Abstract
The fields of phototherapy and of inorganic chemotherapy both have long histories. Inorganic photoactivated chemotherapy (PACT) offers both temporal and spatial control over drug activation and has remarkable potential for the treatment of cancer. Following photoexcitation, a number of different decay pathways (both photophysical and photochemical) are available to a metal complex. These pathways can result in radiative energy release, loss of ligands or transfer of energy to another species, such as triplet oxygen. We discuss the features which need to be considered when developing a metal-based anticancer drug, and the common mechanisms by which the current complexes are believed to operate. We then provide a comprehensive overview of PACT developments for complexes of the different d-block metals for the treatment of cancer, detailing the more established areas concerning Ti, V, Cr, Mn, Re, Fe, Ru, Os, Co, Rh, Pt, and Cu and also highlighting areas where there is potential for greater exploration. Nanoparticles (Ag, Au) and quantum dots (Cd) are also discussed for their photothermal destructive potential. We also discuss the potential held in particular by mixed-metal systems and Ru complexes.
Keywords: chemotherapy, metal, photochemistry
Introduction
Transition metal complexes have proven success as anticancer agents.1 Photoactivated chemotherapy (PACT) provides the opportunity for control over when and where a drug is activated, resulting in a greater specificity of drug action. The use of an inactive precursor or “prodrug” is an important strategy in drug targeting.2 In this perspective, we discuss the photophysical and photochemical processes which can occur following photoexcitation of a metal complex, and the potential of PACT for cancer treatment shown by metal complexes. There are excellent reviews concerning the photophysical properties of metal complexes3,4 and the potential of photoactive metals for medicinal applications.5 The effect of light on metal complexes and the subsequent effects on biomolecules, particularly DNA, is also well-documented.6,7 Here, we focus specifically on inorganic PACT anticancer agents, a few examples of which we recently highlighted.8 We consider the photophysical and photochemical properties which are desirable, discuss the activation pathways available, and summarise the PACT potential shown by the d-block metals (see Figure 1).
Figure 1.
Table of d-block metals, coloured to demonstrate the PACT potential of each metal, based on recent literature. Bold = well-documented photochemical activity.3 Underlined = well-documented anticancer activity.1a Bold + underlined = photochemical + anticancer activity.
Photomedicinal applications of the lanthanides (largely radioimmunotherapy and photodynamic therapy)9 and the anticancer activity of the main group metals10 are both well-documented elsewhere3 and will not be discussed, nor will the use of metal photochemistry for diagnostic (e.g. imaging, DNA footprinting, photoaffinity labelling) rather than therapeutic purposes.
What do metals offer for PACT?
Light can be used to alter the electronic structure of molecules, inducing changes in both physical and chemical properties. The excited state which is generated is typically short-lived; however, as the molecule returns to the ground state, the energy can be dissipated in a wide variety of ways, in the form of light or heat, a chemical modification of the structure or transferral of energy to another species. In contrast to organic species, metals have excited states that are often easily accessible by irradiation with visible and UVA light. Transition metal complexes with d3 and d6 electronic configurations are particularly promising, due to the favourable photophysical properties and the relative non-lability of complexes with these configurations. In particular, d6 transition metal complexes can be used to exemplify the diversity of excited states that can be generated by light excitation, and the chemistry that is associated with their generation.7
The nature of the excited states of metal complexes has been increasingly studied in recent years and several applications have been developed exploiting their photophysics and photochemistry.11 Excitation leads to electronically- and vibrationally-excited states with the same multiplicity as the ground state. The transitions to the excited electronic states are formally classified according to the character of the orbitals involved in the electronic transition, as depicted in Figure 2. Such classification is a simplification in some cases; orbitals may have mixed metal/ligand character depending on the nature of the metal-ligand bond and, furthermore, electronic transitions may involve more than two orbitals at a time.
Figure 2.
Simplified orbital and excited-state diagram for a d6 metal complex with octahedral coordination (strong crystal field is assumed). Each black arrow (↑↓) represents an electron with its associated spin. Coloured arrows ( ) represent the electron involved in each electronic transition. In the singlet state electrons are spin down (
) represent the electron involved in each electronic transition. In the singlet state electrons are spin down ( ), while in the triplet state they are spin up (
), while in the triplet state they are spin up ( ).
).
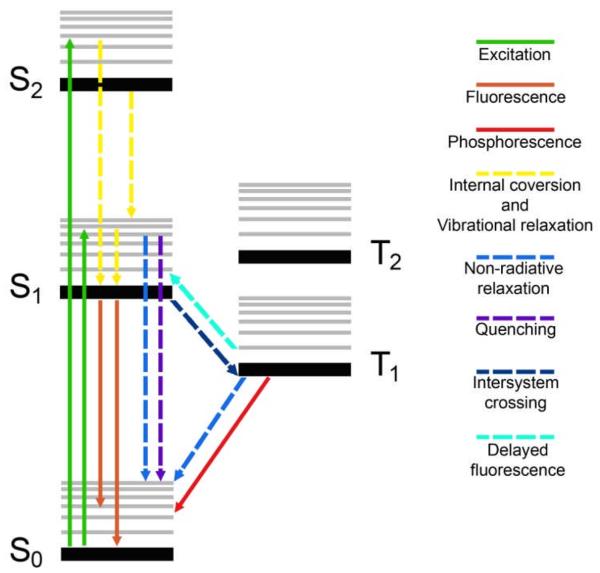
Once these excited states are generated they can undergo a series of physical radiationless processes which ultimately lead to the ground-state electronic structure; intersystem crossing (ISC), internal conversion (IC) vibrational relaxation, intramolecular vibrational redistribution and solvation dynamics (reorganisation of solvent shells). Radiative processes such as fluorescence (singlet-singlet) and phosphorescence (triplet-singlet) result in a return to the ground state, with emission of light of longer wavelength than was used for the excitation. Although, metal complexes generally emit from triplet states (i.e. phosphorescence dominates), their emissive properties differ significantly from those observed in organic chromophores. Due to the efficient intersystem crossing promoted by the metal ion, the lifetime of such triplet states is typically on the 50 ns – 1 μs timescale, much faster that of classical organic compounds (ms), and their emission quantum yield is relatively high.
Photochemical reactions from a photophysically excited metal complex (ligand dissociation, redox processes etc.) can occur at any stage during the decay back to the ground state. The nature of the particular photochemical process is intimately related not only to the nature of the excited state reached by the molecule upon excitation, but also to the energy and nature of closely-lying states and to the availability of the dynamic processes just described, which can determine the population and depopulation of reactive states. Associating the photochemical behaviour of a d6-metal complex with the nature of the lowest lying triplet state (T1, Figure 3) is a reasonable model for most long-lived (ns or longer) inorganic systems. In this way the excited state reactivity of metal complexes can be summarized as follows:
Metal-centred (MC) transitions {i.e. d-d or ligand-field (LF) transitions}. These are orbitally (Laporte)-forbidden, and can also be spin-forbidden if the spin state changes. Consequently, they give rise to weak absorptions (ε ~ 1–20 × 103 M−1cm−1) which can be masked by stronger, formally allowed charge-transfer transitions. Since MC transitions typically populate antibonding orbitals,13 the excited states generated often lead to bond lengthening and favour ligand substitution. Photochemical lability is commonly a feature of complexes in which a MC excited state is lowest in energy, such as those metal complexes which photorelease a bioactive molecule (e.g. CO,14 NO15).
Charge-transfer (CT) transitions {metal-to-ligand (MLCT), ligand-to-metal (LMCT) or to-solvent (TS)}. These give rise to more intense transitions (typically ε ~ 0.01 – 500 × 103 M−1cm −1) and can lead to redox reactions (of both the complex and molecules in the local environment e.g. solvent) and also result in homolytic bond cleavage, reducing the metal centre and generating radicals. Production of radicals under biological conditions is a well-established mechanism for causing damage to cellular components (e.g. DNA).
Ligand-centred (LC) transitions (or interligand (IL) transitions). These generally involve only ligand-centered orbitals and are often seen in large delocalised systems.
Figure 3.
Jabłonski energy diagram. All possible physical processes triggered by light excitation of a d6 metal complex are represented by dotted (– – – radiationless) and solid (——, radiative) lines.12
Steady-state and nanosecond spectroscopic characterisation methods can determine the nature of excited states when electronic state evolution is complete and aid identification of the transitions involved following irradiation. Ultrafast (fs) time-resolved techniques and computational methods show that more complex scenarios are possible and photochemistry (e.g. bond breaking) can occur when electronic state evolution is not complete, from both singlet and triplet states which are accessible through excitation or radiationless processes. For multimetal complexes, there is the possibility for additional metal-metal transfers.
For complexes in which the CT absorption band is well-separated from the MC band, selective irradiation can often control the type of photoreaction: for example for CoIII, irradiation into the LMCT band typically produces photoreduction giving rise to CoII and oxidised ligand, whereas irradiation into the MC (d-d) irradiation causes photosubstitution/aquation.7
Desirable features of photoactivated metal-based drugs
Aspects such as aqueous solubility, cell uptake and stability in biological media are common considerations for any potential drug. When developing photoactivatable metal anticancer agents additional key features which should be considered include the following.
A large difference between cytotoxicity in the presence and absence of irradiation is desirable in order to limit unwanted side-effects, which may also reduce drug efficacy.
The wavelength of activation should ideally lie within a phototherapeutic window of 620–850 nm. This range has the maximum depth penetration into mammalian tissue.5
The dependence of the PACT mechanism on O2. Tumours exhibit varying levels of oxygenation;16 low levels can reduce drug efficacy e.g. of PDT agents (which require O2), and for some photoactive complexes different pathways are favoured in the presence or absence of oxygen.
The quantum yield or efficiency of the photochemical process.
Suitability of the wavelength of light for irradiation (λirr)
As highlighted above, the depth of penetration of light into tissue is important. It depends on both the wavelength and the tissue type;17 highly pigmented tissue can rapidly attenuate light. For activation of photochemotherapeutic compounds, the wavelength of irradiation (λirr) depends on the properties of the photochemical agent and the size of the tumour (and in practice, the availability of light sources). For current clinical application the use of red light (~630 nm) is routine although for superficial tumours shorter wavelengths e.g. blue (420 nm), may be more appropriate.18
Multiphoton excitation is an effective way to extend the wavelength of excitation of a metal complex.19 It imposes constraints on the structure of the compound, and the light source needs to have a high photon density (e.g. a femtosecond laser). Consequently the excitation volume is much smaller than that for one-photon excitation since two photons need to be absorbed by the molecule simultaneously.
Mechanisms of anticancer action
Although often complex, rational improvement is only possible if the mechanism of action is at least partly understood. Where possible in this overview we highlight the mechanism proposed.
The mechanisms of action fall into three broad categories:
1) Photodissociation and/or redox changes: causing direct reaction of the metal with a biological agent e.g. Pt binding to DNA or protein, or photorelease of a bioactive agent e.g. NO, CO. The term ‘photocisplatin’ reagents was coined for rhodium (and related) metal complexes which are thermally inert, but which form covalent bonds with DNA upon irradiation with UV or visible light.20 Different oxidation states often exhibit different ligand binding kinetics, e.g. photoreduction of PtIV to PtII generates a much more labile, reactive species.
2) Photosensitisation: Excited triplet states of metal complexes may be deactivated by reaction with ground-state triplet oxygen, forming highly reactive singlet oxygen (1Δg) (known as a type-II process). In order for this to be possible, the energy of the excited state triplet of the metal complex must be ≥ 22.4 kcal.mol−1 compared to the ground state, since this is the excitation energy of singlet oxygen (1O2).21 Subsequent reactions of singlet oxygen in biological systems result in DNA damage, protein destruction, and cell lysis, destroying the tumour.22-24 Generation of a secondary destructive species (e.g. 1O2, HO•) in this way is known as photosensitisation, and the medical application is photodynamic therapy (PDT).9b,21,25 For PDT applications, the singlet oxygen quantum yield ΦΔ of a given complex is the crucial parameter which determines the efficacy of the system; d0(MgII, AlIII) or d10 (ZnII, CdII) configurations usually have moderate to high values of ΦΔ as do complexes of some of the heavier elements (PdII, PtII).21 Apart from the potential of the metal complex for photobleaching, this can be considered catalytic.
3) Photothermal reaction: Conversion of excited state energy to thermal energy (e.g. nanoparticles, quantum dots). Due to the absence of radiative (emissive) decay, following photoexcitation the energy is released as heat, which then destroys the tumour.
The potential of d-block metals as photoactive anticancer complexes
Several excellent reviews have focussed on the handful of well-developed PACT metal complexes;7,20 here we discuss the general potential shown by d-block elements for PACT, highlighting that several photoactive metals have been scarcely investigated for PACT applications and as such, are worthy of pursuit.
Group 3
The elements of group 3, Sc, Y and La, exist predominantly in the 3+ oxidation state. The PACT potential of Group 3 elements has not yet been investigated.
Group 4
Of Group 4, Ti, in the form TiO2, has been studied extensively for its photochemical properties, particularly in photocatalysis, photoelectrochemical solar energy conversion and self-cleaning/sterilizing applications.26 Crucially, TiO2 can kill cancer cells when irradiated with UVA light.27,28 Sadler et al. have shown that a mononuclear citrate TiIV complex is able to undergo photoreduction (with UVA) to TiIII; as TiIII is a strong reductant, it is capable of initiating free radical reactions in cells.29 Although Ti is undergoing a renaissance as an anticancer agent,30 the heavier elements Zr and Hf have been little studied in this regard.
Group 5
Chakravarty et al. are developing V-based PDT agents, achieving photoactivation at ~800 nm (current PDT agents absorb ~630 nm), with little dark toxicity. OxovanadiumIV complexes containing the heterocyclic bases dpq (dipyrido[3,2-d:2′,3′-f]quinoxaline) and dppz (dipyrido[3,2-d:2′,3′-c]phenazine) have been shown to exhibit DNA cleavage mechanisms involving both singlet oxygen and hydroxyl radicals when activated at 365 nm, with only the HO• radical mechanism operating when irradiated with near-IR light.31 Although VIV (d1) complexes show similarities to CuII (d9) complexes in terms of possessing low-energy visible bands, they do not possess the drawbacks of dark toxicity exhibited by the CuII complexes, caused by in vivo reduction to CuI since the VIV ion in VO2+ is unlikely to undergo redox transformation in a biological medium.32 Others have also reported the photochemical cleavage of DNA by V complexes.33 Currently, the medical applications of Ta are limited to metal implants34 and the poor stability of Nb compounds in aqueous solutions below pH 10 may explain its limited exploration to date35
Group 6
The photochemistry of Cr has been extensively investigated.3a [M(CO)4(α-diimine)] complexes (where M = Cr, Mo, W and for α-diimines such as bpy) have well-characterised photochemical properties, and undergo photochemical substitution of an axial CO ligand when irradiated with UV or visible light.36 Although such studies have not yet targeted medical applications, controlled CO release can be an efficient and advantageous way of promoting cell death.37 Morrison et al. have studied the DNA binding properties of photoactive CrIII diimine complexes such as [Cr(phen)(Cl)2],38 which can photorelease a coordinated ligand (e.g. Cl). Ford and coworkers have developed several CrIIINO complexes which release NO upon light irradiation.39 In particular, the new generation complexes with antenna ligands have improved absorption properties (λmax 650 nm) since absorption at these longer wavelengths is useful for photoactivation in tissues.40
Both porphyrin41 and porphycene42 complexes of [O=MoV-X] show visible light induced dissociation of the axial ligand (X) and reduction to MoIV. For X = Cl, the quantum yields (Φ) for the homolytic bond cleavage and photochemical release of Cl• from the porphycene complex are 0.055 (500 nm), 0.045 (600 nm) and 0.040 (650 nm). WIV polyoxometalates show promise in photoactivatable antibacterial applications.43 However, the application of these Mo and W systems to PACT has yet to be demonstrated.
Group 7
MnIII porphyrins show promise for PDT44 and the photorelease of CO from Mn centres has also been demonstrated.14,45 Radioactive isotopes of both Re and Tc are used in imaging and radiotherapy. Anticancer applications exploiting the photochemical activity of Tc are scarce (presumably because all Tc isotopes are radioactive, making them more hazardous to work with than Re), although Re has been more extensively investigated.3,46
Group 8
This group offers examples of photosensitizers, intercalators, DNA binders and complexes able to photorelease bioactive ligands. Two-photon excitation (2PE) leading to NO release has been explored in depth for Fe nitrosyls,47 and recently Prasad et al. have reported two-photon excitable, water-soluble Fe (NO) release agents which show dark toxicity but which exhibit a slight phototoxic enhancement in HeLa cells.48 Irradiation of a FeIII triazine complex (72 hr, λirr ≥ 455 nm) creates a LMCT excitation, generating a highly oxidising, charge-separated excited state (Fe2+-ligand radical). The triazine radical thus generated is capable of cleaving plasmid DNA by an oxygen-independent pathway, and subsequently decomposes to give N2. 49 An FeIII complex of dpq (selected for its DNA binding properties) and the tetradentate (2,2-bis(3,5-di-tert-butyl-2-hydroxybenzyl)aminoacetic acid)] ligand (which stabilizes the +3 state) causes DNA nicking following irradiation (λirr ≤ 647 nm), again, due to the generation of a charge-separated excited state.50 The mechanism of cleavage is oxygen-dependent, but operates through a superoxide O2•−and HO• radical mechanism rather than by 1O2 generation. Related work has demonstrated oxo-bridged diiron complexes of dpq which are capable of photocleaving bovine serum albumin (λirr 365 nm), again, via the HO• radical pathway.51
The photophysics and photochemistry of Ru has been extensively studied. Both RuIII and RuII show potential; RuIII complexes are reasonably inert to ligand substitution and can be activated by reduction to RuII, which can then react either thermally or photochemically with DNA. The dinuclear complex [{(η6-indan)RuCl}2(μ-2,3-dpp)](PF6)2 undergoes arene loss via an oxygen-independent pathway when irradiated (λirr = 365 nm), releasing a fluorescent marker (indan) and creating a highly reactive Ru species capable of forming both mono- and bifunctional adducts of DNA.52 The use of π-acceptor ligands such as TAP (1,4,5,8-tetraazaphenanthrene) and HAT (1,4,5,8,9,12-hexaazatriphenylene) in RuII complexes shows much photochemical potential and is discussed in the Highlights section.53
Os offers both a rich photochemistry3b and promise in the anticancer field54 but most PACT investigations using Os have been in mixed-metal systems (see Highlights section). The high thermal stability of OsII complexes can make syntheses challenging, but the final product is also more robust to degradation. Promisingly, Os complexes possess low energy MLCT bands55 and the design aspects of DNA cleaving complexes involving Os have been considered, paving the way for development of PACT applications.56
Group 9
CoIII complexes are usually substitution-inert. Thermal or photochemical reduction to the more kinetically labile CoII can induce selective ligand release. Thermal (e.g. hypoxia-activated)57 release is exemplified by a CoIII complex of the matrix metalloproteinase inhibitor Marimastat, a combination which shows excellent anticancer activity.58 Photochemical release has also been used to deliver a therapeutic agent, for example, cis-[CoL(NO2)(ONO)]+ (L = 6-(anthracen-9-ylmethyl)-1,4,8,11-tetraazacyclotetradecane) releases NO (which sensitises hypoxic tissue to γ-radiation59) upon irradiation (λirr = 360 nm).60 Photoreduction is also seen for CoIII and several complexes have been shown to photocleave DNA;61 McFadyen et al. have shown that [CoIII(en)2(dppz)]3+ photocleaves DNA (λirr = 350–400 nm). The mechanism involves reductive quenching of the CoIII* excited state by bases e.g. guanosine (G), producing a radical cation G•+ which eventually results in cleavage of the DNA backbone. Although phototoxicity has been demonstrated in cellulo, the complex also exhibits considerable dark toxicity.62 Stereospecific photocleavage of DNA using chiral CoIII complexes is well-established,63 and in general Co holds considerable potential for future PACT developments.
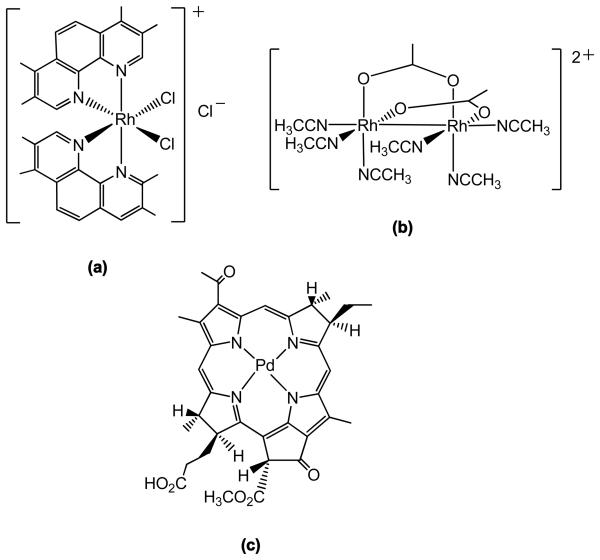
The photochemistry of RhIII complexes is determined by the nature of the lowest lying state. In bis-chelated (polypyridyl) RhIII complexes this is typically a 3MC state; photoexcitation weakens the M–L bond, resulting in photosubstitution. In contrast, excitation of the lowest lying state (3LC) in the tris-chelated (polypyridyl) analogues creates a strong oxidising agent. The complex cis-[Rh(phen)2Cl2]+ illustrates the dependence of the photochemistry on the presence of various species; O2 quenches photoaquation of this species, with formation of singlet oxygen – but the quantum efficiency for photoaquation is increased by the presence of deoxyguanosine (dG), possibly due to reductive quenching.7 The excited states of RhIII polypyridyl complexes tend to be more strongly oxidising than their RuII counterparts, resulting in more efficient reactions for DNA damage. RhIII complexes are relatively thermally inert, but may undergo efficient photosubstitutions, making them potentially useful for PACT. However, in contrast to their RuII counterparts, mononuclear RhIII complexes show little absorption (e.g. MC or CT bands) in the visible region of the electronic spectrum – instead, the spectrum is dominated by intense LC bands in the UV region.7 Despite this, Morrison et al. have shown that methylated derivatives of [cis-Rh(phen)2Cl2]Cl such as OCTBP (see Figure 4) can be activated at wavelengths where there is no apparent absorption, (λirr > 500 nm) due to the direct population of weakly absorbing 3MC states by photoexcitation from the ground state; these complexes exhibit moderate phototoxicity in tumour cells.64 Barton et al. have pioneered the use of complexes such as [Rh(bpy)2(chrysi)]3+ which targets single base DNA mismatches, and upon photoactivation (λirr ~ 340 – 450 nm) cleaves the DNA next to the mismatch.65 This shows potential for PACT since the inability of cells to control and regulate mismatch repair is implicated in several cancers.66 Dinuclear Rh complexes allow extension of the possible wavelength of activation;67 the cytotoxicity of cis-[Rh2(μ-O2CCH3)2(CH3CN)]2+ (Figure 4) increases 34 times upon irradiation, with an LC50 ~12μM in human skin cells when irradiated (λirr = 400–700 nm).68
Figure 4.
(a) OCTBP, a methylated [cis-Rh(phen)2(Cl)2]Cl derivative which shows phototoxicity towards tumour cells (λirr> 500 nm). (b) dinuclear Rh complex which shows 34 × increase in cytotoxicity when irradiated (λirr = 400–700 nm) (c) Pd-based PDT agent TOOKAD currently in clinical trials (structure adapted from reference 71).
Ir complexes exhibit a rich photochemistry,69 however, most photobiological applications focus on the luminescent properties70 and the PACT potential of Ir complexes remains relatively unexplored.
Group 10
Although Ni complexes have been investigated as anticancer agents,72 only a few investigations consider photoactivity;73 for example, [Ni(bpy)2(benzo[1.8]naphthyridinone-2-ol)](PF6)2 binds to DNA in the absence of irradiation, and upon irradiation (λirr 365 nm) photocleaves the DNA through a mechanism involving both 1O2 and HO• radicals.74
Both common oxidation states of Pt show a rich photochemistry;75 many PtII complexes luminesce following irradiation, due to long-lived excited states76 while the photochemistry of PtIV complexes is dominated by dissociative/reductive behaviour. In collaboration with others we have investigated the photochemistry of a range of PtIV diazido complexes, in an effort to develop selective PACT agents.75,77 Briefly, irradiation of the complexes into either the LMCT bands (λirr = 365 nm) or the weaker d-d transitions at longer wavelengths (λirr = 420, 514 nm) can cause photodissociation and/or photodecomposition of the azido ligands and reduction of the PtIV centre to PtII. When the irradiation is undertaken in cell culture, a potent cytotoxic effect is seen, which is not observed either in the dark, or with irradiation of the cells in the absence of the Pt complex. For the PtIV complexes — irradiation at wavelengths where there appears to be essentially no absorbance in the electronic spectrum can still result in photoactivity77a (as observed for Morrison's Rh complexes)64 due to the direct excitation of dissociative low-lying LMCT states (e.g. S1–S4, Figure 5a). Time-dependent DFT calculations show that such transitions involve σ-antibonding orbitals (Figure 5b) which favour ligand dissociation once populated.
Figure 5.
(a) Calculated (TDDFT) and experimental absorption spectrum of cis,trans,cis-[Pt(N3)2(OH)2(NH3)2] in H2O. The excited states are shown as vertical bars with heights equal to their extinction coefficients. Transitions S1–S4 have all dissociative character since they involve (b) the σ-antibonding orbitals LUMO and LUMO+1.
Clearly the action spectrum does not mirror the UV-vis electronic spectrum for these complexes. The use of weaker field donors such as iodide instead of azide typically results in dark toxicity due to thermal reactions with biological reductants (e.g. glutathione).77f Extension of the wavelength of activation of these complexes is a balance between thermal stability and activation with the longest wavelengths possible. In other research, the combination of a phototoxic porphyrin with a cytotoxic Pt unit within the same molecule has been investigated,78 but in general the photochemistry of the Pt centre itself remains largely unexploited for PACT applications.
A PDT photosensitiser containing Pd (TOOKAD)79 is in Phase I/II clinical trials for treatment of prostate cancer (see Figure 4); the complex can be photoactivated to produce 1O2 (λirr = 762 nm, diode laser) and shows rapid clearance from the body, an advantage over conventional PDT agents, which can cause prolonged photosensitivity of the patient following treatment. Contrastingly, a paramagnetic, non-emitting Pd-phthalocyanin complex has shown promise for photothermal therapy (PTT) since it shows considerable absorption at near–IR wavelengths (826 nm);80 in PTT tumour cells are killed by the heat generated from the efficient radiationless decay to ground state of such a Pd derivative.
Group 11
Cu complexes such as [CuII(2-(2-pyridyl)benzthiazole)Br2] have been shown to oxidatively cleave DNA in the presence of O2 without direct irradiation,81 others only cleave DNA upon irradiation,82 with complexes such as [CuII(dppz)((L-lysine)(OClO3)] being activated to cleave DNA with near-IR light by a mechanism involving 1O2 (λirr 700 – 755 nm).83 Binuclear copper complexes [{(R)CuII}2(μ-dtdp)2] (where R = phen or dpq and H2dtdp = 3,3′-dithiodipropionic acid) reportedly cleave DNA when irradiated (λirr = 753 – 799 nm) both in the presence of O2 (through 1O2 and HO• pathways) but also in the absence of O2, due to the generation of sulfur anion radicals.84 It will be of interest to see whether this translates to photocytotoxicity in cellulo or indeed, in vivo.
Irradiation of Ag complexes in the presence of DNA results in metallation but not cleavage,85 and although Ag compounds are often photosensitive little PACT research has been conducted. In contrast, Au compounds are well-known for their anticancer potential;86 if the dark cytotoxicity of AuIII porphyrins87 can be controlled, then AuIII tetraarylporphyrins have potential in PDT.3 The alternative technique of photothermal ablation uses irradiation of gold (or silver) nanoparticles to achieve a cytotoxic effect;88 the absorption of light by the nanoparticles induces localised surface plasmon oscillations, the electrons resonate in response to incoming radiation, causing them to both absorb and scatter light. The light is rapidly converted into heat which destroys the immediate tissue. The use of targeting features such as Paclitaxel (Taxol),89 Herceptin90 or transferrin91 conjugated to nanoparticles enables targeting, imaging and therapy for cancer cells, all with a single agent. Gold nanoparticles have also been shown to cleave DNA upon irradiation (aerobic conditions) with UV (λirr = 312 nm) light.92
Group 12
Aside from the use of Zn-porphyrin and Zn-phthalocyanine derivatives in PDT,21 Cd-based quantum dots (QD) represent the most promising development of group 12 for PACT application.93 QD can be used to generate reactive radicals to kill tumours, at the same time offering highly tunable absorption and emission properties.94 QD which are activated by X-ray – behaving as a PDT agent as well as a fluorescent marker – are particularly promising.95 Explorations of Hg photochemistry do not appear to have been applied yet to biological systems, presumably for toxicity reasons.96
Highlights
In the following section we consider the potential offered by mixed-metal systems and Ru complexes.
• Mixed-metal systems
Combining different metals in a multinuclear complex is a powerful way to exploit the (photo)chemical properties of each metal, in a synergistic fashion.
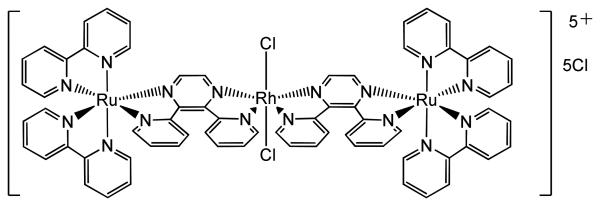
Work on trinuclear complexes [{(bpy)2M(dpp)}2RhCl2]Cl5 (where M = Ru or Os and dpp = 2,3-bis(2-pyridyl)pyrazine, Figure 6) has highlighted that complexes which demonstrate DNA photocleavage (e.g. where M = OsII) do not necessarily exhibit photocytotoxicity in mammalian cell lines.97 In contrast, the RuII analogue showed evidence of cell death for concentrations ≥ 10 μM following irradiation (λirr > 460 nm) with no cytotoxic activity in the dark. The mechanism of the phototoxic effect involves 1MLCT excitation of the RuII followed by conversion to a low-lying reactive 3MMCT state in which charge transfers from RuII – via a bridging π-ligand – to RhIII; the latter acts as an electron acceptor and subsequently cleaves DNA, demonstrating activity both in the presence and absence of oxygen. The analogous system in which Rh is substituted for Ir showed no DNA photocleavage in vitro; 98 promising variants involving Pt are being developed.99 In other work, the mixed-metal system [{RuII(tpm)(dppz)}(μ-dpp[5]){fac-(CO)3ReI(dppz)}]3+ (tpm = tris(1-pyrazolyl)methane, dpp[5] = 4,4′-dipyridylpentane) combines the strong DNA binding ability of the RuII with the DNA photocleavage ability of the ReI centre. Activation (λirr = 355 nm) results in both single- and double-strand cleavage of pBR322 plasmid DNA.100 A potential drawback of some multimetal systems is that a high formal charge is thought to make cell uptake of such complexes more challenging; complexes with lower overall charges which have not been photobiologically investigated may prove promising in this regard.101 However it is notable that some multinuclear PtII complexes with high charge (e.g. BBR3464, 4+ charge) can readily enter cells, so pathways for the transport of charged species into cells do exist.
Figure 6.
Mixed metal Ru-Rh-Ru complex of Brewer et al.
• Polyazaaromatic RuII complexes: photoactive molecules for a gene silencing approach in chemotherapy
Kirsch-De Mesmaeker and coworkers have recently developed a new class of RuII polyazaaromatic complexes containing TAP and HAT ligands, which can selectively form photoadducts with DNA bases and amino acids when their MLCT state is excited.102
Although many RuII polypyridyl complexes have both oxidizing and reducing properties in the excited state, Ru-TAP and Ru-HAT complexes show a more pronounced oxidizing character. When excited with visible light the complexes reported in Table 1 are able to react with DNA bases or oligomers and aminoacids such as tyrosine (Tyr) and tryptophan (Trp), resulting in a series of redox reactions which eventually lead to the formation of photoproducts.
Table 1.
Ground-state and excited-state potentials of the first reduction wave (vs. SCE, acetonitrile) for selected polyazaaromatic RuII derivatives
| Complex | Ered (V/SCE) | E*red(V/SCE) |
|---|---|---|
|
| ||
| [Ru(HAT)3]2+ | −0.62 | +1.49 |
| [Ru(TAP)3]2+ | −0.75 | +1.30 |
| [Ru(HAT)2(phen)]2+ | −0.66 | +1.25 |
| [Ru(HAT)2(bpy)]2+ | −0.76 | +1.17 |
| [Ru(TAP)2(phen)]2+ | −0.83 | +1.15 |
| [Ru(TAP)2(bpy)]2+ | −0.83 | +1.10 |
| [Ru(TAP)2(dppz)]2+ | −0.80 | +1.20 |
|
| ||
| Biomolecule | Ered (V/SCE) | |
|
| ||
| GMP | +1.05 | |
| Tyr | −0.85 | |
| Trp | −0.78 | |
The potentials were obtained as described in reference 102.
For example, in the presence of a guanine residue (G) from guanosine-5′-monophosphate (GMP) or DNA, [Ru(TAP)2(L′)]2+ complexes can undergo the following reactions (where * represents an excited state):
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
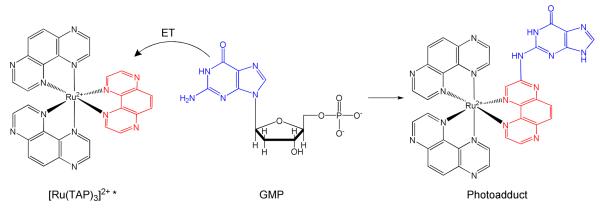
Steady-state and time-resolved studies103 have highlighted that the direct (2) and back (3) electron-transfer process from G is likely to occur through a proton-coupled electron-transfer mechanism (4 and 5). Two TAP and HAT ligands are necessary to achieve oxidation of GMP, while only the highly oxidizing complexes [Ru(TAP)3]2+ and [Ru(HAT)3]2+ can react with adenosine-5′-monophosphate (AMP). In the photoproduct obtained from the reaction between Ru-TAP and Ru-HAT complexes, the guanine is covalently linked to the complex by the exocyclic amino group of the base, which substitutes a H atom in the polyazaaromatic ligand ring (TAP: α-position with respect of the non-coordinating nitrogen, Figure 7.)
Figure 7.
Photoadduct formation during the photoirradiation of [Ru(TAP)3]2+ in the presence of GMP. The photoproduct is obtained after several steps of purification and acidification.
In addition to the photochemistry described, Ru-TAP and Ru-HAT derivatives also damage DNA by 1O2 photosensitization. However, the photoinduced electron transfer process that leads to the formation of stable and specific photoproducts makes this class of complexes potential candidates in PACT, where they can exploit a novel mechanism of action.
In fact, oligonucleotides hybridized with oxidizing Ru polyazaaromatic complexes can irreversibly form photo-induced cross-links with a target strand. This strategy can be used to develop specific anticancer agents based on gene silencing (inhibition of gene expression).
Synthetic oligodeoxynucleotides (ODNs) have been used for gene expression, targeting either double-strand DNA (antigen strategy) or single-strand mRNA (antisense strategy). Their application has been limited by the poor stability of the ODN-DNA/mRNA aggregates and by the activity of enzymes that can recognize and destroy such aggregates.104
Modified ODNs with anchored Ru-TAP and Ru-HAT complexes (Ru-ODNs) can be successfully employed to increase the stability of the ODN aggregates. The Ru-ODN complexes are activated by light and form strong cross-links between the ODN strand and a guanine residue (G) on the target sequence. The so-formed adducts have improved stability and are more resistant to enzyme action.
Several different Ru-ODNs have prepared by attaching covalently one ruthenium polyazaaromatic unit to timidine residues or to the 5′-terminal phosphate group. All these Ru-ODNs have been tested in the presence of their target sequence under light irradiation with the aim of assessing the general principle governing the photoadduct formation.
The percentage of photocross-links is determined by the ionization potential of G (e.g. GG sites exhibit higher reduction oxidation potentials than G sites) and by the distance between the Ru complex and G. Therefore, a minimum distance is required to have a good photocross-linking percentage, otherwise only fluorescence quenching occurs.
Until recently, it was considered necessary to avoid Ru-ODNs containing G to reduce self-inhibition. However, the same authors have developed Ru-ODNs containing guanine residues (Ru-ODN(G)), which are able to self-inhibit when irradiated in the absence of the complementary target strand or in the presence of non-complementary strands (Figure 8).105 In both cases cycRu-ODN(G) photoadducts are formed. In the case of non-complementary (containing G as well) strands self-inhibition reaches an 80 % yield.
Figure 8.
Schematic representation of the behaviour of Ru-ODN(G) (a)in the absence of complementary strands, (b) in the presence of non-complementary strands, and (c) in the presence of complementary target strands (adapted from reference [105]).
On the contrary, when a complementary strand is added and the Ru-ODN(G) is irradiated, selective formation of inter-strand photo-crosslinks is observed and no self-inhibition. The selectivity towards inter-strand adducts in the presence of a complementary target strand can be explained by the increased rigidity of the duplex. The improvement obtained with the Ru-ODNs(G) is relevant for in vivo gene silencing applications, since it reduces the possibility of secondary photoeffects (as for example seen with proteins).
Outlook
Platinum complexes have a proven track record of being well suited to the treatment of cancer and those of other transition metals have much potential. Photoactivation provides scope for another dimension – one of highly controllable activity – of more potent, highly targeted drugs with reduced side-effects.
The availability of a wide array of different metals, each with unique photophysical and photochemical properties gives a broad spectrum from which to choose. This spectrum dramatically widens once the multitude of possible ligands (and ligand arrangements) are considered and the potential for tuning the photochemistry is further expanded by combining several metals within the same complex.
The photoactivation pathways taken by metal complexes can be complicated, and can vary depending on the conditions, e.g. solvent, presence/absence of oxygen, possible reactants (e.g. DNA/proteins) and on the wavelength of light used. Many elegant techniques have been developed by the photochemical community in order to better understand and therefore control the photochemistry of such systems. These include measurements of action spectra, singlet oxygen quantum yields and the use of TD-DFT and ultrafast (fs) time-resolved techniques (e.g. IR) to characterise the excited states and photochemical pathways more accurately. It is anticipated that future improvements in X-ray time-resolved techniques will help to determine transient structures and to validate computational results.
Understanding the variation of the photochemistry in vitro, in cellulo and in vivo is crucial to the development of potential PACT drugs. It has been demonstrated that reactions with biomolecules (e.g. DNA scission in vitro) does not necessarily correspond to the desired biological action in cellulo, and it is anticipated that the translation to in vivo testing will provide an even greater challenge (not least, because of the need to synchronise and optimise arrival of the photoactive complex at the target site with irradiation). Whereas the photophysics and photochemistry of some metal systems (e.g. the Ru pyridyl complexes) have been investigated in detail, there is still much room for exploration in this young and promising field.
Acknowledgements
We thank the MRC (G070162) and EPSRC (EP/G006792/1) for support and members of COST Action D39 for stimulating discussions. L.S. was supported by the Marie Curie Intra European Fellowship 220281 PHOTORUACD within the 7th European Community Framework Programme. We also thank the EDRF for support for Science City/AWM.
Biographies
 Nicola Farrer received her degree (Natural Sciences, 2003) and subsequently her PhD (2007) from Cambridge University, UK. Her doctoral work involved developing readily ionisable, phosphine ligands with a view to detecting catalytic intermediates by electrospray mass spectrometry, and was supervised by Prof. Brian Johnson (Cambridge), with the bulk of the synthetic work undertaken in the lab of Dr. Scott McIndoe (University of Victoria, Canada). She returned to the UK to join the Sadler group as a post-doctoral fellow where her research interests include one- and two-photon photoactivatable metal complexes, multinuclear NMR spectroscopy and chemical applications of photonic crystal fibers.
Nicola Farrer received her degree (Natural Sciences, 2003) and subsequently her PhD (2007) from Cambridge University, UK. Her doctoral work involved developing readily ionisable, phosphine ligands with a view to detecting catalytic intermediates by electrospray mass spectrometry, and was supervised by Prof. Brian Johnson (Cambridge), with the bulk of the synthetic work undertaken in the lab of Dr. Scott McIndoe (University of Victoria, Canada). She returned to the UK to join the Sadler group as a post-doctoral fellow where her research interests include one- and two-photon photoactivatable metal complexes, multinuclear NMR spectroscopy and chemical applications of photonic crystal fibers.
 Luca Salassa received his undergraduate degree (2001) in Italy at the University of Turin, where he also completed his PhD (2004) on fluorescent metal complexes under the supervision of Prof. R. Gobetto. He later joined the University of Montana (USA) as a post-doctoral fellow and studied protein dynamics using long-lived fluorescent probes. After a period in industry, he was awarded an Intraeuropean Marie Curie Fellowship to study photoactivatable ruthenium complexes for anticancer applications in Prof. Sadler's group at the University of Warwick (2008). His research interests are focussed on the photophysics and photochemistry of metal complexes and the use of computational (DFT) and spectroscopic methods for the study of such systems.
Luca Salassa received his undergraduate degree (2001) in Italy at the University of Turin, where he also completed his PhD (2004) on fluorescent metal complexes under the supervision of Prof. R. Gobetto. He later joined the University of Montana (USA) as a post-doctoral fellow and studied protein dynamics using long-lived fluorescent probes. After a period in industry, he was awarded an Intraeuropean Marie Curie Fellowship to study photoactivatable ruthenium complexes for anticancer applications in Prof. Sadler's group at the University of Warwick (2008). His research interests are focussed on the photophysics and photochemistry of metal complexes and the use of computational (DFT) and spectroscopic methods for the study of such systems.
 Peter Sadler obtained his BA, MA and DPhil at the University of Oxford and then spent two years as a Medical Research Council Research Fellow in Molecular Pharmacology at the University of Cambridge and the National Institute for Medical Research. In 1973 he was appointed as a Lecturer in Chemistry at Birkbeck College, University of London, where he subsequently became Reader in Biological Inorganic Chemistry, and Professor of Chemistry. In 1996 he was appointed to the Crum Brown Chair of Chemistry at the University of Edinburgh, and in June 2007 took up a Chair in Chemistry at the University of Warwick where he is also Head of Department. He is a Fellow of the Royal Society of Edinburgh (FRSE) and the Royal Society of London (FRS). His research interests are centred on the chemistry of metals in medicine and the design of novel therapeutic agents.
Peter Sadler obtained his BA, MA and DPhil at the University of Oxford and then spent two years as a Medical Research Council Research Fellow in Molecular Pharmacology at the University of Cambridge and the National Institute for Medical Research. In 1973 he was appointed as a Lecturer in Chemistry at Birkbeck College, University of London, where he subsequently became Reader in Biological Inorganic Chemistry, and Professor of Chemistry. In 1996 he was appointed to the Crum Brown Chair of Chemistry at the University of Edinburgh, and in June 2007 took up a Chair in Chemistry at the University of Warwick where he is also Head of Department. He is a Fellow of the Royal Society of Edinburgh (FRSE) and the Royal Society of London (FRS). His research interests are centred on the chemistry of metals in medicine and the design of novel therapeutic agents.
References
- 1.a) Desoize B. Anticancer Res. 2004;24:1529–1544. [PubMed] [Google Scholar]; b) Sun RW-Y, Ma D-L, Wong EL-M, Che C-M. Dalton Trans. 2007;43:4884–4892. doi: 10.1039/b705079h. [DOI] [PubMed] [Google Scholar]; c) Nguewa P, Cepeda V, Fuertes MA, Castilla J, Alonso C, Perez JM. In: Metal Antitumor Compounds: An Overview, Metal Compounds in Cancer Chemotherapy. Perez JM, Fuertes MA, Alonso C, editors. Research Signpost; Trivandrum, India: 2005. pp. 1–29. [Google Scholar]; d) Fricker SP. Dalton Trans. 2007:4903–4917. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- 2.Denny WA. Eur. J. Med. Chem. 2001;36:577–595. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- 3.For metals a) Cr, Cu, Ru, Rh see Vol I (280) and b) Ln, Rh, Os, Ir, Pt, Au see Vol II (281); Balzani V, Campagna S, editors. Photochemistry and Photophysics of Coordination Compounds I&II”, Top. Curr. Chem. 280 & 281. Springer-Verlag; Berlin Heidelberg: 2007.
- 4.Patmore NJ. Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem. 2008;104:498–528. [Google Scholar]
- 5.Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G. Chem. Rev. 2005;5:2647–2694. doi: 10.1021/cr030707e. [DOI] [PubMed] [Google Scholar]
- 6.Billadeau MA, Morrison H. Met. Ions. Bio. Syst. 1996;33:269–296. [PubMed] [Google Scholar]
- 7.Mesmaeker AK-D, Lecomte J-P, Kelly JM. Top. Curr. Chem. 1996;177:25–76. [Google Scholar]
- 8.Farrer NJ, Sadler PJ. Aust. J. Chem. 2008;61:669–674. [Google Scholar]
- 9.a) Kostova I. Curr. Med. Chem. Anti. Canc. Agents. 2005;5:591–602. doi: 10.2174/156801105774574694. [DOI] [PubMed] [Google Scholar]; b) Mody TD, Sessler JL. J. Porphyr. Phthalocyan. 2001;5:134–142. [Google Scholar]
- 10.Jakupec MA, Keppler BK. Met. Ions Bio. Syst. 2004;42:425–462. [PubMed] [Google Scholar]
- 11.a) Meyer TJ. Acc. Chem. Res. 1989;22:163–170. [Google Scholar]; b) Neumann-Spallart M, Kalyanasundaram K, Grätzel M, Grätzel C. Helv. Chim. Acta. 1980;63:1111–1118. [Google Scholar]; c) Buda M, Kalyuzhny G, Bard AJ. J. Am. Chem. Soc. 2002;124:6090–6098. doi: 10.1021/ja017834h. [DOI] [PubMed] [Google Scholar]; d) Nazeeruddin MK, Zakeeruddin SM, Lagref JJ, Liska P, Comte P, Barolo C, Viscardi G, Schenk K, Grätzel M. Coord. Chem. Rev. 2004;248:1317–1328. [Google Scholar]
- 12. Adapted from http://www.olympusmicro.com/primer/java/jablonski/jabintro/index.html.
- 13.a) Salassa L, Garino C, Salassa G, Gobetto R, Nervi C. J. Am. Chem. Soc. 2008;130:9590–9597. doi: 10.1021/ja8025906. [DOI] [PubMed] [Google Scholar]; b) Salassa L, Garino C, Salassa G, Nervi C, Gobetto R, Lamberti C, Gianolio D, Bizzarri R, Sadler PJ. Inorg. Chem. 2009;48:1469–1481. doi: 10.1021/ic8015436. [DOI] [PubMed] [Google Scholar]
- 14.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann B,E, Green CJ. Circ. Res. 2002;90:e17–e24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 15.Juban EA, Smeigh AL, Monat JE, McCusker JK. Coord. Chem. Rev. 2006;250:1783–1791. [Google Scholar]
- 16.Hambley TW. Aust. J. Chem. 2008;61:647–653. [Google Scholar]
- 17.Brancaleon L, Moseley H. Lasers Med. Sci. 2002;17:173–186. doi: 10.1007/s101030200027. [DOI] [PubMed] [Google Scholar]
- 18.Radu A, Conde R, Fontolliet C, Wagnieres G, Van den Bergh H, Monnier P. Gastrointest Endosc. 2003;57:897–905. doi: 10.1016/s0016-5107(03)70027-3. [DOI] [PubMed] [Google Scholar]
- 19.He GS, Tan L-S, Zheng Q, Prasad PN. Chem. Rev. 2008;108:1245–1330. doi: 10.1021/cr050054x. [DOI] [PubMed] [Google Scholar]
- 20.Loganathan D, Morrison H. Curr. Opin. Drug Discovery Dev. 2005;8:478–486. [PubMed] [Google Scholar]
- 21.Bonnett R. In: Metal Complexes for Photodynamic Therapy, Comprehensive Coordination Chemistry II. McCleverty JA, Meyer TJ, editors. Vol. 9. Elsevier; Oxford: 2004. pp. 945–1003. [Google Scholar]
- 22.Schuitmaker JJ, Baas P, van Leengoed HLLM, van der Meulen FW, Star WM, van Zandwijk N. J. Photochem. Photobiol. B. 1996;34:3–12. doi: 10.1016/1011-1344(96)07342-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z. Technol. Cancer Res. Treat. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CM, Pendse D, Emberton M. Nat. Clin. Pract. Urol. 2009;6:18–30. doi: 10.1038/ncpuro1274. [DOI] [PubMed] [Google Scholar]
- 25.a) Trushina OI, Novikova EG, Sokolov VV, Filonenko EV, Chissov VI, Vorozhtsov GN, Hertzen PA. Photodiag. Photodynamic Ther. 2009;5:256–259. doi: 10.1016/j.pdpdt.2008.09.005. [DOI] [PubMed] [Google Scholar]; b) Sharman WM, Allen CM, van Lier JE. Drug Disc. Today. 1999;4:507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 26.Fujishima A, Rao TN, Tryk DA. J. Photochem. Photobio. C. 2000;1:1–21. [Google Scholar]
- 27.Kalbacova M, Macak JM, Schmidt-Stein F, Mierke CT, Schmuki P. Phys. Status Sol. (RRL) 2008;2:194–196. [Google Scholar]
- 28.Xu J, Sun Y, Zhao YM, Huang JJ, Chen CM, Jiang ZY. Int. J. Photoenergy. 2007;2:1–7. [Google Scholar]
- 29.Paradies J, Crudass J, MacKay F, Yellowlees LJ, Montgomery J, Parsons S, Oswald L, Robertson N, Sadler PJ. J. Inorg. Biochem. 2006;100:1260–1264. doi: 10.1016/j.jinorgbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Tshuva EY, Ashenhurst JA. Eur. J. Inorg. Chem. 2009;15:2203–2218. [Google Scholar]
- 31.Sasmal PK, Saha S, Majumdar R, Dighe RR, Chakravarty AR. Chem. Commun. 2009;13:1703–1705. doi: 10.1039/b822229k. [DOI] [PubMed] [Google Scholar]
- 32.Sasmal PK, Patra AK, Nethaji M, Chakravarty AR. Inorg. Chem. 2007;46:11112–11121. doi: 10.1021/ic7011793. [DOI] [PubMed] [Google Scholar]
- 33.a) Hiort C, Goodisman J, Dabrowiak JC. Biochemistry. 1996;35:12354–12362. doi: 10.1021/bi9606253. [DOI] [PubMed] [Google Scholar]; b) Sam M, Hwang JH, Chanfreau G, Abu-Omar MM. Inorg. Chem. 2004;43:8447–8455. doi: 10.1021/ic0486419. [DOI] [PubMed] [Google Scholar]
- 34.Weeden SH, Schmidt RH. J. Arthroplasty. 2007;22:151–155. doi: 10.1016/j.arth.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Kim GS, Judd DA, Hill CL, Schinazi RF. J. Med. Chem. 1994;37:816–820. doi: 10.1021/jm00032a016. [DOI] [PubMed] [Google Scholar]
- 36.Vłcek A. Coord. Chem. Rev. 2002;230:225–242. [Google Scholar]
- 37.a) Niesel J, Pinto A, N'Dongo HWP, Merz K, Ott I, Gust R, Schatzschneider U. Chem. Commun. 2008:1798–1800. doi: 10.1039/b719075a. [DOI] [PubMed] [Google Scholar]; b) Johnson TR, Mann BE, Clark JE, Foresti R, Green CJ, Motterlini R. Angew. Chem., Int. Ed. 2003;42:3722–3729. doi: 10.1002/anie.200301634. [DOI] [PubMed] [Google Scholar]
- 38.Billadeau MA, Morrison H. J. Inorg. Biochem. 1995;57:249–270. doi: 10.1016/0162-0134(94)00029-a. [DOI] [PubMed] [Google Scholar]
- 39.DeLeo MA, Ford PC. Coord. Chem. Rev. 208:47–59. [Google Scholar]
- 40.DeRosa F, Bu XH, Ford PC. Inorg. Chem. 2005;44:4157–4165. doi: 10.1021/ic048311o. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda Y, Takaki T, Murakami Y. Bull. Chem. Soc. Jpn. 1986;59:1839–1844. [Google Scholar]
- 42.Maeda D, Shimakoshi H, Abe M, Hisaeda Y. Dalton Trans. 2009:140–145. doi: 10.1039/b809262a. [DOI] [PubMed] [Google Scholar]
- 43.Bae E, Lee JW, Hwang BH, Yeo J, Yoon J, Cha HJ, Choi W. Chemosphere. 2008;72:174–181. doi: 10.1016/j.chemosphere.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 44.Banfi S, Cassani E, Caruso E, Cazzaro M. Bioorg. Med. Chem. 2003;11:3595–3605. doi: 10.1016/s0968-0896(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 45.Niesel J, Pinto A, N'Dongo HWP, Merz K, Ott I, Gust R, Schatzschneider U. Chem. Commun. 2008:1798–1800. doi: 10.1039/b719075a. [DOI] [PubMed] [Google Scholar]
- 46.Coleman A, Brennan C, Vos JG, Pryce MT. Coord. Chem. Rev. 2008;252:2585–2595. [Google Scholar]
- 47.a) Conrado CL, Wecksler S, Egler C, Magde D, Ford Peter C. Inorg. Chem. 2004;43:5543–5549. doi: 10.1021/ic049459a. [DOI] [PubMed] [Google Scholar]; b) Wecksler SR, Mikhailovsky A, Korystov D, Ford PC. J. Am. Chem. Soc. 2006;128:3831–3837. doi: 10.1021/ja057977u. [DOI] [PubMed] [Google Scholar]; c) Wecksler SR, Mikhailovsky A, Korystov D, Buller F, Kannan R, Tan L-S, Ford PC. Inorg. Chem. 2007;46:395–402. doi: 10.1021/ic0607336. [DOI] [PubMed] [Google Scholar]
- 48.Zheng Q, Bonoiu A, Ohulchanskyy TY, He GS, Prasad PN. Mol. Pharm. 2008;5:389–398. doi: 10.1021/mp700117s. [DOI] [PubMed] [Google Scholar]
- 49.Maurer TD, Kraft BJ, Lato SM, Ellington AD, Zaleski JM. Chem. Commun. 2000:69–70. [Google Scholar]
- 50.Roy M, Saha S, Patra AK, Nethaji M, Chakravarty AR. Inorg. Chem. 2007;46:4368–4370. doi: 10.1021/ic062056l. [DOI] [PubMed] [Google Scholar]
- 51.Roy M, Bhowmick T, Santhanagopal R, Ramakumar S, Chakravarty AR. Dalton Trans. 2009:4671–4682. doi: 10.1039/b901337g. [DOI] [PubMed] [Google Scholar]
- 52.Magennis SW, Habtemariam A, Novakova O, Henry JB, Meier S. l., Parsons S, Oswald IDH, Brabec V, Sadler PJ. Inorg. Chem. 2007;46:5059–5068. doi: 10.1021/ic062111q. [DOI] [PubMed] [Google Scholar]
- 53.Herman L, Ghosh S, Defrancq E, Kirsch-De Mesmaeker A. J. Phys. Org. Chem. 2008;21:670–681. [Google Scholar]
- 54.Dorcier A, Ang WH, Bolano S, Gonsalvi L, Juillerat-Jeannerat L, Laurenczy G, Peruzzini M, Phillips AD, Zanobini F, Dyson PJ. Organometallics. 2006;25:4090–4096. [Google Scholar]
- 55.Zigler DF, Mongelli MT, Jeletic M, Brewer KJ. Inorg. Chem. Commun. 2007;10:295–298. [Google Scholar]
- 56.Holder AA, Swavey S, Brewer KJ. Inorg. Chem. 2004;43:303–308. doi: 10.1021/ic035029t. [DOI] [PubMed] [Google Scholar]
- 57.Blower PJ, Dilworth JR, Maurer RI, Millen GD, Reynolds CA, Zheng Y. J. Inorg. Biochem. 2001;85:15–22. doi: 10.1016/s0162-0134(00)00228-2. [DOI] [PubMed] [Google Scholar]
- 58.Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW. Chem. Eur J. 2007;13:2974–2982. doi: 10.1002/chem.200601137. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell JB, Wink DA, DeGraff W, Gamson J, Keefer LK, Krishna MC. Cancer Res. 1993;53:5845–5848. [PubMed] [Google Scholar]
- 60.Boyd S, Ghiggino KP, McFadyen WD. Aust. J. Chem. 2008;61:585–591. [Google Scholar]
- 61.Arounaguiri S, Maiya BG. Inorg. Chem. 1996;35:4267–4270. doi: 10.1021/ic9508684. [DOI] [PubMed] [Google Scholar]
- 62.Funston AM, Cullinane C, Ghiggino KP, McFadyen WD, Stylli SS, Tregloan PA. Aust. J. Chem. 2005;58:206–212. [Google Scholar]
- 63.Barton JK, Raphael AL. Proc. Natl. Acad. Sci. 1985;82:6460–6464. doi: 10.1073/pnas.82.19.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loganathan D, Morrison H. Photochem. Photobiol. 2006;82:237–247. doi: 10.1562/2005-01-19-RA-420. [DOI] [PubMed] [Google Scholar]
- 65.Zeglis BM, Barton JK. Nat. Protoc. 2007;2:357–370. doi: 10.1038/nprot.2007.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Junicke H, Hart JR, Kisko J, Glebov O, Kirsch IR, Barton JK. Proc. Natl. Acad. Sci. 2003;100:3737–3742. doi: 10.1073/pnas.0537194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chifotides HT, Dunbar KR. Acc. Chem. Res. 2005;38:146–156. doi: 10.1021/ar0302078. [DOI] [PubMed] [Google Scholar]
- 68.Lutterman DA, Fu PK-L, Turro C. J. Am. Chem. Soc. 2006;128:738–739. doi: 10.1021/ja057620q. [DOI] [PubMed] [Google Scholar]
- 69.Dixon IM, Collin J-P, Sauvage J-P, Flamigni L, Encinas S, Barigelletti F. Chem. Soc. Rev. 2000;29:385–391. [Google Scholar]
- 70.Zhang KY, Lo KK-W. Inorg. Chem. 2009;48:6011–6025. doi: 10.1021/ic900412n. [DOI] [PubMed] [Google Scholar]
- 71.Vakrat-Haglili Y, Weiner L, Brumfeld V, Brandis A, Salomon Y, Mcllroy B, Wilson BC, Pawlak A, Rozanowska M, Sarna T, Scherz A. J. Am. Chem. Soc. 2005;127:6487–6497. doi: 10.1021/ja046210j. [DOI] [PubMed] [Google Scholar]
- 72.Moody L, Holder AA. Ann. Rep. Prog. Chem. Sect. A. 2008;104:477–497. [Google Scholar]
- 73.Talib J, Harman DG, Dillon CT, Aldrich-Wright J, Beck JL, Ralph SF. Dalton Trans. 2009:504–513. doi: 10.1039/b814156h. [DOI] [PubMed] [Google Scholar]
- 74.Prabhakara MC, Bhojya Naik HS. Main Group Chem. 2008;7:97–107. [Google Scholar]
- 75.Bednarski PJ, Mackay FS, Sadler PJ. Anti-Cancer Agents in Med. Chem. 2007;7:75–93. doi: 10.2174/187152007779314053. [DOI] [PubMed] [Google Scholar]
- 76.Adams CJ, Fey N, Harrison ZA, Sazanovich IV, Towrie M, Weinstein JA. Inorg. Chem. 2008;47:8242–8257. doi: 10.1021/ic800850h. [DOI] [PubMed] [Google Scholar]
- 77.a) Mackay FS, Farrer NJ, Salassa L, Tai H-C, Deeth RJ, Moggach SA, Wood P, Parsons S, Sadler PJ. Dalton Trans. 2009:2315–2325. doi: 10.1039/b820550g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Phillips HIA, Ronconi L, Sadler PJ. Chem. Eur J. 2009;15:1588–1596. doi: 10.1002/chem.200802206. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mackay FS, Moggach SA, Collins A, Parsons S, Sadler PJ. Inorg. Chim. Acta. 2009;362:811–819. [Google Scholar]; Ronconi L, Sadler PJ. Chem. Commun. 2008:235–237. doi: 10.1039/b714216a. [DOI] [PubMed] [Google Scholar]; d) Mackay FS, Woods JA, Heringová P, Kašpárková J, Pizarro AM, Moggach SA, Parsons S, Brabec V, Sadler PJ. Proc. Natl. Acad. Sci. 2007;104:20743–20748. doi: 10.1073/pnas.0707742105. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Salassa L, Phillips HIA, Sadler PJ. Phys. Chem. Chem. Phys. 2009 doi: 10.1039/b912496a. DOI:10.1039/b912496a. [DOI] [PubMed] [Google Scholar]; f) Kratochwil NA, Parkinson JA, Bednarski PJ, Sadler PJ. Angew. Chem., Int. Ed. 1999;38:1460–1463. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1460::AID-ANIE1460>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 78.Lottner C, Knuechel R, Bernhardt G, Brunner H. Cancer Lett. 2004;215:167–177. doi: 10.1016/j.canlet.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 79.Weersink RA, Bogaards A, Gertner M, Davidson SRH, Zhang K, Netchev G, Trachtenberg J, Wilson BC. J. Photochem. Photobio. B. 2005;79:211–222. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Bucking M, Dickson EFG, Farahani M, Fischer F, Holmes D, Jori G, Kennedy JC, Kenney ME, Peng X, Pottier RH, Weagle G. J. Photochem. Photobio. B. 2000;58:87–93. doi: 10.1016/s1011-1344(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 81.Maheswari PU, van der Ster M, Smulders S, Barends S, van Wezel GP, Massera C, Roy S, den Dulk H, Gamez P, Reedijk J. Inorg. Chem. 2008;47:3719–3727. doi: 10.1021/ic702306f. [DOI] [PubMed] [Google Scholar]
- 82.Patra AK, Bhowmick T, Roy S, Ramakumar S, Chakravarty AR. Inorg. Chem. 2009;48:2932–2943. doi: 10.1021/ic8017425. [DOI] [PubMed] [Google Scholar]; Goswami TK, Roy M, Nethaji M, Chakravarty AK. Organometallics. 2009;28:1992–1994. [Google Scholar]
- 83.Fortner A, Wang S, Darbha GK, Ray A, Yu H, Ray PC, Kalluru RR, Kim CK, Rai V, Singh JP. Chem. Phys. Lett. 2007;434:127–132. [Google Scholar]
- 84.Lahiri D, Bhowmick T, Pathak B, Shameema O, Patra AK, Ramakumar S, Chakravarty AR. Inorg. Chem. 2009;48:339–349. doi: 10.1021/ic800806j. [DOI] [PubMed] [Google Scholar]
- 85.Zinchenko AA, Chen N, Murata S. Chem. Lett. 2008;37:1096–1097. [Google Scholar]
- 86.Ott I. Coord. Chem. Rev. 2009;253:1670–1681. [Google Scholar]
- 87.Che C-M, Sun RW-Y, Yu W-Y, Ko C-B, Zhu N, Sun H. Chem. Commun. 2003:1718–1719. doi: 10.1039/b303294a. [DOI] [PubMed] [Google Scholar]
- 88.Jain PK, Huang X, El-Sayedand IH, El-Sayed MA. Acc. Chem. Res. 2008;41:1578–86. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 89.Hwu JR, Lin YS, Josephrajan T, Hsu M-H, Cheng F-Y, Yeh C-S, Su W-C, Shieh D-B. J. Am. Chem. Soc. 2009;131:66–68. doi: 10.1021/ja804947u. [DOI] [PubMed] [Google Scholar]
- 90.Wang C, Chen J, Talavage T, Irudayaraj J. Angew. Chem., Int. Ed. 2009;48:2759–2763. doi: 10.1002/anie.200805282. [DOI] [PubMed] [Google Scholar]
- 91.Li J-L, Wang L, Liu X-Y, Zhang Z, Guo H-C, Liu W, Tang S. Cancer Lett. 2009;274:319–326. doi: 10.1016/j.canlet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 92.Hsu M-H, Josephrajan T, Yeh C-S, Shieh D-B, Su W-C, Hwu JR. Bioconj. Chem. 2007;18:1709–1712. doi: 10.1021/bc700222n. [DOI] [PubMed] [Google Scholar]
- 93.Rzigalinski BA, Strobl JS. Toxicol. Appl. Pharmacol. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anas A, Akita H, Harashima H, Itoh T, Ishikawa M, Biju V. J. Phys. Chem. B. 2008;112:10005–10011. doi: 10.1021/jp8018606. [DOI] [PubMed] [Google Scholar]
- 95.Juzenas P, Chen W, Sun YP, Coelho MAN, Generalov R, Generalova N, Christensen IL. Adv. Drug Deliver. Rev. 2008;60:1600–1614. doi: 10.1016/j.addr.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valicsek Z, Lendvay G, Horvath O. J. Phys. Chem. B. 2008;112:14509–14524. doi: 10.1021/jp804039s. [DOI] [PubMed] [Google Scholar]
- 97.Holder AA, Zigler DF, Tarrago-Trani MT, Storrie B, Brewer KJ. Inorg. Chem. 2007;46:4760–4762. doi: 10.1021/ic0619916. [DOI] [PubMed] [Google Scholar]
- 98.Molnar SM, Jensen GE, Vogler LM, Jones SW, Laverman L, Bridgewater JS, Richter MM, Brewer KJ. J. Photochem. Photobiol. A. 1994;80:315–322. [Google Scholar]
- 99.Zhao S, Arachchige SM, Slebodnick C, Brewer KJ. Inorg. Chem. 2008;47:6144–6152. doi: 10.1021/ic7023296. [DOI] [PubMed] [Google Scholar]
- 100.Foxon SP, Phillips T, Gill MR, Towrie M, Parker AW, Webb M, Thomas JA. Angew. Chem., Int. Ed. 2007;46:3686–3688. doi: 10.1002/anie.200604837. [DOI] [PubMed] [Google Scholar]
- 101.Pfennig BW, Norris MR, Zimmerman N, Gallagherand JR, McCloskey AI. Inorg. Chim. Acta. 2009;362:1701–1708. [Google Scholar]
- 102.Elias B, Kirsch-De Mesmaeker A. Coord. Chem Rev. 2006;250:1627–1641. [Google Scholar]
- 103.Ortmans I, Elias B, Kelly JM, Moucheron C, Kirsch-De Mesmaker A. Dalton Trans. 2004:668–676. doi: 10.1039/b313213g. [DOI] [PubMed] [Google Scholar]
- 104.De Mesmaeker A, Häner R, Martin P, Moser HE. Acc. Chem. Res. 1995;28:366–374. [Google Scholar]
- 105.Le Gac S, Rickling S, Gerbaux P, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A. Angew. Chem., Int. Ed. 2009;121:1142–1145. doi: 10.1002/anie.200804503. [DOI] [PubMed] [Google Scholar]