Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator
MTA overexpression has been linked to cancer progression. This study indicates that Lys 626 acetylation of MTA1 is essential for the activation of the Ras–Raf pathway, which mediates the transforming activity of MTA1.
Keywords: MTA1, Lys 626 acetylation, Raf1 activation, Ras, transformation
Abstract
High expression of metastasis-associated protein 1 co-regulator (MTA1), a component of the nuclear remodelling and histone deacetylase complex, has been associated with human tumours. However, the precise role of MTA1 in tumorigenesis remains unknown. In this study, we show that induced levels of MTA1 are sufficient to transform Rat1 fibroblasts and that the transforming potential of MTA1 is dependent on its acetylation at Lys626. Underlying mechanisms of MTA1-mediated transformation include activation of the Ras–Raf pathway by MTA1 but not by acetylation-inactive MTA1; this was due to the repression of Gαi2 transcription, which negatively influences Ras activation. We observed that acetylated MTA1–histone deacetylase (HDAC) interaction was required for the recruitment of the MTA1–HDAC complex to the Gαi2 regulatory element and consequently for the repression of Gαi2 transcription and expression leading to activation of the Ras–Raf pathway. The findings presented in this study provide for the first time—to the best of our knowledge—evidence of acetylation-dependent oncogenic activity of a cancer-relevant gene product.
Introduction
Metastasis-associated protein 1 (MTA1), a component of nuclear remodelling and histone deacetylase (NuRD) complex, is one of the most widely overexpressed gene products in human cancer, including breast cancer (Kumar et al, 2003). MTA1 interacts directly with histone deacetylase 1 (HDAC1) and HDAC2 and represses transactivation activity by recruiting HDAC co-repressor complexes to the target genes (Mazumdar et al, 2001; Balasenthil et al, 2007; Manavathi & Kumar, 2007). However, MTA1 also acts as a coactivator for some target promoters, such as breast carcinoma amplified sequence 3 (BCAS3; Gururaj et al, 2006). Although the expression of MTA1 correlates with tumour progression, the precise role of MTA1 in oncogenesis is not fully understood. Post-translational modifications, such as acetylation of histones and non-histone proteins, have long been accepted as important in the regulation of protein function (Eberharter & Becker, 2002). Among other pathways, the process of oncogenesis is also affected by the activation of the Ras–Raf pathway (Macrae et al, 2005). Activation of the Ras pathway can also be regulated by the G-protein-coupled receptors and involves negative regulation of the ability of Gβγ subunits to activate Ras by the formation of Gαi2–Gβγ (Edamatsu et al, 1998). However, it remains unknown whether the process of Ras activation and transformation can be also influenced by a putative upstream transcriptional regulator of Gαi2. In this study, we explore the role of MTA1 acetylation on its transforming activity. We report that MTA1 acetylation on Lys 626 constitutes the principle mechanism of its transforming activity and that MTA1 is the first upstream modifier of Gαi2 gene expression and promotes Ras signalling.
Results And Discussion
MTA1 is acetylated on Lys 626 in vitro and in vivo
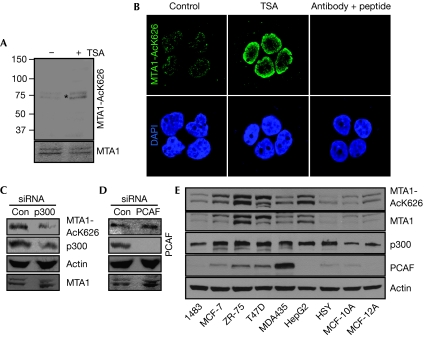
Having established that MTA1 is acetylated in vivo on Lys 626 in the context of the GKSYP motif (Gururaj et al, 2006), we next confirmed the Lys 626 modification by mass spectroscopy (supplementary Fig S1A,B online). Mass spectrometry analysis of the control digest peptide (unmodified) showed that the spectrum is weaker than the spectrum of the modified peptide (acetylated), probably because it exists mainly in forms cleaved at KSYP, which is internal to this peptide (acetylation protects it from cleavage), thus very little of this miscleavage product is observed (supplementary Fig S2 online). Furthermore, to demonstrate the existence of acetylated Lys 626 of MTA1 (MTA1-AcK626) under physiological conditions, we generated and characterized a site-specific antibody. This MTA1-AcK626 antibody recognizes only MTA1 acetylated on Lys 626 in the whole cell lysate, does not cross-react with other acetylated proteins and works well in immunohistochemical staining assay (supplementary Figs S3,S4,S7 online). Treatment with Trichostatin A (Fig 1A,B) increased the level of MTA1 acetylation on Lys 626 in ZR-75 cells.
Figure 1.
Metastasis-associated protein 1 is acetylated on Lys 626. (A) ZR-75 cells were treated with or without TSA (8 h) and cell lysates were probed with the MTA1-AcK626 or MTA1 antibodies. The asterisk indicates an acetylated MTA1 band. (B) ZR-75 cells were treated with and without TSA, fixed in paraformaldehyde and MTA1-AcK626 antiserum was used to perform immunofluorescence. An increased signal with TSA treatment is apparent for MTA1-AcK626 antibody. The signal with the antiserum could be effectively competed out using the Lys 626 peptide. (C,D) Cancer cells were treated with control, p300 or PCAF siRNA for 48 h and cell lysates were probed with the indicated antibodies. (E) Lysates from exponentially growth indicated cell lines were probed with antibodies against MTA1-AcK626, MTA1, p300, PCAF or actin. MTA1, metastasis-associated protein 1; PCAF, p300/cAMP response element-binding protein-associated factor; siRNA, small interfering RNA; TSA, Trichostatin A.
To identify the primary acetyltransferase enzyme responsible for Lys 626 acetylation, we performed an in vitro acetylation experiment with the purified p300 and p300/cAMP response element-binding protein-associated factor (PCAF) enzymes using the wild-type glutathione S-transferase (GST)–MTA1 as a substrate. Reaction products were resolved onto a gel, proteins were transferred to nitrocellulose membrane and western blotted with the MTA1-AcK626 antibody. Results showed that only p300, not PCAF, could acetylate MTA1 (supplementary Fig S5 online). To validate this finding in vivo, we next knocked down either p300 or PCAF and examined the level of MTA1-AcK626. There was a distinct reduction in the levels of MTA1-AcK626 (but not in the total level of MTA1) by p300 small interfering RNA (siRNA), but not by PCAF (Fig 1C,D). Next, we used the MTA1-AcK626 antibody to determine the association between the endogenous levels and acetylation status of MTA1 in various human cancer and two non-cancerous cell lines. We observed a widespread prevalence of MTA1 acetylated on Lys 626 in cancer cells (Fig 1E). In addition, p300 expression increased modestly in five of the seven cancer cell lines compared with the two non-cancer cell lines. By contrast, PCAF expression pattern did not follow the levels of MTA1-AcK626 in cancer cell lines, whereas its levels were very low in non-cancerous cells. Together these findings indicate that MTA1 is acetylated on Lys 626 in physiological settings.
Recruitment of acetylated MTA1 to Gαi2 chromatin
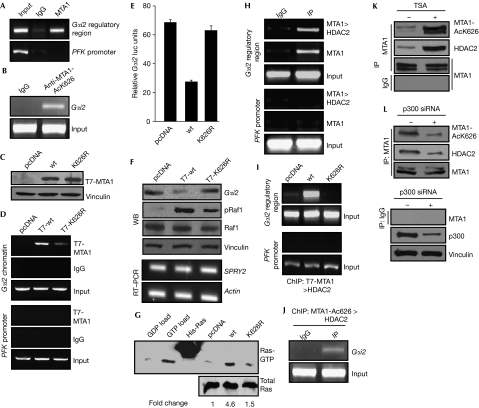
While performing chromatin immunoprecipitation (ChIP)-based screening of MCF-7 cells to identify new targets of MTA1, we identified several targets (Gururaj et al, 2006), including a 675 bp fragment that mapped approximately 9.5 kb upstream from the Gαi2 transcription start site. Gαi2 gene product is an established negative modifier of Ras activation owing to its ability to prevent Gβγ subunits from activating Ras by forming an inhibitory Gαi2–Gβγ complex and its downregulation promotes Ras activation owing to the release of the Gβγ subunits from the inhibitory Gαi2–Gβγ complex (Crespo et al, 1994). Next, we showed that MTA1-bound chromatin in MCF-7 cells contained the Gαi2 regulatory fragment (Fig 2A). Interestingly, endogenous acetylated MTA1, acetylated on Lys 626, is also bound to the Gαi2 regulatory chromatin, as detected by the comparative ChIP analysis using the MTA1-AcK626 antibody and IgG control (Fig 2B). To assess the significance of Lys 626 modification on the ability of MTA1 to potentially regulate the expression of Gαi2, we next generated stable pooled clones of Rat1 fibroblasts expressing MTA1 or MTA1-K626R (Fig 2C). We observed that MTA1 is recruited effectively to the Gαi2 gene chromatin. However, the recruitment level of MTA1-K626R to Gαi2 was significantly lower than observed for MTA1 (Fig 2D). Accordingly, MTA1, but not MTA1-K626R, effectively inhibited Gαi2 luc-reporter activity (Fig 2E). The noted differential regulation of Gαi2 luc-reporter activity by MTA1 mutants was functional because MTA1 expression in Rat1 clones was accompanied by downregulation of Gαi2 protein and upregulation of phosphorylated Raf1 as compared with cells expressing the control vector, whereas the levels of phosphorylated Raf1 were considerably lower in T7-MTA1-K626R cells as compared with T7-MTA1-expressing cells (Fig 2F). To understand whether acetylated MTA1 could affect other target genes that hinge on the Ras pathway, we examined the status of Sprouty homologue 2 (SPRY2), a negative regulator of the Ras pathway (Hanafusa et al, 2002), in various Rat1 clones. The results showed that there was no change in the expression levels of SPRY2 (Fig 2F, lower panels).
Figure 2.
Enhanced recruitment of acetylated metastasis-associated protein 1 to the Gαi2 regulatory region. (A) MTA1-bound chromatin from MCF-7 cells associates with the regulatory region of Gαi2. (B) ChIP analysis of the recruitment of acetylated MTA1 to the Gαi2 regulatory region in Rat1 cells. (C) Lysates from pooled stable Rat1 clones of pcDNA, wild-type (wt) MTA1 and MTA1-K626R were probed with T7 antibody. (D) Differential recruitment of MTA1 mutants onto the Gαi2 regulatory region. Exponentially growing pcDNA, T7-MTA1 and T7-MTA1-K626R Rat1 stable clones were analysed by ChIP using T7 antibody. (E) Repression of Gαi2 promoter activity by MTA1 or MTA1-K626R. Rat1 cells were transfected with pGL2-Gαi2 vector and either pcDNA, MTA1-wt or MTA1-K626R plasmids. (F) Western blot (WB) analysis for Gαi2 and phosphorylated Raf1 and RT–PCR analysis for SPRY2 in Rat1 clones expressing pcDNA, MTA1 or MTA1-K626R. (G) Status of Ras activation in Rat1 clones expressing MTA1 or its mutants. Lysates from MTA1 Rat1 clones were used for the Ras activation assay as described in the Methods section. The bead efficiency was determined by incubating lysates with excess GDP (GDP load) or GTP (GTP load) followed by incubation with Raf–RBD beads. (H) Analysis of MTA1 recruitment to Gαi2 by ChIP in ZR-75 cells. (I) Rat1 clones expressing MTA1 or MTA1-K626R were subjected to ChIP analysis with T7 antibody followed by HDAC2 antibody. (J) Double ChIP performed in Rat1 cells with MTA1-AcK626 antibody followed by HDAC2 antibody. (K,L) Western blot analysis for acetylated MTA1, MTA1 and HDAC2 after immunoprecipitation with MTA1 in ZR-75 cells in the presence and absence of either TSA or p300 siRNA. ChIP, chromatin immunoprecipitation; HDAC2, histone deacetylase2; IP, immunoprecipitation; MTA1, metastasis-associated protein 1; RT–PCR, reverse transcriptase–PCR; siRNA, small interfering RNA; SPRY2, Sprouty homologue 2; TSA, Trichostatin A; wt, wild type.
Interaction of Lys 626 acetylated MTA1 with HDACs
As repression of Gαi2 expression is known to activate Ras activation (Edamatsu et al, 1998), we tested the status of activated Ras by using glutathione S-transferase-tagged Raf1–Ras-binding domain beads and immunoblotting with Ras antibody in the Rat1 stable clones. We observed that the overexpression of MTA1, but not MTA1-K626R, in Rat1 cells leads to a significant stimulation of activated Ras level (Fig 2G). As MTA1 interacts directly with HDACs (Mazumdar et al, 2001), the above findings suggested a role of Lys 626 on MTA1 in its interaction with HDACs. To test this, we performed double ChIP of endogenous MTA1 and HDAC2 (first, ChIP with MTA1 and second, ChIP with HDAC2) in ZR-75 cells to show co-recruitment of the two molecules to the Gαi2 regulatory region. The results show a distinct recruitment of HDAC2 to the Gαi2 regulatory region (Fig 2H). We next determined the effect of the acetylation status of MTA1 and its putative interaction with HDAC2 on its ability to be recruited to the Gαi2 regulatory sequence by using a double ChIP assay. HDAC2 was recruited to the Gαi2 regulatory region in Rat1 cells that expressed wild-type MTA1 (MTA1-wt) but not in cells that expressed MTA1-K626R (Fig 2I), suggesting the in vivo existence of an endogenous MTA1–HDAC2 complex and its interaction with Gαi2 chromatin. To confirm this, we next performed a sequential double ChIP in the Rat1 cells using the MTA1-AcK626 and HDAC2 antibodies. The results suggested that the acetylated MTA1–HDAC2 complex is indeed recruited to the Gαi2 regulatory chromatin, as detected by the comparative ChIP analysis using the MTA1-AcK626 antibody and IgG control (Fig 2J).
The above data suggested that MTA1-K626 acetylation might participate in the formation of a co-repressor complex, presumably by improving the ability of MTA1 to interact with HDAC2. Next, we determined the ability of increased acetylated MTA1 to interact with HDAC2. By co-immunoprecipitation assay, we confirmed an enhanced interaction between the acetylated MTA1 and HDAC2 on Trichostatin A treatment (Fig 2K), whereas such association was decreased on p300 siRNA treatment in the ZR-75 cells (Fig 2L). Consistent with this idea, we also noticed that HDAC2 interacts efficiently with the transiently expressed MTA1, but not with MTA1-K626R (data not shown). These results imply a potential role of acetylation modification in recruiting HDAC2 to MTA1.
Repression of Gαi2 expression by acetylated MTA1
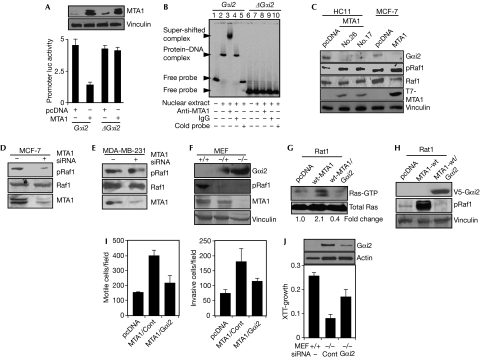
To determine the significance of MTA1 recruitment onto the Gαi2 regulatory region in the noted MTA1 repression of Gαi2 in Rat1/MTA1 cells, we next assessed the functionality of MTA1-interacting Gαi2 sequence. Results from MTA1 co-transfection studies involving Gαi2 promoter-luc reporter or ΔGαi2 promoter-luc reporter lacking the Gαi2 regulatory region showed that MTA1-mediated repression of Gαi2 expression requires the Gαi2 regulatory region (Fig 3A), suggesting that MTA1 behaves as a co-repressor of the Gαi2 gene. Furthermore, electrophoretic mobility shift assay demonstrated that the MTA1 protein does form a complex with the PCR product generated from the Gαi2 regulatory region, but not from the mutant ΔGαi2 promoter region, and such an MTA1–DNA complex could be super-shifted by the MTA1 antibody but not by IgG (Fig 3B, lanes 1–5). Consistent with these findings, MTA1 overexpression in HC11 murine mammary epithelial (Bagheri-Yarmand et al, 2004) and MCF-7 cells (Mazumdar et al, 2001) was accompanied by reduced Gαi2 protein and increased activated Raf levels (Fig 3C). Conversely, MTA1 knockdown in MCF-7 cells led to decreased levels of phosphorylated Raf1 (Fig 3D). As MDA-MB-231 cells contain a naturally mutated Ras and thus lack upstream regulation by MTA1, we next used these cells to show that knockdown of MTA1 had no effect on the activated Raf1 (Fig 3E). These observations suggest a regulatory role of Gαi2 in the noted Raf1 stimulation by MTA1.
Figure 3.
Metastasis-associated protein 1 inhibits Gαi2 expression. (A) Effect of MTA1 on Gαi2 and ΔGαi2-luc activity in MCF-7 cells. (B) Electrophoretic mobility shift assay analysis of MTA1 binding to the Gαi2 regulatory region using PCR product encompassing the Gαi2 regulatory and ΔGαi2 region in ZR-75 cells. (C) Western blot analysis of Gαi2, phosphorylated Raf1 and Raf1 using cell lysates from wild-type (wt) MTA1 overexpressing mouse mammary HC11 cells and MCF-7 cells. (D) Effect of MTA1 knockdown by siRNA on the levels of activated Raf1 in MCF-7 cells. Either MTA1 siRNA or non-specific siRNA was transfected into MCF-7 cells. (E) Lack of effect of MTA1 knockdown on the level of phosphorylated Raf1 in MDA-MB-231 cells. MDA-MB-231 cells were analysed as described for panel D. (F) Western blot analysis of MTA1 and Gαi2 in MEFs from wild-type (+/+), heterozygous (+/−) and homozygous (−/−) mice for MTA1 deletion. (G) Effect of reintroduction of V5-Gαi2 in the pooled Rat1 clones that express wild-type MTA1. Double stable clones of V5-Gαi2 were generated in wild-type MTA1-overexpressing Rat1 cells. (H) Status of Ras activation in Rat1 clones expressing MTA1 or MTA1/Gαi2. Ras activation was assayed in the double stable clones. (I) Effects of the overexpression of MTA1 or MTA1/Gαi2 on cell migration and invasion in Rat1 cells. (J) XTT-based cell growth assay of MTA1−/− MEFs after Gαi2 knockdown. MEF, murine embryonic fibroblast; MTA1, metastasis-associated protein 1; siRNA, small interfering RNA; wt, wild type; XTT, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
To establish a causative relationship between the levels of MTA1 and Gαi2 expression, we showed that loss of MTA1 expression in murine embryonic fibroblasts (MEFs) from homozygous mice resulted in a distinct upregulation of Gαi2 levels (Fig 3F). To provide proof-of-principle confirmation that Gαi2 is a direct target of MTA1 and that MTA1 stimulates the Ras pathway, we generated V5-Gαi2 stable clones in the background of Rat1 fibroblasts expressing wild-type MTA1 (double stable pooled clone) and evaluated the status of activated Ras in these clones. We observed that the overexpression of Gαi2 in MTA1 clones led to a significant reduction in the levels of activated Ras and its downstream effector, phosphorylated Raf1 (Fig 3G,H). In addition, motility and invasiveness in the wild-type MTA1-overexpressing Rat1 cells were compromised by the overexpression of Gαi2 (Fig 3I). Similarly, we observed that Gαi2 knockdown in MTA1−/− MEFs leads to a partial reversal of the slower growth rate as compared with MTA1−/− MEFs treated with the control siRNA (Fig 3J). Together these results suggest that Gαi2 gene chromatin is a direct target of MTA1 and that MTA1 might stimulate the Ras pathway, in part, by repressing the expression of Gαi2, which in turn is a negative regulator of the Raf pathway.
Oncogenic activity of acetylated MTA1
To assess the role of MTA1-K626 acetylation in transformation, we next studied the biological characteristics of Rat1 fibroblasts expressing MTA1 or MTA1-K626R (Fig 2C). The Rat1/MTA1 clones exhibited morphological characteristics reminiscent of transformed cells. Overexpression of MTA1 enhanced the growth rate of Rat1 cells in the monolayer culture compared with vector-transfected cells, and the growth rate of the Rat1/MTA1-K626R mutant clone was between that of the wild-type and vector control clones (Fig 4A). The growth rate of MTA1−/− MEFs was also slower than the MEFs derived from normal mice (supplementary Fig S6 online). Interestingly, overexpression of wild-type MTA1, but not MTA1-K626R, showed enhanced motility (Fig 4B) and invasiveness (Fig 4C) as compared with vector control cells, whereas wild-type MTA1 strongly promoted the transformation of Rat1 cells compared with cells expressing pcDNA or MTA1-K626R in a foci-forming assay (Fig 4D). We observed that Rat1/MTA1 cells (seven of the eight animals), but not Rat1/pcDNA or Rat1/MTA1-K626R cells (none of the eight animals), formed palpable fibrosarcomas (Fig 4E,F). These findings confirm that MTA1 is a bona fide oncogene and its ability to transform Rat1 cells might be dependent, at least in part, on its Lys 626 acetylation.
Figure 4.
Acetylated metastasis-associated protein 1 has oncogenic activity and stimulates Ras pathway. (A) Growth characteristics of Rat1/pcDNA, Rat1/wild-type MTA1 and Rat1/MTA1-K626R clones. (B) The effect of MTA1 mutants on cell migration in Rat1 cells. Five-thousand cells of pcDNA, wild-type MTA1 and MTA1-K626R stable clones in Rat1 cells were loaded onto an insert of Boyden chamber with a pore size of 8 μm. (C) The effect of MTA1 mutants on cell invasiveness in Rat1 cells. (D) The effect of MTA1 mutants on the foci-formation ability of Rat1 cells. Approximately, 2,000 cells per plate of pcDNA, wild-type MTA1 and MTA1-K626R Rat1 stable clones. (E) In vivo tumour formation by wild-type MTA1 clones. Approximately, 5 × 106 cells of Rat1/pcDNA, wild-type MTA1 or MTA1-K626R clones were bilaterally injected into the mammary fat pads of eight athymic nude mice. (F) Tumours derived from the experiment in (E) were embedded into paraffin; sections were made and H&E staining was used to identify the tumours. Histological characteristics of tumours No. 1 and No. 2, malignant histiocytomas with giant cells; No. 3, abundance of blood vessels of the tumour tissue; No. 4, active mitosis (arrows); and No. 5 and No. 6, fusiform fibrosarcoma tumour cells arranged in various orientations. (G) MTA1-AcK626, MTA1 and pERK staining in primary breast cancer samples. Breast cancer tissue microarrays were stained with MTA1-AcK626, pERK and MTA1, and the staining was scored as low and high. (H) Model elucidating the mechanism of MTA1 regulation of the Ras pathway. ERK, extracellular signal-regulated kinase; H&E, haematoxylin and eosin; MTA1, metastasis-associated protein 1; wt, wild type.
MTA1-mediated activation of the Ras pathway
To assess the relevance of our findings in cancer tissues and to characterize the efficiency of MTA1-AcK626 to detect acetylated MTA1 in paraffin-embedded tissues, we next determined whether an association exists between the status of MTA1, MTA1-AcK626 and components of the Ras pathway, such as extracellular signal-regulated kinase (ERK) activation in a cohort of 380 premenopausal breast cancer patients (Ryden et al, 2005). The expression of MTA1 was first evaluated from 0 (negative) to 4 (strongest intensity), but was later divided into three groups: 0 or 1, 2, and 3 or 4. Representative illustration of strongest MTA1-AcK626, MTA1 and phosphorylated ERK are shown in Fig 4G, and the overall data summary is presented in supplementary Table S1 online. In general, we observed easily detectable nuclear staining of MTA1-AcK626, MTA1 and phosphorylated ERK proteins and a strong relationship between MTA1-AcK626, MTA1 and ERK activation in the breast tumour samples (supplementary Table S1). Together, these results suggest a causal relationship between the upregulation of MTA1-AcK626 and the Ras pathway under physiological settings.
Speculation
The findings presented in this study show that MTA1 is a bona fide oncogene and a new regulator of Ras activation, with roles in transformation. As many of the breast tumours also overexpress BCAS3, another direct target of MTA1 in oestrogen receptor (ER)-positive setting (Gururaj et al, 2006), our findings now suggest that the noted MTA1–Gαi2–Ras connections probably have a wider influence in breast cancer, as this pathway will be active in both ER− and ER+ settings. The underlying mechanism of MTA1 regulation of the Ras pathway, at least in part, involves transcriptional repression of the Gαi2 protein, which normally inhibits Ras stimulation (Fig 4H). Notably, MTA1 is also one of the direct downstream targets of c-MYC and has an essential role in the transformation activity of c-MYC (Zhang et al, 2005). Our findings presented here now suggest that in addition to MTA1 deregulation, its acetylation status might also be crucial for the MTA1 transforming activity. In addition, MTA1 seems to be an important upstream modifier of Gαi2 expression and, consequently, could influence the activation of the Ras pathway, at least in part. This study provides evidence of acetylation-dependent transcriptional repression of a negative modifier of Ras activation, providing clues about the transformation activity of MTA1.
Methods
Mouse xenograft studies. For the tumorigenesis studies, 4-week-old female athymic nude mice (Charles River) were bilaterally injected with 5 × 106 cells of either Rat1/pcDNA, MTA1-wt or MTA1-K626R clones into the mammary fat pads. Tumours were allowed to grow for 5 weeks and tumour size was measured every 15 days. All animal procedures were performed in compliance with the Institutional Animal Care and Use Committee and the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals.
Breast tumour material. A total of 564 premenopausal breast cancer patient samples were assayed and analysed by G.L. as per Malmö University Hospital policy. All patients received radical masectomy or breast-conserving surgery followed by radiotherapy, and less than 2% were given adjuvant polytherapy.
ChIP, immunohistochemistry and immunofluorescence. Microscopy-based assays and ChIP were performed using established methods as described previously (Mazumdar et al, 2001). See the supplementary information online for details.
Supplementary Information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank R.R. Singh for cloning and A.H. Talukder for help with reporter assays. This study was supported by National Institutes of Health (NIH) grant CA98823 (to R.K.). Work in the N.H.L. laboratory is supported by NIH grant CA120316
Footnotes
The authors declare that they have no conflict of interest.
References
- Bagheri-Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R (2004) Metastasis-associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development 131: 3469–3479 [DOI] [PubMed] [Google Scholar]
- Balasenthil S, Gururaj AE, Talukder AH, Bagheri-Yarmand R, Arrington T, Haas BJ, Braisted JC, Kim I, Lee NH, Kumar R (2007) Identification of Pax5 as a target of MTA1 in B-cell lymphomas. Cancer Res 67: 7132–7138 [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind JS (1994) Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature 369: 418–420 [DOI] [PubMed] [Google Scholar]
- Eberharter A, Becker PB (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep 3: 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edamatsu H, Kaziro Y, Itoh H (1998) Expression of an oncogenic mutant G alpha i2 activates Ras in Rat-1 fibroblast cells. FEBS Lett 440: 231–234 [DOI] [PubMed] [Google Scholar]
- Gururaj AE, Singh RR, Rayala SK, Holm C, den Hollander P, Zhang H, Balasenthil S, Talukder AH, Landberg G, Kumar R (2006) MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc Natl Acad Sci USA 103: 6670–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H, Torii S, Yasunaga T, Nishida E (2002) Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 4: 850–858 [DOI] [PubMed] [Google Scholar]
- Kumar R, Wang RA, Bagheri-Yarmand R (2003) Emerging roles of MTA family members in human cancers. Semin Oncol 30: 30–37 [DOI] [PubMed] [Google Scholar]
- Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F (2005) A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell 8: 111–118 [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R (2007) Metastasis tumor antigens, an emerging family of multifaceted master coregulators. J Biol Chem 282: 1529–1533 [DOI] [PubMed] [Google Scholar]
- Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, Kumar R (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol 3: 30–37 [DOI] [PubMed] [Google Scholar]
- Ryden L, Jirstrom K, Bendahl PO, Ferno M, Nordenskjold B, Stal O, Thorstenson S, Jonsson PE, Landberg G (2005) Tumor-specific expression of vascular endothelial growth factor receptor 2 but not vascular endothelial growth factor or human epidermal growth factor receptor 2 is associated with impaired response to adjuvant tamoxifen in premenopausal breast cancer. J Clin Oncol 23: 4695–4704 [DOI] [PubMed] [Google Scholar]
- Zhang XY, DeSalle LM, Patel JH, Capobianco AJ, Yu D, Thomas-Tikhonenko A, McMahon SB (2005) Metastasis-associated protein 1 (MTA1) is an essential downstream effector of the c-MYC oncoprotein. Proc Natl Acad Sci USA 102: 13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.