New data show that epithelial to mesenchymal transition induces stem cell properties and prevents apoptosis and senescence. Zinc finger enhancer binding transcription factors are crucial EMT activators, suggesting that the ZEB/miR-200 feedback loop is the molecular motor of cellular plasticity in development and disease.
Keywords: ZEB1, miR-200, EMT, stemness, cancer, metastasis
Abstract
Epithelial-to-mesenchymal transition (EMT) is a fundamental process in development and disease. Zinc-finger enhancer binding (ZEB) transcription factors (ZEB1 and ZEB2) are crucial EMT activators, whereas members of the miR-200 family induce epithelial differentiation. They are reciprocally linked in a feedback loop, each strictly controlling the expression of the other. Now data show that EMT not only confers cellular motility, but also induces stem-cell properties and prevents apoptosis and senescence. Thus the balanced expression of ZEB factors and miR-200 controls all these processes. We therefore propose that the ZEB/miR-200 feedback loop is the molecular motor of cellular plasticity in development and disease, and in particular is a driving force for cancer progression towards metastasis by controlling the state of cancer stem cells.
See Glossary for abbreviations used in this article.
Glossary.
- bHLH
basic helix–loop–helix
- BRG1
BRM/SW12-related genes
- CDK
cyclin-dependent kinase
- CtBP
C-terminal binding protein
- EGFR
epithelial growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- ES cell
embryonic stem cell
- FAP1
FAS-associated phosphatase 1
- FGF
fibroblast growth factor
- HDAC
histone deacetylase
- iPS
induced pluripotent stem cells
- MET
mesenchymal-to-epithelial transition
- miR/miRNA
microRNA
- NSCLC
non-small-cell lung cancer
- PARP
poly(ADP-ribose) polymerase 1
- pCAF
p300/CBP-associated factor
- PI3K
phosphatidylinositol-3 kinase
- shRNA
short hairpin RNA
- TGF-β
transforming growth factor beta
- USH
(FOG2) Friend of GATA2
- UTR
untranslated region of the mRNA
- ZEB
zinc-finger enhancer binding protein
- ZFH
zinc-finger homeodomain
Introduction
Controlled activation of stemness and cellular motility are of outstanding importance in embryonic development and adult tissue homeostasis. Uncontrolled activation or maintenance of these properties is associated with the pathogenesis of various diseases, in particular cancer. Pure epithelial and mesenchymal phenotypes mark the extreme endpoints of stationary and highly mobile cell types, respectively. Epithelial-to-mesenchymal transition (EMT) is a reversible embryonic programme that allows partial or complete transition between these extreme phenotypes and is essential for embryonic processes such as gastrulation. However, if EMT is aberrantly activated it is a trigger for tumour progression and metastasis (Thiery et al, 2009). It is now known that EMT activation is also associated with the maintenance of stem-cell properties (Mani et al, 2008). EMT is activated by key signalling pathways, including the TGF-β, Notch and FGF pathways, which converge in the stimulation of EMT activators—a group of transcription factors repressing epithelial gene expression. This group includes members of the Snail, the bHLH and the ZFH families (ZEB1 and ZEB2; Thiery et al, 2009).
MicroRNAs (miRNAs) are small non-coding RNAs that can silence their cognate target genes by binding specifically to mRNAs and inhibiting their translation (Bartel, 2004). The key region of the 18–24-nucleotide-long mature miRNAs is the so-called ‘seed sequence', which determines target specificity. So far, approximately 800 miRNAs have been identified in humans. They are potent regulators of gene expression and control diverse cellular processes. miRNAs have also been shown to function either as tumour suppressors or as oncogenes by repressing the expression of important cancer-related genes (Esquela-Kerscher & Slack, 2006).
This article highlights a recently described feedback loop between ZEB factors and members of the miR-200 family of miRNAs. New findings indicate that this loop controls not only EMT, but also other crucial cellular processes, such as stemness, senescence and survival, indicating its central role in embryonic development and in the pathogenesis of many diseases, in particular malignant tumour progression.
Molecular basis: the ZEB/miR-200 feedback loop
ZEB factors (ZEB1 and ZEB2, encoded by the ZFHX1a and ZFHX1b genes) are transcriptional repressors that comprise two widely separated clusters of C2H2-type zinc fingers binding to paired CAGGTA/G E-box-like promoter elements. They induce EMT by suppressing the expression of many epithelial genes, including E-cadherin (Vandewalle et al, 2009). Their repressive function is exerted through binding to different co-repressors, such as CtBPs, HDACs and BRG1 (Browne et al, 2010; Sanchez-Tillo et al, 2010). A central activator of ZEB factors is the TGF-β signalling pathway, indicating that they are crucial intracellular mediators of TGF-β-induced EMT. Enforced expression of ZEB factors in epithelial cells results in a rapid EMT associated with a breakdown of cell polarity, loss of cell–cell adhesion and induction of cell motility. Vice versa, a knockdown of ZEB factors in undifferentiated cancer cells induces a mesenchymal-to-epithelial transition (MET). It is no wonder that these potent factors, if aberrantly overexpressed, have central roles in tumour progression. ZEB1 (also called δ-EF1) is a crucial EMT activator in many human cancers, including prostate, colon, breast and pancreatic (Aigner et al, 2007; Graham et al, 2008; Spaderna et al, 2006; Wellner et al, 2009), and it suppresses the expression of basement membrane components (Spaderna et al, 2006) and cell polarity factors (Aigner et al, 2007; Spaderna et al, 2008). Moreover, expression of ZEB1 promotes metastasis of tumour cells in a mouse xenograft model, indicating a role for ZEB1 in invasion and metastasis of human tumours (Spaderna et al, 2008).
The second family member, ZEB2 (also called SIP1), was initially described as a factor collaborating with the TGF-β signalling pathway by interacting with SMAD factors, and was also shown to induce tumour cell invasion (Comijn et al, 2001). In contrast to Snail factors, ZEB factors can also interact with transcriptional co-activators, such as p300 and pCAF, and can subsequently switch to a transcriptional activator under still poorly defined conditions (Postigo et al, 2003; van Grunsven et al, 2006).
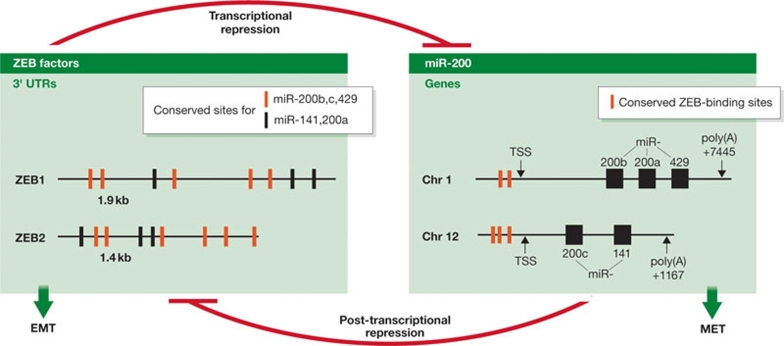
miRNAs are known to control central cellular processes, therefore we and others had hypothesized that EMT might also be regulated by miRNAs. Data from several research groups including our own, all independently investigating EMT from different angles, pointed to the involvement of one family of miRNAs, the miR-200 family that includes miR-200a, miR-200b, miR-200c, miR-141 and miR-429 (Burk et al, 2008; Christoffersen et al, 2007; Gregory et al, 2008a; Hurteau et al, 2007; Korpal et al, 2008; Park et al, 2008). By compiling these published data it became clear that members of the miR-200 family can revert an EMT and are powerful inducers of epithelial differentiation. The five miRNAs are located within two clusters on separate chromosomes (Fig 1). They can be further divided into two subgroups according to their seed sequences—subgroup I: miR-141 and miR-200a; subgroup II: miR-200b,c and miR-429—which indicate slight differences in their target gene sets.
Figure 1.
The ZEB/miR-200 double-negative feedback loop. ZEB factors transcriptionally repress the genes of the miR-200 family members located in two clusters by binding to highly conserved recognition sequences in their promoters. miR-200 family members inhibit expression of ZEB at the post-transcriptional level by binding to highly conserved target sites in their 3′ UTRs. Chr, chromosome; EMT, epithelial-to-mesenchymal transition; MET, mesenchymal-to-epithelial transition; miR, microRNA; TSS, transcriptional start site; UTR, untranslated region; ZEB, zinc-finger enhancer binding.
How can miR-200 family members exert such a strong EMT-reverting effect? The simple answer lies in their most prominent target factors: all studies so far have described ZEB1 and ZEB2 as the crucial targets of miR-200 family members (Burk et al, 2008; Christoffersen et al, 2007; Gregory et al, 2008a; Hurteau et al, 2007; Korpal et al, 2008; Park et al, 2008). The ZEB1 3′ UTR contains eight miR-200 binding sites (five for subgroup II members and three for subgroup I members), and the ZEB2 3′ UTR contains nine binding sites (six for subgroup II members and three for subgroup I members; Fig 1).
Notably, in addition to the inhibitory effect of miR-200 on ZEB1, we also found a reverse interrelation. Knockdown of ZEB1 led to an increase in the expression of all miR-200 family members (Burk et al, 2008). We can demonstrate that ZEB1 directly inhibits transcription of miR-141 and miR-200c genes by binding to highly conserved sites in their common promoter (Fig 1). This finding was corroborated and extended by showing that all miR-200 members are transcriptional targets of ZEB1 and ZEB2 (Bracken et al, 2008). These data indicate that ZEB factors and miR-200 family members not only have opposite functions, but also reciprocally control the expression of each other. We have therefore proposed a close functional link between both factor groups in a double-negative feedback loop: the ZEB/miR-200 feedback loop (Burk et al, 2008; Fig 1). The consequence of such a reciprocal loop is that the activation of one group of factors would strongly affect the expression and effects of the other group. As ZEB factors are strong EMT inducers, the consequence of miR-200 overexpression is the reduced expression of ZEB factors and subsequent epithelial differentiation. Depending on the extracellular signals, such a loop could easily switch from one to the other side and stabilize either an epithelial or mesenchymal phenotype.
Effects: control of central cellular processes
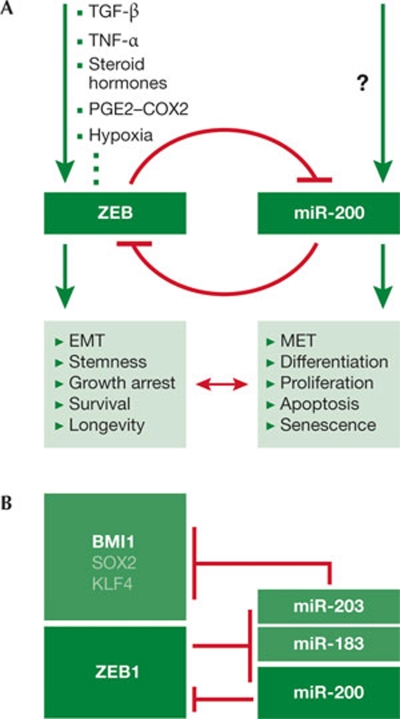
New data indicate that ZEB factors and miR-200 family members not only regulate EMT, but also control other crucial cellular functions and states, such as stemness—differentiation; longevity—senescence; cell-cycle arrest—proliferation; survival—apoptosis (Fig 2A). Thus the ZEB/miR-200 loop might be a central switch that regulates important intracellular decision processes. We focus on these new aspects of ZEB factors and miR-200 family members, as their roles in controlling typical EMT-associated properties, such as loss of cell–cell adhesion and gain of cellular motility, are already extensively described (Gregory et al, 2008b; Peinado et al, 2007; Vandewalle et al, 2009).
Figure 2.
Processes controlled by the ZEB/miR-200 feedback loop. (A) ZEB factors are induced by the indicated factors resulting in activation of the listed processes. Owing to the feedback loop, miR-200 members induce opposite processes. Little is known about factors that activate the expression of miR-200 family members. (B) An example showing that ZEB and miR-200 not only block expression of each other, but also ZEB1 suppresses transcription of miR-203 and miR-183, which together with miR-200 inhibit expression of stem-cell factors, linking the induction of EMT and the maintenance of stemness (Wellner et al, 2009). EMT, epithelial-to-mesenchymal transition; MET, mesenchymal-to-epithelial transition; miR, microRNA; TGF-β, transforming growth factor beta; TNF-α, tissue necrosis factor alpha; ZEB, zinc-finger enhancer binding.
Stemness: differentiation
Is the transition or partial transition from an epithelial to a mesenchymal phenotype molecularly linked to a dedifferentiation programme? If so, this could indicate that certain mesenchymal properties are related to a stemness phenotype. There is increasing evidence that EMT activators and miRNAs also regulate embryonic development and have a role in controlling stemness in both normal stem cells and cancer stem cells (Peter, 2009). We previously postulated a potential link between EMT activation and stemness maintenance in invading tumour cells (Brabletz et al, 2005b). Such a link was described at the molecular level, showing that the EMT activator Twist can induce stem-cell properties in mammary epithelial cells (Mani et al, 2008; Morel et al, 2008). We have recently shown that ZEB1 increases stem-cell properties and the tumorigenic capacity in pancreatic cancer cells (Wellner et al, 2009), and ES cells have been shown to express ZEB1 (Ben-Porath et al, 2008), which is reduced on their differentiation. Moreover, ES-cell differentiation is associated with the increased expression of miR-200 family members (Bar et al, 2008; Wellner et al, 2009), whereas Shimono et al (2009) showed that both normal mammary stem cells and breast cancer stem cells have reduced expression of miR-200 family members, which results in increased expression of the stem-cell factor and oncoprotein BMI1. Moreover, the overexpression of miR-200c in normal stem cells or cancer stem cells reduces their clonogenic or tumour-initiating capacities (Shimono et al, 2009). We found that ZEB1 not only inhibits the expression of miR-200, but also of miR-183 and miR-203, which together target BMI1 and possibly other stemness-associated factors, such as SOX2 and KLF4 (Wellner et al, 2009). Interestingly, some of the stem-cell factors can also induce pluripotent stem (iPS) cells, and further work will show whether the ZEB/miR-200 loop is actually involved in the generation of iPS cells. Finally, the counterpart of miR-200 in Drosophila, miR-8, inhibits Wnt signalling—a pathway known to activate the stemness/progenitor cell phenotype in many tissues (Kennell et al, 2008). These data are supported by the finding that the Wnt-pathway effector β-catenin is a target of miR-200a (Xia et al, 2010). Together these data indicate that EMT and stemness are linked at the molecular level and are controlled by the proposed ZEB/miR-200 loop (Fig 2). Such a miRNA-mediated link between EMT and stemness is probably not a special property of selected tumours, but could be a basal regulatory principle already active in early embryonic and organ development.
Longevity: senescence
Senescence and apoptosis are safeguard programmes that protect cells from neoplastic transformation in response to cellular stress and DNA damage; EMT activators have been shown to be involved in the control of both processes and particularly are able to prevent senescence. For example, Ansieau and co-workers showed that Twist1 can override activated RAS-induced senescence and can cooperate for oncogenic transformation (Ansieau et al, 2008). In addition, mouse embryonic fibroblasts from ZEB1-null mice revealed proliferation defects and underwent premature replicative senescence (Liu et al, 2008). It was thereby shown that ZEB1 maintains proliferation by transcriptional repression of p21 and p15INK4b, two CDK inhibitors taking part in TGF-β signalling. Recently, Ohashi and colleagues also detected that ZEB1 cooperates with mutant p53 to overcome senescence induced by EGFR signalling in oesophageal cancer cells (Ohashi et al, 2010). However, in contrast to ZEB1, ZEB2 can induce replicative senescence in cancer cell lines through transcriptional repression of hTERT expression (Lin & Elledge, 2003). Although these data indicate that ZEB1 and ZEB2 might have opposite effects in controlling senescence, it is not clear whether these differences are dependent on the specific cellular background or how they can be explained at the molecular level. As expression of ZEB factors is inhibited by miR-200 family members, these members also indirectly control the senescence state of the cell.
Survival: apoptosis
Controlled apoptosis is important for the homeostasis of tissues with high turnover rates, such as many epithelial tissues. It is also the ultimate mechanism to protect the organism from neoplastic transformation after DNA damage induced by toxic agents. Stem cells have a higher survival capacity than differentiated cells and are more resistant to apoptotic stimuli, as they are the only cells that guarantee tissue reconstitution. It is already known that the induction of EMT is generally associated with reduced apoptosis and increased cell survival. In consequence, cells that have undergone EMT are more resistant to toxic stress, including chemotherapy and radiotherapy (Thiery et al, 2009). For example, both Snail1 and Twist1 were shown to confer resistance to cell death and induce drug resistance in cancer cell lines (Li et al, 2009a; Vega et al, 2004). Similarly, the long-term exposure of breast cancer cells to TGF-β, a highly potent inducer of ZEB1 expression, induces EMT and inhibits apoptosis (Gal et al, 2008). We have shown that the shRNA-mediated knockdown of ZEB1 in pancreatic and colorectal cancer cell lines not only affects their stem-cell properties, but also increases the sensitivity of the cells to chemotherapeutic agents such as gemcitabine. Moreover, drug-resistant clones of the gemcitabine-sensitive pancreatic cancer cell line BXPC3 strongly upregulate ZEB1 and reduce expression of miR-200c (Wellner et al, 2009). These data are in line with publications by other groups showing that ZEB1 and the associated EMT phenotype confer resistance to chemotherapeutics—such as gemcitabine, 5-FU and cisplatin—in breast and pancreatic cancer cells (Arumugam et al, 2009; Shah et al, 2007; Wang et al, 2009), and that ZEB1 depletion sensitizes NSCLC cells as well as head-and-neck cancer cells to the EGFR inhibitor erlotinib (Buck et al, 2007; Haddad et al, 2009; Witta et al, 2006). Furthermore, expression of ZEB2 protects bladder and squamous cell carcinoma cells from cisplatin- and UV-induced apoptosis by suppressing mitochondrial depolarization and cleavage of PARP and caspase 3 (Sayan et al, 2009). Cancer cells are often dependent on certain oncogenic mutations that prevent apoptosis, a phenomenon called ‘oncogene addiction'. It was shown recently that pancreatic and lung cancer cells that express ZEB1 can overcome K-RAS-dependent oncogene addiction owing to persisting survival signals after sh-mediated knockdown of oncogenic K-RAS (Singh et al, 2009).
The miR-200 side of the feedback loop favours apoptosis and sensitivity to toxic agents. Re-expression of miR-200 family members in breast cancer cell lines restores sensitivity to doxorubicin by increasing the expression of pro-apoptotic genes (Tryndyak et al, 2010). Forced expression of miR-200c reverses resistance to chemotherapy in female reproductive cancer cells (Cochrane et al, 2010) and to EGFR-mediated therapy in bladder cancer cells (Adam et al, 2009). Furthermore, isoflavones, known as natural cancer protective agents, induce the expression of miR-200 family members and subsequently reverse the resistance of pancreatic cancer cells to gemcitabine (Li et al, 2009b). Recently, a direct pro-apoptotic function of miR-200c was discovered by showing that this miRNA targets the apoptosis inhibitor FAP1, thereby sensitizing tumour cells to apoptosis (Schickel et al, 2010).
Growth arrest: proliferation
It is known that the EMT state is often associated with reduced proliferative activity. We made observations that the EMT/stemness phenotype is associated with the growth arrest of invasive tumour cells (Brabletz et al, 2001; Jung et al, 2001). It became clear that EMT activators directly affect the cell-cycle machinery. It was shown that Snail1 represses cyclin D2 transcription and thereby impairs cell-cycle progression (Vega et al, 2004). Moreover, ZEB2 was shown to impair G1–S cell-cycle progression by repressing cyclin D1 transcription (Mejlvang et al, 2007). By contrast, recent publications indicate that the switch to an epithelial state (MET) induced by miR-200 members is associated with higher proliferative activity in tumour cells. Overexpression of miR-200 family members increases the proliferation of pancreatic cancer cells (Kent et al, 2009) and nasopharynx carcinoma cells (Zhang et al, 2010). Interestingly, Uhlmann and co-workers described that only the miR-200b/c/429 cluster, not the miR-141/200a cluster, increases the G2/M population and proliferation in breast cancer cells (Uhlmann et al, 2010). These data are corroborated by findings in Drosophila. In this case, the miR-200 counterpart miR-8 enhances cell growth and subsequent whole body size by targeting the p85α inhibitor USH/FOG2, thereby leading to activation of the PI3K pathway (Hyun et al, 2009). Together, these data show that EMT is often associated with growth arrest. miR-200 members can stimulate proliferation, indicating why re-expression of miR-200 family members and the subsequent MET might be crucial for metastatic colonization.
Consequences: out of control in disease
All the features and processes controlled by the ZEB/miR-200 loop are fundamental cellular processes (a list of miR-200 targets, reflecting the importance of this miR family, is shown in Table 1). In particular, all these properties are important for the regulated maintenance of a stem-cell phenotype. As such, an aberrant and uncontrolled shift to one side of this loop can have severe consequences for the cell and the organism, and might have an important role in disease pathogenesis. For example, EMT activators such as ZEB1 have been shown to be crucial for the development of organ fibrosis (Kalluri & Weinberg, 2009; Lopez-Novoa & Nieto, 2009). The most relevant disease to the ZEB/miR-200 loop is cancer, in particular in the light of two emerging concepts of cancer initiation and malignant progression. First, on the basis of genetic alterations, aberrant EMT activation is thought to trigger cancer cell motility and thus induce tumour-cell dissemination and metastasis; second, the sustained maintenance of stem-cell properties is the basis of tumour development and progression in the ‘cancer stem cell' concept.
Table 1. Validated targets of miR-200 family members.
| Target | miR-200 member | References |
|---|---|---|
| ZEB1 | All family members | Hurteau et al, 2007; Gregory et al, 2008a; Park et al, 2008; Burk et al, 2008; Korpal et al, 2008 |
| ZEB2 | All family members | Gregory et al, 2008a; Park et al, 2008; Korpal et al, 2008 |
| TGF-β2 | miR-141, miR-200c | Burk et al, 2008 |
| ERBB receptor feedback inhibitor 1 (ERRFI1) | miR-200c | Adam et al, 2009 |
| Friend of GATA 2 (FOG2) | All family members miR-8 (Drosophila) | Hyun et al, 2009 |
| Polycomb ring finger oncogene (BMI1) | miR-200c | Shimono et al, 2009; Wellner et al, 2009 |
| WAS protein family member 3 (WASF3, WAVE3) | miR-200b | Sossey-Alaoui et al, 2009 |
| β-catenin (CTNNB1) | miR-200a | Xia et al, 2010 |
| Class III beta-tubulin (TUBB3) | miR-200c | Cochrane et al, 2010 |
| Phospholipase C gamma 1 (PLCG1) | miR-200b/c, miR-429 | Uhlmann et al, 2010 |
| FAS-associated phosphatase 1 (FAP1) | miR-200c | Schickel et al, 2010 |
miR, microRNA; ZEB, zinc-finger enhancer binding.
On the basis of our own studies of colorectal cancer, we postulated that EMT and stem-cell properties are combined in invasive cancer cells—which often co-express EMT and stem-cell markers—and proposed the ‘migrating cancer stem cell' concept (Brabletz et al, 2005b). Mani et al (2008) showed that EMT and stemness features are indeed linked in breast epithelium and breast cancer cells. The ZEB/miR-200 feedback loop can explain such a link at the molecular level. Switching to the ZEB side of this loop—for example, induction by cytokines such as TGF-β secreted in the invasive tumour environment—can trigger the combined activation of many features crucial for invasion and metastasis of cancer cells. Classical EMT-associated features support tumour cell motility and dissemination, and the inhibition of the apoptotic machinery prevents anoikis and allows the cells to survive under the stressful conditions of a new environment. The associated stem-cell properties, including the prevention of senescence, allow unlimited reconstitution of the tumour at a distant site, ultimately leading to metastasis. In addition, there is increasing evidence that these properties are the reason for radiotherapy and chemotherapy resistance, which results in the selection of surviving cancer cells with an EMT and stemness phenotype after therapy.
However, one fact remains to be considered. Most carcinomas have a more or less differentiated phenotype and only few cancer cells, particularly at the invasive fronts, show the EMT phenotype that favours dissemination. Instead, most metastases re-acquire the differentiated state of the primary tumour, indicating that tumour progression is a highly dynamic process based on the plasticity of tumour cells (Brabletz et al, 2001, 2005a,Brabletz et al, 2001, 2005a). Fixation of tumour cells in one phenotypical state could therefore inhibit tumour progression. It has been shown that forced and irreversible overexpression of miR-200b abrogated the metastatic capacity of lung cancer cells (Gibbons et al, 2009). However, if the EMT state confers necessary traits to metastasize, why do metastases re-differentiate? Because the EMT/stemness phenotype of tumour cells that favours dissemination is associated with a growth arrest of invasive and disseminating tumour cells (Brabletz et al, 2001; Jung et al, 2001; Vega et al, 2004), such tumour stem cells must consequently switch back to the differentiation and proliferation mode in order to grow. Thus, the switch to the miR-200 side of the loop could be crucial for the growth of both the primary tumour and the metastasis. Recent publications support this view. Expression of miR-200 is indeed associated with an increased metastatic potential of breast cancer cell lines in an isogenic mouse model (Dykxhoorn et al, 2009). Moreover, overexpression of miR-200 is associated with increased malignancy of human ovarian cancers (Bendoraite et al, 2010).
Thus, a fixation towards neither the ZEB nor the miR-200 sides of the loop alone might favour tumour progression and metastasis. Rather, it is the aberrant ability of the tumour cell to use this loop and switch from one side to the other that allows it to adapt to the demanding conditions of the changing environment. We therefore suggest that the ZEB/miR-200 loop is the molecular motor of tumour-cell plasticity and is the crucial prerequisite for cancer progression. This view is fostered more and more by publications showing that, in an increasing number of human cancer types, the expression of ZEB factors and miR-200 family members correlates with their clinical behaviour (Table 2).
Table 2. Expression of ZEB factors and miR-200 family members in human cancers.
| Cancer type | Associated features | References |
|---|---|---|
| ZEB1 | ||
| Lung | Correlates with lack of E-cadherin in NSCLC Correlates with resistance to celecoxib and erlotinib in NSCLC | Dohadwala et al, 2006 Reckamp et al, 2008 |
| Colorectal | Increased levels in cancer cells of invasive regions | Spaderna et al, 2006; Aigner et al, 2007 |
| Pancreatic | Correlates with poor prognosis Correlates with lack of E-cadherin | Wellner et al, 2009 Arumugam et al, 2009 |
| Gallbladder | Increased levels in invasive tumour cells | Adachi et al, 2009 |
| Breast | Increased levels in undifferentiated cancer cells of invasive ductal and lobular cancers Strongly increased levels in triple negative cancers | Aigner et al, 2007 Graham et al, 2009 |
| Ovarian | Increased levels in metastasis | Elloul et al, 2010 |
| Endometrial | Increased levels in highly aggressive type II cancers | Spoelstra et al, 2006; Singh et al, 2008; Hurt et al, 2008 |
| Prostate | Correlates with high Gleason score | Graham et al, 2008 |
| ZEB2 | ||
| Pancreatic | Correlates with lack of E-cadherin | Imamichi et al, 2007 |
| Stomach | Increased levels in intestinal type with low E-cadherin | Rosivatz et al, 2002 |
| Bladder | Correlates with poor outcome | Sayan et al, 2009 |
| Ovarian | Increased levels in effusions | Elloul et al, 2005 |
| miR-200 | ||
| Pancreatic | miR-200 increased levels in benign PanIN compared with normal duct miR-200 reduced in pancreatic neuroendocrine tumours | du Rieu et al, 2010 Olson et al, 2009 |
| Liver | miR-200c reduced in benign liver tumours | Ladeiro et al, 2008 |
| Stomach | miR-141 reduced in gastric cancer | Du et al, 2009 |
| Breast | Low expression of miR-200 in basal type compared with other types of breast cancer Reduced expression of miR-200a/c in metastases compared with primary tumour | Burk et al, 2008; Gregory et al, 2008a Iliopoulos et al, 2009 |
| Ovarian | Low expression of miR-200a/b/429 correlates with poor survival miR-200 upregulated in ovarian cancer High expression of miR-200 correlates with poor prognosis | Hu et al, 2009 Nam et al, 2008 Bendoraite et al, 2010 |
miR, microRNA; NSCLC, non-small-cell lung cancer; ZEB, zinc-finger enhancer binding.
Conclusions
New data show that EMT not only confers cellular motility, but also induces stem-cell properties, and prevents apoptosis and senescence. These processes provide the basis for cellular plasticity in development and adult tissue homeostasis. Increasing evidence shows that they are regulated by a balanced expression of ZEB factors and miR-200 family members, which are reciprocally linked in the ZEB/miR-200 feedback loop. We propose that this feedback loop is a molecular motor of cellular plasticity in development and disease, and in particular is a driving force for cancer progression towards metastasis. However, many questions remain to be answered (Sidebar A). The clarification of these points might lead to new therapeutic options for diseases such as fibrosis and cancer.
Sidebar A | In need of answers.
What is the relevance of the ZEB/miR-200 feedback loop in normal development?
What is the individual function and the expression pattern of the five miR-200 family members in normal development and tumours?
How is expression of miR-200 family members induced?
What is the specific function of ZEB1, ZEB2 and the other EMT inducers?
Can the direction of the ZEB/miR-200 loop be manipulated by drugs?
Acknowledgments
This work was supported by grants to T.B. from the Deutsche Forschungsgemeinschaft (BR 1399/5-1, BR 1399/6-1 and SFB850) and the European Union (MCSC contract no. 037297).
References
- Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M (2009) Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett 583: 430–436 [DOI] [PubMed] [Google Scholar]
- Adam L et al. (2009) miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res 15: 5060–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner K et al. (2007) The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 26: 6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S et al. (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14: 79–89 [DOI] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W (2009) Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 69: 5820–5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M et al. (2008) MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells 26: 2496–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40: 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendoraite A et al. (2010) Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol 116: 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart L, Knuechel R, Kirchner T (2001) Variable beta-catenin expression in colorectal cancer indicates a tumor progression driven by the tumor environment. Proc Natl Acad Sci USA 98: 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T (2005a) Invasion and metastasis in colorectal cancer: epithelial–mesenchymal transition, mesenchymal–epithelial transition, stem cells and beta-catenin. Cells Tissues Organs 179: 56–65 [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005b) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5: 744–749 [DOI] [PubMed] [Google Scholar]
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial–mesenchymal transition. Cancer Res 68: 7846–7854 [DOI] [PubMed] [Google Scholar]
- Browne G, Sayan AE, Tulchinsky E (2010) ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle 9: 886–891 [DOI] [PubMed] [Google Scholar]
- Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, Iwata KK, Gibson NW, Cagnoni P, Haley JD (2007) Loss of homotypic cell adhesion by epithelial–mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther 6: 532–541 [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9: 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen NR, Silahtaroglu A, Orom UA, Kauppinen S, Lund AH (2007) miR-200b mediates post-transcriptional repression of ZFHX1B. RNA 13: 1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DR, Howe EN, Spoelstra NS, Richer JK (2010) Loss of miR-200c: a marker of aggressiveness and chemoresistance in female reproductive cancers. J Oncol 2010: 821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7: 1267–1278 [DOI] [PubMed] [Google Scholar]
- Dohadwala M et al. (2006) Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E2 induces transcriptional repressors ZEB1 and Snail in non-small cell lung cancer. Cancer Res 66: 5338–5345 [DOI] [PubMed] [Google Scholar]
- Du J, Yang S, An D, Hu F, Yuan W, Zhai C, Zhu T (2009) BMP-6 inhibits microRNA-21 expression in breast cancer through repressing δEF1 and AP-1. Cell Res 19: 487–496 [DOI] [PubMed] [Google Scholar]
- du Rieu MC et al. (2010) MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem 56: 603–612 [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, Martinvalet D, Song E, Lim B, Lieberman J (2009) miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS ONE 4: e7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103: 1631–1643 [DOI] [PubMed] [Google Scholar]
- Elloul S, Vaksman O, Stavnes HT, Trope CG, Davidson B, Reich R (2010) Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin Exp Metastasis 27: 161–172 [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6: 259–269 [DOI] [PubMed] [Google Scholar]
- Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A (2008) Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene 27: 1218–1230 [DOI] [PubMed] [Google Scholar]
- Gibbons DL et al. (2009) Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev 23: 2140–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O'Regan RM (2008) Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res 68: 2479–2488 [DOI] [PubMed] [Google Scholar]
- Graham TR, Yacoub R, Taliaferro-Smith L, Osunkoya AO, Odero-Marah VA, Liu T, Kimbro KS, Sharma D, O'Regan RM (2009) Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat 123: 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ (2008a) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601 [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bracken CP, Bert AG, Goodall GJ (2008b) MicroRNAs as regulators of epithelial–mesenchymal transition. Cell Cycle 7: 3112–3118 [DOI] [PubMed] [Google Scholar]
- Haddad Y, Choi W, McConkey DJ (2009) Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res 15: 532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN, Wang X (2009) A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol 114: 457–464 [DOI] [PubMed] [Google Scholar]
- Hurt EM, Saykally JN, Anose BM, Kalli KR, Sanders MM (2008) Expression of the ZEB1 (deltaEF1) transcription factor in human: additional insights. Mol Cell Biochem 318: 89–99 [DOI] [PubMed] [Google Scholar]
- Hurteau GJ, Carlson JA, Spivack SD, Brock GJ (2007) Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res 67: 7972–7976 [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN (2009) Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139: 1096–1108 [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN (2009) MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal 2: ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi Y, Konig A, Gress T, Menke A (2007) Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene 26: 2381–2385 [DOI] [PubMed] [Google Scholar]
- Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, Niedobitek G, Brabletz T, Kirchner T (2001) The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D(1), and p16(INK4A) and is a region of low proliferation. Am J Pathol 159: 1613–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial–mesenchymal transition. J Clin Invest 119: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell JA, Gerin I, MacDougald OA, Cadigan KM (2008) The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci USA 105: 15417–15422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent OA et al. (2009) A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther 8: 2013–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y (2008) The miR-200 family inhibits epithelial–mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283: 14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucmann-Rossi J (2008) MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology 47: 1955–1963 [DOI] [PubMed] [Google Scholar]
- Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, Chen ZQ, Liu XP, Xu ZD (2009a) Twist1-mediated adriamycin-induced epithelial–mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res 15: 2657–2665 [DOI] [PubMed] [Google Scholar]
- Li Y, VandenBoom TG, II, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH (2009b) Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res 69: 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-Y, Elledge SJ (2003) Multiple tumor suppressor pathways negatively regulate telomerase. Cell 113: 881–889 [DOI] [PubMed] [Google Scholar]
- Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC (2008) Zeb1 links epithelial–mesenchymal transition and cellular senescence. Development 135: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1: 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA et al. (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E (2007) Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 18: 4615–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS ONE 3: e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S (2008) MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res 14: 2690–2695 [DOI] [PubMed] [Google Scholar]
- Ohashi S et al. (2010) Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res 70: 4174–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D (2009) MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev 23: 2152–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22: 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Peter ME (2009) Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 8: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL (2003) Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J 22: 2453–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckamp KL et al. (2008) Tumor response to combination celecoxib and erlotinib therapy in non-small cell lung cancer is associated with a low baseline matrix metalloproteinase-9 and a decline in serum-soluble E-cadherin. J Thorac Oncol 3: 117–124 [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF (2002) Differential expression of the epithelial–mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 161: 1881–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A (2010) ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 29: 3490–3500 [DOI] [PubMed] [Google Scholar]
- Sayan AE et al. (2009) SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci USA 106: 14884–14889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickel R, Park SM, Murmann AE, Peter ME (2010) miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell 38: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE (2007) Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol 14: 3629–3637 [DOI] [PubMed] [Google Scholar]
- Shimono Y et al. (2009) Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138: 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J (2009) A gene expression signature associated with ‘K-Ras addiction' reveals regulators of EMT and tumor cell survival. Cancer Cell 15: 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M et al. (2008) ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol 21: 912–923 [DOI] [PubMed] [Google Scholar]
- Sossey-Alaoui K, Bialkowska K, Plow EF (2009) The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem 284: 33019–33029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, Jung A, Kirchner T, Brabletz T (2006) A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 131: 830–840 [DOI] [PubMed] [Google Scholar]
- Spaderna S et al. (2008) The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res 68: 537–544 [DOI] [PubMed] [Google Scholar]
- Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK (2006) The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res 66: 3893–3902 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial–mesenchymal transitions in development and disease. Cell 139: 871–890 [DOI] [PubMed] [Google Scholar]
- Tryndyak VP, Frederick AB, Igor PP (2010) E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer 126: 2575–2583 [DOI] [PubMed] [Google Scholar]
- Uhlmann S, Zhang JD, Schwager A, Mannsperger H, Riazalhosseini Y, Burmester S, Ward A, Korf U, Wiemann S, Sahin O (2010) miR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene 29: 4297–4306 [DOI] [PubMed] [Google Scholar]
- van Grunsven LA, Taelman V, Michiels C, Opdecamp K, Huylebroeck D, Bellefroid EJ (2006) deltaEF1 and SIP1 are differentially expressed and have overlapping activities during Xenopus embryogenesis. Dev Dyn 235: 1491–1500 [DOI] [PubMed] [Google Scholar]
- Vandewalle C, Van Roy F, Berx G (2009) The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci 66: 773–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18: 1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH (2009) Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 69: 2400–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U et al. (2009) The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 11: 1487–1495 [DOI] [PubMed] [Google Scholar]
- Witta SE et al. (2006) Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res 66: 944–950 [DOI] [PubMed] [Google Scholar]
- Xia H, Ng SS, Jiang S, Cheung WK, Sze J, Bian XW, Kung HF, Lin MC (2010) miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem Biophys Res Commun 391: 535–541 [DOI] [PubMed] [Google Scholar]
- Zhang L et al. (2010) microRNA-141 is involved in a nasopharyngeal carcinoma-related genes network. Carcinogenesis 31: 559–566 [DOI] [PubMed] [Google Scholar]