KO mice and WT controls. B: Analysis of scotopic ERG a-wave and b-wave in the conditional VEGF KO mice and controls at P21 after dox induction at E10-12, E13-15, or E16-18. * and **: p<0.05 and p<0.001, respectively. ns: not significant. Inducible disruption of the RPE-derived VEGF at various times resulted in regulatable change of rod photoreceptor function. Significant loss of rod function was observed in P21 conditional VEGF KO mice with dox induction before embryonic day 15.
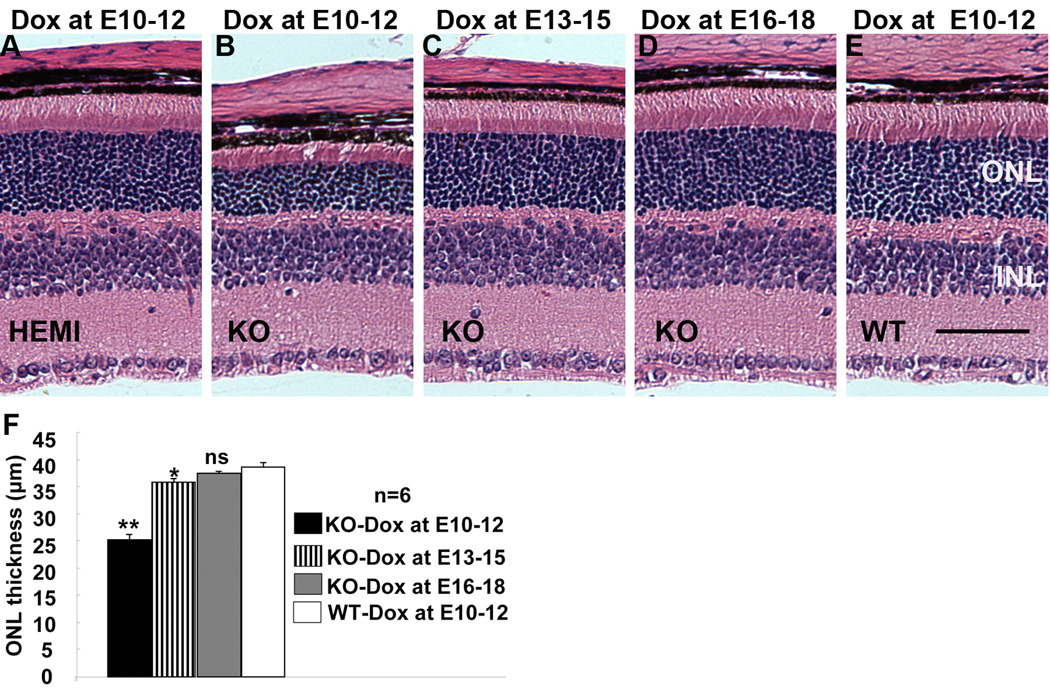
To determine whether the reduction in rod photoreceptor function correlated with the loss of rod photoreceptors, we further examined retinal morphology in conditional VEGF KO mice with dox induction at various times. Compared with WT controls, dox induction at E10-12 caused a significant reduction (34.8%) in the thickness of photoreceptor outer nuclear layer (ONL), with an apparent shortened layer of photoreceptor outer and inner segments (Figure 4B, F), in P21 conditional VEGF KO mice. As expected, dox induction at E13-15 demonstrated a less dramatic change in retinal morphology in P21 conditional VEGF KO mice. Induction at E13-15 resulted in a 7.3% reduction of photoreceptor ONL thickness in P21 conditional VEGF KO mice (Figure 4C, F). No significant change in retinal morphology was observed if dox induction occurred after E15 (Figure 4D, F). These results suggest that the loss of photoreceptor function is a direct consequence of photoreceptor loss in the conditional VEGF KO mice.
Figure 4.
Retinal morphology in P21 conditional VEGF KO mice after dox induction at various time for two days. A–E: H & E stained P21 retinal sections from homozygous and hemizygous (HEMI) conditional VEGF KO mice and WT controls with dox induction at E10-12, E13-15, or E16-18. ONL: outer nuclear layer. INL: inner nuclear layer. Scale bar: 40 µm. F: Average ONL thickness 0.5 mm from optic nerve. * and **: p<0.05 and p<0.001, respectively. ns: not significant. Inducible disruption of the RPE-derived VEGF at various times resulted in regulatable loss in rod photoreceptor function and photoreceptor ONL thickness in P21 conditional VEGF KO mice with dox induction before E15.
Integrity of the RPE and retinal vasculature
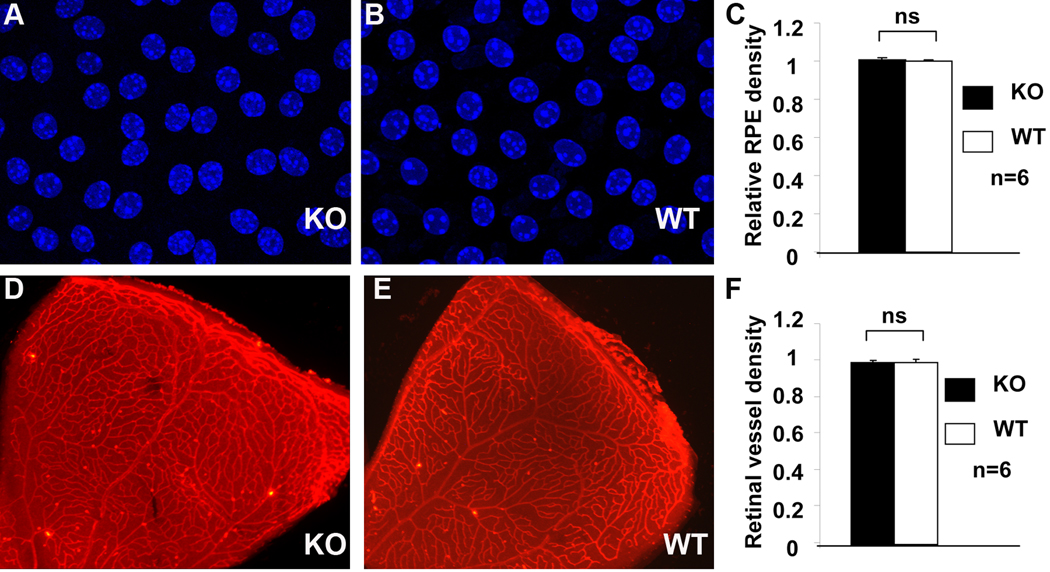
To determine whether the loss of the RPE-derived VEGF affects the integrity of the RPE and retinal vasculature, we also examined the RPE and retinal vessels, a measurement of the RPE and retinal vascular integrity, in conditional VEGF KO mice with dox induction at E10-12. We did not detect any loss of pigmentation in the RPE (Figure 4), a critical parameter of RPE integrity, in the conditional VEGF KO mice at P21. We further determined if the loss of the RPE-derived VEGF affected RPE density, another representation of RPE integrity. Confocal microscopic analysis of RPE flat-mounts demonstrated that there was no significant change in the size of the RPE cells, as well as the number of RPE cells in P21 conditional VEGF KO mice with dox induction at E10-12 (Figure 5A–C).
Figure 5.
RPE and retinal vessel density in conditional VEGF KO mice with dox induction at E10-12. A–C: Confocal microscopy analysis of RPE density with DAPI stained RPE/choroid flat-mounts at P21. D–F: Fluorescent microscopic analysis of retinal vessel density with lectin-stained retina at P10. No significant difference in RPE or retinal vessel density was observed in conditional VEGF KO mice with dox induction at E10-12.
To distinguish whether the abnormality in retinal morphology and function in E10-15 induced conditional VEGF KO mice were derived from a possible defect in retinal vascular development, we also examined the density of retinal vessels, a measurement of the integrity of retinal vasculature. Using lectin-staining method (Bai et al. 2009), we examined the pattern and density of retinal vessels after the RPE-derived VEGF was disrupted. Compared with WT mice, no detectable difference in the density and the pattern of P10 retinal vessels (Figure 5D–F) were observed in conditional VEGF KO mice with dox induction at E10-12 (Figure 5D–F). This result suggests that the RPE-derived VEGF is not required for the development of retinal vasculature.
Ultrastructural integrity of conditional VEGF KO mice with dox induction after E15
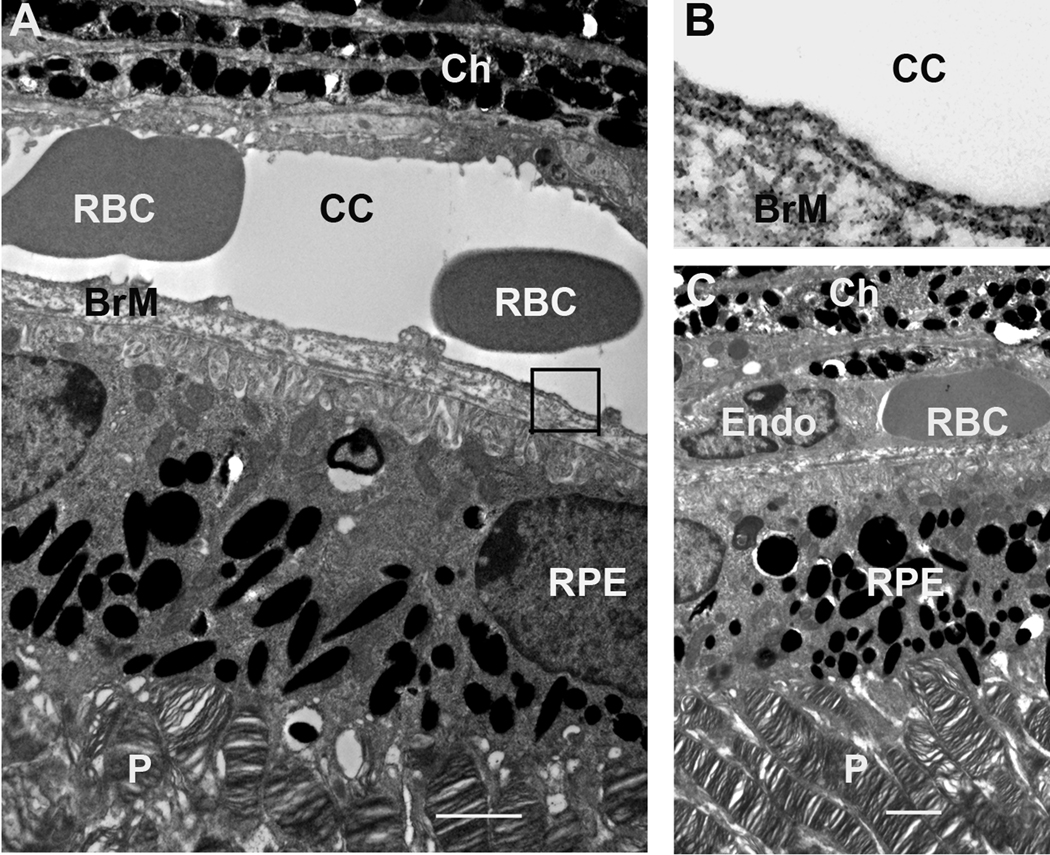
To ascertain if the loss of VEGF after E15 did not cause any developmental defects in the conditional VEGF KO mice, we performed transmission electron microscopic analysis in 1-month-old conditional VEGF KO mice with dox induction at E16-18. As expected, the conditional VEGF KO mice demonstrated normal ultrastructures in the RPE and its pigmentation, photoreceptor outer segment, and choriocapillaris (Figure 6). No detectable alteration in the degree of endothelial fenestrations was observed in the conditional VEGF KO mice (Figure 6A–B). In summary, the loss of RPE-derived VEGF after E15 did not cause any subtle developmental defects in the outer retina and choroid of the conditional VEGF KO mice.
Figure 6.
TEM analysis of the outer retina and choroid from 1-month-old conditional VEGF KO mice with dox induction at E16-18. A, C: Outer retina and choroid. B: Enlarged boxed area in A showing fenestrations. The scale bars in A and C equal to 2 µm. Ch: choroid; RBC: red blood cells; cc: choriocapillaris; BrM: Bruch’s membrane; Endo; endothelial cells; P: photoreceptors. Disruption of RPE-derived VEGF after E15 did not result in detectable ultrastructural changes in choroid, fenestration, RPE, and photoreceptors in mice.
DISCUSSION
Although little is known and much is yet to be revealed about the development of choroidal vasculature, previous studies suggest that fibroblast growth factors (FGFs) and VEGF may be involved in the formation of choroidal vessels (Zhao & Overbeek 2001, Rousseau et al. 2003, Gogat et al. 2004, Yi et al. 1998). In mammals VEGF and its receptors are highly expressed in the RPE and the underlying mesenchyme during the development of choriodal vasculature (Gogat et al. 2004, Zhao & Overbeek 2001, Yi et al. 1998). As VEGF is strongly expressed in the RPE from E10.5 until at least P7 in mice (Saint-Geniez et al. 2006, Zhao & Overbeek 2001), it was assumed that the RPE-derived VEGF was required for the formation and maturation of choroidal vasculature, before and after birth. For the first time, our study established a time line for the RPE-derived VEGF in this process. We demonstrated that the RPE-derived VEGF was required for choroidal formation from the beginning of the ocular vascular development to embryonic day 15. This timepoint coincides with a potential involvement of FGF-2 after E12.5 in choroidal development (Rousseau et al. 2003), suggesting that VEGF and FGFs may work together for proper formation of choroidal vasculature. As disruption of both FGF-1 and FGF-2 did not cause a severe defect in vascular development (Miller et al. 2000), it is likely that VEGF may be a major stimulator for initial choroidal development. However, RPE-derived VEGF appeared to be not required for choroidal maturation after E15. It is unclear what growth factor(s) are responsible for the growth of chorodial vessels after E15. The VEGF expressed in choroid could be a potential stimulator for its maturation through an autocrine mechanism. More studies are needed to determine the mechanisms of choroidal development.
Analysis of choroidal vascular density in P5 mice demonstrated a similar density of choroidal vessels as that examined at weanling age (data not shown). We elected to perform most of our analyses in weanling aged mice due to the fact that we were able to relate the morphological changes in choroidal vasculature and rod photoreceptors to retinal function. The peak for the appearance of rod photoreceptor cells were close to the birth day (P0) in mice (Cepko et al. 1996). In this study photoreceptor loss was observed in conditional VEGF KO mice with dox induction before E15. This result suggests that the loss of photoreceptors is likely a direct consequence of a change in choroidal integrity. However, we cannot exclude a possibility that RPE-derived VEGF may play a directly role in early stage of photoreceptor development or the development of photoreceptor progenitor cells. We previously used a VMD2-Cre line and generated conditional VEGF KO mice for Müller cells (Bai et al. 2009). While this VMD2-Cre line showed a much stronger Cre activity in neural retina during embryonic development (Ueki et al. 2009), the conditional VEGF KO mice did not cause any developmental defects in choroidal and retinal vasculatures and retinal morphology and function under normal conditions (Bai et al. 2009). Therefore, the presence of a residual Cre activity in neural retina in our VRC2 mice (Figure 2A–B), which in turn resulted in a marginal reduction of VEGF in the retina, is unlikely to caused defects in choroidal vasculature and retinal morphology and function. Since VEGF is a potential survival factor for photoreceptors (Saint-Geniez et al. 2008), we cannot completely exclude a possibility that down-regulation of VEGF in the Müller cells could partially account for photoreceptor loss in conditional VEGF KO mice with defects in choroidal vasculature. Our results that the loss of the RPE-derived VEGF did not cause any apparent changes in retinal vascular density suggested that the RPE-derived VEGF does not play a significant role in retinal vascular development under normal conditions.
A previous study demonstrated that the RPE-derived VEGF is required for choroidal development at E10.5 (Marneros et al. 2005) and the loss of RPE-derived VEGF causes dramatic defects in the eye, including absence of choriocapillaris, occurrence of microphthalmia, abnormal RPE and its pigmentation, loss of photoreceptors and INL layer neurons, and completely diminished visual function. Our study supports their conclusion about the role of the RPE-derived VEGF in choroidal development. However, it is worthwhile to point out the differences in the results. We have not observed the loss of INL neurons as well as any abnormality in the RPE and its pigmentation in our conditional VEGF KO mice. Occasionally we observed micropthalmia in the conditional VEGF KO mice with dox induction at E10 (data not shown), which is likely an inherent nature of transgenics (Jaenisch 1988) that yielded a Cre expression pattern more closely resemble to that in their study (Marneros et al. 2005). Nevertheless, we did not observe the loss of INL neurons in conditional VEGF KO mice with microphthalmia. Given that abnormal development of choroid did not cause INL neuron loss in a previous study (Rousseau et al. 2000), the loss of INL neurons in their study may be a consequence of VEGF disruption in the neural retina, due to Cre expression controlled by the promoter for tyrosinase-related protein-1 (TRP-1) promoter (Marneros et al. 2005, Mori et al. 2002). The temporal and spatial Cre expression conferred by TRP-1 promoter may also result in the defect in the RPE it pigmentation in their study (Marneros et al. 2005).
Numerous studies have documented the biochemical and pharmacological functions of VEGF, the cellular mechanisms of VEGF action in the retina are virtually uninvestigated. The use of cell-specific gene KO approach extends our knowledge to a more defined cellular targets or tissues. However, in some cases such as the one discussed here, investigating the function of a gene may be dependent on our ability to turn on or off a molecular switch for a gene at a desired time during the lifespan of an animal. An inducible gene activation or inactivation system provides a superior advantage. While our inducible system is not ideal and may have some inherent problems due to the leakiness of the system and the timing of the promoter activity (Le et al. 2008, Zhu et al. 2010, Ueki et al. 2009), we were capable of establishing a mouse model of inducible RPE-specific VEGF KO with regulatable phenotypes in choroidal vasculature and retinal morphology and function. In addition to this study, a major benefit of having such a novel genetic system is its usefulness in the analysis of post-developmental function of the RPE-derived VEGF, a current focus of our laboratory.
This study utilizes immunostained choroidal vessels for semi-quantitative analysis of mouse choroidal vascular density. Choroid/RPE interaction provides approximately 70 to 80 percent of retinal blood circulation and thus is vital to the function and maintenance of the retina. Many attempts have been made to quantify choroidal vascular density. Immunohistostaining of alkaline phosphatase for choroidal vessels is effective with human and primates (McLeod et al. 2002, Otsuji et al. 2002). However, the method does not work well with mice (Dr. G. Lutty, personnel communication). Corrosion cast for mouse choroidal vessels (Majji et al. 2000) is not easily reproducible, at least not in our hands. The leaked fluorescently conjugated dextran interferes with visualization of choroidal vessels in fluorescin angiography. In summary, the methods for imaging and quantify mouse choroidal vessels are not well established. The experimental procedure described here allows us to visualize choroidal vascular density in a very short time. The images obtained in this study (Figure 2) gave a similar readout as that derived from corrosion cast (Majji et al. 2000). Although this semi-quantitative method may not be suitable for detecting subtle changes in the choroidal vasculature, a weakness of all other methods described above, this method can be used to detect and quantify a moderate change in choroidal vascular density, as well as the size of choroidal vessels, in a timely fashion.
ACKNOWLEDGEMENTS
We thank W. Zheng and Y.W. Le for technical assistance, Dr. N. Ferrara and Genentech Inc. for providing floxed VEGF mice, and J. Wilkerson and Image Core Facility at the Oklahoma Medical Research Foundation for transmission electron microscopy, and Core Facilities for services supported by NIH grants P20RR17703, P20RR024215, P30EY12190 and a grant from Research to Prevent Blindness, Inc. This study was supported by AHAF grant M2008-059, FFB grant BR-CMM-0808-0453-UOK; ADA grant 1-10-BS-94, OCAST grant HR09-058, and Unrestricted Research Award from Hope for Vision.
Footnotes
Disclosure: Authors declare no conflict interests relevant to this study.
References
- Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Archives of ophthalmology. 1995;113:1538–1544. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ma J, Guo J, Wang J, Zhu M, Chen Y, Le Y. Müller cell-derived VEGF is a significant contributor to ischemia-induced retinal neovascularization. J Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. The American journal of pathology. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J, Wylie CK, Gole G. Quantification of oxygen-induced retinopathy in the mouse. Investigative ophthalmology & visual science. 1997;38:1168–1174. [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development (Cambridge, England) 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Gogat K, Le Gat L, Van Den Berghe L, et al. VEGF and KDR gene expression during human embryonic and fetal eye development. Investigative ophthalmology & visual science. 2004;45:7–14. doi: 10.1167/iovs.02-1096. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bonin AL, Freundlieb S, Bujard H. Inducible gene expression systems for higher eukaryotic cells. Curr Opin Biotechnol. 1994;5:516–520. doi: 10.1016/0958-1669(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Developmental biology. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science (New York, N.Y. 1988;240:1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Jarve A, et al. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26:12439–12446. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Ash JD, Al-Ubaidi MR, Chen Y, Ma J, Anderson RE. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol Vis. 2004;10:1011–1018. [PubMed] [Google Scholar]
- Le Y, Gagneten S, Larson T, Santha E, Dobi A, v Agoston D, Sauer B. Far-upstream elements are dispensable for tissue-specific proenkephalin expression using a Cre-mediated knock-in strategy. Journal of neurochemistry. 2003;84:689–697. doi: 10.1046/j.1471-4159.2003.01573.x. [DOI] [PubMed] [Google Scholar]
- Le Y, Sauer B. Conditional gene knockout using cre recombinase. Methods Mol Biol. 2000;136:477–485. doi: 10.1385/1-59259-065-9:477. [DOI] [PubMed] [Google Scholar]
- Le Y, Zheng L, Zheng W, Agbaga M, Zhu M, Ash JD, Anderson RE. Mouse opsin promoter controlled expression of Cre recombinase in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- Le YZ, Zheng W, Rao PC, Zheng L, Anderson RE, Esumi N, Zack DJ, Zhu M. Inducible expression of cre recombinase in the retinal pigmented epithelium. Investigative ophthalmology & visual science. 2008;49:1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majji AB, Cao J, Chang KY, Hayashi A, Aggarwal S, Grebe RR, De Juan E., Jr Age-related retinal pigment epithelium and Bruch's membrane degeneration in senescence-accelerated mouse. Investigative ophthalmology & visual science. 2000;41:3936–3942. [PubMed] [Google Scholar]
- Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. The American journal of pathology. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Investigative ophthalmology & visual science. 2002;43:1986–1993. [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Molecular and cellular biology. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes/metabolism reviews. 1997;13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Mori M, Metzger D, Garnier JM, Chambon P, Mark M. Site-specific somatic mutagenesis in the retinal pigment epithelium. Investigative ophthalmology & visual science. 2002;43:1384–1388. [PubMed] [Google Scholar]
- Nishijima K, Ng YS, Zhong L, et al. Vascular Endothelial Growth Factor-A Is a Survival Factor for Retinal Neurons and a Critical Neuroprotectant during the Adaptive Response to Ischemic Injury. The American journal of pathology. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004 doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- Otsuji T, McLeod DS, Hansen B, Lutty G. Immunohistochemical staining and morphometric analysis of the monkey choroidal vasculature. Experimental eye research. 2002;75:201–208. doi: 10.1006/exer.2002.2020. [DOI] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Progress in retinal and eye research. 2008 doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau B, Dubayle D, Sennlaub F, Jeanny JC, Costet P, Bikfalvi A, Javerzat S. Neural and angiogenic defects in eyes of transgenic mice expressing a dominant-negative FGF receptor in the pigmented cells. Experimental eye research. 2000;71:395–404. doi: 10.1006/exer.2000.0892. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Larrieu-Lahargue F, Bikfalvi A, Javerzat S. Involvement of fibroblast growth factors in choroidal angiogenesis and retinal vascularization. Experimental eye research. 2003;77:147–156. doi: 10.1016/s0014-4835(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS ONE. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Investigative ophthalmology & visual science. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- Schwesinger C, Yee C, Rohan RM, et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. The American journal of pathology. 2001;158:1161–1172. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. The Journal of clinical investigation. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investigative ophthalmology & visual science. 1996;37:290–299. [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Ash JD, Zhu M, Zheng L, Le Y. Expression of Cre recombinase in retinal Müller cells. Vision Research. 2009;49:615–621. doi: 10.1016/j.visres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cepko CL. Flk-1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci. 1996;16:6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Mai LC, Uyama M, Yew DT. Time-course expression of vascular endothelial growth factor as related to the development of the retinochoroidal vasculature in rats. Exp Brain Res. 1998;118:155–160. doi: 10.1007/s002210050267. [DOI] [PubMed] [Google Scholar]
- Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001;7:277–282. [PubMed] [Google Scholar]
- Zheng L, Anderson RE, Agbaga MP, Rucker EB, 3rd, Le YZ. Loss of BCL-XL in rod photoreceptors: Increased susceptibility to bright light stress. Investigative ophthalmology & visual science. 2006;47:5583–5589. doi: 10.1167/iovs.06-0163. [DOI] [PubMed] [Google Scholar]
- Zhu M, Ueki Y, Ash JD, Zheng L, Le YZ. Unexpected transcriptional activity of human VMD2 promoter. Adv Exp Med Biol. 2010 doi: 10.1007/978-1-4419-1399-9_24. In Press. [DOI] [PubMed] [Google Scholar]