Abstract
Background
A blood-based biomarker of Alzheimer disease (AD) would be superior to CSF and neuroimaging measures in terms of cost, invasiveness and feasibility for repeated measures. We previously reported blood ceramides varied in relation to timing of memory impairment in a population-based study. The present objective was to examine whether plasma ceramides varied by AD severity in a well-characterized clinic sample and were associated with cognitive decline and hippocampal volume loss over one year.
Methods
Participants included 25 normal controls (NC), 17 amnestic Mild Cognitive Impairment (MCI), and 21 early probable AD. A thorough neuropsychological battery and neuroimaging with hippocampal volume determination were conducted at baseline and one year later. Plasma ceramides were assayed at baseline using HPLC-coupled electrospray ionization tandem mass spectrometry.
Results
While all saturated ceramides were lower in MCI compared to AD at baseline, Ceramides C22:0 and C24:0 were significantly lower in the MCI group compared to both NC and AD groups (p<0.01). Ceramide levels did not differ (p>0.05) in AD versus NC. There were no cross-sectional associations between ceramides C22:0 and C24:0 and either cognitive performance or hippocampal volume among any group. However, among the MCI group, higher baseline ceramide C22:0 and C24:0 levels were predictive of cognitive decline and hippocampal volume loss one year later.
Conclusion
Results suggest that very long-chain plasma ceramides C22:0 and C24:0 are altered in MCI and predict memory loss and right hippocampal volume loss among subjects with MCI. These plasma ceramides may be early indicators of AD progression.
Keywords: Ceramides, Lipids, Biomarker, Plasma, Mild cognitive impairment, Hippocampal volume
1. Introduction
Accumulating evidence suggests that ceramide metabolism may be perturbed early in the pathogenesis of AD [1-4] with prominent changes in the very long-chain C22:0, C24:0 and C24:1 species [1]. The exact mechanisms by which ceramide levels become perturbed in AD is currently an active area of research, and a number of studies have identified pathogenic links between ceramides and amyloid-beta (Aβ). Increased levels of ceramides may accelerate the formation of pathogenic forms of amyloid by increasing β- and γ-cleavage of APP [5-7] by mechanisms that involve stabilization of secretase activity [6,8]. It has also been demonstrated that Aβ can induce ceramide formation by an oxidative-stress mediated activation of neutral sphingomyelinase (nSMase) and inhibiting ceramide formation protects neurons from Aβ-induced death [1,9,10]. These results suggest that a disruption of ceramide metabolism may be an early and critical event involved in Aβ production and neuronal dysfunction associated with AD.
Cumulatively, studies of ceramides in brain tissues and CSF have suggested that levels are increased early in AD [1,2,4,11]. We previously reported that low serum levels of long-chain and very long-chain ceramides were cross-sectionally associated with memory impairment and high levels predicted the accelerated onset of memory impairment during a 10-year follow-up [12]. These findings suggested that blood ceramide levels may be useful as biomarkers that could predict the onset and progression of AD. Therefore, we sought to further evaluate the clinical utility of blood ceramides using a well-defined clinic population of normal controls, amnestic MCI and early probable AD cases followed for one year. Further, we do not yet understand the neuropathological correlate of serum ceramides in AD. Therefore, we also examined the association between plasma ceramides and baseline hippocampal volume, as well as annual hippocampal volume loss, in this cohort to determine whether the plasma lipids were related to a well-known and well-characterized neuropathological marker of AD.
2. Methods
2.1. Participants
Participants were community-dwelling volunteers who enrolled in a longitudinal study examining the utility of DTI and arterial spin labeling as biomarkers of AD progression. Recruiting methods have previously been described [13]. Briefly, participants were recruited from the Clinical Core of the Johns Hopkins Alzheimer's Disease Research Center and memory clinics associated with Johns Hopkins Medicine. Participants included: (1) Normal Controls (NC): cognitively normal individuals with a Clinical Dementia Rating (CDR) [14] of 0; (2) Amnestic Mild Cognitive Impairment (MCI): non-demented participants with mild memory problems, CDR=0.5, and met criteria for amnestic MCI, single or multiple domains [15]; (3) Alzheimer's disease (AD): participants with mild-moderate AD, CDR=1, and met NINCDS/ADRDA [16] criteria for probable AD. All participants were required to be older than 55 years, have no history of a neurological disease other than AD or of a major psychiatric illness, and have an informant who could provide information about their daily function. Seventy-five participants completed the baseline examination, 25 per group, and 71 participants completed the 1-year follow-up examination (25 NC, 23 MCI, and 23 AD). Informed consent was obtained prior to the initiation of the study in accordance with the requirements of the Johns Hopkins Institutional Review Board. Consent procedures followed the guidelines endorsed by the Alzheimer's Association for participation of cognitively impaired individuals [17].
2.2. Assessments
Each participant received a detailed medical evaluation at baseline and one-year later, including: (1) a medical, psychiatric and neurologic history; (2) a neuropsychological battery; (3) a physical, psychiatric and neurological examination; (4) an assessment of clinical severity using the CDR scale; (5) MRI scan; and (6) a blood draw. However, as the blood draw was an optional add-on to the imaging study, 8 MCI and 4 AD participants did not provide blood at baseline. There were no differences in demographic or health-related characteristics between those with and without available blood.
The neuropsychological battery included the Mini-Mental State Exam (MMSE) [18]; Alzheimer's Disease Assessment Scale – cognitive portion (ADAS-Cog) [19]; California Verbal Learning Test (CVLT) [20]; the Logical Memory Story A from the Wechsler Memory Scale (WMS)[21]; Trail Making Test (TMT) Parts A (TMT-A) and B (TMT-B) [22]; and the total score on the Controlled Word Association test for category and letter fluency [23].
2.3. MRI Acquisition
MRI images were acquired using a SENSE head coil on a 3.0 Tesla (3T) scanner (Philips Medical Systems, Best, The Netherlands) equipped with explorer gradients (40 mT/m) at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute. At each scanning session, a high-resolution T1-weighted image Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE) scan and a DTI scan was acquired. The MPRAGE scan was conducted according to the protocol of the Alzheimer's Disease Neuroimaging Initiative (ADNI) [24].
2.3.1. Hippocampal Volume Measurement
Right and left hippocampal volumes were calculated by voxel counting on binary hippocampal images segmented on T1-MRI data at baseline and one year. A template-based segmentation algorithm was used to segment the binary hippocampal images for the baseline scans. Briefly, a representative elderly normal subject was selected as the template and left and right hippocampi were segmented manually. A total of 38 landmarks were placed on the hippocampus similar to the procedure described in Csernansky et al. [25]. First, the head and the tail landmarks were placed to identify the principal axis of the structure. Nine slices at equal distance from each other and perpendicular to this principal axis were then identified and a total of four landmarks were placed at the anterior, inferior, posterior, and superior midpoints in each slice. Using these landmarks, the subvolume images were then created around the hippocampi and the landmarks were used to calculate a rigid transformation [26] between the template and individual subject subvolumes. The large deformation diffeomorphic metric mapping (LDDMM) landmark matching algorithm [27] was used to register the subvolumes further [28]. These transformations were subsequently used to move the template hippocampi segmentation onto each subject's MR scan and to create an initial hippocampus segmentation for the subject. The alternating kernel mixture (AKM) [29] method was used to segment the subject subvolume image into Grey Matter (GM)/white matter (WM)/CSF regions. Using this AKM segmentation, any mislabeled WM and CSF voxels were removed from the initial hippocampus segmentation. To calculate segmentations at one year, each subject's baseline scan and baseline hippocampi segmentation was used as the template. The baseline segmentation was transformed onto other time point scans using LDDMM image matching. In the statistical analyses, the right and left hippocampi were examined separately. In cross-sectional analyses, to control for head size, the outcome measure was the ratio of the right or left hippocampal volume to the intracranial volume (ICV). In longitudinal analyses, change in hippocampi volumes alone were examined as each person is their own control.
2.4. Measurement of Plasma Lipids
Non-fasting blood was drawn at baseline; plasma was isolated and frozen at -80°C until processing. Total lipids were extracted from plasma samples according to a modified Bligh and Dyer procedure [30]. Briefly, each sample was homogenized at room temperature in 10 volumes of deionized water, then in 3 volumes of 100% methanol containing 30 mM ammonium acetate, and vortexed. Four volumes of chloroform were added and the mixture was vortexed and centrifuged at 1,000g for 10 minutes. The bottom (chloroform) layer was removed, dried and resuspended in 100% methanol for LC/MS/MS.
Ceramides were detected and quantified by LC/MS/MS using multiple reaction monitoring (MRM). The procedure is based on high performance liquid chromatography (HPLC) for temporal resolution of compounds with subsequent introduction into the mass spectrometer for detection and quantification by mass/charge. Samples were injected using a PAL autosampler into a PerkElmer HPLC equipped with a phenomenex, luna 100×2 mm, 5 μm, C18 column coupled with guard column containing identical packing material (Phenomenex, Torrance, CA). For a typical run, the LC column was first pre-equilibrated for 0.5 min with the first mobile phase consisting of 85% methanol, 15% H20, and 5 mM ammonium formate. The column was then eluted with the second mobile phase consisting of 99% methanol, 1% formic acid, and 5 mM ammonium formate at the flow rate of 100.0 μl /min. The eluted sample was then injected into the ion source. The detection, and quantitation of each analyte was carried out by ESI/MS/MS in MRM mode monitoring the parent compound, and products by ion scan (m/z 264.4, 266.4).
2.5. Covariates
Ceramides were log transformed to obtain a normal distribution prior to the initiation of any analyses. We first assessed the association between several health and demographic characteristics and plasma ceramides using t-tests for dichotomous variables and Pearson's correlations for continuous variables. Covariates examined included age, race, sex, and education; presence of an APOE ε4 allele; current and/or history of self-reported medical conditions including hypertension, diabetes, myocardial infarction, stroke, angina, hypercholesterolemia, cancer, and major depression; current medication use including statins and other lipid-lowering drugs, anti-hypertensives, and dementia medications; and other health-related measures including systolic and diastolic blood pressure, body mass index, and depressive symptoms measured by the Geriatric Depression Scale. As none of these variables, including statin use, presence of an APOE ε4 allele, and vascular conditions were associated with plasma ceramides (p<0.05), group comparisons are presented as univariate analyses.
2.6. Statistical Analysis
ANOVAs and Fischer's exact tests were used to examine group differences (NC, MCI, AD) in demographic and other health-related characteristics. Group differences in the plasma lipids were examined with ANOVAs and pairwise associations with t-tests and bonferroni correction. Linear regression was used to examine the association between baseline plasma ceramides and baseline cognitive test score or hippocampal volume (right or left) to ICV ratio, as well as change in these outcomes at the one-year follow-up. Models with outcomes of cognitive test scores controlled for age and education and those for hippocampus volume controlled for age. When longitudinal change was the outcome, the baseline hippocampal volume (right or left) or cognitive test score was also added to the models. The a priori p-value was set at p<0.05. All analyses were conducted using STATA Version 10.0 (StataCorp, College Station, TX).
3. Results
3.1. Participant characteristics and descriptive of ceramide levels
The baseline demographic, health and clinical characteristics of participants with available baseline blood, by diagnostic group, are shown in Table 1. There were no demographic differences between groups with regard to age, sex, race, and education. In addition, the occurrence of vascular factors such as hypertension, hypercholesterolemia, and myocardial infarction did not differ between the groups. The AD group had a higher percentage of persons with an APOE E4 allele and, as expected, was more likely to be taking anti-dementia medications and had markedly lower scores across neuropsychological tests compared to MCI and NC (p<0.001).
Table 1. Baseline demographic, health and neuropsychological characteristics of participants with available baseline blood (n=63), by diagnostic group.
|
NC (n=25) Mean(SD)/n(%) |
MCI (n=17) Mean(SD)/n(%) |
AD (n=21) Mean(SD)/n(%) |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 74.4 (7.0) | 74.5 (5.6) | 74.8 (7.0) | 0.843 |
| Female | 14 (56.0%) | 5 (29.4%) | 6 (28.6%) | 0.111 |
| Caucasian vs. Other | 24 (96.0%) | 15 (88.2%) | 16 (76.2%) | 0.133 |
| Education (years) | 16.2 (2.6) | 15.9 (2.9) | 15.2 (3.8) | 0.230 |
| Any APOE E4 allele | 5 (20.0%) | 7 (43.8%) | 12 (57.1%) | 0.032 |
| Health Characteristics | ||||
| Current Hypertension | 14 (56.0%) | 8 (47.1%) | 9 (42.9%) | 0.733 |
| Current Hypercholesterolemia | 9 (36.0%) | 9 (52.9%) | 13 (68.4%) | 0.071 |
| Current or History of Angina | 1 (4.0%) | 2 (11.8%) | 2 (9.5%) | 0.609 |
| Current or History of Atrial Fibrillation | 1 (4.0%) | 3 (18.8%) | 2 (9.5%) | 0.312 |
| History of Heart Attack | 0 | 2 (11.8%) | 1 (4.8%) | 0.182 |
| Current Diabetes | 2 (8.0%) | 2 (11.8%) | 3 (14.3%) | 0.881 |
| Geriatric Depression Scale (15-item) | 0.8 (1.3) | 1.5 (1.3) | 1.9 (1.9) | 0.123 |
| Current Dementia Medication | 0 | 2 (12.5%) | 19 (90.5%) | <0.001 |
| Current Statin Use | 8 (32.0%) | 8 (50.0%) | 14 (66.7%) | 0.062 |
| Clinical Characteristics | ||||
| CDR Rating | 0.0 (0.1) | 0.5 (0.1) | 1.1 (0.4) | <0.001 |
| CDR Sum of Boxes | 0.0 (0.1) | 1.2 (0.6) | 6.0 (2.5) | <0.001 |
| MMSE | 28.8 (1.2) | 27.2 (2.1) | 22.2 (3.1) | <0.001 |
NC=Normal Control; MCI=Mild Cognitive Impairment; AD=Alzheimer's Disease, SD=Standard Deviation
Of the 63 participants at baseline with available blood, all participants had cognitive tests and 57 had available scans for neuroimaging at baseline. There were no differences in demographic or other health-related characteristics between those with and without hippocampal volume measurements. Two MCI and one AD participant dropped out and were not followed at one year, leaving 60 participants for longitudinal analysis of cognitive tests. One NC and two AD participants did not have hippocampal volumes at one year, leaving 54 participants for longitudinal analyses of hippocampal volumes.
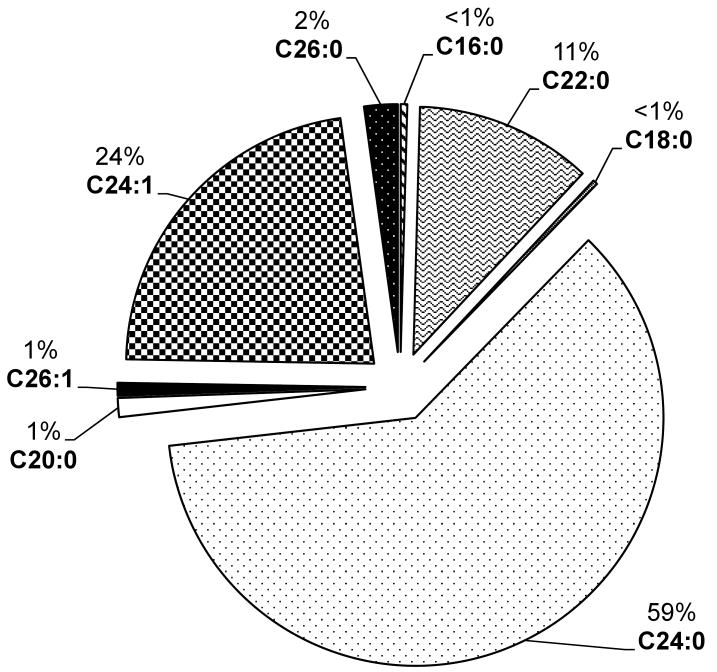
The major ceramide species detected in plasma were C24:0, C24:1 and C22:0. Other ceramides including C16:0, C18:0, C20:0, C26:0 and C26:1, were also detected in smaller concentrations (Fig. 1). The effect of several demographic and health-related factors (see those listed in Table 1) on plasma log ceramide levels at baseline were examined; none were found to be associated with the plasma ceramides at p<0.05.
Fig 1.
Percent ceramide species in plasma
3.2. Very long chain plasma ceramides are altered in MCI
Plasma log ceramides C22:0 (F=7.17, p=0.002), C24:0 (F=8.07, p=<0.001), and C26:0 (F=6.62, p=0.003) were significantly lower in the MCI group compared to both the NC and AD groups using ANOVA for overall group differences and t-tests with bonferroni correction for pairwise associations (Table 2). Mean Ceramides C16:0, C18:0 and C20:0, were also lower in the MCI group compared to the AD group, but not when compared to the NC group. There were no group differences for the unsaturated ceramides C24:1 and C26:1. For all analyses, controlling for variables including age, statin use, the APOE ε4 allele, or vascular conditions, did not did not alter any of the associations. As plasma log ceramides C22:0 and C24:0 were significantly lower in the MCI group compared to both NC and AD at p<0.01, all additional analyses focused on these two lipids.
Table 2. Group differences in plasma log ceramides.
| NC (n=25) Mean (SD) |
MCI (n=17) Mean (SD) |
AD (n=21) Mean (SD) |
F | p | MCI vs. NC | MCI vs. AD | NC vs. AD | |
|---|---|---|---|---|---|---|---|---|
| Ceramides | ||||||||
| C16:0 | 12.62 (0.26) | 12.43 (0.51) | 12.74 (0.30) | 3.51 | 0.036 | 0.334 | 0.031 | 0.705 |
| C18:0 | 12.25 (0.37) | 12.03 (0.59) | 12.47 (0.39) | 4.69 | 0.013 | 0.378 | 0.010 | 0.274 |
| C20:0 | 13.64 (0.28) | 13.44 (0.46) | 13.83 (0.30) | 5.84 | 0.005 | 0.228 | 0.003 | 0.211 |
| C22:0 | 15.94 (0.58) | 15.12 (1.18) | 16.03 (0.66) | 7.17 | 0.002 | 0.006 | 0.003 | 1.000 |
| C24:1 | 16.54 (0.22) | 16.49 (0.37) | 16.60 (0.21) | 0.85 | 0.434 | |||
| C24:0 | 17.66 (0.39) | 17.05 (0.80) | 17.71 (0.47) | 8.07 | <0.001 | 0.003 | 0.002 | 1.000 |
| C26:1 | 13.28 (0.26) | 13.16 (0.39) | 13.29 (0.28) | 1.11 | 0.337 | |||
| C26:0 | 14.25 (0.25) | 14.02 (0.35) | 13.34 (0.22) | 6.62 | 0.003 | 0.033 | 0.002 | 0.768 |
NC=Normal Control; MCI=Mild Cognitive Impairment; AD=Alzheimer's Disease, SD=Standard Deviation
All units are in log cycles per second (cps)
3.2.1. There are no cross-sectional associations between plasma levels of ceramides C22:0 and C24:0 and cognitive test performance or hippocampal volume
We first examined the association between plasma ceramides C22:0 and C24:0 and cognitive test performance on each individual test controlling for age and education. Including all groups, there were no associations between these plasma ceramides and any cognitive test. When stratifying by group, the only association was among NC with higher log ceramides C22:0 (b = -27.89, p = 0.003) and C24:0 (b = -41.19, p = 0.002) associated with better performance (time to completion) on Trails B controlling for age, education, and Trails A. We also examined the plasma ceramides and both the left and right hippocampal volume to ICV ratios and found no association at the p<0.05 level.
3.2.1. Baseline plasma log ceramides C22:0 and C24:0 predict cognitive decline and hippocampal volume loss
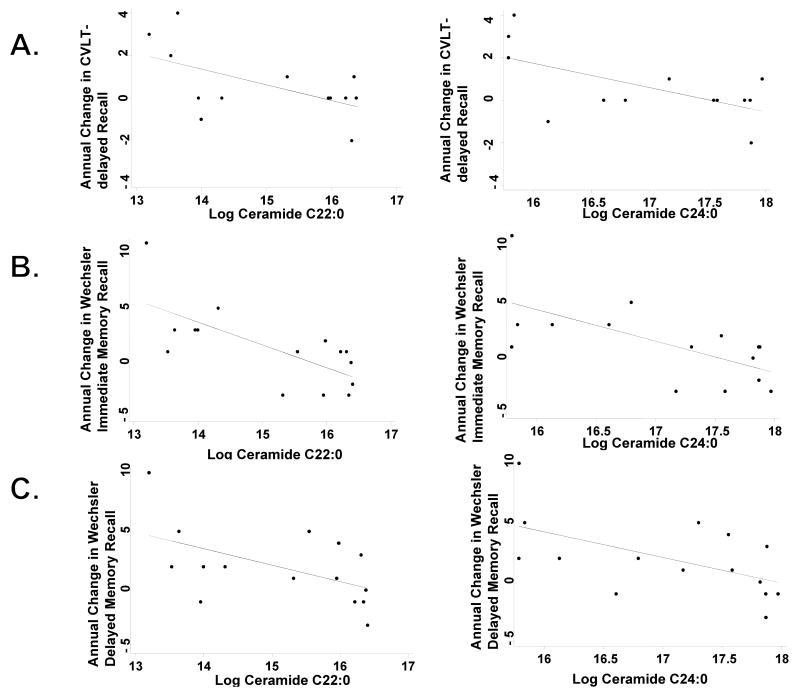
Using linear regression and controlling for age, education, and baseline cognitive test score, increasing log ceramides C22:0 and C24:0 among the MCI group, was associated with worsening performance on the MMSE, CVLT-long delayed free recall, Wechsler Logical Memory Immediate and Delayed Recall, and Trails A and Trails B (Table 3 and Fig. 2). While there were some trends for a relationship between baseline ceramides and cognitive change among NC and AD groups, these were not consistent and not significant at the p<0.05 level. Similarly, increasing baseline log ceramides C22:0 (b = -18.76, p = 0.041) and C24:0 (b = - 26.52, p = 0.055) were also associated with greater right hippocampal volume loss in the MCI group using linear regression and controlling for age and baseline hippocampal volume (Table 4). Again, there was no association between baseline ceramides and right hippocampal volume loss in the NC or AD groups. Ceramides did not predict left hippocampal volume loss in any group.
Table 3. Baseline plasma log ceramides predicting annual change in cognitive tests controlling for baseline age, education and cognitive test score.
| Baseline | Normal (n=25) | MCI (n=15) | AD (n=20) | |||
|---|---|---|---|---|---|---|
| Log Ceramides | b | p-value | b | p-value | b | p-value |
| CDR Sum of Boxes | ||||||
| C22:0 | 0.04 | 0.475 | 0.09 | 0.726 | -0.34 | 0.677 |
| C24:0 | 0.06 | 0.435 | 0.14 | 0.703 | -0.95 | 0.373 |
| MMSE | ||||||
| C22:0 | 0.60 | 0.055 | -0.71 | 0.165 | 0.07 | 0.971 |
| C24:0 | 0.79 | 0.091 | -1.27 | 0.080 | 0.03 | 0.990 |
| ADAS-COG | ||||||
| C22:0 | 0.42 | 0.527 | -0.16 | 0.750 | -3.04 | 0.421 |
| C24:0 | 0.07 | 0.467 | -0.27 | 0.708 | -3.39 | 0.503 |
| CVLT-Immediate Recall | ||||||
| C22:0 | 2.63 | 0.403 | -2.08 | 0.336 | 2.85 | 0.278 |
| C24:0 | 3.89 | 0.392 | -3.36 | 0.281 | 3.57 | 0.307 |
| CVLT-Long Delay Free Recall | ||||||
| C22:0 | 0.26 | 0.723 | -0.76 | 0.073 | 0.01 | 0.986 |
| C24:0 | 0.75 | 0.480 | -1.16 | 0.052 | -0.21 | 0.790 |
| Wechsler –Immediate Recall | ||||||
| C22:0 | -1.05 | 0.248 | -2.10 | 0.007 | -1.49 | 0.120 |
| C24:0 | -1.21 | 0.363 | -2.82 | 0.016 | -2.30 | 0.070 |
| Wechsler – Delayed Recall | ||||||
| C22:0 | -1.61 | 0.070 | -1.55 | 0.053 | 0.07 | 0.923 |
| C24:0 | -2.17 | 0.092 | -2.31 | 0.040 | 0.29 | 0.760 |
| TMT-A | ||||||
| C22:0 | 3.91 | 0.262 | 5.19 | 0.011 | 9.40 | 0.579 |
| C24:0 | 4.43 | 0.382 | 7.52 | 0.012 | -0.60 | 0.979 |
| TMT-B | ||||||
| C22:0 | 8.10 | 0.060 | 4.50 | 0.006 | 14.43 | 0.464 |
| C24:0 | 10.10 | 0.114 | 6.80 | 0.004 | 2.56 | 0.924 |
CVLT = California Verbal Learning Test; TMT = Trail Making Test, parts A (TMT-A) or B (TMT-B)
Fig. 2.
Correlations between baseline plasma log ceramides C22:0 and C24:0 and A) CVLT-delayed recall; B) Logical Memory Story A from the Wechsler Memory Scale, Immediate Recall; and C) Logical Memory Story A from the Wechsler Memory Scale, Delayed Recall
Table 4. Baseline plasma log ceramides predicting change in hippocampal volume, controlling for baseline age and hippocampal volume.
| Baseline | Normal (n=22) | MCI (n=15) | AD (n=17) | |||
|---|---|---|---|---|---|---|
| Log Ceramides | b | p-value | b | p-value | b | p-value |
| Left Hippocampus | ||||||
| C22:0 | -5.81 | 0.470 | -1.44 | 0.833 | -26.89 | 0.285 |
| C24:0 | -11.52 | 0.318 | -3.53 | 0.730 | -31.54 | 0.363 |
| Right Hippocampus | ||||||
| C22:0 | -4.06 | 0.286 | -18.76 | 0.041 | -4.66 | 0.884 |
| C24:0 | -5.76 | 0.289 | -26.52 | 0.055 | -7.01 | 0.869 |
4. Discussion
Findings from biochemical studies in brain and CSF suggest that perturbed sphingolipid metabolism may be an early event involved in the pathogenesis of AD [1,2,4,8]. In particular, levels of very-long chain ceramides are increased in brain early in the course of AD. A recent epidemiological study also suggests that serum levels of these ceramides are altered in individuals with memory impairment and may predict incident impairment [12]. In the present study of well-characterized patients, we provide additional evidence that plasma ceramides C22:0 and C24:0 are perturbed in amnestic MCI, considered the earliest stage of AD. While plasma ceramides did not appear to be cross-sectionally associated with cognitive performance or hippocampal volume, plasma ceramide levels in the MCI group were predictive of one-year change in cognitive test performance and hippocampal volume loss, suggesting that these blood-based lipids may predict changes of cognitive status that relate to hippocampal volume loss and be predictors of Alzheimer progression.
Biochemical studies using human autopsy brain tissues and CSF have demonstrated increased levels of very-long chain ceramides in the middle frontal gyrus and white matter of AD patients; some of which were observed in the earliest stage of AD (CDR=0.5) [1,2]. Consistent with these findings, gene expression patterns of enzymes participating in the sphingolipid metabolic pathway are also perturbed in mild AD and further suggests that disruptions of ceramide metabolism may be early pathogenic events in AD [11]. Increased levels of total ceramides measured in CSF of subjects with moderate, compared with mild or severe, AD has also been reported [4]. Based on these studies, we expected to find elevated plasma ceramides in both MCI and early AD. However, when we compared plasma ceramides across groups, the MCI group had lower mean plasma ceramide levels compared to both normal controls and AD patients. Notably, this finding is consistent with that observed in an epidemiological study in which low serum ceramides levels were cross-sectionally association with memory impairment [12]. An explanation for these findings was recently provided in a study of how plasma and CSF ceramides in HIV infected subjects was associated with cognitive function. In this study it was found that plasma ceramides showed an inverse relationship to CSF ceramides (Norman Haughey, personal communication). Thus, the lower plasma ceramide levels observed in the MCI group may be indicative of higher brain ceramide levels, which have consistently been found in previous AD studies. Ongoing research will further examine the plasma-CSF ceramide correlation in a group of normal control, MCI and AD patients to further understand the utility of plasma ceramides as a biomarker of AD progression.
One difficulty in developing blood-based biomarkers for AD is in interpreting the relationship between alterations in these biomarkers and neurodegenerative pathology. We therefore examined the cross-sectional and longitudinal predictive association between baseline ceramides and hippocampal atrophy, a well-known, and well-characterized neuropathological marker of AD. While there was not a cross-sectional association between the plasma ceramides and hippocampal volume, high levels of plasma ceramides were associated with greater right hippocampal atrophy among the MCI cases. We do not understand why there was laterality to this association (i.e. it was not also found on the left side). However, consistent longitudinal findings between high levels of plasma ceramides and declines on multiple tests of memory further suggest the involvement of the hippocampus is not simply due to chance.
In the present study, MCI had lower mean levels of all unsaturated ceramides compared to the AD group. However, findings were strongest for the saturated ceramides with 22 and 24-carbon chain lengths, such that mean levels were also lower compared to the NC group. Ceramides C22:0 and C24:0 are the most abundant in plasma (Fig. 1) and, as mentioned above, have previously been observed to be altered in brain tissue of AD patients. As the basic biological role of these differing chain lengths, and the importance of saturation is not understood, we currently do not have an explanation for our lack of results with the unsaturated compounds and future studies will need to focus on reasons for the observed specificity to saturated ceramides.
Based on the observed findings presented here, plasma ceramides do not appear to be good diagnostic biomarkers for separating MCI cases from normal controls or patients with AD. This is due to the clear overlap in ceramide levels between groups and because it is not possible to determine where, on the U-shaped curve, an individual is at the time o the blood draw. For example, among the MCI group, we do not know from the baseline plasma levels whether a person is in transition from NC to MCI or MCI to AD. Serial measures of plasma ceramides over time are needed to determine how ceramides change with disease progression and whether change in these lipids are a better predictor of progression than baseline levels. While the present findings are intriguing, additional research is clearly needed, especially in a study with a larger sample size and longer duration. Nonetheless, the potential identification of a blood-based biomarker that could be used as a routine procedure and be easily incorporated into patient care in either developed or developing counties, where most AD cases will occur in the next half-century, [31]would be highly advantageous.
Acknowledgments
This research was supported by the National Institute on Aging grants R21 AG028754 and 2P50AG005146-22A1, and by the National Institute of Neurological Disorders and Stroke grant R21NS060271-01 and grants from GlaxoSmithKline and the George and Cynthia Mitchell Fund.
Footnotes
Disclosure: All authors report no conflicts of interest. The corresponding author had full access to all the data in the study and had final responsibility of the decision to submit for publication.
Disclosure Statement: While funding for the neuroimaging and participant follow-up was partially obtained through a grant from GlaxoSmithKline, the authors had access to the data at all times and retain the data. Funding for the plasma lipids were obtained from NIH grants. All authors report no conflicts of interests with regards to GlaxoSmithKline or any other organization. All participants provided informed consent and the study was approved by the Johns Hopkins University Institutional Review Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, D MH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 3.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.010. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, et al. Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 5.Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 7.Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, et al. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 9.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer's disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochem. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielke MM, Kozauer NA, Chan KCG, George M, Toroney J, Zerrate M, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. NeuroImage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Alzheimers Association. Research consent for cognitively impaired adults: recommendations for institutional review boards and investigators. Alzheimer Dis Assoc Disord. 2004;18:171–175. doi: 10.1097/01.wad.0000137520.23370.56. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 2:S13–21. [PubMed] [Google Scholar]
- 20.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1988. [Google Scholar]
- 22.Reitan R. Trail Making Test: Manual for administration, scoring and interpretation. Indianapolis: Department of Neurology, Indiana University Medical Center; 1958. [Google Scholar]
- 23.Benton A, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- 24.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umeyama S. Least-squares estimation of transformation parameters between two point patterns. IEEE Trans Pattern Analysis and Machine Intelligence. 1991;13:376–380. [Google Scholar]
- 27.Joshi SC, Miller MI. Landmark matching via large deformation diffeomorphisms. Image Processing, IEEE Trans Image Process. 2000;9:1357–1370. doi: 10.1109/83.855431. [DOI] [PubMed] [Google Scholar]
- 28.Beg MF, Miller MI, Trouve A, Younes L. Computing Large Deformation Metric Mappings via Geodesic Flows of Diffeomorphisms. Int J Comput Vision. 2005;61:139–157. [Google Scholar]
- 29.Priebe CE, Marchette DJ. Alternating kernel and mixture density estimates. Comput Stat Data Anal. 2000;35:43–65. [Google Scholar]
- 30.Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]