Abstract
Introduction
Adipose derived stromal cells (ASCs) are a potential cell source for the successful healing of skeletal defects. In this study we sought to investigate the potential for cranial suture-derived mesenchymal cells (SMCs) to promote the osteogenic differentiation of ASCs. Various reports have previously examined the unique in vitro attributes of SMCs; this study sought to extend those findings.
Methods
SMCs were isolated from wild-type mice (N=30) from both the fusing posterofrontal (PF) and patent sagittal (SAG) sutures. Cells were placed in transwell inserts with human ASCs (N=5 patients) with osteogenic differentiation medium with or without rNoggin (10-400 ng/ml). Specific gene expression of osteogenic markers and Hedgehog pathway were assayed; standard osteogenic assays (alkaline phosphatase and Alizarin red staining) were performed. All assays were performed in triplicate.
Results
Both PF and SAG SMCs induced osteogenic differentiation of ASCs (*p<0.05). PF SMCs induced ASC-osteogenesis to a greater degree than SAG SMCs (*p<0.05). This was accompanied by an increase in BMP expression (*p<0.05). Finally, rNoggin mitigated the pro-osteogenic effects of co-culture accompanied by a reduction in Hedgehog signaling (*p<0.05).
Conclusions
SMCs secrete paracrine factors that induce osteodifferentiation of multipotent stromal cells (hASCs). Cells derived from the fusing PF suture do this to a significantly greater degree than cells from the patent SAG suture. Enhanced BMP and Hedgehog signaling may underlie this paracrine effect.
Keywords: Co-culture, Adipose-Derived Stem Cell, Skeletal Tissue Engineering, Skeletal Tissue Regeneration, Suture-derived mesenchyme, Cranial suture fusion, Cranial suture patency, Craniosynostosis
INTRODUCTION
Adipose-derived stromal cells (ASCs) are a promising cell source for use in skeletal regenerative medicine. ASCs maintain a capacity for differentiation along multiple mesodermal lineages, including osteogenic and chondrogenic differentiation (1-4). Moreover, ASCs are easily harvested with minimal morbidity, and easily expanded and maintained in vitro (5). Numerous factors have been observed to enhance the osteodifferentiation of ASCs, including soluble cytokines, mechanical stimuli, pulsed DC electrical fields and other factors (3, 6, 7). In contrast, the use of another cell type to drive osteogenic differentiation of ASCs via co-culture has not been explored in detail. In a candidate fashion, we chose to isolate and co-culture calvarial-derived cells whose osteogenic potential has been thoroughly investigated. Our fundamental question was whether one could enhance hASC osteogenesis via a co-culture system and secondarily what molecular mechanisms may underlie this phenomenon.
Our laboratory has specifically studied the murine skull as a paradigm of programmed suture fusion. The posterofrontal (PF) suture is the only suture in the mouse skull to undergo fusion, and does so in a tightly regulated fashion within the first weeks of life (8). This lies in contrast to the sagittal (SAG) suture, which remains patent throughout the animal’s lifetime. The focused and comparative study of these two sutures has elucidated differences between these two distinct sutures. First, growth and differentiation factors have been shown to be differentially expressed between fusing and patent cranial sutures. These include, but are not limited to, fibroblast growth factor (FGF) (9, 10), transforming growth factor (TGF)-β (11, 12), bone morphogenetic protein antagonists (specifically Noggin) (13), and Hedgehog signaling (14). Next, embryonic origins have been shown to have a cellular impact on the cranial vault and cranial suture biology (15). The PF suture is bounded on either side by frontal bone, of neural crest (NC) origin, while the SAG suture lies between paraxial mesoderm derived parietal bones (16). In contrast, both PF and SAG suture mesenchyme are of NC origin (16). Fundamental differences exist between bone forming cells derived from NC as compared mesodermal origin, including enhanced osteodifferentiation in NC derived skeletal cells (17). This has been shown both in the cranial (17) as well as appendicular skeleton (18). Furthermore, since Virchow first discussed the possibility, it has been discovered that mechanical force and cellular tension significantly differ between fusing and patent sutures (19-21). Thus, studies that surgically create intrauterine constraint, for example, produce craniosynostosis (22, 23). Finally, numerous studies have elucidated the importance of paracrine signaling in cranial suture biology (24-29). These studies have primarily focused on the role of paracrine signaling from suture-associated dura mater unto overlying suture mesenchyme (24, 25, 30). Reciprocal signaling derived from suture mesenchyme or alternatively from expanding osteogenic fronts may also play an important role in suture ossification and fusion (31).
In recent years our laboratory has utilized the isolation and culture of suture-derived mesenchymal cells (SMCs) to more thoroughly explore the cellular and molecular mechanisms of cranial suture fusion. SMCs isolated from the PF suture have been observed to have unique properties in vitro, including a robust capacity for osteogenic differentiation (10). Moreover, they elaborate significantly more osteogenic cytokines such as BMPs, FGFs and TGF-βs (10, 31). In fact, PF SMCs uniquely respond to various cytokines important in cranial suture biology, including bFGF as well as TGF-β1 (10, 12, 32). The paracrine effects of SMCs on other osteo-competent cells have not yet been studied. Thus, our study sought to enhance hASC osteogenesis via a co-culture system with SMCs. Our hypotheses were two fold: firstly that SMCs serve a paracrine function in vivo to regulate cranial bone ossification, thus in vitro they would likely elaborate pro-osteogenic paracrine factors that enhance hASC osteogenesis. Secondarily, we anticipated that SMCs derived from the fusing PF suture would enhance hASC osteogenesis to a greater degree than SMCs derived from the patent SAG suture. After verifying these findings, we sought to elucidate the molecular mechanisms that may underlie this paracrine interaction.
MATERIALS AND METHODS
Chemicals and supplies
Dulbecco’s Modified Eagles Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from GIBCO Life Technologies, (Carlsbad, CA). β-glycerophosphate and ascorbic acid were purchased from Sigma Aldrich, (St. Louis, MO). All cell culture wares were purchased from Corning Inc, (San Mateo, CA). Recombinant mouse Noggin was purchased from R&D Systems. Unless otherwise specified, all other chemicals were purchased from Sigma-Aldrich.
Tissue harvest and primary cell culture
Posterofrontal (PF) and sagittal (SAG) suture-derived mesenchymal cells (SMCs) were harvested from four-day-old CD-1 mice (N=30) (Charles River Laboratories, Wilmington, MA) as previously described (10). Sutures with 500-micron bony margins were dissected with great care to remove all dura mater and pericranial tissue. Ten sutures were plated per dish, so that in total three distinct populations of SMCs were derived from 30 calvaria. Human adipose-derived stromal cells were isolated from human lipoaspirate from five female patients under the age of 50, from the flank and thigh subcutaneous depots as previously described (33). Human ASCs were kept separate by patient, yielding 5 distinct cell populations. Cells were cultured in standard growth medium (SGM), containing DMEM, 10% FBS, and 100 IU/mL of penicillin/streptomycin and were maintained at 37 °C in an atmosphere of 5% CO2. SMCs of passage one only were utilized; ASCs of passage one and two only were utilized. Cells were passaged at subconfluency by trypsinization.
Proliferation and assessments
Proliferation of PF and SAG SMCs was assessed by bromodeoxyuridine (BrdU) incorporation as previously described (10). Briefly, cells were grown in 96 well plates with or without recombinant Noggin (10-200 ng/ml), seeded at a density of 1,000 cells / well. After 2 or 4d in culture, BrdU labeling was performed for a period of 8 hours. Next, BrdU incorporation was quantified using photometric ELISA (Roche Applied Science, Indianapolis, Ind.). Three distinct populations (10 mice per population) of PF and SAG SMCs were utilized, yielding 3 distinct data sets that were pooled for analysis.
Osteogenic differentiation and assessments
For osteogenic differentiation, SMCs were seeded on 12-well transwell inserts (0.22 um pore-size) at a density of 35,000 cells per insert. ASCs were seeded underneath in 12-well plates at a density of 50,000 cells per well. Upon attachment, transwell inserts were combined and medium was replenished with osteogenic differentiation medium (ODM). Alkaline phosphatase staining and quantification were performed as previously described (10) on day 3 in triplicate wells for each condition. Alizarin red staining and quantification were performed as previously described (10) on day 7 in triplicate wells for each condition. Specific gene expression was assayed by quantitative RT-PCR on days 3 and 7 (see below). For analysis of the effects of SMCs on hASCs, 3 distinct populations of PF and SAG SMCs were utilized, yielding 3 distinct data sets that were pooled for analysis (N=3). For the converse analysis of the effects of hASCs on SMCs, 5 distinct populations of hASCs from 5 different patients were utilized, yielding 5 distinct data sets that were pooled for analysis (N=5). For select experiments, rNoggin was added to ODM (100-400 ng/ml) and these cells were maintained for up to 7d in culture. For rNoggin experiments, a single representative PF SMC population and a single representative hASC population was shown (N=1), however experiments were performed in triplicate wells.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from cells as previously described (10). Isolation was performed with the RNeasy Mini Kit (Qiagen Sciences, Maryland) on days 3 and 7 of osteogenic differentiation. Reverse transcription was performed with Taqman Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System and Sybr Green PCR Master Mix (Applied Biosystems). Specific primers for the genes examined were designed based on their PrimerBank (http://pga.mgh.harvard.edu/primerbank/) sequence and are listed in Supplemental Table 1. The levels of gene expression were determined by normalizing to values of Gapdh and performed in triplicate wells.
Statistical analysis
Means and standard deviations were calculated from numerical data, as presented in the text, figures and figure legends. N=3 unless otherwise stated. In figures, bar graphs represent means, whereas error bars represent one standard deviation. Statistical analysis was performed using an ANOVA when multiple factors were being compared: in Fig. 2 a one-factor ANOVA was utilized (treatment group), in Fig. 3 a two factor ANOVA was used (cell type and treatment group), in Fig. 4 a two factor ANOVA was used (cell type and treatment group), in Fig. 5 a two factor ANOVA was used (Noggin treatment and co-culture treatment). In addition, a post-hoc Students’s two-tailed t-test was used with the Bonferroni standardization for multiple group testing. Inequality of standard deviations was either confirmed or excluded by the Levene’s test, in which p<0.05 was considered significant. For ANOVA, *p≤ 0.05 were considered to be significant. For specific post-hoc tests, the p value considered significant is presented in the figure legends.
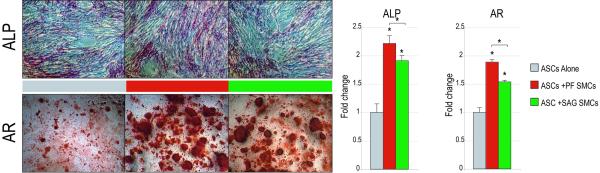
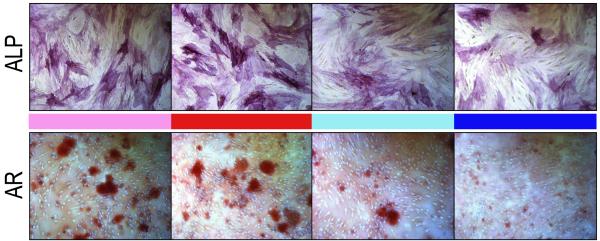
Figure 2. SMCs enhance the osteogenic differentiation of hASCs.
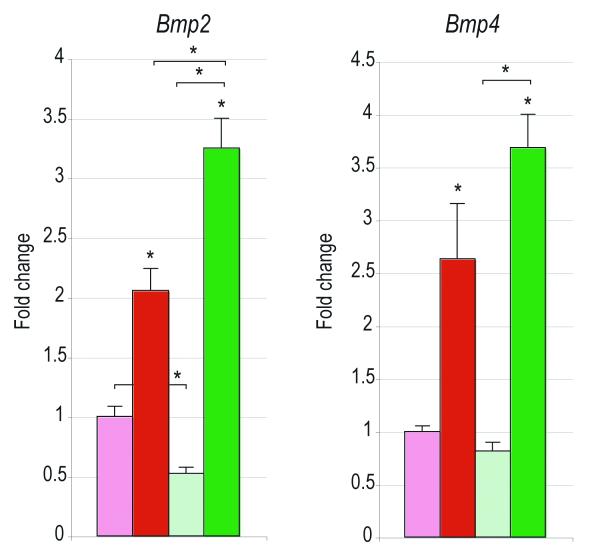
ASCs were cultured either alone (grey) or in co-culture with either PF (red) or SAG SMCs (green); osteogenic differentiation was performed. (A) Alkaline phosphatase (ALP) staining and quantification at 3d differentiation (ANOVA F=69.96, *p=0.0001, post-hoc ASC:ASC+PF SMCs *p=0.0005, ASC:ASC+SAG SMCs *p=0.0013). Alizarin red (AR) staining and quantification at 7d differentiation (ANOVA F=201.53, *p=0.0001, post-hoc ASC:ASC+PF SMCs, *p=0.0001, ASC:ASC+SAG SMCs, *p=0.0003). (B) From left to right, RUNX2, ALP, COL1A1, OPN expression at 7d differentiation as assessed by qRT-PCR. (RUNX2 ANOVA F=33.42, *p=0.0001; COL1A1 F=103.89, *p=0.0001; OPN ANOVA F=116.59, *p=0.0001) (C) From left to right, BMP2 and BMP4, expression at 7d differentiation as assessed by qRT-PCR (ANOVA F=106.71, 509.58, *p=0.0001, for BMP2 and BMP4, respectively). N=3 distinct SMC populations which yielded 3 distinct data sets that were pooled for analyses. Statistics were calculated by one-factor ANOVA (*p<0.05) followed by a post-hoc Student’s t-test with Bonferroni correction (*p<0.017).
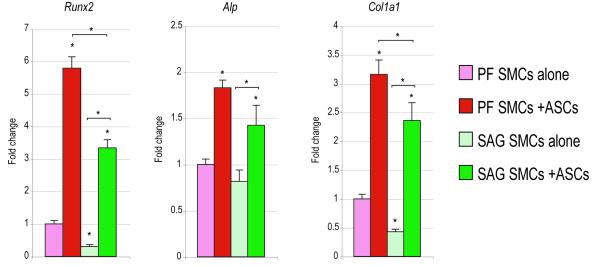
Figure 3. ASCs enhance the osteogenic differentiation of SMCs.
SMCs were cultured either alone (light red and green bars) or in co-culture with ASCs (dark red and green bars); osteogenic differentiation was performed. (A) Alkaline phosphatase (ALP) staining at 3d differentiation. Alizarin red (AR) staining and quantification at 7d differentiation. (ANOVA F=194.92, *p=0.0001; for post-hoc comparison of PF compared to PF:ASC, *p=0.0002; for post-hoc comparison of SAG alone compared to SAG:ASC, *p=0.0001) (B) From left to right, Runx2, Alp, Col1a1 expression at 7d differentiation as assessed by qRT-PCR. (ANOVA F=376.52, 35.53, 111.59, respectively, *p=0.0001 for all genes) (C) From left to right, Bmp2, Bmp4 expression at 7d differentiation as assessed by qRT-PCR. (F=l69.46, 60.06, *p=0.0001, 0.0001 for Bmp2 and Bmp4, respectively). N=5 distinct hASC populations from 5 patients, which yielded 5 distinct data sets that were pooled for analyses. Statistics were calculated by two-factor ANOVA followed by a post-hoc Student’s t-test with Bonferroni correction. For ANOVA *p<0.05 was considered significant, for post-hoc test *p<0.0083 was considered significant.
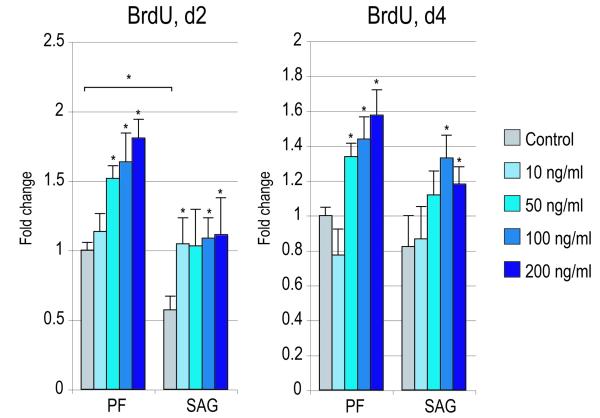
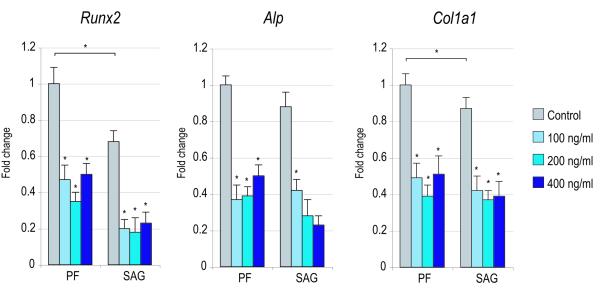
Figure 4. Effects of recombinant Noggin in SMCs.
SMCs were isolated from PF and SAG sutures and exposed to recombinant Noggin. (A) Cellular proliferation after 2 and 4d with or without rNoggin by BrdU incorporation (N=6) (for 2d BrdU ANOVA: F=41.43, 7.54, *p=0.0001, 0.0001 for PF and SAG SMCs respectively; for 4d BrdU ANOVA: F=51.75, 13.23, *p=0.0001, 0.0001, respectively). (B) Osteogenic gene expression after 3d with or without rNoggin by qRT-PCR (from left to right: Runx2, Alp, Col1a1). (ANOVA among PF SMCs: F=47.24, 69.89, 38.01, respectively, *p=0.0001 for all genes; ANOVA among SAG SMCs: F=42.65, 48.49, 33.34, respectively, *p=0.0001 for all genes) (C) Gene expression of members of the Hedgehog signaling pathway after 3d with or without rNoggin by qRT-PCR (from left to right: the Hedgehog ligand Indian Hedgehog (Ihh), the transmembrane receptor Patched1 (Ptch1), the zinc finger transcription factor Gli1, another member of the Gli family, Gli3 which functions to antagonize Hedgehog, and Rab23 a GTPase and Hedgehog antagonist). (ANOVA for PF SMCs, F=12.60, 4.67, 28.88, *p=0.002, 0.036, 0.0001, for Ihh, Ptc1, Gli1, respectively; ANOVA for SAG SMCs, F=12.05, 9.94, 11.39, *p=0.002, 0.004, 0.0001, respectively). Error bars represent one standard deviation; N=3 distinct SMC populations which yielded 3 distinct data sets that were pooled for analyses. Statistics were calculated by two-factor ANOVA followed by a post-hoc Welch’s t-test. For ANOVA *p<0.05 was considered significant, for post-hoc analysis *p<0.002 for 4A and *p<0.0031 for 4B,C.
Figure 5. Effects of rNoggin on co-culture osteogenic differentiation.
PF SMC:ASC co-cultures were next performed in the absence (red bars) or presence (blue bars) of recombinant mouse Noggin (200 ng/ml). (A) Alkaline phosphatase and Alizarin red staining at 3 and 7d differentiation, respectively. (B) ALP and AR quantification after 3 and 7d. (ANOVA F=35.59, 109.05, *p=0.0001, 0.0001, respectively; post-hoc with Bonferroni correction *p=0.0002, 0.0011, respectively). (C) After 3d, osteogenic gene expression was assayed by qRT-PCR as previously performed. From left to right: ALP, COL1A1, OCN, BMP2, BMP4, BMPR1B. (ANOVA F=49.48, 185.81, 30.17, 35.53, 31.05, 11.42 for ALP, COL1A1, OCN, BMP2, BMP4, BMPR1B, respectively; *p=0.0001 for all genes with the exception of BMPR1B *p=0.003) (for post hoc comparison of ASC+SMC:ASC+SMC+NOG, *p=0.0002, 0.0001, 0.0015, 0.0001, 0.0037, 0.0004, 0.0125, 0.019, for ALP, AR, ALP, COL1A1, OCN, BMP2, BMP4, BMPR1B, respectively). Error bars represent one standard deviation; N=1 representative SMC population and N=1 representative hASC population. Experiments were performed in triplicate wells (N=3) that yielded 3 distinct data sets that were pooled for analyses. Statistics were calculated by two-factor ANOVA followed by a post-hoc Student’s t-test with Bonferroni correction. For ANOVA, *p<0.05 was considered significant, for post-hoc comparisons *p<0.0083 was considered significant.
RESULTS
SMCs induce osteogenic differentiation of ASCs
First, we hypothesized that SMCs when placed in co-culture would enhance the osteogenic differentiation of hASCs (see Fig. 1 for method of co-culture). Alkaline phosphatase (ALP) staining and quantification was performed at 3d differentiation (Fig. 2A). Staining showed an increase in the number of ALP positive cells when ASCs were cultured in the presence of either PF or SAG SMCs. This was subsequently quantified, and showed an approximate 2-fold increase in ALP activity among ASCs when cultured in the presence of SMCs (*p<0.017). Alizarin red staining of bone nodules was performed at 7d differentiation, where calcified deposits appear red by microscopy. This revealed a similar enhancement of hASC osteogenesis via co-culture with either PF or SAG SMCs (Fig. 2A). Quantification by photometric analysis verified a significant increase in bone nodule formation upon hASC culture in the presence of SMCs (approximately 2- and 1.5-fold with PF and SAG SMCs, respectively, *p<0.017). Interestingly, SMCs derived from the fusing PF suture showed a greater degree of stimulation of hASC osteogenesis than those derived from the patent SAG suture (red as compared to green bars, Fig. 2A). This phenomenon was observed during both early (ALP staining) and later osteogenesis (Alizarin red staining) (*p<0.017).
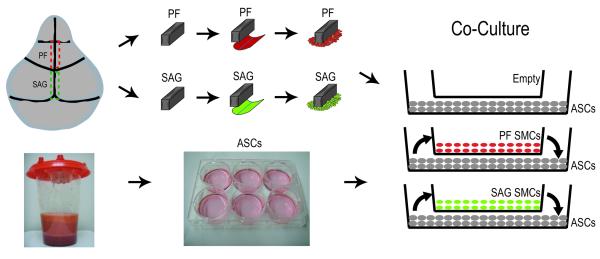
Figure 1. Suture-derived mesenchymal cell (SMC) and hASC harvest and co-culture.
Although contiguous, the posterofrontal (PF) suture lies anterior to the sagittal (SAG) suture, which lies posterior. SMCs were derived from both the fusing PF (red) and patent SAG (green) sutures. PF and SAG suture were microdissected with 500 micron bony margins, pericranium was removed from the ectocranial surface and dura mater was removed from the endocranial surface of sutures and SMC migration occurred over a period of one week in culture. Next, hASCs were obtained from human lipoaspirate by enzymatic digestion and centrifugation. Finally, using transwell inserts, co-culture assays were performed. SMCs were plated in transwell inserts, while ASCs were plated on the bottom well. Thus, soluble factors were allowed to diffuse between SMC and ASC populations and visa versa.
Next, osteogenic gene expression was assayed of hASCs cultured alone, hASCs cultured with PF SMCs or hASCs cultured with SAG SMCs after 7d differentiation (Fig. 2B,C). Overall, both PF and SAG SMCs were observed to have a pro-osteogenic effect on hASCs by qRT-PCR. This included a significant increase in the master osteogenic transcription factor Runt-related transcription factor-2 (RUNX2), as well as extracellular matrix components such as Type I Collagen (COL1A1), and Osteopontin (OPN) (Fig. 2B,C). PF SMCs induced expression of all markers in hASCs by approximately two- to four-fold (red bars, Fig. 2B,C) (*p<0.017). This effect was also significant in SAG SMCs for some genes, but the magnitude of increase was less as compared to PF SMCs (compare red and green bars, Fig. 2B,C) (post-hoc *p=0.0041, 0.0028, 0.0001, 0.0011 for RUNX2, ALP, COL1A1, OPN, respectively). In fact, SAG SMCs were observed to have a more variable effect on hASC gene expression (green bars Fig. 2B,C). No significant effect on COL1A1 expression was observed with SAG SMC co-culture (post-hoc *p=0.12), while RUNX2 and OPN were increased in expression upon SAG SMC co-culture with hASCs (Fig. 2B,C) (post-hoc *p=0.018, 0.0014, respectively). Thus in summary, PF SMCs showed a significant induction of osteogenic specific gene expression among hASCs when placed in co-culture. This increase in osteogenic differentiation was of less magnitude when SMCs from the patent SAG suture were co-cultured with hASCs.
Bone morphogenetic proteins (BMPs) have been demonstrated to be of significant importance in the osseous differentiation of ASCs. We examined the effects of co-culture of PF/SAG SMCs on BMP signaling in hASCs (Fig. 2C). Results showed an approximate 3- and 5-fold induction of BMP2 and BMP4 among hASCs when cultured in the presence of PF SMCs (red bars, Fig. 2C) (*p=0.017). In contrast, with SAG SMC co-culture, no increase in BMP2 transcript abundance was observed, and only a 2-fold induction of BMP4 was measured (green bars, Fig. 2C) (*p=0.017). Thus, PF SMCs when placed in a co-culture environment, induced hASC osteodifferentiation accompanied by increased BMP transcripts. In contrast, SMCs derived from the patent SAG suture enhanced osteogenesis and BMP expression to a lesser degree.
ASCs induce osteogenic differentiation of SMCs
Having observed the paracrine effects of SMCs on hASCs, the converse relationship was assayed. In the same co-culture environment (with SMCs placed in transwell insert atop of hASCs) the effects of hASCs on SMC osteodifferentiation and gene expression were next evaluated (Fig. 3). Results showed that in a similar fashion hASCs induced osteogenic differentiation of SMCs. This was observed across both SMC types (PF and SAG). An overall increase in the intensity of ALP and Alizarin red (AR) staining was observed (left, Fig. 3A). This induction of AR staining was quantified and yielded an approximate 60-100% increase across both PF and SAG SMCs (right, Fig. 3A) (*p<0.0083). Specific gene expression for osteogenic markers was next assayed by quantitative RT-PCR (Fig. 3B). All markers, including Runx2, Alp and Col1a1 were observed to be significantly increased in SMCs in the presence of hASCs (Fig. 3B) (*p<0.0083). Again, this was observed both among PF and SAG SMCs (red and green bars Fig. 3B) (*p<0.0083). Finally, as before, Bone morphogenetic protein (BMP) expression was evaluated, including Bmp2 and Bmp4 (Fig. 3C). As shown previously in ASCs, the expression of both BMP ligands was increased in the co-culture environment as compared to SMC culture alone (Fig. 3C) (*p<0.0083). Thus and in aggregate, a reciprocal relationship was observed between hASCs and SMCs in culture: both cell types were observed to enhance the osseous differentiation of the other cell type. This was in both cases accompanied by increased BMP expression.
Effects of Noggin on SMCs in vitro
In our co-culture model, increased BMP expression seemed to accompany the pro-osteogenic effect of each cell population on the other. Thus, we next asked whether the BMP antagonist Noggin could mitigate the pro-osteogenic effect of co-culture. First, the specific effects of Noggin were assayed in SMCs alone (Figure 4). Cellular proliferation was assayed by bromodeoxyuridine (BrdU) incorporation after 2 and 4d exposure to recombinant mouse Noggin (10-200 ng/mL) (Figure 4A). At concentrations of 50-200 ng/mL, rNoggin exposure increased BrdU incorporation in both PF and SAG SMC populations (*p<0.002). Next, osteogenic markers were assayed by qRT-PCR after 3d treatment with rNoggin (Figure 4B). As expected, exposure to the BMP antagonist significantly decreased expression of all markers examined (Runx2, Alp, Col1a1) (*p<0.0031). Thus exposure to rNoggin was observed to increase BrdU incorporation in SMCs while at the same timing repressing osteogenic gene expression.
We next inquired as to whether antagonism of BMP signaling via recombinant Noggin effected Hedgehog signaling in SMCs. Interestingly, rNoggin exposure resulted in global changes to Hedgehog expression. Indian Hedgehog (Ihh) and effectors of Hedgehog signaling including Patched1 (Ptch1) and Gli1 were reduced in transcript abundance by rNoggin (Figure 4C) (*p<0.0031). Conversely, Hedgehog antagonists were dramatically increased in expression by exposure to rNoggin, including Gli3 and Rab23 (*p<0.0031). Thus, rNoggin effected both PF and SAG SMCs similarly: in both cell populations rNoggin exposure increased cell proliferation at the expense of osteogenic gene expression, and decreased Hedgehog signaling.
Noggin inhibits the pro-osteogenic effect of SMC-ASC co-culture
Noggin was observed to repress SMC osteogenesis when cultured alone, we next hypothesized that rNoggin would likewise inhibit paracrine mediated osteogenesis onto hASCs. As already observed, co-culture of PF SMCs significantly enhanced hASC osteogenesis (Figure 5). Both the intensity of the ALP and Alizarin red staining was increased with SMC:ASC co-culture (Figure 5A). Quantification of ALP and AR activity likewise revealed a significant increase with co-culture (pink and red bars, Figure 5B) (*p<0.0083). Osteogenic gene expression revealed similar findings (Figure 5C, light red as compared to dark red bars). This included an approximate 1.5-4 fold induction in expression of all markers examined by 3 days differentiation (*p<0.0083). Next the same co-culture assay was performed in the presence of recombinant Noggin (200 ng/ml). This single concentration was chosen as it appeared to have the maximal inhibitory effect on expression of Runx2 in SMCs (Figure 4B). Notably, the pro-osteogenic effect of co-culture was decreased with the addition of rNoggin to culture medium (Figure 5A-C, blue bars) (*p<0.0083). For example, some gene markers such as BMP2 and BMP4 were indeed up-regulated in hASCs by co-culture, but to a much lesser degree in the presence of rNoggin (Figure 5, compare light blue to dark blue bars). In the presence of Noggin, SMCs decreased gene expression of ALP, and COL1A1 was appreciable. Thus, not only does Noggin have a significant and repressive effect on osteogenic differentiation in SMCs, recombinant Noggin substantially mitigated the pro-osteogenic effect we observed with co-culture.
DISCUSSION
The study of suture fusion in the mouse continues to reveal signaling pathways of importance in cranial suture biology (8). Our laboratory has specifically compared the posterofrontal (PF) suture and sagittal sutures as exemplars of physiologic suture fusion and patency, respectively (8). Our study of suture-derived mesenchymal cells (SMCs) has yielded insights into signaling pathways and known (10, 12, 32) and unknown importance (34) in suture biology. This study sought to further clarify the differences between PF and SAG SMCs in terms of their ability to induce osteogenic differentiation in a paracrine fashion on an osteo-competent cell population.
Not surprisingly, we observed that SMCs enhanced the osteogenic differentiation of human adipose-derived stromal cells. To some extent, an osteoprogenitor population (in this case SMCs) would be expected to stimulate an osteogenic response in an osteocapable cell population (in this case hASCs). These findings may have clinical relevance for the future use of ASCs for tissue engineering.
Interestingly, we observed that cells derived from the fusing PF suture (PF SMCs) stimulated ASC osteogenesis to a significantly greater degree than those derived from the patent SAG suture (SAG SMCs) by all markers examined. This observation is in agreement with our previous studies in which we observed PF SMCs to retain a more robust in vitro potential for osteogenic differentiation in comparison to SAG SMCs (10). Moreover, PF SMCs elaborate greater amounts of various potentially pro-osteogenic factors such as FGFs, TGF-β, and BMPs (10, 31, 32). This study, then, extends our knowledge concerning how PF and SAG SMCs differ in their fundamental in vitro biology. Cells derived from the PF suture retain a greater ability in vitro to stimulate osteogenesis of another cell type in a paracrine fashion. Moreover, in preliminary studies we have extended these observations to find that PF SMCs induce osteogenesis in calvarial-derived osteoblasts as well, and do this to a greater degree than SAG SMCs.
Does this in vitro observation when comparing PF and SAG SMCs have in vivo significance? The coordinated growth of calvarial bones has been postulated to rely on the controlled availability of pro-growth and pro-osteogenic factors (15). Paracrine factors secreted from dura mater have been shown to coordinate skull growth in an anatomic-specific fashion (24, 25, 30). For example, numerous growth factors have been identified by in situ and immunohistochemical analysis to reside in suture-associated dura mater during programmed suture fusion (29, 35-39). These findings have later been verified by qRT-PCR as well as microarray analysis (40-42). The paracrine relationship between dura and suture has been explored by co-culture assays similar to our own presented in the current study (25, 41, 43, 44). Moreover, the importance of this paracrine relationship has been verified by operative techniques which artificially manipulated the suture:dura interface (26, 45), or by viral vector-mediated manipulation of suture:dura signaling (11, 46). Just as PF and SAG associated dura mater exhibit intrinsic differences, so too have PF and SAG SMCs been documented to retain strikingly unique in vitro properties (10, 12, 32). Collectively, these data suggest that cranial suture fate (i.e. fusion versus patency) rests not solely with differences between underlying dura mater, but may part and parcel be explained by differences in mesenchymal cell properties. Alternatively, early paracrine stimulation by dura mater (i.e. at the embryonic stage) may still explain differences in SMCs derived from fusing and patent sutures.
BMP signaling is of central importance in bone biology and skeletogenesis, first recognized to stimulate ectopic bone formation (47). Although BMP ligand expression has not been observed to differ between physiologically fusing and patent sutures, the BMP antagonist Noggin is only present in patent murine sutures (13). Moreover, artificial manipulation of Noggin signaling has been observed to alter in vivo cranial suture fate (13). First, we found that BMP expression differs in PF and SAG SMCs: Bmp2 was more highly expressed in PF as compared to SAG SMCs when cultured alone. Moreover, previous studies in SMCs have also found differential BMP signaling between PF and SAG derived cells: while PF SMCs express higher levels of Bmpr1b, SAG SMCs express higher levels of BMP inhibitors, such as Bmp3 and Noggin (31). This led to the hypothesis that the disparity in PF as compared to SAG SMC induced osteogenesis lay in a difference in BMP signaling. Results showed, interestingly, that co-culture of SMCs with ASCs led to an increase in expression of BMP ligands in both cell types in comparison to either cell type when cultured alone. This lent further credence to the hypothesis that the pro-osteogenic effect of co-culture was owing to paracrine BMP signaling. This seemed to be correct, as the BMP antagonist Noggin blocked the pro-osteogenic effect of co-culture. These sets of experiments did not, however, prove that rNoggin addition to co-culture directly inhibited osteogenic differentiation by competitive inhibition of BMP signaling, or via a secondary signaling pathway. Noggin not only binds with equal avidity to BMP-2 and BMP-4 and competitively inhibits their interaction with the BMP receptor type 1A, ALK-3 (48). Noggin also inhibits the osteoclastogenic cytokine RANK-L (the receptor activator for NF-kB ligand). This suggests that Noggin is not only a direct inhibitor of osteoblast differentiation (by BMP antagonism), but also an indirect regulator of osteoclast formation (49). Moreover, recent studies have shown that Noggin binds to another BMP antagonist with high affintity (Sclerostin), and that these two BMP antagonists attenuate the activity of the other (50). Thus, Noggin is no doubt more pleotrophic than once realized. In this study, we found that Noggin significantly effects Hedgehog expression in both PF and SAG SMCs. It seems reasonable to speculate that other signaling pathways of importance in osteogenic differentiation may also be regulated by Noggin addition to medium, such as FGF, TGF-β, and Wnt signaling pathways. Thus, we speculate that other known osteogenic agents, such as Hedgehog family members (Sonic and Indian), in synergy with BMP exert the pro-osteogenic effects of co-culture.
Hedgehog signaling is of newly recognized importance in cranial suture biology. Hedgehog signaling is most well studied in cartilage biology, but also has significant functions in osteogenic differentiation. In numerous bone forming cells, Hedgehog signaling has been shown to promote osteogenic differentiation in vitro, including cell lines (MC3T3-E1, C310T1/2, KS483), primary osteoblasts, adipose-derived stromal cells, and bone marrow mesenchymal cells (51-56). Moreover, it is known that mice with genetic deficiency in Ihh have severe skeletal defects, including foreshortened limbs due to a defect in endochondral ossification (57). In addition, the Ihh −/− mouse has a cranial suture phenotype with a reduction in cranial dermal bone size, although this has not been fully studied (57). BMP and Hedgehog signaling interact in numerous organ systems and cell types to dictate phenotypic changes (23, 56, 58, 59). Patterns of hedgehog expression have been documented in animal cranial sutures (14, 23). Our data suggest an intriguing, potential relationship between Noggin and Hedgehog signaling in SMCs. Noggin was noted to significantly induce expression of Hedgehog antagonists (Gli3, Rab23), while reducing transcript abundance for members of Hedgehog signaling (Ihh, Gli1, Ptch1). One may wonder whether abnormal cranial suture patency via forced Noggin misexpression is via repression of BMP, and/or alternatively repression of Hedgehog signaling (13). More in vivo study is warranted to verify these speculations.
Several limitations do exist toward the broader generalization of these results to the application for regenerative medicine purposes. First, it did appear that experimental variability existed within our study. This can be best appreciated when comparing data between Figures 2 and Figures 5. For example, in Figure 2, alkaline phosphatase activity was increased approximately 110% upon PF SMC co-culture with hASCs, however in the additional set of experiments performed in Figure 5, ALP was increased only 30% above baseline. We believe this can be explained by variations in the baseline osteogenic differentiation of hASCs, which has been shown to vary from cell batch to batch. The baseline differences in the osteogenic differentiation of ASCs has been examined by our laboratory and others. Differences in age of the patient, gender, anatomic site of ASC derivation, passage number and freeze-thaw conditions have all been shown to create disparity in the osteogenic differentiation of ASCs (60-65). For our study, we isolated hASCs from five female patients. All patients were under 50 years of age and all hASCs were derived from the flank and thigh regions. All cells were passage one or two, and were passaged on sub-confluence. In this strategy, we attempted to minimize potential hASC heterogeneity. It does appear, however, that significant heterogeneity exists within the ASC population. This can be observed by clonal analysis, examination of cell surface markers or even on the structural level of electron microscopy (66-69). It may be that sorting ASCs by cell surface markers or transcriptional activity may narrow the heterogeneity of this multipotent stromal cell population and be of advantage for tissue engineering purposes.
CONCLUSION
Our study indicates that SMCs secrete paracrine factors that induce osteodifferentiation of multipotent mesenchymal stromal cells (hASCs). Interestingly, cells derived from the fusing PF suture do this to a greater degree than cells derived from the patent SAG suture.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grants R01 DE-13194, R21 DE-019274, the Hagey Laboratory for Pediatric Regenerative Medicine and the Oak Foundation to M.T.L.; National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302-01 to B.L.; Genentech Foundation Fellowship to A.W.J. We thank Y. Xu for her expert reading of the manuscript.
Footnotes
Financial Disclosure None of the authors of this manuscript have any commercial associations or financial disclosures that might pose or create a conflict of interest with information presented in the submitted manuscript PRS-D-09-02108R2. None of the authors have any associations, consultancies, stock ownership, or other equity interests, patent licensing arrangements, or payments for conducting or publicizing the study described in the manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Malladi P, Chiou M, et al. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue engineering. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 3.Malladi P, Xu Y, Yang GP, et al. Functions of Vitamin D, Retinoic Acid, and Dexamethasone on Mouse Adipose-Derived Mesenchymal Cells (AMCs) Tissue engineering. 2005 doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 4.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Current opinion in molecular therapeutics. 2005;7:300–305. [PubMed] [Google Scholar]
- 6.Xu Y, Hammerick KE, James AW, et al. Inhibition of Histone Deacetylase Activity in Reduced Oxygen Environment Enhances the Osteogenesis of Adipose-derived Stromal Cells. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammerick KE, James AW, Huang Z, et al. Pulsed DC Electric Fields enhance osteogenesis in adipose-derived stromal cells. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0267. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahar DE, Longaker MT, Quarto N. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev Biol. 2005;280:344–361. doi: 10.1016/j.ydbio.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald JA, Mehrara BJ, Spector JA, et al. In vivo modulation of FGF biological activity alters cranial suture fate. The American journal of pathology. 2001;158:441–452. doi: 10.1016/s0002-9440(10)63987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James AW, Xu Y, Wang R, et al. Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- 11.Mehrara BJ, Spector JA, Greenwald JA, et al. Adenovirus-mediated transmission of a dominant negative transforming growth factor-beta receptor inhibits in vitro mouse cranial suture fusion. Plast Reconstr Surg. 2002;110:506–514. doi: 10.1097/00006534-200208000-00022. [DOI] [PubMed] [Google Scholar]
- 12.James AW, Xu Y, Lee JK, et al. Differential effects of TGF-beta1 and TGF-beta3 on chondrogenesis in posterofrontal cranial suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2009;123:31–43. doi: 10.1097/PRS.0b013e3181904c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren SM, Brunet LJ, Harland RM, et al. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Rice DP, Kettunen PJ, et al. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 15.Lenton KA, Nacamuli RP, Wan DC, et al. Cranial suture biology. Current topics in developmental biology. 2005;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Iseki S, Maxson RE, et al. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Malladi P, Zhou D, et al. Molecular and cellular characterization of mouse calvarial osteoblasts derived from neural crest and paraxial mesoderm. Plast Reconstr Surg. 2007;120:1783–1795. doi: 10.1097/01.prs.0000279491.48283.51. [DOI] [PubMed] [Google Scholar]
- 18.Leucht P, Kim JB, Amasha R, et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- 19.Henderson JH, Longaker MT, Carter DR. Sutural bone deposition rate and strain magnitude during cranial development. Bone. 2004;34:271–280. doi: 10.1016/j.bone.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Hirukawa K, Miyazawa K, Maeda H, et al. Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Archives of oral biology. 2005;50:367–372. doi: 10.1016/j.archoralbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Peptan AI, Lopez A, Kopher RA, et al. Responses of intramembranous bone and sutures upon in vivo cyclic tensile and compressive loading. Bone. 2008;42:432–438. doi: 10.1016/j.bone.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschner RE, Gannon FH, Xu J, et al. Craniosynostosis and altered patterns of fetal TGF-beta expression induced by intrauterine constraint. Plast Reconstr Surg. 2002;109:2338–2346. doi: 10.1097/00006534-200206000-00028. discussion 2347-2354. [DOI] [PubMed] [Google Scholar]
- 23.Jacob S, Wu C, Freeman TA, et al. Expression of Indian Hedgehog, BMP-4 and Noggin in craniosynostosis induced by fetal constraint. Annals of plastic surgery. 2007;58:215–221. doi: 10.1097/01.sap.0000232833.41739.a5. [DOI] [PubMed] [Google Scholar]
- 24.Bradley JP, Levine JP, McCarthy JG, et al. Studies in cranial suture biology: regional dura mater determines in vitro cranial suture fusion. Plast Reconstr Surg. 1997;100:1091–1099. doi: 10.1097/00006534-199710000-00001. discussion; 1100-1092. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald JA, Mehrara BJ, Spector JA, et al. Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency. J Bone Miner Res. 2000;15:2413–2430. doi: 10.1359/jbmr.2000.15.12.2413. [DOI] [PubMed] [Google Scholar]
- 26.Levine JP, Bradley JP, Roth DA, et al. Studies in cranial suture biology: regional dura mater determines overlying suture biology. Plast Reconstr Surg. 1998;101:1441–1447. doi: 10.1097/00006534-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Spector JA, Greenwald JA, Warren SM, et al. Dura mater biology: autocrine and paracrine effects of fibroblast growth factor 2. Plast Reconstr Surg. 2002;109:645–654. doi: 10.1097/00006534-200202000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Opperman LA, Sweeney TM, Redmon J, et al. Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn. 1993;198:312–322. doi: 10.1002/aja.1001980408. [DOI] [PubMed] [Google Scholar]
- 29.Bradley JP, Han VK, Roth DA, et al. Increased IGF-I and IGF-II mRNA and IGF-I peptide in fusing rat cranial sutures suggest evidence for a paracrine role of insulin-like growth factors in suture fusion. Plast Reconstr Surg. 1999;104:129–138. [PubMed] [Google Scholar]
- 30.Bradley JP, Levine JP, Blewett C, et al. Studies in cranial suture biology: in vitro cranial suture fusion. Cleft Palate Craniofac J. 1996;33:150–156. doi: 10.1597/1545-1569_1996_033_0150_sicsbv_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Malladi P, Chiou M, et al. Isolation and characterization of posterofrontal/sagittal suture mesenchymal cells in vitro. Plast Reconstr Surg. 2007;119:819–829. doi: 10.1097/01.prs.0000255540.91987.a0. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, James AW, Longaker MT. Transforming Growth Factor-beta1 Stimulates Chondrogenic Differentiation of Posterofrontal Suture-Derived Mesenchymal Cells In Vitro. Plast Reconstr Surg. 2008;122:1649–1659. doi: 10.1097/PRS.0b013e31818cbf44. [DOI] [PubMed] [Google Scholar]
- 33.Quarto N, Wan DC, Longaker MT. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs) Bone. 2008 doi: 10.1016/j.bone.2008.01.026. Accepted. [DOI] [PubMed] [Google Scholar]
- 34.James AWT, Bruggman SAAA, Xu Y, Carre AL, Hamilton K, Korach KS, Longaker MT. Estrogen / Estrogen Receptor Alpha Signaling in Mouse Posterofrontal Cranial Suture Fusion. PLoS ONE. 2009 doi: 10.1371/journal.pone.0007120. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth DA, Longaker MT, McCarthy JG, et al. Studies in cranial suture biology: Part I. Increased immunoreactivity for TGF-beta isoforms (beta 1, beta 2, and beta 3) during rat cranial suture fusion. J Bone Miner Res. 1997;12:311–321. doi: 10.1359/jbmr.1997.12.3.311. [DOI] [PubMed] [Google Scholar]
- 36.Mehrara BJ, Steinbrech DS, Saadeh PB, et al. Expression of high-affinity receptors for TGF-beta during rat cranial suture fusion. Annals of plastic surgery. 1999;42:502–508. doi: 10.1097/00000637-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Mehrara BJ, Mackool RJ, McCarthy JG, et al. Immunolocalization of basic fibroblast growth factor and fibroblast growth factor receptor-1 and receptor-2 in rat cranial sutures. Plast Reconstr Surg. 1998;102:1805–1817. doi: 10.1097/00006534-199811000-00001. discussion 1818-1820. [DOI] [PubMed] [Google Scholar]
- 38.Most D, Levine JP, Chang J, et al. Studies in cranial suture biology: up-regulation of transforming growth factor-beta1 and basic fibroblast growth factor mRNA correlates with posterior frontal cranial suture fusion in the rat. Plast Reconstr Surg. 1998;101:1431–1440. doi: 10.1097/00006534-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Mehrara BJ, Most D, Chang J, et al. Basic fibroblast growth factor and transforming growth factor beta-1 expression in the developing dura mater correlates with calvarial bone formation. Plast Reconstr Surg. 1999;104:435–444. doi: 10.1097/00006534-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Nacamuli RP, Song HM, Fang TD, et al. Quantitative transcriptional analysis of fusing and nonfusing cranial suture complexes in mice. Plast Reconstr Surg. 2004;114:1818–1825. doi: 10.1097/01.prs.0000143578.41666.2c. [DOI] [PubMed] [Google Scholar]
- 41.Nacamuli RP, Fong KD, Lenton KA, et al. Expression and possible mechanisms of regulation of BMP3 in rat cranial sutures. Plast Reconstr Surg. 2005;116:1353–1362. doi: 10.1097/01.prs.0000182223.85978.34. [DOI] [PubMed] [Google Scholar]
- 42.Kwan MD, Wan DC, Wang Z, et al. Microarray analysis of the role of regional dura mater in cranial suture fate. Plast Reconstr Surg. 2008;122:389–399. doi: 10.1097/PRS.0b013e31817d6244. [DOI] [PubMed] [Google Scholar]
- 43.Warren SM, Greenwald JA, Nacamuli RP, et al. Regional dura mater differentially regulates osteoblast gene expression. J Craniofac Surg. 2003;14:363–370. doi: 10.1097/00001665-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Spector JA, Greenwald JA, Warren SM, et al. Co-culture of osteoblasts with immature dural cells causes an increased rate and degree of osteoblast differentiation. Plast Reconstr Surg. 2002;109:631–642. doi: 10.1097/00006534-200202000-00033. discussion 643-634. [DOI] [PubMed] [Google Scholar]
- 45.Roth DA, Bradley JP, Levine JP, et al. Studies in cranial suture biology: part II. Role of the dura in cranial suture fusion. Plast Reconstr Surg. 1996;97:693–699. doi: 10.1097/00006534-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Song HM, Fong KD, Nacamuli RP, et al. Mechanisms of murine cranial suture patency mediated by a dominant negative transforming growth factor-beta receptor adenovirus. Plast Reconstr Surg. 2004;113:1685–1697. doi: 10.1097/01.prs.0000117363.43699.5b. [DOI] [PubMed] [Google Scholar]
- 47.Wan M, Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651–657. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 48.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signaling inhibition by Noggin, a novel twelve-membered cystine knot protein. The Journal of bone and joint surgery. 2003;85-A(Suppl 3):52–58. doi: 10.2106/00004623-200300003-00010. [DOI] [PubMed] [Google Scholar]
- 49.Wu XB, Li Y, Schneider A, et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. The Journal of clinical investigation. 2003;112:924–934. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler DG, Yu C, Geoghegan JC, et al. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem. 2004;279:36293–36298. doi: 10.1074/jbc.M400521200. [DOI] [PubMed] [Google Scholar]
- 51.Spinella-Jaegle S, Rawadi G, Kawai S, et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 52.Jemtland R, Divieti P, Lee K, et al. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;32:611–620. doi: 10.1016/s8756-3282(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Aikawa T, Iwamoto-Enomoto M, et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 54.Edwards PC, Ruggiero S, Fantasia J, et al. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene therapy. 2005;12:75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- 55.van der Horst G, Farih-Sips H, Lowik CW, et al. Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone. 2003;33:899–910. doi: 10.1016/j.bone.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Yuasa T, Kataoka H, Kinto N, et al. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193:225–232. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- 57.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes & development. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abzhanov A, Rodda SJ, McMahon AP, et al. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- 59.Kawai S, Sugiura T. Characterization of human bone morphogenetic protein (BMP)-4 and -7 gene promoters: activation of BMP promoters by Gli, a sonic hedgehog mediator. Bone. 2001;29:54–61. doi: 10.1016/s8756-3282(01)00470-7. [DOI] [PubMed] [Google Scholar]
- 60.van Beek EA, Bakker AH, Kruyt PM, et al. Intra- and interindividual variation in gene expression in human adipose tissue. Pflugers Arch. 2007;453:851–861. doi: 10.1007/s00424-006-0164-4. [DOI] [PubMed] [Google Scholar]
- 61.Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Annals of plastic surgery. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 62.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Annals of plastic surgery. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levi B, James AW, Glotzbach J, et al. Depot specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2009 doi: 10.1097/PRS.0b013e3181e5f892. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 64.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 65.Thirumala S, Gimble JM, Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. Journal of tissue engineering and regenerative medicine. 2009 doi: 10.1002/term.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 67.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 68.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem cells (Dayton, Ohio) 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 69.Hattori H, Sato M, Masuoka K, et al. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2–12. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.