SUMMARY
Neuromuscular synapse formation requires a complex exchange of signals between motor neurons and skeletal muscle fibers, leading to the accumulation of postsynaptic proteins, including acetylcholine receptors in the muscle membrane and specialized release sites, or active zones in the presynaptic nerve terminal. MuSK, a receptor tyrosine kinase that is expressed in skeletal muscle, and Agrin, a motor neuron-derived ligand that stimulates MuSK phosphorylation, play critical roles in synaptic differentiation, as synapses do not form in their absence, and mutations in MuSK or downstream effectors are a major cause of a group of neuromuscular disorders, termed congenital myasthenic syndromes (CMS). How Agrin activates MuSK and stimulates synaptic differentiation is not known and remains a fundamental gap in our understanding of signaling at neuromuscular synapses. Here, we report that Lrp4, a member of the LDLR family, is a receptor for Agrin, forms a complex with MuSK and mediates MuSK activation by Agrin.
INTRODUCTION
Understanding how signals are exchanged at neuromuscular synapses is fundamental to understanding the principles that govern the formation and function of synapses in the peripheral and central nervous systems (Sanes and Lichtman, 2001). The discovery of genes critical for forming and maintaining neuromuscular synapses has not only provided insight into the normal mechanisms for synapse formation but also led to the identification of genes that are responsible for a group of neurological disorders, termed congenital myasthenic syndromes (CMS), and for understanding how mutations in these genes leads to deficits in neuromuscular synapse (Engel and Sine, 2005). Further, because molecules and mechanisms that direct the formation and maintenance of neuromuscular synapses are likely to be utilized in the central nervous system, understanding how neuromuscular synapses form and work remains a paradigm for gaining insight into the types of signaling mechanisms that regulate synapse formation and function in the central nervous system as well.
The formation of the neuromuscular synapse is a multi-step process requiring coordinated interactions between motor neurons and muscle fibers, which eventually lead to the formation of a highly specialized postsynaptic membrane and a highly differentiated nerve terminal (Arber et al., 2002; Burden, 1998; Kummer et al., 2006; Sanes and Lichtman, 2001). As a consequence, acetylcholine receptors (AChRs) become highly concentrated in the postsynaptic membrane and arranged in perfect register with active zones in the presynaptic nerve terminal, insuring for fast, robust and reliable synaptic transmission. The signals and mechanisms responsible for this process are poorly understood but require the neurally derived ligand Agrin, and MuSK, a receptor tyrosine kinase expressed in muscle (Glass and Yancopoulos, 1997). Defects in this signaling pathway, which lead to a reduced number of AChRs at synapses, are responsible for a variety of congenital neuromuscular disorders, termed CMS (Beeson et al., 2008; Engel et al., 2008; Engel and Sine, 2005).
Efforts to identify the muscle receptor for Agrin have led to several false starts, including reports that α-dystroglycan was an Agrin receptor (Bowe et al., 1994; Campanelli et al., 1994; Gee et al., 1994; Glass et al., 1996b). Agrin, however, stimulates synaptic differentiation without binding α-dystroglycan (Hopf and Hoch, 1998; Jacobson et al., 1998; Meier et al., 1996). MuSK, though essential for Agrin signaling (DeChiara et al., 1996), does not bind Agrin (Glass et al., 1996a). Moreover, Agrin stimulates MuSK tyrosine phosphorylation in differentiated muscle, but not in other cell types transfected with a MuSK expression vector (Glass et al., 1996a). Together, these data demonstrate that MuSK is essential to transmit the Agrin signal but indicate that additional components, collectively termed MASC (myotube-associated specificity component) are required for Agrin to bind and activate MuSK (Glass et al., 1996a).
In a genetic screen for genes that regulate early development in the mouse, mice carrying mutations in lrp4 were found to have severe defects in neuromuscular synapse formation, leading to neonatal lethality (Weatherbee et al., 2006). Indeed, lrp4 mutant mice display defects in presynaptic and postsynaptic differentiation that are strikingly similar to those found in MuSK mutant mice. Moreover, lrp4 is expressed preferentially by muscle synaptic nuclei, raising the possibility that Lrp4 plays a role in the Agrin/MuSK signaling pathway (Weatherbee et al., 2006). Here, we study how Lrp4 regulates neuromuscular synapse formation and show that Lrp4 selectively binds neural isoforms of Agrin and is required for Agrin to stimulate MuSK phosphorylation. Moreover, we show that Lrp4 self-associates, forms a complex with MuSK and can reconstitute Agrin-stimulated MuSK phosphorylation in non-muscle cells that express Lrp4 and MuSK. Together, these experiments indicate that Lrp4 is the long-sought and elusive receptor for Agrin and has a critical role in activating MuSK and stimulating neuromuscular synapse formation.
RESULTS
Lrp4 is required for Agrin to stimulate MuSK phosphorylation
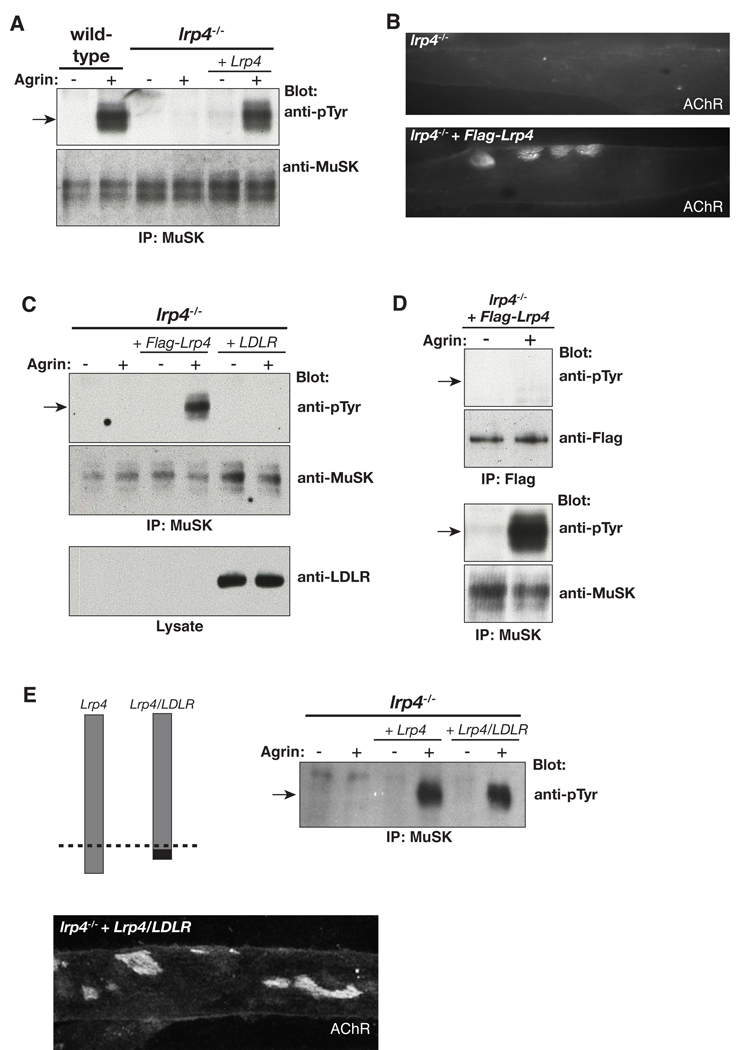
To determine how Lrp4 regulates neuromuscular synapse formation, we generated muscle cell lines from lrp4mitt mutant mice, using methods described previously (Herbst and Burden, 2000). We stimulated wild-type and lrp4 mutant myotubes with Agrin and found that Agrin failed to stimulate MuSK tyrosine phosphorylation in lrp4 mutant cells (Figure 1A). We measured MuSK protein expression and found that total and cell surface MuSK protein expression were normal in lrp4 mutant myotubes (Figure S1), indicating that Lrp4 is not required to express or present MuSK on the cell surface.
Figure 1. Lrp4 is required for Agrin to stimulate MuSK phosphorylation.
(A) Agrin stimulates MuSK tyrosine phosphorylation in wild-type but not in lrp4 mutant myotubes. (A, B) Restoring Lrp4 expression in lrp4 mutant myotubes rescues Agrin-stimulated MuSK phosphorylation as well as AChR clustering. (C) Expression of LDLR fails to rescue MuSK phosphorylation. (D) Flag-Lrp4 is not tyrosine phosphorylated by Agrin stimulation, indicating that Lrp4 is not itself a substrate for MuSK. (E) A Lrp4/LDLR chimera, in which the LDLR cytoplasmic domain was exchanged for the Lrp4 cytoplasmic domain, rescues Agrin-stimulated MuSK phosphorylation and AChR clustering.
To determine whether the loss of Lrp4 was solely responsible for these defects, we infected lrp4 mutant muscle cells with a retroviral vector that restored expression of wild-type Lrp4. Figure 1A shows that expression of Flag-Lrp4 in lrp4 mutant myotubes rescued Agrin-stimulated MuSK phosphorylation. Moreover, restoring Lrp4 expression rescued Agrin-stimulated clustering of acetylcholine receptors (AChRs) (Figure 1B). We infected lrp4 mutant muscle cells with a low density lipoprotein receptor (LDLR) expression vector and found that LDLR cannot substitute for Lrp4, demonstrating specificity within the LDLR family (Figure 1C). Further, we found that Lrp4 is not detectably tyrosine phosphorylated by Agrin (Figure 1D), indicating that Lrp4 is not a substrate for MuSK, consistent with the idea that Lrp4 acts upstream rather than downstream from MuSK. To determine whether the Lrp4 cytoplasmic domain is required for Agrin-responsiveness, we expressed an Lrp4/LDLR chimeric protein, composed of the ecto- and transmembrane-domain from Lrp4 and the cytoplasmic domain from LDLR, in lrp4 mutant myotubes. Agrin stimulation led to wild-type levels of MuSK phosphorylation and AChR clustering (Figure 1E), indicating that the ecto- and transmembrane-domain from Lrp4 are sufficient to confer Agrin-responsiveness. Together, these data demonstrate that Lrp4 is critical for Agrin to activate MuSK and provide a clear explanation for the complete absence of synaptic differentiation in lrp4 mutant mice, as MuSK phosphorylation is required for presynaptic and postsynaptic differentiation (DeChiara et al., 1996; Herbst and Burden, 2000; Zhou et al., 1999). Further, these findings show that Lrp4 acts upstream from MuSK, raising the possibility that Lrp4 is a receptor for Agrin.
Lrp4 is a receptor for Agrin
To determine whether Agrin binds Lrp4, we expressed Lrp4 in BaF3 cells, a non-adherent pro-B cell line, and measured binding of BaF3 cells to substrate-attached Agrin. This experimental arrangement closely mimics the manner in which Agrin, an extracellular matrix protein, is normally presented to and engages its receptor in muscle cells. The agrin gene is expressed in many cell types, but only neurons, including motor neurons, express ‘neural’ splice forms that contain eight (Agrin B8), eleven or nineteen amino acids near the C-terminus (Sanes and Lichtman, 2001).
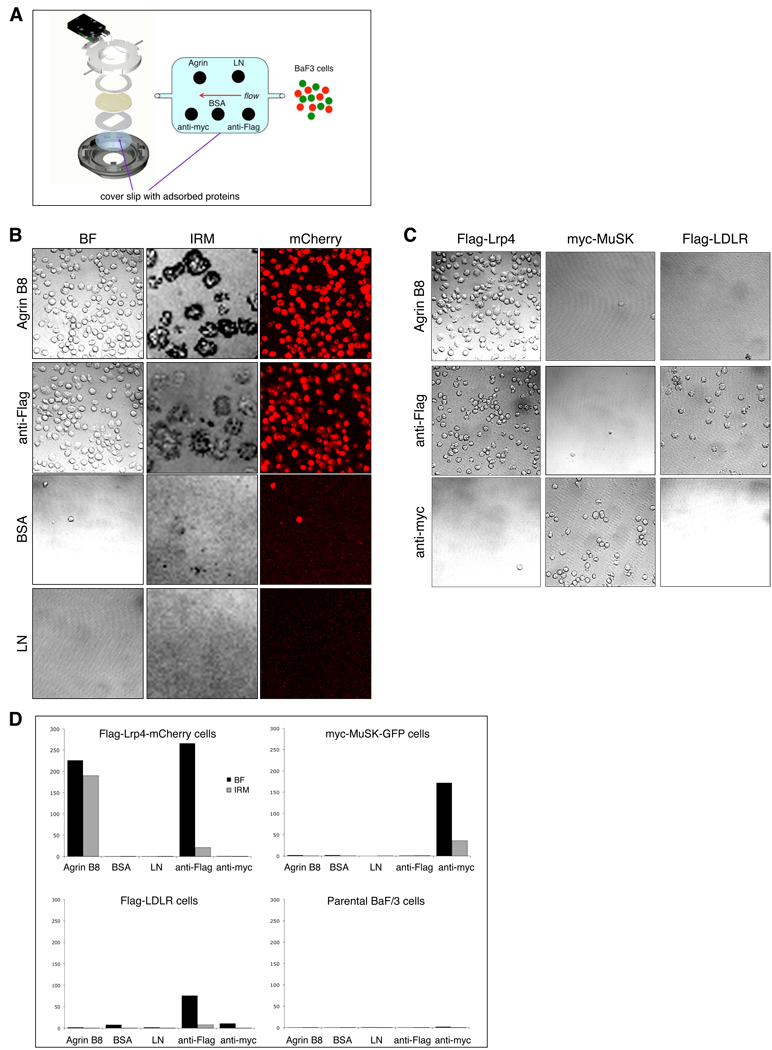
BaF3 cells, expressing either Flag-Lrp4-mCherry, myc-MuSK-GFP. Flag-LDLR or CCR7-GFP, a chemokine receptor, or parental BaF3 cells, were perfused through a chamber containing a glass coverslip on which a ~50 kd recombinant fragment of Agrin B8, BSA or laminin had been adsorbed, and the number of cells that remained attached to each substrate, following washing, was determined (Figure 2A). As positive controls, antibodies to epitope tags, introduced in the ectodomain of Lrp4 (Flag), LDLR (Flag) and MuSK (myc), were also adsorbed to the glass coverslip. We measured the number of attached cells in three ways: (1) bright field microscopy; (2) fluorescence emitted from mCherry or GFP; (3) interference reflection (IRM), caused by close adhesion of cells to substrate (Grakoui et al., 1999; Verschueren, 1985). By all criteria, BaF3 cells expressing Lrp4, or Lrp4 and MuSK, bound selectively to Agrin B8 (Figure 2B, D). Moreover, binding between Agrin B8 and Lrp4 was specific, since parental BaF3 cells, as well as BaF3 cells expressing MuSK, LDLR or CCR7 failed to bind Agrin B8 (Figure 2C, D). The strong IRM signal caused by adhesion of Lrp4-expressing cells to Agrin is consistent with a high affinity association between Agrin B8 and Lrp4 but could be due to a large number of lower affinity interactions cooperating to mediate adhesion (Dustin, 1997).
Figure 2. Lrp4 is a receptor for Agrin.
(A) BaF3 cells, expressing Flag-Lrp4-mCherry, myc-MuSK-GFP, Flag-LDLR, CCR7-GFP, or parental BaF3 cells, were perfused through a chamber containing a glass coverslip on which Agrin B8, BSA or laminin (LN) had been adsorbed. (B) Bright field (BF), fluorescence (mCherry) and interference reflection microscopy (IRM) were used to determine the number of cells that bound to each substrate. The darkest area of the IRM image marks the close apposition of BaF3 cells to Agrin or antibodies. By all criteria, BaF3 cells expressing Lrp4, or Lrp4 and MuSK, bound selectively to Agrin. (C) Binding of Lrp4 to Agrin is specific, since BaF3 cells expressing myc-MuSK-GFP or Flag-LDLR, as well as parental BaF3 cells and cells expressing CCR7-GFP, fail to bind Agrin. Antibodies to epitope tags, introduced into the ectodomain of Lrp4 (Flag), MuSK (myc) and LDLR (Flag), were also adsorbed to the glass coverslip and served as positive and negative controls. (D) Quantitation of the number (mean ± s.e.m.) of Flag-Lrp4-mCherry, myc-MuSK-GFP, Flag-LDLR and parental BaF3 cells that bound to Agrin B8, BSA, LN, anti-Flag and anti-myc, as determined by BF and IRM optics.
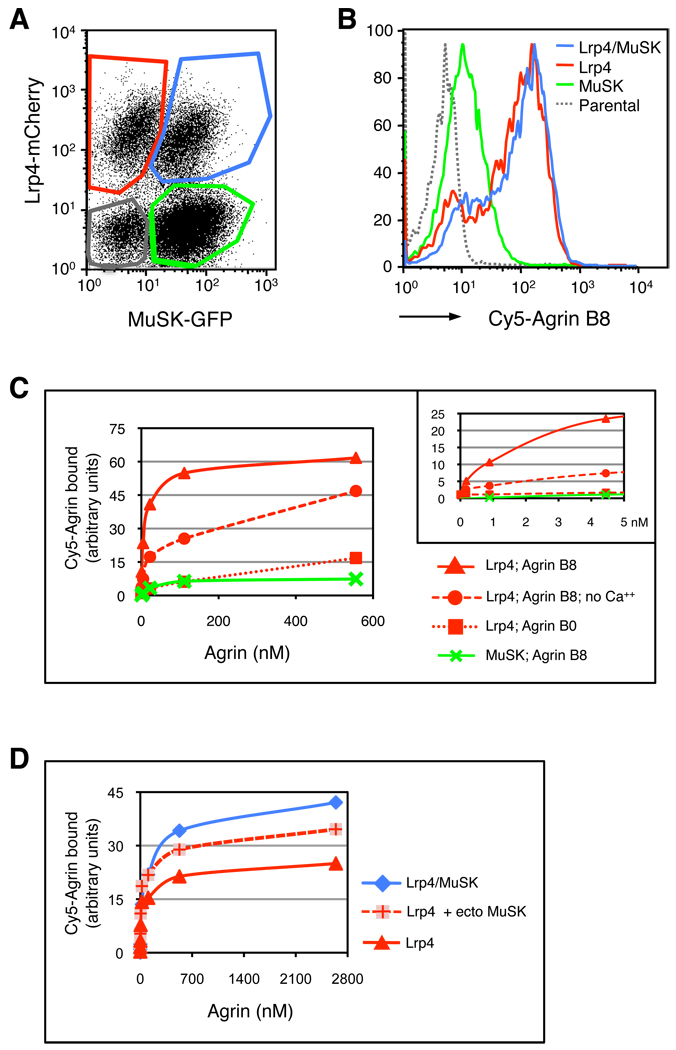
To study binding between Agrin and Lrp4 further, we conjugated purified Agrin with Cy5, and incubated Cy5-Agrin with cells expressing Lrp4, MuSK or Lrp4 and MuSK, as well as parental BaF3 cells. Following washing, we measured the amount of Cy5-Agrin bound to each cell type by flow cytometry (Figure 3A, B). Cy5-Agrin B8 bound selectively, in a saturable and dose-dependent manner, to BaF3 cells that expressed Lrp4 (Figure 3C). Half-maximal binding was attained at 6 nM Agrin B8 (6 ± 1 nM, n=3), typical for the binding affinity between many growth factors and their receptors. Moreover, the extent of Cy5-Agrin binding was linearly correlated well with the amount of Lrp4 expression in individual cells (Figure S2, p < 0.005), consistent with the idea that Agrin binds directly to Lrp4.
Figure 3. Lrp4 binds selectively and with high affinity to neural isoforms of Agrin.
(A) The graph shows the gates for distinguishing cells expressing: Lrp4-mCherry, MuSK-GFP, Lrp4-mCherry plus MuSK-GFP, as well as parental BaF3 cells, as measured by flow cytometry. (B) The histograms depict binding of Cy5-Agrin B8 to the gated populations of cells shown in (A) at 100 nM Agrin. Cy5-Agrin B8 bound preferentially to cells expressing Lrp4-mCherry or Lrp4-mCherry plus MuSK-GFP and weakly to cells expressing MuSK-GFP alone. (C) Lrp4-expressing cells bound Agrin B8 with ~100-fold higher affinity (~6 nM) than Agrin B0. Binding between Agrin B8 and Lrp4 is strengthened ~20-fold in the presence of Ca++. The inset shows binding at the lower concentrations of Agrin. The 95 kd and 50 kd forms of Agrin B8 bound similarly to cells expressing Lrp4 or Lrp4 and MuSK (data not presented). Agrin binding to parental cells was minimal (B) and was subtracted from all values. (D) Co-expression of MuSK-GFP potentiates binding of Cy5-Agrin B8 to cells expressing Lrp4-mCherry; addition of the soluble ectodomain from MuSK (2 µM) also enhances binding of Cy5-Agrin B8 to Lrp4-mCherry expressing cells.
Neural isoforms of Agrin, including Agrin B8, stimulate MuSK phosphorylation and acetylcholine receptor clustering, whereas non-neural isoforms (Agrin B0) are far less (~1000-fold) potent (Ferns et al., 1993; Gesemann et al., 1995; Glass et al., 1996a; Hopf and Hoch, 1998). To determine whether this selectivity is mediated by isoform-selective binding to Lrp4, we measured binding of Cy5-Agrin B8 and Cy5-Agrin B0 to Lrp4-expressing cells. Figure 3 shows that Cy5-Agrin B8 bound to Lrp4-expressing cells with a ~100-fold higher affinity than Cy5-Agrin B0 (Figure 3C), providing an explanation for the selective activation of MuSK by neural isoforms of Agrin in vivo.
Agrin stimulates AChR clustering in a calcium-dependent manner (Borges et al., 2002; Megeath and Fallon, 1998). We measured binding of Cy5-Agrin to Lrp4-expressing BaF3 cells in the presence or absence of calcium and found that calcium increases Agrin-binding by ~20-fold, indicating that the association between Agrin and Lrp4 is enhanced but not dependent upon calcium (Figure 3C). Together with other experiments (Borges et al., 2002), these data suggest that calcium acts to increase the affinity of Agrin for Lrp4 but also at additional steps in the pathway that follow MuSK activation and lead to AChR clustering.
At the higher concentrations of Agrin, we detected a low level of Cy5-Agrin binding to cells expressing MuSK alone, suggesting that MuSK may bind Agrin with low affinity (Figure 3C). Consistent with this idea, cells expressing Lrp4 and MuSK bound more Cy5-Agrin than cells expressing Lrp4 alone, but only at high concentrations of Agrin (Figure 3D). We further tested the idea that MuSK may potentiate Agrin-binding by supplying soluble MuSK ectodomain to Lrp4-expressing cells just prior to incubation with Cy5-Agrin. Figure 3 shows that the MuSK ectodomain enhanced binding of Cy5-Agrin to Lrp4-expressing cells, suggesting that the ectodomains of Lrp4 and MuSK form a complex (Figure 3D) (see below). Together, these data suggest that Lrp4 binds Agrin with high affinity but that a complex of Lrp4/MuSK has a higher binding capacity at high concentrations of Agrin, as may be present in the synaptic cleft.
Lrp4 self-associates and forms a complex with MuSK
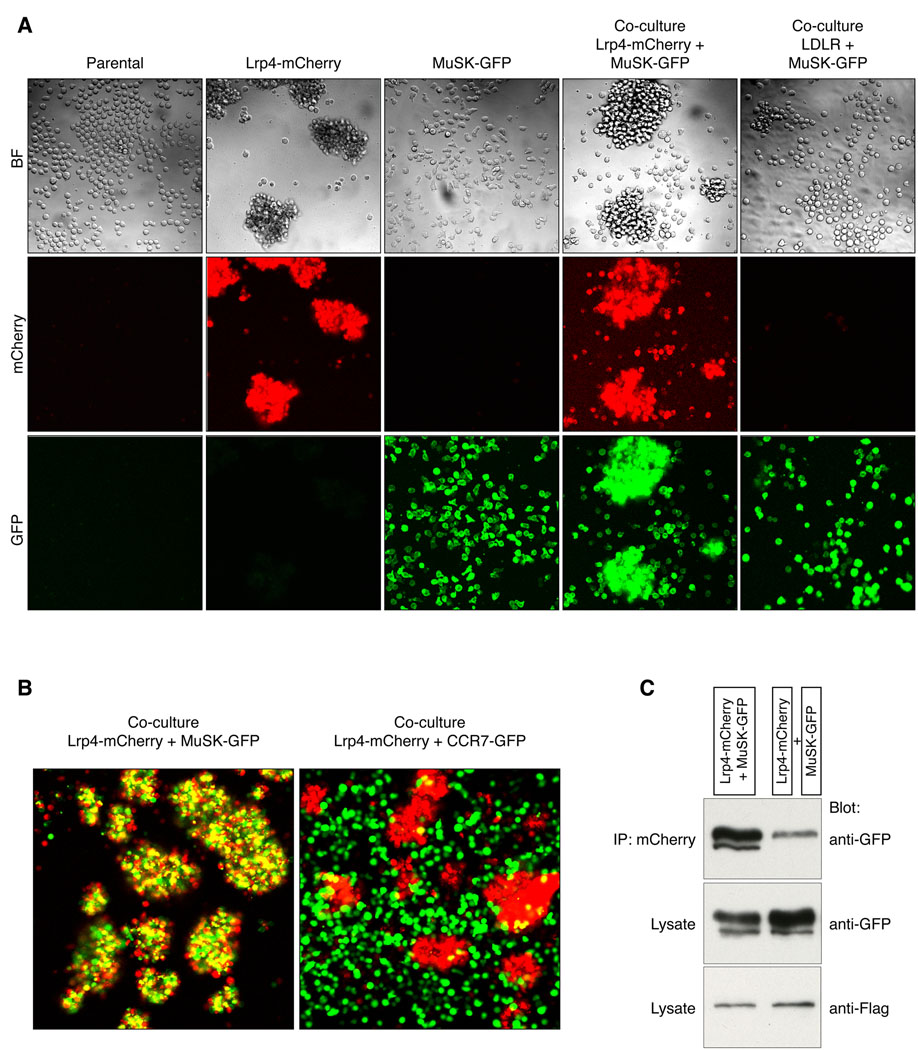
Parental BaF3 cells, as well as BaF3 cells expressing MuSK, LDLR or CCR7, grow in suspension as single cells (Figure 4A). In contrast, Lrp4-expressing cells form aggregates (Figure 4A). Since Lrp4-expressing cells do not aggregate with parental BaF3 cells or CCR7-expressing cells (Figure 4B), the aggregation of Lrp4-expressing cells is caused by Lrp4 self-association. To determine whether Lrp4 also binds MuSK, we mixed MuSK- and Lrp4-expressing cells. Figure 4 shows that MuSK-expressing cells, which grow as solitary cells when cultured alone, co-aggregate with Lrp4-expressing cells (Figure 4B). Binding between Lrp4 and MuSK is specific, since Lrp4-expressing cells fail to co-aggregate with BaF3 cells expressing CCR7 (Figures 4B, S3). These findings, together with experiments showing that the soluble ectodomain of MuSK potentiates Agrin-binding to Lrp4-expressing cells (Figure 3D), support the idea that Lrp4 and MuSK can interact and form a complex.
Figure 4. Lrp4 self-associates and forms a complex with MuSK.
(A) BaF3 cells, as well as BaF3 cells expressing myc-MuSK-GFP or CCR7-GFP, grow in suspension as single cells. Flag-Lrp4-mCherry-expressing cells, however, form aggregates, indicating that Lrp4 binds Lrp4. Although myc-MuSK-GFP-expressing cells grow as solitary cells when cultured alone, they co-aggregate with Flag-Lrp4-mCherry expressing cells, indicating that MuSK binds Lrp4. (B) Binding between Lrp4 and MuSK is specific, since myc-MuSK-GFP-expressing cells fail to aggregate with themselves, and BaF3 cells expressing CCR7-GFP fail to co-aggregate with Flag-Lrp4-mCherry-expressing cells. (C) Lrp4 and MuSK interact in cis, as they co-immunoprecipitate when expressed together in the same cell.
These experiments demonstrate that Lrp4 can bind to MuSK in trans. MuSK and Lrp4, however, are not normally expressed in adjacent cells, since both proteins are expressed in muscle and not in motor neurons (Valenzuela et al., 1995; Weatherbee et al., 2006). To determine whether Lrp4 can associate with MuSK in the same cell, we expressed FLAG-Lrp4-mCherry and myc-MuSK-GFP in BaF3 cells. To minimize trans-interactions between Lrp4 and MuSK, we disrupted cell aggregates, which are labile and disrupted by gentle pipetting, prior to lysis. Lrp4 was immunoprecipitated from cell lysates, using antibodies to mCherry, and MuSK was detected by probing Western blots of the immunoprecipitate with antibodies to GFP. Figure 4 shows that MuSK and Lrp4, expressed in the same cell, form a complex, in a manner that does not depend upon Agrin (Figure 4C). Under these conditions, when expressed in adjacent cells, MuSK co-immunoprecipitated poorly with Lrp4 (Figure 4C). Together, these experiments demonstrate that Lrp4 and MuSK associate in the same cell.
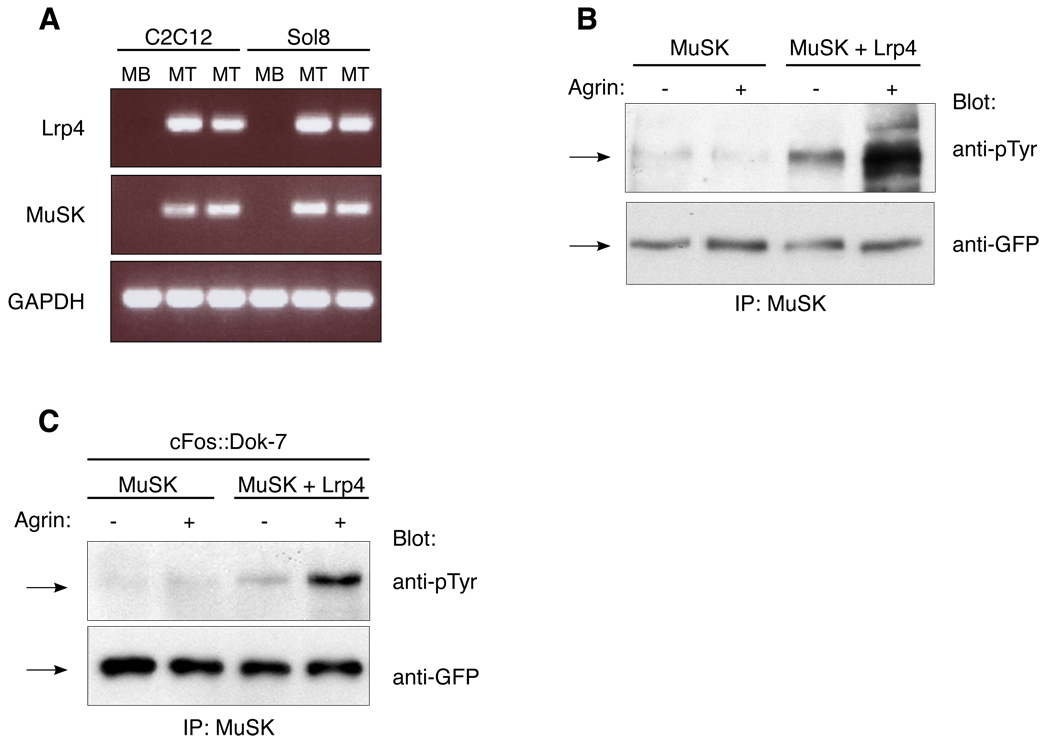
Agrin stimulates MuSK phosphorylation in non-muscle cells expressing Lrp4
Agrin stimulates MuSK phosphorylation in cultured myotubes but neither in myoblasts nor other cell types transfected with a MuSK expression vector, indicating that additional myotube-specific activities are required for Agrin to stimulate MuSK phosphorylation (Glass et al., 1996a). Our data raised the possibility that Lrp4 may be one such myotube-specific activity. Consistent with this idea, we found that lrp4 is expressed in myotubes but poorly, if at all in myoblasts (Figure 5A). To determine whether Lrp4 is sufficient to reconstitute Agrin-stimulated MuSK phosphorylation, we stimulated BaF3 cells that expressed MuSK and Lrp4, or MuSK alone, with Agrin and measured MuSK tyrosine phosphorylation. Agrin stimulation led to a substantial increase in MuSK phosphorylation in BaF3 cells that expressed MuSK and Lrp4 but not in cells that expressed MuSK alone (Figure 5B). These data demonstrate that Lrp4 is sufficient to confer responsiveness to Agrin.
Figure 5. Agrin stimulates MuSK phosphorylation in non-muscle cells expressing Lrp4.
(A) Lrp4, like MuSK, is expressed in C2 and Sol8 myotubes but poorly, if at all in myoblasts. (B) Agrin stimulates phosphorylation of MuSK-GFP in BaF3 cells expressing Lrp4-mCherry and MuSK-GFP but not in cells expressing MuSK-GFP alone. (C) Agrin stimulates MuSK phosphorylation of in BaF3 cells expressing Lrp4, MuSK-GFP and Dok-7 but not in cells expressing MuSK-GFP and Dok-7 without Lrp4. MuSK was immunoprecipitated from BaF3 cells with antibodies to MuSK, and Western blots were probed with antibodies to phosphotyrosine or GFP.
Dok-7 is a muscle-specific adaptor protein, which stabilizes/enhances MuSK phosphorylation in muscle and is expressed in myotubes but not in myoblasts (Okada et al., 2006). Thus, we considered the possibility that Dok-7 may function as a second MASC and confer Agrin-responsiveness in non-muscle cells. We introduced Dok-7 into BaF3 cells that expressed MuSK alone, or Lrp4 and MuSK, and measured MuSK phosphorylation following Agrin stimulation. Because Dok-7 overexpression in muscle can stimulate MuSK phosphorylation even in the absence of Agrin, we expressed low levels of Dok-7 using a weak c-Fos basal promoter cassette (Simon and Burden, 1993) (Figure S4). We found that Agrin stimulation led to a substantial increase in MuSK phosphorylation in BaF3 cells that expressed Lrp4, MuSK and Dok-7, but not in cells that expressed only MuSK and Dok-7 (Figure 5C). Thus, Dok-7 is insufficient to confer Agrin responsiveness without Lrp4 expression, indicating that Dok-7 does not function as a MASC.
In the absence of Agrin stimulation, MuSK phosphorylation was greater in cells that expressed Lrp4 and MuSK than MuSK alone (Figure 5B, C). These data indicate Lrp4 is sufficient to increase the basal level of MuSK phosphorylation, which potentially underlies the requirement for Lrp4 in Agrin-independent, MuSK-dependent muscle prepatterning (see below) (Arber et al., 2002; Kummer et al., 2006; Weatherbee et al., 2006).
DISCUSSION
Agrin stimulates synaptic differentiation by activating MuSK, but identification of the receptor for Agrin has proved elusive (Kleiman and Reichardt, 1996; Kummer et al., 2006; Sealock and Froehner, 1994). Here, we report that Lrp4 is a functional receptor for Agrin, forms a complex with MuSK and mediates Agrin-stimulated MuSK activation.
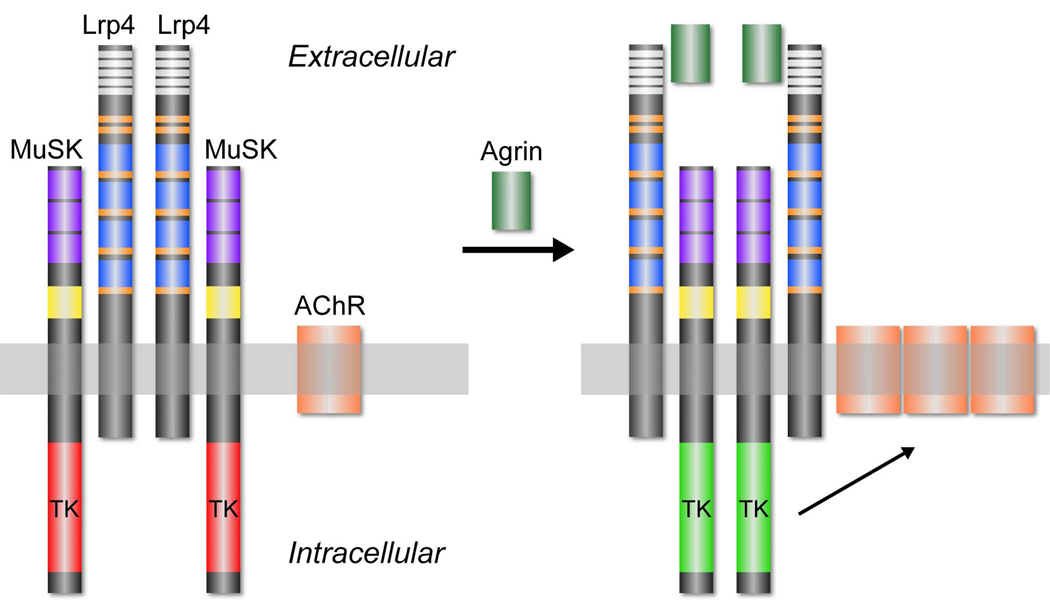
Our data indicate that Lrp4 self-associates and forms an Agrin-independent complex with MuSK (Figure 6). We reason that Agrin may alter the conformation of this preformed complex, reorienting adjacent MuSK molecules and promoting trans-phosphorylation. Because the most N-terminal Ig-like domain in MuSK is essential for Agrin to stimulate MuSK phosphorylation (Stiegler et al., 2006; Zhou et al., 1999), this Ig-like domain may interact with Lrp4 and participate in this proposed rearrangement. Further biochemical and structural studies will be necessary to test this model and to learn how Agrin-binding is converted to MuSK phosphorylation.
Figure 6.
Cartoon illustrating interactions between neural Agrin, Lrp4 and MuSK. Lrp4 self-associates and interacts with MuSK in the absence of Agrin (left). The arrangement of Lrp4 and MuSK within this complex is not known, and the complex may be oligomeric rather than dimeric, as depicted. Moreover, the domains responsible for the Lrp4-Agrin and Lrp4-MuSK interactions are not known. Neural Agrin binds to the preformed complex and triggers a reorganization or reorientation of MuSK, promoting trans-phosphorylation and kinase activation (right). Once phosphorylated, MuSK activates a signaling pathway that leads to synaptic differentiation, including clustering of AChRs. Domain coloring for Lrp4: white, LDLa; orange, EGF-like; blue, b-propeller. Domain coloring for MuSK: purple, immunoglobulin-like; yellow, Frizzled-like cysteine-rich; red or green, tyrosine kinase (TK).
Lrp4-expressing BaF3 cells bind Agrin B8 with an affinity of ~6 nM, whereas half-maximal MuSK phosphorylation and AChR clustering in muscle is achieved at ~40 pM Agrin (Ferns et al., 1993; Gesemann et al., 1995; Hopf and Hoch, 1998). These data suggest that MuSK phosphorylation may saturate at low levels of Agrin-binding, consistent with previous studies showing that a brief pulse of Agrin triggers a pathway that increases MuSK phosphorylation autonomously of Agrin (Mittaud et al., 2004). Alternatively, the affinity of Agrin for Lrp4 in muscle may be increased by post-translational modifications or additional interactions that occur in muscle but not in BaF3 cells. Nonetheless, BaF3 cells expressing Lrp4, like muscle, display a strong preference for neural isoforms of Agrin, as Lrp4-expressing BaF3 cells bound Agrin B8 with a 100-fold higher affinity than Agrin B0. Thus, the critical determinants for selective binding of neural forms of Agrin are intrinsic to Lrp4 and present on Lrp4 expressed in non-muscle cells. Our data provide strong evidence that Lrp4 binds neural Agrin directly. In particular, the amount of Agrin bound to Lrp4-expressing cells correlates well with the level of Lrp4 expression. Moreover, an Lrp4/LDLR chimera, in which the cytoplasmic domain of Lrp4 was exchanged for the cytoplasmic domain of LDLR, rescues Agrin-stimulated MuSK phosphorylation and AChR clustering in lrp4 mutant myotubes. Since LDLR is not thought to regulate transcription independent from cholesterol uptake (Brown and Goldstein, 1997), these data indicate that that this chimera responds to Agrin by binding neural Agrin directly. Nonetheless, we cannot formally exclude the possibility that BaF3 cells express a latent Agrin-binding protein that binds Agrin only following expression of Lrp4. Unfortunately, purification of the properly folded ectodomain from Lrp4, important for such binding studies, has proven recalcitrant to the methods that we have employed to express and purify Agrin and MuSK.
During development, as motor axons first approach muscle, MuSK is activated in an Agrin-independent manner in the prospective synaptic zone, thereby directing motor axons to form synapses in the central region of muscle (Arber et al., 2002; Kim and Burden, 2008; Kummer et al., 2006). This muscle prepatterning is dependent upon Lrp4 and highly sensitive to MuSK dosage (Lin et al., 2001; Weatherbee et al., 2006). These results indicate that Lrp4 functions together with MuSK in prepatterning muscle and at later stages in development when Agrin is supplied by motor neurons to stabilize nascent synapses (Kummer et al., 2006). Our results demonstrate that BaF3 cells expressing Lrp4 and MuSK show a higher level of MuSK basal phosphorylation than BaF3 cells expressing MuSK alone. Because Lrp4 self-associates and interacts with MuSK, these data suggest that Lrp4 self-association may promote trans-phosphorylation and activation of MuSK, contributing to the low level of Agrin-independent MuSK activation that is critical for muscle prepatterning.
Mutations in lrp4, which truncate Lrp4 and lead to a loss of the cytoplasmic domain without disruption of the ectodomain, cause syndactyly without apparent defects in neuromuscular synapses (Drogemuller et al., 2007; Johnson et al., 2005). Our findings show that the cytoplasmic domain of Lrp4 is dispensable for Agrin-responsiveness. Together, these data suggest that the Lrp4 cytoplasmic domain has important signaling and/or anchoring functions in certain tissues but not at neuromuscular synapses.
Myasthenia gravis is an autoimmune disease that causes dysfunction of the neuromuscular synapse (Fambrough et al., 1973; Vincent and Drachman, 2002). Seventy percent of patients with myasthenia gravis carry auto-antibodies to AChRs and a separate ten percent carry auto-antibodies to MuSK (Vincent and Leite, 2005). Twenty percent of patients with myasthenia gravis are sero-negative for antibodies to AChR and MuSK, suggesting that they carry antibodies against other synaptic components (Vincent and Leite, 2005). It will be interesting to learn whether these patients carry auto-antibodies to Lrp4.
In addition to neuromuscular synapses, Lrp4, Agrin and MuSK are expressed in the central nervous system (Garcia-Osta et al., 2006; Ksiazek et al., 2007; Sanes and Lichtman, 2001; Tian et al., 2006; Weatherbee et al., 2006). Agrin binds the alpha3 subunit of the Na/K ATPase in neurons and regulates Na/K ATPase activity (Hilgenberg et al., 2006). Our results raise the possibility that neuronal Lrp4 may also function as a neuronal receptor for Agrin, associate with MuSK and have a role, analogous to its role at neuromuscular synapses, in the central nervous system. Lrp4 is also widely expressed in other developing tissues, but how Lrp4 participates in differentiation in these tissues is poorly understood (Weatherbee et al., 2006). Since many of these tissues do not express MuSK nor depend upon Agrin for their differentiation, our findings raise the possibility that Lrp4 binds ligands that are structurally akin to Agrin and associates with other receptor tyrosine kinases, like MuSK, in these tissues.
EXPERIMENTAL PROCEDURES
Lrp4 mutant myoblasts and BaF3 cells were infected with retroviral vectors, which conferred expression of Flag-Lrp4-mCherry (NM_172668), myc-MuSK-GFP (NM_031061), CCR7-GFP (NM_007719) and Flag-LDLR (NM_000527). The myc epitope tag was positioned between the second and third Ig-like domains in MuSK (Herbst and Burden, 2000); the Flag epitope tag was positioned twenty four amino acids following the initiator methionine in Lrp4. The Lrp4/LDLR chimera is composed of the ecto- and transmembrane-domain from murine Lrp4 and the cytoplasmic domain from human LDLR, which is substantially shorter (51 versus 161 amino acids) and dissimilar from the Lrp4 cytoplasmic domain (6% identity; 9% similarity). Expressing-cells were selected for resistance to puromycin (pBabe for myoblasts) or by flow cytometry (pMXs for BaF3 cells). We used flow cytometry to compare Flag-Lrp4-mCherry levels in BaF3 cells expressing Flag-Lrp4-mCherry plus myc-MuSK-GFP or Flag-Lrp4-mCherry alone. We measured mCherry expression and stained for surface Flag-Lrp4-mCherry with a Cy5-conjugated antibody to Flag and found that total and cell surface Lrp4 expression were ~two-fold lower in Lrp4/MuSK than Lrp4-only cells. Dok-7 (NM_172708), containing a HA epitope tag at the carboxy terminus, was introduced into BaF3 cells with a pBabe retroviral vector in which a cassette, containing a weak enhancer (−148/−95) from the acetylcholine receptor δ subunit gene fused to the c-Fos basal promoter (Simon and Burden, 1993), was inserted upstream from the internal SV40 promoter.
Adhesion of BaF3 cells to immobilized substrates was measured as described previously (Dustin et al., 2007). Coverslips were spotted with proteins (0.1 picomole in 1 µl) and mounted in a flow chamber (Bioptechs, Bulter, PA) on a Zeiss 510 confocal microscope and maintained at 37°C. Cells were allowed to bind for 30 min, and images (bright field, fluorescent and differential interference contrast) were captured before and after washing with media. 50 kd C-terminal fragments of Agrin B0 and B8, containing Six-His and myc epitope tags at their N-terminus and a HA tag at the C-terminus (Hopf and Hoch, 1998), as well as the ectodomain from MuSK, were purified from media of baculovirus-infected insect cells (Stiegler et al., 2006). The 95 kd C-terminal fragment of Agrin B8 was purchased from R&D Systems (Minneapolis, MN); laminin was purchased from Invitrogen (Carlsbad, CA). Purified Agrin was conjugated with Cy5 and incubated simultaneously with MuSK-GFP-, Lrp4-mCherry- and MuSK-GFP/Lrp4-mCherry-expressing cells, as well as parental BaF3 cells, for 30 min to 1 hr on ice. After washing, the Cy5-Agrin signal was quantified by flow cytometry. Cells were analyzed using a LSR II flow cytometer and FlowJo software.
MuSK phosphorylation and surface expression were measured as described previously (Herbst and Burden, 2000; Stiegler et al., 2006). LDLR was detected by probing Western blots with antibodies (#52818) to the C-terminus of LDLR (Abcam, Cambridge, MA). To study association between Lrp4 and MuSK, Flag-Lrp4-mCherry was immunoprecipitated with rabbit antiserum against mCherry, and myc-MuSK-GFP was detected by probing Western blots with antibodies to GFP.
Supplementary Material
Acknowledgements
We thank Katherine Anderson, Lee Niswander and Scott Weatherbee for providing us with lrp4 mutant mice and David Russell for the human LDLR plasmid. We are grateful to Jihua Fan for excellent technical assistance and Peter Lopez for help with the flow cytometry analysis. The flow cytometry core is supported by an NCI Cancer Center Support Grant (P30 CA016087). This work was supported with funds from the NIH (NS36193 to S.J.B., R21 GM075180 to M.L.D., NS053414 to S.R.H.) and the Robert Packard Center for ALS research (S.J.B.).
Footnotes
None of the authors have a financial interest related to this work.
REFERENCES
- Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Curr Opin Neurobiol. 2002;12:100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]
- Beeson D, Webster R, Cossins J, Lashley D, Spearman H, Maxwell S, Slater CR, Newsom-Davis J, Palace J, Vincent A. Congenital myasthenic syndromes and the formation of the neuromuscular junction. Ann N Y Acad Sci. 2008;1132:99–103. doi: 10.1196/annals.1405.049. [DOI] [PubMed] [Google Scholar]
- Borges LS, Lee Y, Ferns M. Dual role for calcium in agrin signaling and acetylcholine receptor clustering. J Neurobiol. 2002;50:69–79. doi: 10.1002/neu.10020. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Deyst KA, Leszyk JD, Fallon JR. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994;12:1173–1180. doi: 10.1016/0896-6273(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Burden SJ. The formation of neuromuscular synapses. Genes Dev. 1998;12:133–148. doi: 10.1101/gad.12.2.133. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Drogemuller C, Leeb T, Harlizius B, Tammen I, Distl O, Holtershinken M, Gentile A, Duchesne A, Eggen A. Congenital syndactyly in cattle: four novel mutations in the low density lipoprotein receptor-related protein 4 gene (LRP4) BMC Genet. 2007;8:5. doi: 10.1186/1471-2156-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. Adhesive bond dynamics in contacts between T lymphocytes and glass-supported planar bilayers reconstituted with the immunoglobulin-related adhesion molecule CD58. J Biol Chem. 1997;272:15782–15788. doi: 10.1074/jbc.272.25.15782. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Starr T, Coombs D, Majeau GR, Meier W, Hochman PS, Douglass A, Vale R, Goldstein B, Whitty A. Quantification and modeling of tripartite CD2-, CD58FC chimera (alefacept)-, and CD16-mediated cell adhesion. J Biol Chem. 2007;282:34748–34757. doi: 10.1074/jbc.M705616200. [DOI] [PubMed] [Google Scholar]
- Engel AG, Shen XM, Selcen D, Sine SM. Further observations in congenital myasthenic syndromes. Ann N Y Acad Sci. 2008;1132:104–113. doi: 10.1196/annals.1405.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol. 2005;5:308–321. doi: 10.1016/j.coph.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973;182:293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Tsokas P, Pollonini G, Landau EM, Blitzer R, Alberini CM. MuSK expressed in the brain mediates cholinergic responses, synaptic plasticity, and memory formation. J Neurosci. 2006;26:7919–7932. doi: 10.1523/JNEUROSCI.1674-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996a;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Glass DJ, DeChiara TM, Stitt TN, DiStefano PS, Valenzuela DM, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation and is a functional receptor for agrin. Cold Spring Harb Symp Quant Biol. 1996b;61:435–444. [PubMed] [Google Scholar]
- Glass DJ, Yancopoulos GD. Sequential roles of agrin, MuSK and rapsyn during neuromuscular junction formation. Curr Opin Neurobiol. 1997;7:379–384. doi: 10.1016/s0959-4388(97)80066-9. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Herbst R, Burden SJ. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. Embo J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenberg LG, Su H, Gu H, O'Dowd DK, Smith MA. Alpha3Na+/K+-ATPase is a neuronal receptor for agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Hopf C, Hoch W. Tyrosine phosphorylation of the muscle-specific kinase is exclusively induced by acetylcholine receptor-aggregating agrin fragments. Eur J Biochem. 1998;253:382–389. doi: 10.1046/j.1432-1327.1998.2530382.x. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Montanaro F, Lindenbaum M, Carbonetto S, Ferns M. alpha-Dystroglycan functions in acetylcholine receptor aggregation but is not a coreceptor for agrin-MuSK signaling. J Neurosci. 1998;18:6340–6348. doi: 10.1523/JNEUROSCI.18-16-06340.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005;14:3523–3538. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman RJ, Reichardt LF. Testing the agrin hypothesis. Cell. 1996;85:461–464. doi: 10.1016/s0092-8674(00)81245-3. [DOI] [PubMed] [Google Scholar]
- Ksiazek I, Burkhardt C, Lin S, Seddik R, Maj M, Bezakova G, Jucker M, Arber S, Caroni P, Sanes JR, et al. Synapse loss in cortex of agrin-deficient mice after genetic rescue of perinatal death. J Neurosci. 2007;27:7183–7195. doi: 10.1523/JNEUROSCI.1609-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Megeath LJ, Fallon JR. Intracellular calcium regulates agrin-induced acetylcholine receptor clustering. J Neurosci. 1998;18:672–678. doi: 10.1523/JNEUROSCI.18-02-00672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Gesemann M, Cavalli V, Ruegg MA, Wallace BG. AChR phosphorylation and aggregation induced by an agrin fragment that lacks the binding domain for alpha-dystroglycan. Embo J. 1996;15:2625–2631. [PMC free article] [PubMed] [Google Scholar]
- Mittaud P, Camilleri AA, Willmann R, Erb-Vogtli S, Burden SJ, Fuhrer C. A single pulse of agrin triggers a pathway that acts to cluster acetylcholine receptors. Mol Cell Biol. 2004;24:7841–7854. doi: 10.1128/MCB.24.18.7841-7854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sealock R, Froehner SC. Dystrophin-associated proteins and synapse formation: is alpha-dystroglycan the agrin receptor? Cell. 1994;77:617–619. doi: 10.1016/0092-8674(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Simon AM, Burden SJ. An E box mediates activation and repression of the acetylcholine receptor delta-subunit gene during myogenesis. Mol Cell Biol. 1993;13:5133–5140. doi: 10.1128/mcb.13.9.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler AL, Burden SJ, Hubbard SR. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J Mol Biol. 2006;364:424–433. doi: 10.1016/j.jmb.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian QB, Suzuki T, Yamauchi T, Sakagami H, Yoshimura Y, Miyazawa S, Nakayama K, Saitoh F, Zhang JP, Lu Y, et al. Interaction of LDL receptor-related protein 4 (LRP4) with postsynaptic scaffold proteins via its C-terminal PDZ domain-binding motif, and its regulation by Ca/calmodulin-dependent protein kinase II. Eur J Neurosci. 2006;23:2864–2876. doi: 10.1111/j.1460-9568.2006.04846.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park JS, Stark JL, Gies DR, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Verschueren H. Interference reflection microscopy in cell biology: methodology and applications. J Cell Sci. 1985;75:279–301. doi: 10.1242/jcs.75.1.279. [DOI] [PubMed] [Google Scholar]
- Vincent A, Drachman DB. Myasthenia gravis. Adv Neurol. 2002;88:159–188. [PubMed] [Google Scholar]
- Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr Opin Neurol. 2005;18:519–525. doi: 10.1097/01.wco.0000180660.57801.3f. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.