Abstract
This review illustrates notable recent progress in the field of medicinal bioinorganic chemistry with many new approaches to the design of innovative metal-based anticancer drugs emerging. Current research addressing the problems associated with platinum drugs has focused on other metal-based therapeutics that have different modes of action, and on prodrug and targeting strategies in an effort to diminish the side-effects of cisplatin chemotherapy.
Keywords: bioinorganic, anticancer drugs, cisplatin, transition metals, ruthenium, osmium, platinum, metal-based drugs photoactivation, redox
1. Introduction
Metals, and in particular transition metals, offer potential advantages over the more common organic-based drugs, including; a wide range of coordination numbers and geometries, accessible redox states, ‘tune-ability’ of the thermodynamics and kinetics of ligand substitution, as well as a wide structural diversity. Medicinal inorganic chemistry is a thriving area of research [1-4], initially fueled by the discovery of the metallopharmaceutical, cisplatin about 40 years ago. Today, over 30 years after its approval as a chemotherapeutic agent, cisplatin is still one of the world’s best selling anticancer drugs. It is mainly used in the treatment of ovarian, head and neck, bladder, cervical and lymphomas cancers. Over the past decades, a large number of cisplatin analogs have been screened as potential antitumor agents, but of these only two, carboplatin and oxaliplatin, have entered world-wide clinical use [5].
Regardless of the achievements of current platinum drugs, there are some major drawbacks: they are efficient only for a limited range of cancers, some tumors can have acquired or intrinsic resistance, and they often cause severe side-effects like nausea, bone marrow suppression and kidney toxicity. Although currently about ten other platinum compounds are in clinical trials, the cisplatin derivatives have not been able to address sufficiently many of the disadvantages associated with cisplatin. There is, therefore, a need for new approaches that are purposefully designed to circumvent these drawbacks. The field of metal-based anticancer drug design can be divided into two different approaches; classical and non-classical chemotherapeutics [6,7].
Cisplatin’s mode of action involves distortion to DNA. Here we use the term classical chemotherapeutics to refer to drugs that target DNA. Classical drugs based on other metals can address the problems associated with platinum drugs and are attracting increasing interest. At present, a number of metal-based compounds are known to have promising antiproliferative effects in a wide range of tumors with novel modes of DNA binding; this will be discussed in the section “Classical non-platinum metal compounds”.
Recent progress in the field of cell biology has resulted in the discovery of receptors and growth factors that are up-regulated in cancer cells. These provide new targets for anticancer drug design. Examples of non-classical chemotherapeutics with proteins and enzymes as targets are emerging; this will be discussed in the section “Non-classical metal compounds”.
In addition, there is continuing interest in designing drugs that can be activated selectively in the tumor by cellular processes or controlled external activation. Extensive studies on the behavior of metal complexes under biologically-relevant conditions have revealed some promising approaches for targeted anticancer drug design with classical and non-classical targets (section 4).
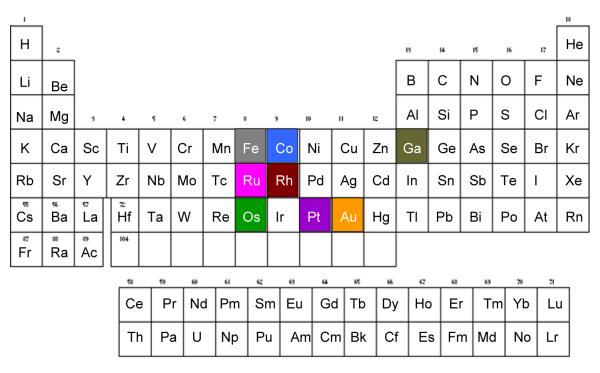
The metals discussed in this review are highlighted in Figure 1.
FIGURE 1.
Periodic table with color coding for the metals discussed in this review
Classical non-platinum metal compounds
Nuclear DNA is considered to be the ultimate target of cisplatin and related platinum therapeutics. Prior to DNA attack, cisplatin undergoes aquation. Although the activated (aquated) cisplatin can interact with other biomolecules, its antitumor activity derives from its capability to form bifunctional DNA cross-links, which causes the DNA to distort (kink, Figure 2a [8]). The platinum-induced kink in the DNA is considered to be the critical lesion leading to a chain of events which includes protein recognition (e.g. by HMG) and eventual apoptosis [9].
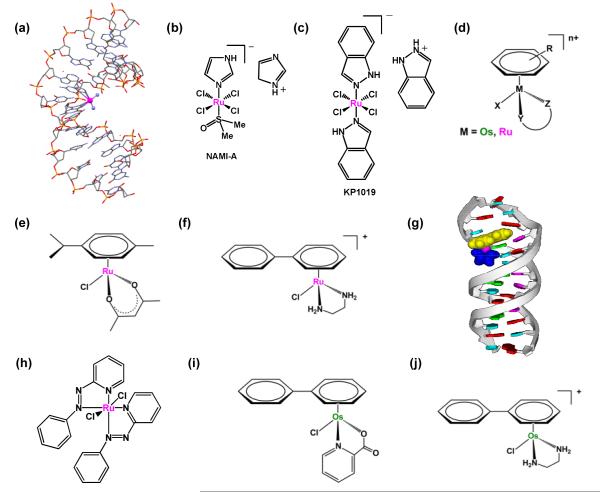
FIGURE 2.
Examples of metal-based compounds that target DNA. (a) X-ray structure showing the kinking of DNA by cisplatin (pdb1aio) (b) NAMI-A. (c) KP1019. (d) General structure of organometallic ruthenium(II) and osmium(II) arene complexes. (e, f) Examples of cytotoxic RuII arene complexes. (g) model showing intercalation of a RuII arene complex into DNA. (h) [γ-Ru(azpy)2Cl2]. (i, j) Examples of cytotoxic OsII arene complexes.
In this section some examples of other metal-based therapeutics that target DNA are given. Their exploration may result in drugs that produce distinctly different lesions on DNA and overcome acquired or inherent cisplatin resistance, or may prove to be more active towards tumors which are non-responsive to current chemotherapy.
Ruthenium
Ruthenium compounds containing RuII or RuIII are considered to be suitable candidates for anticancer drug design, since they exhibit a similar spectrum of kinetics for their ligand substitution reactions as platinum(II). A number of ruthenium compounds have been shown to display promising anticancer activity and two ruthenium(III) complexes have entered clinical trials, trans-[RuCl4(DMSO)(Im)]ImH (NAMI-A, where Im = imidazole, Figure 2b), and trans-[RuCl4(Ind)2]IndH (KP1019, where Ind = indazole, Figure 2c). NAMI-A is more active against metastases than against primary tumors [10]. In contrast, the structurally-similar KP1019 is active against primary tumors [11]. It is believed that the activity of the ruthenium(III) compounds is dependent on the in vivo reduction to the more reactive ruthenium(II) species [12]. This has led to increased interest in the anticancer potential of ruthenium(II) compounds. Much work has focused on the anticancer potential of half-sandwich Ru(II) arene complexes of the type, [(η6-arene)Ru(YZ)(X)], where YZ is a bidentate chelating ligand and X is a good leaving group e.g. Cl, Figure 2d). These half-sandwich “piano-stool” complexes offer great scope for design, with the potential to vary each of the building blocks (arene, chelated ligand YZ, and monodentate ligand X) to allow modifications of thermodynamic and kinetic parameters.
Some of the half-sandwhich Ru(II) arene complexes display promising in vitro and in vivo anticancer activity [13] (Figure 2e and f). These monofunctional compounds bind coordinatively to N7 of guanine in DNA which can be complemented by intercalative binding of an extended arene as well as specific hydrogen-bonding interactions between the chelating ligand and C6O of guanine [14]. These additional interactions result in unique modes of binding to duplex DNA and structural distrortions that differ significantly from those caused by cisplatin [15] (Figure 2g). This may explain why these compounds are not cross-resistant with cisplatin. Indeed, it has been found that increasing the size of the coordinated arene increases their activity in the human ovarian cancer cell line [13]. Changing the chelating ligand in these ruthenium arene complexes also appears to have an enormous effect on their kinetics and even changes their nucleobase selectivity [16]. It is believed that in vivo the aquation of the chloro complex is largely suppressed in intracellular fluids where high chloride concentrations are found (100 mM), whereas in the cell nucleus, where the chloride concentration is lower (4 mM), the complex forms predominately the active aqua species [17].
Another interesting class of ruthenium compounds containing arylazopyridine (azpy) ligands shows promising cytotoxic activity that is structurally-dependent. Three of the five possible isomers of [Ru(azpy)2Cl2] (α, β, λ, Figure 2h) have been reported, where the α isomer represents the cis,trans,cis, β the cis,cis,cis and γ the trans,cis,cis orientation of the chlorides, the pyridine and the azo nitrogens, respectively. The α and λ isomers show higher toxicities compared to the β isomer [18]. Indeed, DFT calculations suggest that the ability of the isomers to intercalate into DNA decreases from λ >α > β isoforms [19]. Recently several isomeric forms of multinuclear ruthenium complexes with bridging azpy ligands have been reported [20]; t he γ/γ isomeric form exhibits the highest cytotoxicity, over 30-fold higher than cisplatin in breast cancer cells.
Osmium
The anticancer potential of osmium, the heavier congener of ruthenium has recently been explored. Osmium complexes have a reputation for being either toxic (OsO4) or substitution-inert (OsII and OsIII complexes), and as a consequence their therapeutic potential has been little explored. However, Sadler and coworkers have synthesized some osmium(II) arene complexes with cancer cell cytotoxicity that is comparable to the clinical drugs carboplatin and cisplatin (Figure 2i and j) [21,22]. This was achieved by systematically varying the nature of the chelating ligand to fine-tune both the kinetics and thermodynamics of reactions of the osmium compounds in aqueous solution [23,24]. The OsII arenes are believed to interact with DNA in a similar fashion as their ruthenium analogs, i.e. binding to N7 of guanine in combination with H-bonding and non-covalent arene DNA interaction. Interestingly, binding of OsII arenes to calf thymus DNA gives rise to a large unwinding of double-helical DNA, unlike cisplatin which causes DNA bending [25]. These osmium complexes do not display cross-resistance with cisplatin towards cancer cells, suggesting promise for addressing the problem of intrinsic or acquired resistance in chemotherapy.
Non-classical metal compounds
The traditional platinum-based therapeutics and the organometallic complexes discussed in the previous section owe their anticancer activity to their non-repairable interaction with DNA and make use of the fast replication and mitotic processes of malignant cells. Drugs that are able to target cellular signaling pathways over-expressed in cancer cells provide attractive approaches for anticancer drug design. Although less studied than the metallodrug-DNA interactions, some examples of metallodrugs that interact with specific proteins and enzymes are given below.
Gold
Gold complexes are well known pharmaceuticals, mainly for their application as drugs to treat rheumatoid arthritis. Some tetrahedral Au(I) phosphine complexes display a wide spectrum of anticancer activity in vivo, especially in towards cisplatin-resistant cell lines [26]. Their cytotoxicity is mediated by their ability to inhibit mitochondrial human glutathione reductase (hGR) and thioredoxin reductase (hTrxR) irreversibly [27]. In particular, phosphol-containing gold(I) complexes (Figure 3a) are highly potent, nanomolar inhibitors of both hGR and hTrxR [28]. hTrxR is associated with many cellular processes such as antioxidant defence and redox homeostasis, and is found at elevated levels in human tumor cell lines.
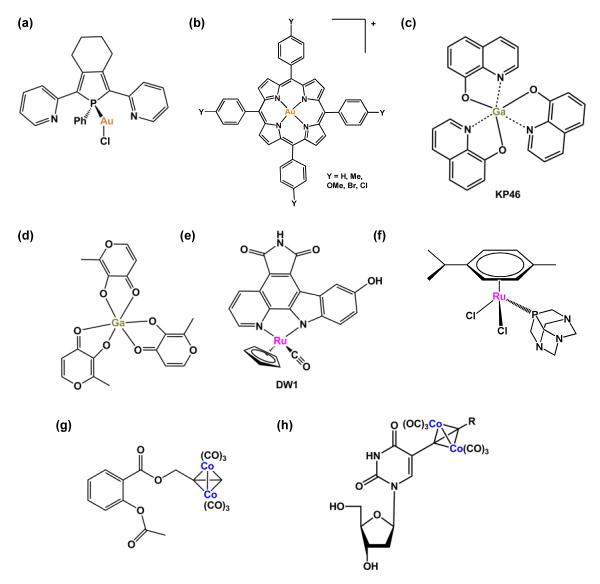
FIGURE 3.
Examples of metal-based anticancer drugs that target proteins and enzymes. (a) Gold(I) phosphole complex. (b) Gold(III) meso-tetraarylporphyrins complexes. (c) KP46. (d) Gallium tris-maltolate. (e) DW1. (f) RuII arene complex containing a monodentate phosphaadamantane (pta) ligand. (g) Cobalt-alkyne analogue of the anti-inflammatory drug aspirin. (h) Hexacarbonyl dicobalt complex containing a nucleoside ligand.
Gold(III) is isoelectronic and isostructural with Pt(II), therefore gold(III) analogues of Pt(II) drugs were investigated for their biological potential soon after the appearance of cisplatin in the clinic. Unfortunately they were found to be relatively unstable as well as easily reduced to metallic gold under physiological conditions [29]. In recent years, however, several gold(III) compounds that incorporate ligands to increase the stability to the gold(III) centre have been reported. For example, gold(III) porphyrins(Figure 3b) exhibit in vitro and in vivo activity in hepatocellular and nasopharyngeal carcinoma [30]. Other Gold(III) compounds with promising biological activity include gold(III) bipyridyl compounds [31], dinuclear gold(III) oxo complexes [32] and gold(III) dithiocarbamates [33]. For these compounds the mitochondria and the proteasome are thought to be targets [34].
Gallium
The chemical behavior of gallium(III) is similar to that of ferric iron (FeIII), but differs in that GaIII is non-reducible under physiological conditions whereas FeIII is readily reduced to FeII. This difference provides therapeutic potential for gallium(III). Currently, two compounds, gallium tris-8-quinolinolate (KP46) and gallium tris-maltolate (Figure 3c and d) are being investigated in clinical trials [35]. Their mechanism of action is associated with the inhibition of ribonucleotide reductase (RR). The enzyme RR catalyses the conversion of ribonucleotides to deoxyribonucleotides and is produced during the transition from the G1 to the S phase of the cell cycle as a prerequisite for DNA replication, and is highly expressed in tumor cells.
Ruthenium
Interestingly, Meggers et al. have developed kinetically-inert organometallic complexes that act as scaffolds to mimic the organic enzyme inhibitor staurosporine, a potent inhibitor of various kinases. In these metal-based inhibitors the carbohydrate unit of staurosporine is replaced with ruthenium fragments (Figure 3e) [36]. Structural variation by substitution of the ligands on the metal results in picomolar protein kinase inhibitors. They are highly toxic towards human melanoma cells. The ruthenium staurosporine bioconjugate was recently investigated as an inhibitor for glycogen synthase kinase (GSK-3β), a major regulator of p53 localization and expression. The ruthenium staurosporine complex acts as a potent p53 activator and induces apoptosis in otherwise chemoresistant melanoma cells [37].
A family of organometallic RuII compounds containing monodentate phosphaadamantane (pta) ligands, exhibits moderate in vitro activity and some compounds show no activity in healthy cells up to millimolar concentrations. The pta compounds show little activity against primary tumors in vivo, although they exhibit some capacity to reduce lung metastases derived from a mammary carcinoma xenograft grown in mice [38]. The cytotoxicity of the RuII pta compound, [Ru(η6-p-cymene)Cl2(pta)] (Figure 3f), in EAC cells is thought to be mediated by mitochondrial and JNK-p53 pathways [39].
Cobalt
Some hexacarbonyl dicobalt complexes exhibit promising activity against several human cancer cell lines [40]. In particular, a cobalt-alkyne analogue of the anti-inflammatory drug, aspirin, is potently active in breast cancer cell lines (Figure 3g). Its toxicity has been attributed to its ability to inhibit cyclooxygenase (COX-1 and 2) [41]. This appears to be a promising approach, since inhibition of cyclooxygenase delays tumor growth as well as improves response to conventional cancer therapies.
Another hexacarbonyl dicobalt series of compounds containing nucleoside ligands (Figure 3h) displays antiproliferative activities with IC50 values in the range of 5–50 μM in human breast cancer cell lines [42].
The family of cysteine cathepsin proteases has recently been validated as an important enzymatic class to target in cancer. More specifically, cathepsin B and L have been shown to be involved in multiple stages of tumor development. Metal-based compounds reported to show promising inhibition of cathepsin B include linear gold(I) complexes containing thiolate and phosphine ligands [43], dinuclear palladium complexes (biphosphinic palladacycle complexes) [44], and several oxorhenium(V) complexes [45].
Other metal-based drugs in preclinical or early phase of clinical development not mentioned in the previous two sections contain vanadium, rhodium, copper, bismuth and lanthanide metals [46-48].
Prodrug stategies
Delivery of metal-based drugs to their targets poses one of the biggest challenges in cancer chemotherapy. A drug needs to be sufficiently reactive to bind to the biological target, but not so reactive that it will be deactivated by the many biomolecules encountered on the way. One strategy involves the design of prodrugs. Prodrugs are drug derivatives that can undergo a transformation in vivo to release the active species, with improved physiochemical, biopharmaceutical and pharmacokinetic properties. For metal-based therapeutics, this prodrug activation may be realized by a photochemical process [49], oxidation or reduction of the metal, or ligand substitution. This requires extensive knowledge of ligand substitution rates, redox potentials, photochemistry and choice of metal but also the effect of the other coordinated ligands in the complex.
In recent years, the focus has been on making prodrugs selective. In order to achieve this, the prodrug has to be activated by specific physiological characteristics of tumors, like the reducing environment of the cell, pH, or cancer cell permeability. Some examples of different prodrug strategies including tumor-selectivity and drug delivery strategies will be discussed in the following paragraphs.
Photoactivation as a prodrug strategy
The activation of a metal-based prodrug can be realised by photochemical means. The advantages of using light as an external stimulus is that it allows local treatment of the tumor, so minimizing side-effects. The poor penetration of tissue by short-wavelength light is, however, a serious limitation: longer wavelength light (red) penetrates more deeply than UVA light. For a photochemical treatment to be effective in the clinic, the wavelength of activation should be within the range 350-900 nm; shorter wavelengths cause damage to tissue and higher wavelengths usually carry insufficient energy to activate the prodrug. Currently used in the clinic, Photodynamic therapy (PDT) treats readily accessible tumors (i.e. skin, neck, bladder) by the administration of a non-toxic photosensitizer and subsequent irradiation of the tumor site [50]. Upon irradiation, the photosensitizer, typically a porphyrin, becomes excited and this energy is transferred to ground-state triplet oxygen (3O2) to form highly reactive singlet oxygen (1O2), leading to cell death. Metal-bound porphyrins can have marked effects on the tumor-localizing properties of the photosensitizer. Indeed, metal-containing photosensitizers for cancer treatment are currently well represented in clinical trials: including a lutetium porphyrin derivative (Lutetium texaphyrin) [51,52], CGP55847 (Zn), Photosense (Al), and Purlytin (Sn) [53]. All these photo-sensitizers need oxygen to generate their cytotoxic effects; however cancer cells are often deficient in oxygen.
Rhodium
Recently, several Rh and Ru complexes have been shown to exhibit oxygen-independent light-induced anticancer activity. For example, Barton et al. have shown that several octahedral RhIII complexes with extended diimine ligands (Figure 4a), bind only weakly to DNA in the absence of light but on photoactivation bind to DNA and cleave the DNA backbone with high specificity for mismatched DNA [54,55]. Recently the effects of variation of the ancillary ligand on the ability of these compounds to target DNA mismatches in vitro and in vivo have been explored [56].
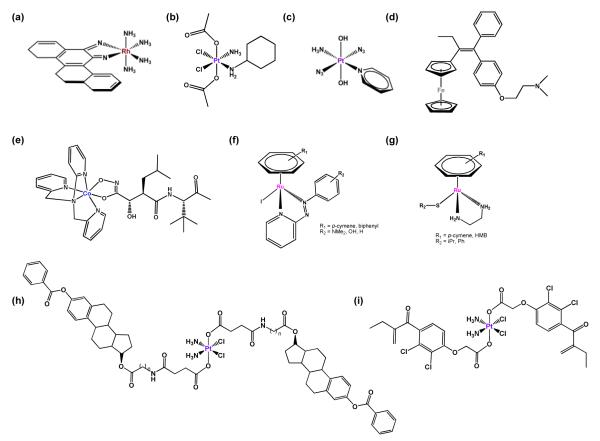
FIGURE 4.
Prodrug strategies. (a) Example of a photoactivatable octahedral RhIII complex with an extended diimine ligand. (b) Satraplatin. (c) Example of a photoactivatable trans-azido platinum(IV) prodrug. (d) Ferrocifen. (e) Cobalt-marimastat bioconjugate. (f) RuII arene complexes containing iodo and phenylplazopyridine ligand. (g) RuII arene complexes containing a thiolate ligand. (h) Pt(IV)-estradiol bioconjugate. (i) Ethacraplatin.
Platinum
The general kinetic inertness of PtIV complexes compared to PtII complexes has been widely exploited in the design of potential prodrugs. The PtIV compound satraplatin, [Pt(cha)Cl2(OAc)2(NH3)] (where cha is cyclohexylamine, Figure 4b), has recently been abandoned in phase III clinical trials for the treatment of hormone-refractory prostate cancer [57]. Substitution reactions of PtIV complexes under physiological conditions are very slow or do not take place at all. Therefore, intracellular reduction to PtII is thought to be essential for cytotoxic activity. This reduction may be achieved by cellular reducing agents (discussed in the next section) or by irradiation with light, allowing site-specific activation of the drugs [58].
Trans-dihydroxido platinum(IV) prodrugs containing two azido ligands in trans or cis positions relative to each other are non-toxic in the dark but show cytotoxicity towards various human cancer cell lines upon irradiation [59,60]. In particular, a PtIV diazido complex containing pyridine trans to ammonia, trans,trans,trans-[Pt(N3)2(OH)2(py)(NH3)] (Figure 4c), is up to 80 times more cytotoxic than cisplatin in ovarian cancer cells upon irradiation, whilst inactive and stable towards biological reductants in the dark. One photoactivation pathway for these complexes (amongst several possibilities) involves ligand-to-metal charge transfer (LMCT) from the azido ligands to PtIV, resulting in reduction to the reactive PtII species. The produces azidyl (N3·) radicals which rapidly combine and decompose to produce nitrogen gas. Aquation of the PtII compound and subsequent binding to DNA (and possibly proteins) leads to its cytotoxic effect. Notably, the mechanism of DNA platination by the trans-azide complex is different from cisplatin and cell death is not solely dependent on activation of the caspase 3 pathway [61]. It is apparent that photoactivation can produce excited states with unusual reactivity and provide routes to intervening in biological pathways not available to ground-state drugs.
Redox activation as a prodrug strategy
The redox behavior of metal complexes offers chemical reactivity that is not accessible to purely organic molecules and therefore can yield novel metallodrugs with new mechanisms of drug action. For the prodrug to be successful, the metal complex should possess biologically-accessible redox potentials. Tuning their properties to the reducing environments of tumor cells may lead to novel targeted strategies.
Iron
Well established examples are ferrocene derivatives of the breast cancer drug tamoxifen (Figure 4d) [62,63]. They display potent activity against both estrogen-dependent and estrogen-independent breast cancer cells, whereas tamoxifen alone is active only against estrogen-dependent cells. The activity of the ferrocene tamoxifen derivatives (ferrocifens) in estrogen-independent cells is attributed to the redox properties of the FeII complex, leading to oxidative damage to DNA. The mechanism of action of ferrocifens in estrogen-dependent cells is likely to be similar to that of tamoxifen itself. It is noteworthy that one of the most active ferrocene derivatives exhibits less toxicity than tamoxifen itself. A recent study on ferrocene tamoxifen derivatives with modified side chains has established the minimal structural requirements to obtain cytotoxicity [64].
Cobalt
A prodrug approach for the inhibition of enzymes by cobalt complexes has been explored by Hambley et al. [65] Their strategy involves the complexation of the matrix metalloproteinase (MMP) inhibitor marimastat to a CoIII complex to achieve selective delivery of MMP to the tumor (Figure 4e). The resulting CoIII complex provides an inert carrier system for the transportation of the inhibitor. The prodrug is activated by a bioreduction pathway producing a labile CoII complex, which results in the release of the MMP inhibitor. The hypoxic nature of the tumor should prevent oxidation back to the cobalt(III) complex thus achieving selective release in tumor cells. Indeed, increased cytotoxicity was observed for the cobalt prodrug in in vivo tumors in mice compared to the MMP inhibitor alone. Unfortunately, both the inhibitor and the prodrug promote metastasis.
Ruthenium
Half-sandwich ruthenium arene complexes containing iodo and phenylazopyridine ligands (Figure 4f) exhibit remarkable inertness towards ligand substitution in aqueous solution, but are highly toxic in human ovarian and lung cancer cells. Whereas azopyridine ligands alone are difficult to reduce, these azopyridine RuII complexes have reduction potentials that are biologically accessible. The toxicity of these complexes can be attributed to their ability to induce redox reactions inside the cell leading to reactive oxygen species (ROS). Interestingly, the redox cycle involves the oxidation of the strong reducing agent glutathione present in millimolar concentrations, in the presence of micromolar concentrations of the ruthenium complex [66].
Organometallic ruthenium arene complexes containing thiolato ligands (e.g. Figure 4g) can be activativated by oxidation [67]. Under physiologically-relevant conditions, the monodentate thiolato ruthenium complex is capable of efficient acceptance of an oxygen atom e.g. from an intermediate in glutathione (GSH) oxidation. This shows that GSH can act as a source of reactive oxygen species, able to induce oxidation of organometallic complexes which are themselves stable in air [68]. Ruthenium sulfenate adducts appear to be more labile than the parent thiolato complexes (e.g. towards DNA binding) perhaps through protonation of the sulfenato oxygen atom.
Platinum
The relative kinetic inertness of six-coordinate octahedral PtIV complexes can be used as a prodrug approach in order to overcome some of the problems associated with (four-coordinate. square-planar) cisplatin. For this, intracellular reduction to PtII is essential for activation and cytotoxic activity. The two extra ligands in the axial positions allow the inclusion of different kinds of bioactive ligands which may lead to platinum anticancer complexes with improved efficacy.
In one such approach, an attempt to target specifically estrogen-positive, ER(+), malignancies, such as breast and ovarian cancers, led to the design of several estrogen-tethered platinum(IV) complexes (Figure 4h) [69]. This is a cancer drug target because estrogen-receptor-positive breast cancer cells treated with estrogen are more sensitive to cisplatin. Upon intracellular reduction, the complex releases cisplatin and two molecules of estrogen derivative. The latter induces upregulation of the high-mobility group domain protein HMGB1 in a human breast cancer cell line. The HMGB1 protein shields platinated DNA from nucleotide-excision repair.
In an attempt to overcome cisplatin resistance, a similar approach has been used to inhibit glutathione-S-transferase (GST), the main cellular defence against xenobiotics. For this, Dyson et al. attached the GST inhibitor ethacrynic acid to a PtIV complex (ethacraplatin, Figure 4i) [70].
Effort in recent years has also been given to the design of novel drug delivery systems that are capable of increasing the cellular uptake as well as directed delivery of metallodrugs to tumor cells only. Interesting approaches include attachment of a platinum drug to a pH-sensitive polymer [71], attachment of a PtIV prodrug non-covalently to single-walled carbon nanotubes [72], encapsulation of platinum drugs in lipid-based nanocapsules [73] and attachment of the SV4-40T antigen nuclear localization signal to metallocenes [74].
Conclusions
Metal coordination complexes offer a very versatile platform for the design of novel anticancer agents. Their properties can be quite distinct from those of purely organic compounds. Particularly attractive for study are the first, second and third row transition metals, with their variable oxidation states, coordination numbers and ability to bind to a wide variety of types of ligands (e.g. halides, O, S, N, P, C). In particular metals in the second and third rows often exchange their ligands relatively slowly, on minutes-to-hours timescales which allow at least some of the original ligands to remain bound to the metal en route to the target site. In general (with some exceptions) because they can undergo ligand exchanges, metal complexes are prodrugs, ligand substitution can activate the metal complex towards binding to target molecules. A key element in the design process is the control of both the thermodynamics (state of equilibrium) and kinetics of ligand substitution events which can occur in vivo, for example the aquation of metal-chloride bonds as the concentration diminishes from extracellular to intracellular (cytoplasmic and nuclear) compartments.
Of interest too are both metal-centered and ligand-centered redox processes. The former can trigger activation by ligand release (e.g. reduction of substitution inert CoIII to labile CoII), and the latter the initiation of the production of reactive oxygen species (e.g. azopyridine RuII arenes) as part of the cytotoxic mechanism. The possibility of using light to activate metal complexes selectively in tumor cells is also an intriguing one. Reactions of excited-state metal complexes can be distinctly different from those of ground-state complexes, giving rise to the possibility of interfering in biochemical pathways with highly reactive novel species.
Cisplatin and the successive generations of platinum-based anticancer drugs (carboplatin and oxaliplatin) have demonstrated that metal coordination complexes can play an important part in anticancer treatment regimes in the clinic. The exploration of other transition metal complexes, as well as targeting and activation strategies, should lead to future generations of drugs which can overcome some of the disadvantages associated with cisplatin therapy, including the reduction of side-effects, widening the spectrum of activity, and resistance.
Finally it is worth emphasizing the subtlety with which ligands control the reactivity of transition metal ions, and also the reciprocal effects which metal ions can have on the properties of ligands. Both the metal and the ligands can play important roles in the recognition of target sites. The subtle effects exerted by ligands can be both steric and electronic in nature. Modern theoretical methods (e.g. Amsterdam Density Functional Theory) and techniques (e.g. high resolution electrospray mass spectrometry, multinuclear polarization transfer NMR spectroscopy) are likely aid our understanding of the chemical and biochemical reactivity of metal complexes and the construction of meaningful structure-activity relationships. For this purpose, studies of the chemistry of metal complexes under physiologically-relevant conditions (e.g. biological screening conditions) become very important.
Acknowledgements
We thank the MRC, EPSRC, EC (COST and Marie Curie) and ERDF (Science City and AWM) for support, and our co-workers for collaborations.
References
- 1.Hambley TW. Metal-Based Therapeutics. Science. 2007;318(5855):1392–1393. doi: 10.1126/science.1150504. [DOI] [PubMed] [Google Scholar]
- 2.Sadler PJ, et al. Metals in medicine. In: Bertini I, Gray HB, Stiefel EI, Selverstone VL, editors. Biological inorganic chemistry: structure and reactivity. University Science Books; U.S.: 2007. pp. 95–135. [Google Scholar]
- 3.Sessler JL, et al. Medicinal inorganic chemistry. In: Sessler JL, Doctrow SR, McMurry T, Lippard SJ, editors. ACS Symp. Ser.; 903. CAPLUS; 2005. p. 453. [Google Scholar]
- 4.Storr T, et al. Design of targeting ligands in medicinal inorganic chemistry. Chem. Soc. Rev. 2006;35(6):534–544. doi: 10.1039/b514859f. [DOI] [PubMed] [Google Scholar]
- 5.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 6.Bergamo A, Sava G. Ruthenium complexes can target determinants of tumor malignancy. Dalton. Trans. 2007;(13):1267–1272. doi: 10.1039/b617769g. [DOI] [PubMed] [Google Scholar]
- 7.Fricker SP. Metal based drugs: from serendipity to design. Dalton. Trans. 2007;(43):4903–4917. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- 8.Sherman SE, et al. X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3)2{d(pGpG)}] Science. 1985;230(4724):412–417. doi: 10.1126/science.4048939. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 10.Rademaker-Lakhai JM, et al. A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004;10(11):3717–3727. doi: 10.1158/1078-0432.CCR-03-0746. [DOI] [PubMed] [Google Scholar]
- 11.Jakupec MA, et al. KP1019 (FFC14A) from bench to bedside: preclinical and early clinical development- an overview. Int. J. Clin. Pharmacol. Ther. 2005;43(12):595–596. doi: 10.5414/cpp43595. [DOI] [PubMed] [Google Scholar]
- 12.Clarke MJ, et al. Non-platinum chemotherapeutic metallopharmaceuticals. Chem. Rev. 1999;99(9):2511–2533. doi: 10.1021/cr9804238. [DOI] [PubMed] [Google Scholar]
- 13.Aird RE, et al. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer. 2002;86(10):1652–1657. doi: 10.1038/sj.bjc.6600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, et al. Organometallic ruthenium(II) diamine anticancer complexes: arene-nucleobase stacking and stereospecific hydrogen-bonding in guanine adducts. J. Am. Chem. Soc. 2002;124(12):3064–3082. doi: 10.1021/ja017482e. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, et al. Induced-fit recognition of DNA by organometallic complexes with dynamic stereogenic centers. Proc. Natl. Acad. Sci. U. S. A. 2003;100(25):14623–14628. doi: 10.1073/pnas.2434016100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, et al. Highly selective binding of organometallic ruthenium ethylenediamine complexes to nucleic acids: novel recognition mechanisms. J. Am. Chem. Soc. 2003;125(1):173–186. doi: 10.1021/ja027719m. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, et al. Kinetics of aquation and anation of Ruthenium(II) arene anticancer complexes, acidity and X-ray structures of aqua adducts. Chem. Eur. J. 2003;9(23):5810–5820. doi: 10.1002/chem.200304724. [DOI] [PubMed] [Google Scholar]
- 18.Hotze ACG, et al. Structure-dependent in vitro cytotoxicity of the isomeric complexes [Ru(L)2Cl2] (L=o-tolylazopyridine and 4-methyl-2-phenylazopyridine) in comparison to [Ru(azpy)2Cl2] J. Biol. Inorg. Chem. 2004;9(3):354–364. doi: 10.1007/s00775-004-0531-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen JC, et al. Electronic structures and SARs of the isomeric complexes alpha -, beta -, gamma - [Ru(mazpy)2Cl2] with different antitumor activities. THEOCHEM. 2005;728(1-3):93–101. [Google Scholar]
- 20.Anna C.G. Hotze, et al. Dinuclear double-stranded metallosupramolecular ruthenium complexes: potential anticancer drugs. Angew. Chem. Int. Ed. Engl. 2006;45(29):4839–4842. doi: 10.1002/anie.200601351. [DOI] [PubMed] [Google Scholar]
- 21.Peacock AFA, Sadler PJ. Medicinal organometallic chemistry: designing metal arene complexes as anticancer agents. Chem. Asian J. 2008;3(11):1890–1899. doi: 10.1002/asia.200800149. [DOI] [PubMed] [Google Scholar]
- 22.van Rijt SH, et al. Organometallic osmium(II) arene anticancer complexes containing picolinate derivatives. Inorg. Chem. 2009;48(4):1753–1762. doi: 10.1021/ic8020222. [DOI] [PubMed] [Google Scholar]
- 23.Peacock AFA, et al. Tuning the reactivity of osmium(II) and ruthenium(II) arene complexes under physiological conditions. J. Am. Chem. Soc. 2006;128(5):1739–1748. doi: 10.1021/ja055886r. [DOI] [PubMed] [Google Scholar]
- 24.Peacock AFA, et al. Tuning the hydrolytic aqueous chemistry of osmium arene complexes with N,O-chelating ligands to achieve cancer cell cytotoxicity. J. Am. Chem. Soc. 2007;129(11):3348–3357. doi: 10.1021/ja068335p. [DOI] [PubMed] [Google Scholar]
- 25.Kostrhunova H, et al. DNA interactions of monofunctional organometallic osmium(II) antitumor complexes in cell-free media. J. Med. Chem. 2008;51(12):3635–3643. doi: 10.1021/jm701538w. [DOI] [PubMed] [Google Scholar]
- 26.Barnard PJ, Berners-Price SJ. Targeting the mitochondrial cell death pathway with gold compounds. Coord. Chem. Rev. 2007;251(13+14):1889–1902. [Google Scholar]
- 27.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007;7(4):392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Urig S, et al. Undressing of phosphine gold(I) complexes as irreversible inhibitors of human disulfide reductases. Angew. Chem. Int. Ed. 2006;45(12):1881–1886. doi: 10.1002/anie.200502756. [DOI] [PubMed] [Google Scholar]
- 29.Shaw CF., III Gold-based therapeutic agents. Chem. Rev. 1999;99(9):2589–2600. doi: 10.1021/cr980431o. [DOI] [PubMed] [Google Scholar]
- 30.Che C-M, et al. Gold(III) porphyrins as a new class of anticancer drugs: cytotoxicity, DNA binding and induction of apoptosis in human cervix epitheloid cancer cells. Chem. Commun. 2003;(14):1718–1719. doi: 10.1039/b303294a. [DOI] [PubMed] [Google Scholar]
- 31.Li CK-L, et al. Anticancer cyclometalated [AuIIIm(C~N~C)mL]n+ compounds: synthesis and cytotoxic properties. Chem. Eur. J. 2006;12(20):5253–5266. doi: 10.1002/chem.200600117. [DOI] [PubMed] [Google Scholar]
- 32.Gabbiani C, et al. Structural characterization, solution studies, and DFT calculations on a series of binuclear gold(III) oxo complexes: relationships to biological properties. Inorg. Chem. 2008;47(7):2368–2379. doi: 10.1021/ic701254s. [DOI] [PubMed] [Google Scholar]
- 33.Saggioro D, et al. Gold(III)-dithiocarbamato complexes induce cancer cell death triggered by thioredoxin redox system inhibition and activation of ERK pathway. Chem. Biol. 2007;14(10):1128–1139. doi: 10.1016/j.chembiol.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Bindoli A, et al. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009;253(11+12):1692–1707. [Google Scholar]
- 35.Jakupec MA, Keppler BK. Gallium in cancer treatment. Curr. Top. Med. Chem. 2004;4(15):1575–1583. doi: 10.2174/1568026043387449. [DOI] [PubMed] [Google Scholar]
- 36.Meggers E, et al. Exploring chemical space with organometallics: ruthenium complexes as protein kinase inhibitors. Synlett. 2007;(8):1177–1189. [Google Scholar]
- 37.Smalley Keiran, S.M., et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67(1):209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 38.Bergamo A, et al. Modulation of the metastatic progression of breast cancer with an organometallic ruthenium compound. Int. J. Oncol. 2008;33(6):1281–1289. [PubMed] [Google Scholar]
- 39.Chatterjee S, et al. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg. Chem. 2008;13(7):1149–1155. doi: 10.1007/s00775-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 40.Ott I, et al. Alkyne hexacarbonyl dicobalt complexes in medicinal chemistry and drug development. Expert Opin. Ther. Pat. 2008;18(3):327–337. [Google Scholar]
- 41.Ott I, et al. Antitumor-active cobalt-alkyne complexes derived from acetylsalicylic acid: studies on the mode of drug action. J. Med. Chem. 2005;48(2):622–629. doi: 10.1021/jm049326z. [DOI] [PubMed] [Google Scholar]
- 42.Sergeant CD, et al. Metallo-nucleosides: synthesis and biological evaluation of hexacarbonyl dicobalt 5-alkynyl-2′-deoxyuridines. Org. Biomol. Chem. 2008;6(1):73–80. doi: 10.1039/b713371e. [DOI] [PubMed] [Google Scholar]
- 43.Gunatilleke SS, et al. Inhibition of cathepsin B by Au(I) complexes: a kinetic and computational study. J. Biol. Inorg. Chem. 2008;13(4):555–561. doi: 10.1007/s00775-008-0344-0. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira CR, et al. Pre-clinical antitumour evaluation of biphosphinic palladacycle complex in human leukaemia cells. Chem.-Biol. Interact. 2009;177(3):181–189. doi: 10.1016/j.cbi.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 45.Mosi R, et al. Rhenium inhibitors of cathepsin B (ReO(SYS)X (Where Y = S, py; X = Cl, Br, SPhOMe-p)): Synthesis and mechanism of Inhibition. J. Med. Chem. 2006;49(17):5262–5272. doi: 10.1021/jm060357z. [DOI] [PubMed] [Google Scholar]
- 46.Desoize B. Metals and metal compounds in cancer treatment. Anticancer Res. 2004;24(3A):1529–1544. [PubMed] [Google Scholar]
- 47.Kostova I. Lanthanides as anticancer agents. Curr. Med. chem. Anticancer Agents. 2005;5(6):591–602. doi: 10.2174/156801105774574694. [DOI] [PubMed] [Google Scholar]
- 48.Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. 2007;340(3):117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- 49.Farrer NJ, Sadler PJ. Photochemotherapy: targeted activation of metal anticancer complexes. Aust. J. Chem. 2008;61(9):669–674. [Google Scholar]
- 50.Henderson BW, et al. Photodynamic Therapy: Basic Principles and Clinical Applications. 1992.
- 51.Detty MR, et al. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47(16):3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 52.Kostenich G, et al. Photosensitization by the near-IR-absorbing photosensitizer lutetium texaphyrin: spectroscopic, in vitro and in vivo studies. J. Porphyrins Phthalocyanines. 1998;2(4-5):383–390. [Google Scholar]
- 53.Sharman WM, et al. Photodynamic therapeutics: basic principles and clinical applications. Drug Discovery Today. 1999;4(11):507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 54.Cordier C, et al. Insertion of a bulky rhodium complex into a DNA cytosine-cytosine mismatch: An NMR solution study. J. Am. Chem. Soc. 2007;129(40):12287–12295. doi: 10.1021/ja0739436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Junicke H, et al. A rhodium(III) complex for high-affinity DNA base-pair mismatch recognition. Proc. Natl. Acad. Sci. U. S. A. 2003;100(7):3737–3742. doi: 10.1073/pnas.0537194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ernst RJ, et al. DNA mismatch binding and antiproliferative activity of rhodium metalloinsertors. J. Am. Chem. Soc. 2009;131(6):2359–2366. doi: 10.1021/ja8081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy H, et al. Current status and future prospects for satraplatin, an oral platinum analogue. Clin. Cancer Res. 2008;14(6):1633–1638. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- 58.Hall MD, et al. Basis for design and development of platinum(IV) anticancer complexes. J. Med. Chem. 2007;50(15):3403–3411. doi: 10.1021/jm070280u. [DOI] [PubMed] [Google Scholar]
- 59.Bednarski PJ, et al. Light-activated destruction of cancer cell nuclei by platinum diazide complexes. Chem. Biol. 2006;13(1):61–67. doi: 10.1016/j.chembiol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Mackay FS, et al. A photoactivated trans-diammine platinum complex as cytotoxic as cisplatin. Chem. Eur. J. 2006;12(11):3155–3161. doi: 10.1002/chem.200501601. [DOI] [PubMed] [Google Scholar]
- 61.Mackay FS, et al. A potent cytotoxic photoactivated platinum complex. Proc. Natl. Acad. Sci. U. S. A. 2007;104(52):20743–20748. doi: 10.1073/pnas.0707742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vessieres A, et al. Modification of the estrogenic properties of diphenols by the incorporation of ferrocene. Generation of antiproliferative effects in vitro. J. Med. Chem. 2005;48(12):3937–3940. doi: 10.1021/jm050251o. [DOI] [PubMed] [Google Scholar]
- 63.Hillard E, et al. Ferrocene-mediated proton-coupled electron transfer in a series of ferrocifen-type breast-cancer drug candidates. Angew. Chem. Int. Ed. 2006;45(2):285–290. doi: 10.1002/anie.200502925. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen A, et al. Synthesis and structure-activity relationships of ferrocenyl tamoxifen derivatives with modified side chains. Chem. Eur. J. 2009;15(3):684–696. doi: 10.1002/chem.200801108. [DOI] [PubMed] [Google Scholar]
- 65.Failes TW, et al. Studies of a cobalt(III) complex of the MMP inhibitor marimastat: a potential hypoxia-activated prodrug. Chem. Eur. J. 2007;13(10):2974–2982. doi: 10.1002/chem.200601137. [DOI] [PubMed] [Google Scholar]
- 66.Dougan SJ, et al. Catalytic organometallic anticancer complexes. Proc. Natl. Acad. Sci. U. S. A. 2008;105(33):11628–11633. doi: 10.1073/pnas.0800076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petzold H, et al. Metal and ligand control of sulfenate reactivity: arene ruthenium thiolato-mono-S-oxides. Angew. Chem. Int. Ed. 2008;47(16):3008–3011. doi: 10.1002/anie.200705342. [DOI] [PubMed] [Google Scholar]
- 68.Petzold H, Sadler PJ. Oxidation induced by the antioxidant glutathione (GSH) Chem. Commun. 2008;(37):4413–4415. doi: 10.1039/b805358h. [DOI] [PubMed] [Google Scholar]
- 69.Barnes KR, et al. Synthesis, characterization, and cytotoxicity of a series of estrogen-tethered platinum(IV) complexes. Chem. Biol. 2004;11(4):557–564. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 70.Ang WH, et al. Rational design of platinum(IV) compounds to overcome glutathione-S-transferase mediated drug resistance. J. Am. Chem. Soc. 2005;127(5):1382–1383. doi: 10.1021/ja0432618. [DOI] [PubMed] [Google Scholar]
- 71.Campone M, et al. Phase I and pharmacokinetic trial of AP5346, a DACH-platinum-polymer conjugate, administered weekly for three out of every 4 weeks to advanced solid tumor patients. Cancer Chemother. Pharmacol. 2007;60(4):523–533. doi: 10.1007/s00280-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 72.Feazell RP, et al. Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J. Am. Chem. Soc. 2007;129(27):8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamelers IHL, et al. High cytotoxicity of cisplatin nanocapsules in ovarian carcinoma cells depends on uptake by caveolae-mediated endocytosis. Clin. Cancer Res. 2009;15(4):1259–1268. doi: 10.1158/1078-0432.CCR-08-1702. [DOI] [PubMed] [Google Scholar]
- 74.Noor F, et al. Enhanced cellular uptake and cytotoxicity studies of organometallic bioconjugates of the NLS peptide in Hep G2 cells. Chembiochem. 2009;10(3):493–502. doi: 10.1002/cbic.200800469. [DOI] [PubMed] [Google Scholar]