1. INTRODUCTION
The vertebrate retina is a specialized neural network that contains very sensitive signal transducers—the rod and cone photoreceptors. Rods function in near darkness (scotopic) and are responsible for vision in dim light, while cones operate in bright light (photopic) and provide high acuity color vision. In the human retina, rods are the dominant photoreceptor cell type and comprise about 95% of all photoreceptor cells, while cones comprise only 5%. Yet in a bright light environment, normal cone function is essential for visual perception since rods become saturated and rendered nonfunctional.
Retinitis pigmentosa (RP) and Age-related macular degeneration (AMD) are two major causes of visual loss due to photoreceptor degeneration. In RP, rod degeneration results in night blindness and loss of peripheral vision. Inevitably, cones are also lost following the disappearance of rods through unknown mechanisms, resulting in blindness.1 AMD is the leading cause of visual impairment and legal blindness in elderly people in the Western world.2 In AMD, central vision is compromised initially, and although rod cell death occurs prior to cone loss, it is the subsequent cone cell death that eventually leads to complete visual loss.3–5 During the last decade, significant advances have been accomplished in understanding the mechanisms leading to rod cell death; however, those underlying cone cell loss are still poorly delineated. This is due to the paucity of cones in the mammalian retina that makes the study of normal cone function and disease-related processes difficult.
The neural retinal leucine zipper (Nrl) knockout (KO, −/−) mouse has a pure-cone retina due to a cell fate switch from rod to S cone during retinal development.6 Electroretinography (ERG) analysis of Nrl−/− mice reveals that the amplitude of light-adapted ERG responses elicited by maximum stimulus did not change significantly up to 31 weeks of age, suggesting the cones in these mouse retinas survive without rod function.6 In this study, we describe an age-dependent retinal degeneration in the pure-cone Nrl−/− mice lacking G protein-coupled receptor kinase 1 (GRK1). The Nrl−/−Grk1−/− mice provide a useful model for studying the molecular, cellular and biochemical pathways of cone cell death in AMD and other retinal diseases.
2. METHODS
2.1. Animals
Nrl−/−Grk1−/− mice were generated by crossing the Nrl−/− mice6 with the Grk1−/− mice7 as described previously.8 The Nrl−/− were generously provided by Anand Swaroop and Alan Mears (University of Michigan), and the Grk1−/− mice were provided by Jason Chen (University of Utah). Both the Nrl−/− and the Nrl−/−Grk1−/− mice were born and maintained in total darkness. All animals were treated according to the guidelines established by the Institute for Laboratory Animal Research.
2.2. Immunoblot Analysis
Three mice from each genotype and each age group were killed at mid-day under room light. From each animal, retina was dissected from one eye and the other eye was prepared for histological and immunohistochemistry analysis (data not shown). The retinas were flash frozen on dry ice and kept at −80°C until use. Frozen retinas were homogenized and an equal amount of proteins from each retina was resolved on replicate 11.5% SDS-PAGE and transferred to PVDF membranes (Millipore Corp., Bedford, MA). The blots were incubated with rabbit polyclonal antibodies against mouse cone arrestin (mCAR/LUMIj),9 S or M opsin8 followed by a horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody and visualized by an Enhanced Chemiluminescence (ECL) Kit (Amersham, Arlington Heights, IL).10
2.3. Electroretinography (ERG)
ERGs were recorded as previously described.11 Mice were dark-adapted overnight, and their eyes were dilated with topical administration of phenylephrine (2.5%) and tropicamide (0.5%). Mice were anesthetized via an intraperitoneal injection of ketamine HCl (100 mg/kg) and xylazine HCl (10 mg/kg), and the cornea anesthetized with 0.5% tropical tetracaine. The mouse was placed in an aluminum foil-lined Faraday cage and a DLT fiber electrode placed on the right cornea. A platinum reference electrode was placed on the lower eyelid and another ground electrode on the ipsilateral ear. Photic stimuli of 10-μs duration were delivered through one arm of a coaxial cable using a Grass PS22 xenon flash. The cable delivered the flash 5 mm from the surface of the cornea, and flashes were attenuated with neutral density filters held to a window of fixed f-stop. Dark-adapted maximum responses (mesopic ERG) were measured using the non-attenuated light stimulus (100). Photopic ERGs were measured using the same stimulus with a 6-foot candle (fc) white background light delivered through the other arm of the coaxial cable. The half-amplitude bandwidth of the system was 0.01 – 100 Hz. Responses were recorded on a Coopervision/Nicolet Electrovisual Diagnostic System, and amplified potentials were displayed on a storage oscilloscope for viewing and photographic recording.
3. RESULTS
3.1. Delayed photoresponse recovery in Nrl−/−Grk1−/− mice
GRK1 is responsible for light-dependent phosphorylation of both S and M cone opsins in the mouse retina.8 To evaluate the physiological effect of the GRK1 deletion on phototransduction in cones, we recorded ERGs from Nrl−/− and Nrl−/−Grk1−/− mice.
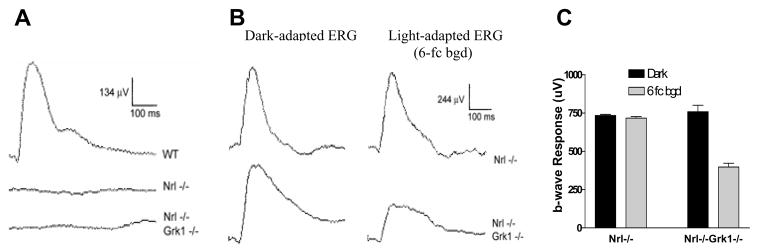
As shown in Figure 1A, both Nrl−/− and Nrl−/−Grk1−/− mice have no ERG response to low intensity light stimulation, which caused a strong rod response in wildtype (WT) mouse, consistent with the pure-cone phenotype of the Nrl−/− mouse.6 Strong ERG responses were elicited in both Nrl−/− and Nrl−/−Grk1−/− mice with high intensity light (Figure 1B). Interestingly, the amplitude of the b-wave is reduced by approximately 50% with 6-fc white background light (6-fc bgd) in the Nrl−/−Grk1−/− mouse, in contrast to that of the Nrl−/− mouse whose b-wave amplitude is not significantly affected by the background light (Figure 1B & C).
Figure 1.
Electroretinography. A. Dark-adapted ERG responses with low stimulus intensity (10−3) from WT, Nrl−/− and Nrl−/−Grk−/− mice. B. Dark- and light-adapted ERGs with high stimulus intensity (100) from Nrl−/− and Nrl−/−Grk1−/− mice. C. Graph representation of the b-wave amplitude from ERG recordings of Nrl−/− and Nrl−/−Grk1−/− mice. The data represent mean ± Standard Deviation (SD) of six mice of each genotype.
We also investigated the time course of recovery of dark-adapted ERGs in Nrl−/− and Nrl−/−Grk1−/− mice. Paired-flash ERG responses of dark-adapted mice were recorded using high intensity (100) white flashes at inter-stimulus intervals (ISIs) specified in seconds (s) to the left of the traces (Figure 2). Complete recovery of the ERG responses in the Nrl−/− mice was achieved in about 0.625 sec, but for the Nrl−/−Grk1−/− mice, the ERG responses were not totally recovered even after 5 sec, suggesting a delayed recovery of cone responses in Nrl−/−Grk1−/− mice.
Figure 2.
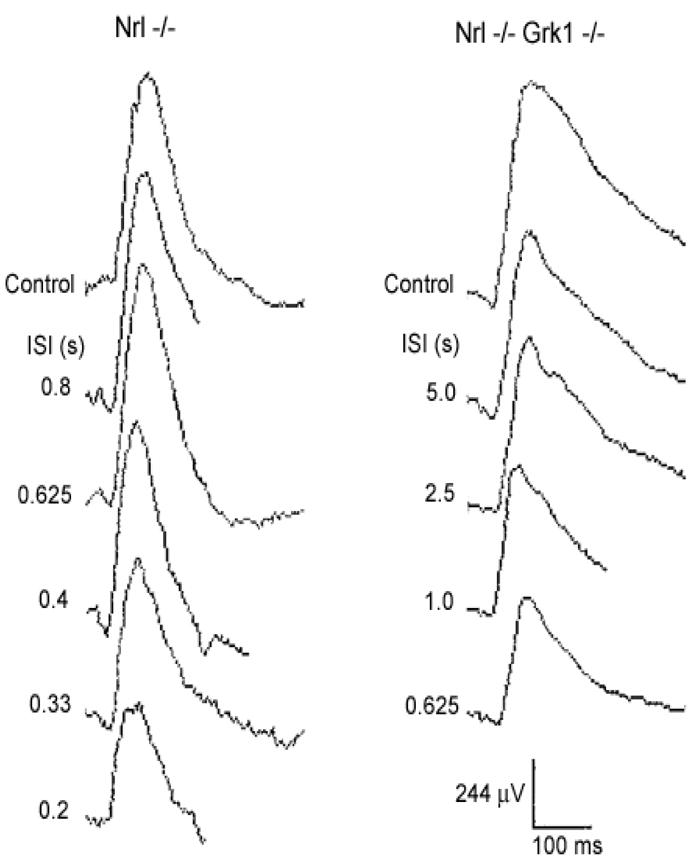
Recovery of ERG responses after a conditioning flash in Nrl−/− and Nrl−/−Grk1−/− mice. A high intensity white flash was delivered after a conditioning flash at the same intensity at inter-stimulus intervals (ISIs) specified in seconds (s) to the left of the traces. Responses obtained without the preceding conditioning flashes are marked as controls.
3.2. Age-dependent cone degeneration in the Nrl−/−Grk1−/− mouse retina
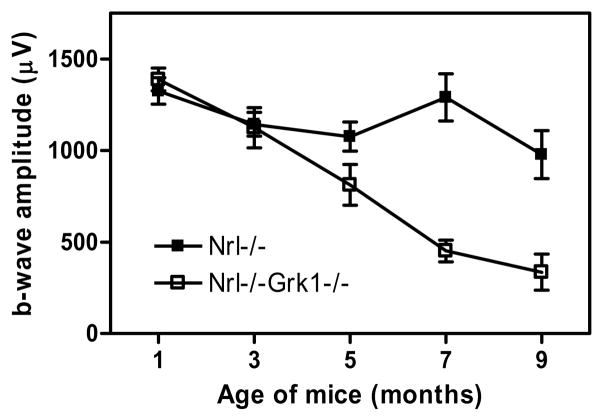
Morphological studies show that the outer nuclear layer (photoreceptor layer) of the Nrl−/−Grk1−/− mice is significantly thinner than that of the age-matched Nrl−/− mice, and is progressively thinner with advancing age, suggesting an age-dependent photoreceptor degeneration in the Nrl−/−Grk1−/− mouse retina (X. Zhu et al., in preparation). ERG analyses of retinal function of mice reared in total darkness reveal that the b-wave amplitude of mesopic ERGs decreases rapidly with increasing age in the Nrl−/−Grk1−/− mice, while that of the Nrl−/− mice remains unchanged until 9 months of age (Figure 3). Animals reared in 12:12 hr cyclic light and bright constant light have similar ERG b-wave amplitude to those of the same genotype raised in total darkness (X. Zhu et al., in preparation), suggesting that the functional decrease and degeneration of the Nrl−/−Grk1−/− photoreceptors are dependent on age but independent of light under these conditions, in contrast to the light-induced rod degeneration in the Grk1−/− mouse retina.7
Figure 3.
Maximum b-wave amplitude of dark-adapted ERG responses in Nrl−/− and Nrl−/−Grk1−/− mice.
3.3. Both S and M cones die with age in the Nrl−/−Grk1−/− mouse retina
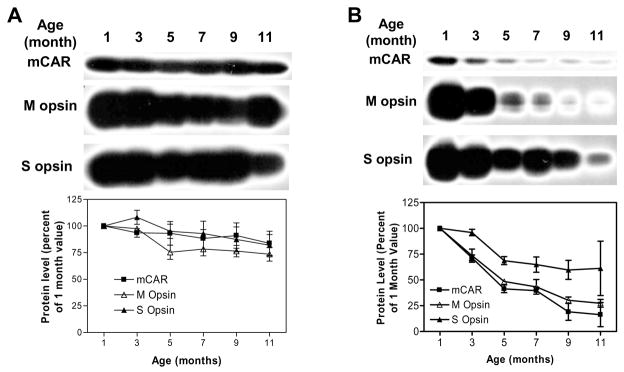
To determine if both S and M cones degenerate in the Nrl−/−Grk1−/− mouse retina, we determined protein expression levels by immunoblot analyses for S and M opsins, as well as mCAR, which is expressed in all cones.8, 9 The expression levels of all three photoreceptor-specific proteins decrease dramatically with age in the Nrl−/−Grk1−/− mouse retina but do not change significantly in the Nrl−/− mouse retina up to 11 months of age (Figure 4). These results suggest that both S and M cones degenerate with age in the Nrl−/−Grk1−/− mouse retina.
Figure 4.
Immunoblot analysis of cone arrestin, S and M Opsin. Nrl−/− (A) and Nrl−/−Grk1−/− mice (B) reared in total darkness were killed, and one retina was dissected and frozen. Frozen retinas were homogenized and equal amount of proteins were applied to a SDS-PAGE. In each panel, a representative immunoblot and a histogram representing quantitative data (Mean ± SEM) from 3 immunoblots are shown per age group.
4. DISCUSSION
Morphological and biochemical analysis of the Nrl−/− retina suggest a complete lack of rods and increased number of S cones.6 Single cell recordings reveal that the Nrl−/− photoreceptors have similar responses to the cones of the rhodopsin−/− mice,12, 13 which are used as a cone animal model for studying cone function.14 Therefore, the photoreceptors of the Nrl−/− mouse retina are functionally and biochemically cones.
In biochemical experiments utilizing these pure-cone retinas, both S and M opsins are phosphorylated following light exposure and cone arrestin preferentially binds to light-activated, phosphorylated cone opsins, suggesting that cones in the Nrl−/− mouse retina may have a similar signal shutoff pathway to that of the WT rods.8
GRK1 is expressed in all rods and cones in the rod-dominant human, monkey and mouse8, 15–17 and in cone-dominant chicken retina.18 GRK1 phosphorylation of light-activated rhodopsin is required for normal inactivation of rhodopsin in vivo.7, 19 Although a cone-specific GRK (GRK7) exists in other species,16, 20–22, GRK1 is the only opsin kinase found in the mouse retina16, 22, 23 and is responsible for light-dependent phosphorylation of both S and M cone opsins.8 ERG analysis of cone photoresponses of the Grk1−/− mouse reveals that GRK1 plays a critical role in the inactivation of murine cone phototransduction.17 In the studies presented here, we demonstrate that when the Grk1 gene is simultaneously knocked out in the Nrl−/− mice, the pure-cone retinas of the Nrl−/−Grk1−/− animals have delayed photoresponse recovery. Both their retinal function and photoreceptor-specific protein expression levels decrease dramatically with age, indicating that lack of GRK1 function in cones leads to cone cell degeneration, in addition to the delayed photoresponse recovery reported previously.
In another study using single cell recordings of the Nrl−/−Grk1−/− photoreceptors, Pugh and collaborators have confirmed the critical role of GRK1 in cone phototransduction shutoff and have shown that the shutoff of M opsin is more dramatically delayed than that of S opsin in the Nrl−/−Grk1−/− retina.24 These results suggest a potential alternative shutoff pathway for S opsin in the Nrl−/−Grk1−/− mouse retina. Our biochemical experiments show that both S and M opsins decrease with age, but S opsin decrease appears to be slower than that of M opsin in the Nrl−/−Grk1−/− retina (Figure 4B). Further immunohistochemistry studies are needed to accurately quantitate if M cones die earlier or more rapidly than S cones in the Nrl−/−Grk1−/− retina. Because the cone cell degeneration in the Nrl−/−Grk1−/− mouse retina is light-independent, we postulate that GRK1 may play other roles that are related to regulation of cellular proliferation and/or apoptosis in cone photoreceptors. Experiments are underway to define the molecular mechanisms leading to cone cell death in the Nrl−/−Grk1−/− mouse retina. These mice provide an excellent model for studying the molecular pathways of cone photoreceptor degeneration and for testing preventive and therapeutic strategies for rescuing cone function, thus preserving vision, in various retinal degenerative diseases.
Acknowledgments
We thank Mary D. Allen for over a decade of generous support to our vision program. CMC is the Mary D. Allen Chair in Vision Research, Doheny Eye Institute (DEI). These studies were also supported, in part, from the NEI Core Vision Research Center grant EY03040 (DEI), the Tony Gray Foundation and Dorie and Fred Miller. The authors also wish to thank Drs. Alan Mears and Anand Swaroop for providing the Nrl−/− mice and Dr. Ching-Kang Jason Chen for the Grk1−/− mice.
References
- 1.Hicks D, Sahel J. The implications of rod-dependent cone survival for basic and clinical research. Invest Ophthalmol Vis Sci. 1999;40:3071–3074. [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 4.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 5.Reme CE, Grimm C, Hafezi F, Iseli HP, Wenzel A. Why study rod cell death in retinal degenerations and how? Doc Ophthalmol. 2003;106:25–29. doi: 10.1023/a:1022423724376. [DOI] [PubMed] [Google Scholar]
- 6.Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 7.Chen CK, Burns ME, Spencer M, et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci U S A. 1999;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Brown B, Li A, et al. GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J Neurosci. 2003;23:6152–6160. doi: 10.1523/JNEUROSCI.23-14-06152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Li A, Brown B, et al. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Mol Vis. 2002;8:462–471. [PubMed] [Google Scholar]
- 10.Zhu X, Craft CM. Modulation of CRX transactivation activity by phosducin isoforms. Mol Cell Biol. 2000;20:5216–5226. doi: 10.1128/mcb.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Hao W, Rife L, et al. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 12.Nikonov SS, Daniele LL, Mears AJ, Swaroop A, Pugh EN. Functional properties of photocurrents of single photoreceptors of the Nrl−/− mouse. ARVO Abstract. 2003 [Google Scholar]
- 13.Nikonov SS, Daniele L, Zhu X, et al. Photoreceptors of the Nrl knockout mouse: Are they cones. The eighth Annual Vision Research Conference. 2004 Abstract. [Google Scholar]
- 14.Jaissle GB, May CA, Reinhard J, et al. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci. 2001;42:506–513. [PubMed] [Google Scholar]
- 15.Zhao X, Huang J, Khani SC, Palczewski K. Molecular forms of human rhodopsin kinase (GRK1) J Biol Chem. 1998;273:5124–5131. doi: 10.1074/jbc.273.9.5124. [DOI] [PubMed] [Google Scholar]
- 16.Weiss ER, Ducceschi MH, Horner TJ, et al. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci. 2001;21:9175–9184. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyubarsky AL, Chen C, Simon MI, Pugh EN., Jr Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J Neurosci. 2000;20:2209–2217. doi: 10.1523/JNEUROSCI.20-06-02209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Yokoyama K, Whitten ME, et al. A novel form of rhodopsin kinase from chicken retina and pineal gland. FEBS Lett. 1999;454:115–121. doi: 10.1016/s0014-5793(99)00764-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH- terminal truncation mutant. Science. 1995;267:374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- 20.Hisatomi O, Matsuda S, Satoh T, et al. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett. 1998;424:159–164. doi: 10.1016/s0014-5793(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 21.Weiss ER, Raman D, Shirakawa S, et al. The cloning of GRK7, a candidate cone opsin kinase, from cone- and rod- dominant mammalian retinas. Mol Vis. 1998;4:27. [PubMed] [Google Scholar]
- 22.Chen CK, Zhang K, Church-Kopish J, et al. Characterization of human GRK7 as a potential cone opsin kinase. Mol Vis. 2001;7:305–313. [PubMed] [Google Scholar]
- 23.Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci U S A. 2004;101:11707–11712. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikonov SS, Daniele L, Zhu X, et al. Photorecptors of Nrl−/− mice co-express functional S- and M-opsins having distinct inactivation mechanisms. J Gen Physiol. 2005 doi: 10.1085/jgp.200409208. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]