Abstract
Background & Aims
Gastrointestinal stromal tumors (GIST) are related to interstitial cells of Cajal (ICC) and often contain activating Kit or Pdgfra mutations. Inhibitors of Kit/Pdgfra signaling such as imatinib mesylate have increased progression-free survival in metastatic GIST but are not curative. In mouse models we investigated whether Kitlow adult ICC progenitors could represent an inherently Kit/Pdgfra inhibitor-resistant reservoir for GIST.
Methods
KitlowCd44+Cd34+ cells were isolated and characterized after serial clonal re-derivation. Tumorigenic potential of spontaneously transformed cells was investigated in nude mice. The KitlowCd44+Cd34+ cells responsiveness to Kit activation and blockade was studied by enumerating them in KitK641E mice (a GIST model), in mice with defective Kit signaling, and pharmacologically.
Results
Single isolated KitlowCd44+Cd34+ cells were clonogenic and capable of self-renewal and differentiation into ICC. In nude mice, spontaneously transformed cells formed malignant tumors expressing GIST markers. The KitlowCd44+Cd34+ cells were resistant to in vitro Kit blockade, including by imatinib, and occurred in normal numbers in mice with reduced Kit signaling. In KitK641E mice, the mutant ICC stem cells were grossly hyperplastic but remained imatinib-resistant. In contrast, the cancer-initiating cell-targeting drug salinomycin blocked the proliferation of KitlowCd44+Cd34+ cells and increased their sensitivity to imatinib.
Conclusions
KitlowCd44+Cd34+ progenitors are true stem cells for normal and hyperplastic ICC and give rise to GIST. Resistance to Kit/Pdgfra inhibitors is inherent in GIST and is due to the native ICC stem cells lack of dependence on Kit for survival, which is maintained after the acquisition of oncogenic Kit mutation. Cancer stem cell drugs may target these cells.
Keywords: Cancer-initiating cell, imatinib, neoplasm
INTRODUCTION
Gastrointestinal stromal tumors (GIST), the most common gastrointestinal sarcomas1, originate from the lineage of interstitial cells of Cajal (ICC).2 ICC regulate gastrointestinal motility.3 The majority of GIST (75–80%) have activating mutations in the receptor tyrosine kinase Kit,2 the receptor for stem cell factor (SCF or Kit ligand; Kitl) and an obligatory differentiation and survival factor for ICC.4,5 Approximately 50% of GIST relapse or metastasize after resection.6,7 Treatments with tyrosine kinase inhibitors such as imatinib mesylate have substantially increased progression-free survival after resection of localized primary GIST and remain the mainstay of treatment for metastatic GIST.7 However, they are not curative and must be maintained indefinitely to prevent progression, which often leads to drug resistance.1 This has led to the hypothesis that GIST “tumor stem cells” exist that are not dependent on Kit, and, therefore, cannot be eradicated by Kit blockade alone.1,8
Recently we identified progenitors for ICC in the postnatal murine stomach9. These rare cells do not resemble ICC and express as little as one-tenth as much Kit on their surface (Kitlow). They also express Cd44, which occurs on ICC,9 mesenchymal stem cells10 and some cancer stem cells;11 Cd34, which is found in 60–70% of GIST,1 and receptors for insulin (Insr) and insulin-like growth factor-I (IGF-I; Igf1r). They differentiate into intermediate, Kit+Cd44+Cd34+ Insr−Igf1r− cells with ICC morphology and then into mature, Kit+Cd44+Cd34−Insr−Igf1r−ICC in response to membrane-bound SCF produced under the control of insulin and IGF-I,9,12 an action not shared by secreted SCF.9 In contrast, the proliferation of the ICC progenitors can be directly stimulated with secreted SCF or IGF-I.9 Thus, Kit signaling may be required for the differentiation of ICC progenitors but not for their survival. Therefore, we hypothesized that these cells could be the precursors of GIST and that they would be resistant to Kit inhibitors.
METHODS
Animals
Experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols were IACUC-approved. KitK641E:Neo/K641E:Neo (KitK641E) mice,13 KitK641E/+ mice lacking the neomycin resistance cassette (see Supplemental Methods) and wild-type littermates were from our breeding programs. NOD/LtJ, W/Wv, Sl/Sld and C57BL/6J mice were from The Jackson Laboratory. BALB/c and H-2Kb-tsA58 breeders were from Harlan and Charles River Laboratories, respectively. Athymic NCr-nu/nu mice were from NCI-Frederick.
Isolation and characterization of ICC progenitors
ICC progenitors were first isolated by identifying a KitlowCd34+ clone by flow cytometry and RT-PCR in low-density primary cultures of Kit+ cells obtained by immunomagnetic enrichment (MACS) from gastric muscles of 6 homozygous, 14-day-old H-2Kb-tsA58 transgenic mice (Immortomice) harboring the temperature-sensitive SV40 large T antigen (tsTAg) (clone D22; see Supplemental Methods, Table S1). Cells were maintained in Clonetics® Smooth Muscle Growth Medium-2 (SmGM-2 , Lonza) under conditions permissive for the tsTAg (33°C, 100 U/mL interferon-γ [IFNγ]) and further purified by MACS and fluorescence-activated cell sorting (FACS; Beckman Coulter EPICS Elite).12 The KitlowCd44+Cd34+ phenotype of the purified line (D2211B) was verified by flow cytometry (Becton Dickinson LSR II; Supplemental Table S2). 2xSCS70 cells were derived from single, propidium iodide (PI; Invitrogen; 10 μg/mL)-excluding D2211B cells by serial cloning by FACS (2 cycles).
Wild-type ICC progenitors (2xSCS2F10) were prospectively isolated by serial FACS-cloning of PI− cells (2 cycles) from primary cell cultures of gastric muscles of five 7-day-old C57BL6/J mice. Before sorting, ICC progenitors in these primary cultures were enriched by extensive culturing (150 days) with media previously found to support the growth of 2xSCS70 cells in the absence of the tsTAg (NeuroCult® Neural Stem Cell Proliferation Medium [NSCPM; STEMCELL Technologies]; Supplemental Figure S1) plus 5% cell-free chick embryo extract (CEE; Accurate Chemical). The KitlowCd44+Cd34+ phenotype of the clonally-derived cells was verified by flow cytometry.
For proliferation studies, PI-excluding cells were either sorted directly into multi-well plates by FACS or their numbers for plating were determined by flow cytometry using fluorescent beads.14 Treated cells were enumerated by flow cytometry or by colorimetry (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega).
Protein and mRNA expresssion
Whole-mounts of gastric and small intestinal muscles, cultured cells and cryosections of tumor samples were immunolabeled and imaged by published techniques9,12,15 (Supplemental Methods, Table S3). Flow cytometry was performed by using established techniques with minor modifications9,12,14 (Supplemental Methods, Table S4, Figure S2). Western immunoblotting and RT-PCR were performed by standard techniques (see Supplemental Methods, Table S5).
Statistical analyses
Data are expressed as means±standard error or median[interquartile range] and analyzed by Student s t test, ANOVA (one- or two-way; with all-pairwise multiple comparisons) or nonparametric alternatives. P<0.05 was considered significant.
RESULTS
Conditionally immortalized cells (D22 and D2211B) were clonally derived from gastric KitlowCd34+ ICC precursors9 of juvenile Immortomice. They had fibroblast-like morphology and expressed very little Kit under conditions permissive for the tsTAg. After heat-inactivation of the tsTAg (nonpermissive condition: 39.5 °C, no IFNγ; Supplemental Figure S1A-C), proliferation was reduced and a subset of cells differentiated into network-forming, bipolar or multipolar cells with ICC-like morphology (Figure 1A). This heterogeneity persisted in a sub-line twice clonally re-derived from a single cell (2xSCS70). Culturing in NeuroCult® Neural Stem Cell Proliferation Medium (NSCPM; STEMCELL Technologies) permitted the maintenance of these cells independent of the tsTAg (Supplemental Figure S1D) for an estimated number of 180 population doublings without compromising their ability to differentiate. Immunocytochemistry of this clone performed under nonpermissive conditions in four different generations showed that the morphologically differentiated cells expressed cell surface Kit and thus were identified as bona fide ICC, whereas the remaining undifferentiated cells expressed very little Kit (Figure 1B). These observations indicated that the isolated KitlowCd34+ precursors were clonogenic and self-renewing while giving rise to ICC. Therefore, the ICC progenitors can be operationally defined as ICC stem cells.
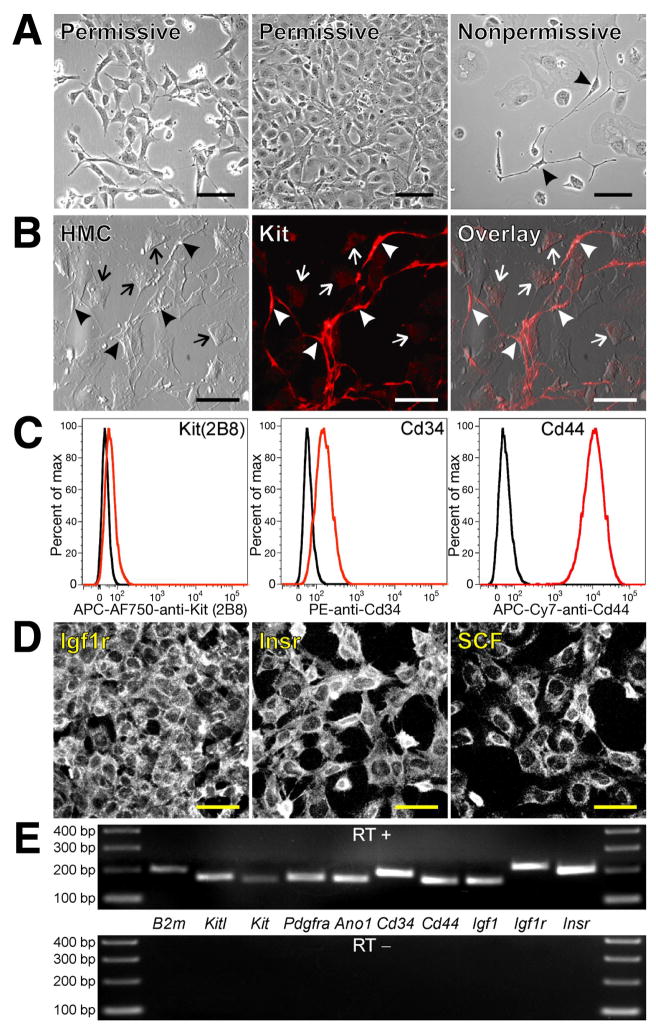
Figure 1. Clonally-derived, conditionally immortalized ICC progenitors maintain their undifferentiated phenotype in culture and give rise to ICC.
(A) Morphology of D2211B cells grown under permissive or nonpermissive conditions. Arrowheads mark ICC-like cells. Scale bars, 100 μm. (B) Cell surface expression of Kit in the clonally-derived sub-line 2xSCS70; nonpermissive conditions; representative of 14 cultures from 4 passages). Scale bars, 50 μm. Arrowheads mark Kitbright ICC-like cells. Undifferentiated cells remained Kitlow (arrows). HMC, Hoffman modulation contrast. (C) Flow cytometry of proliferating D2211B cells. Black histograms show isotype controls. APC-AF750, allophycocyanin-Alexa Fluor 750 tandem conjugate; PE, phycoerythrin; APC-Cy7, allophycocyanin-cyanine 7 tandem conjugate. Isolated ICC progenitors maintained a KitlowCd34+Cd44+ phenotype. (D) Surface immunolabeling of D2211B cells under permissive conditions. Scale bars, 50 μm. (E) RT-PCR analysis of D2211B cells grown under permissive conditions. The reverse transcriptase was omitted from the control experiment (RT−). B2m, beta-2 microglobulin used as reference. Each reaction is a representative of at least two experiments performed in different passages.
Flow cytometry, immunocytochemistry and RT-PCR showed that the isolated undifferentiated ICC progenitors retained the full phenotype of native gastric ICC progenitors (KitlowCd44+Cd34+Insr+Igf1r+ and Cd45−F4/80−Cd11b−Cd31−)9 and also expressed the novel ICC and GIST marker anoctamin 1 (Ano1)15–17 (Figure 1C-E). They also expressed Kitl/SCF and mRNA for IGF-I (Igf1), i.e., growth factors that promote proliferation of GIST cells by autocrine mechanisms18,19 and mRNA for platelet-derived growth factor α (Pdgfra), an alternative target of oncogenic mutations in GIST.20
Using the media that permitted the maintenance of the Immortomouse-derived ICC stem cells in the absence of the tsTAg, we also isolated from long-term primary cultures of gastric muscles of juvenile C57BL/6J mice a clonogenic cell line (2xSCS2F10) by repeat single-cell sorting. 2xSCS2F10 cells had the same features as the Immortomouse-derived cells including a KitlowCd44+Cd34+ phenotype, expression of Ano1, Kitl, Pdgfra, Insr and Igf1r and the ability to differentiate into Kit+Cd44+Cd34− ICC after an estimated 166 population doublings (Supplemental Figure 3). This finding provides additional support to the existence of KitlowCd44+Cd34+ ICC precursors in the gastric musculature.
We tested the in vivo differentiation potential of the isolated ICC stem cells in an allogeneic transplantation model by injecting 31–34 million 2xSCS70 cells intraperitoneally into 4 adult, 14-day diabetic NOD/LtJ mice, a model of ICC injury.12,21 Two vehicle-injected mice served as controls. The transplanted cells did not affect the progression of diabetes and 2 mice died 30–32 days after the onset of diabetes. In the surviving animals, the injected cells fate was tracked by detecting the major histocompatibility complex (MHC) class I protein H-2Kb, which is expressed by the 2xSCS70 cells, possibly because of the previous exposure to IFNγ (Figure 2A), but not by the host (H-2Kd). 20 days after injection, numerous undifferentiated, Kitlow donor cells were found in the serosa, mainly in the lesser curvature (Figure 2B). In this region we also detected occasional H-2Kb+Kit+ cells with ICC-like morphology that did not incorporate into the host s ICC network (Figure 2C). In addition, both mice also had areas where H-2Kb+ cells extensively incorporated into myenteric and intramuscular ICC networks (Figure 2D, Supplemental Figure S4). Overall, incorporation was significant but highly variable (Figure 2E). While the immunodetection system used caused nonspecific staining of capillaries, macrophages and some Kit− fibroblast-like cells, no staining of Kit+ ICC was seen in the sham-injected controls (Figure 2F and Supplemental Figure S4). Thus, isolated ICC stem cells can also differentiate into ICC in vivo and, similarly to mesenchymal stem cells, likely possess immunosuppressive properties.10
Figure 2. Isolated, clonally-derived ICC stem cells transplanted into diabetic NOD/LtJ mice differentiate into ICC.
(A) Expression of H-2Kb detected by flow cytometry in 2xSCS70 cells. PE-Cy5-SA, streptavidin coupled with PE-Cy5 tandem conjugate. Black histograms show controls stained with PE-Cy5-SA only. (B) Undifferentiated KitlowH-2Kb+ donor cells (arrowheads) on the serosal surface of the lesser curvature of the stomach of a transplanted mouse. All ICC (arrows) in this region were from the host. (C) An H-2Kb+Kit+ dividing cell with prominent ICC-like features in the same mouse. (D) Extensive incorporation of H-2Kb+ donor cells into Kit+ ICC networks in the distal corpus. Asterisks mark nonspecifically stained capillaries. (E) Quantitative estimate of colocalization of Kit and H-2Kb immunoreactivities in cell- and sham-transplanted mice. Overlap of red and green pixels was normalized to the number of green (Kit+) pixels in each confocal stack following equalization and binarization of the images (see Supplemental Methods). (F) Nonspecific staining of capillaries (asterisks) by mouse anti-H-2Kb monoclonal antibody and AF594-anti-mouse IgG in a sham-transplanted NOD/LtJ mouse. Background staining in the H-2Kb image was gamma-enhanced to demonstrate lack of nonspecific staining of Kit+ ICC. Scale bars are 50 μm.
After long-term culturing in NSCPM, 2xSCS70 cells (maintained under nonpermissive conditions) and 2xSCS2F10 cells underwent spontaneous transformation as evidenced by reduced dependence on growth factors and acquisition of abnormal karyotype (Supplemental Figure S5). To test their tumorigenic potential, 5×106 cells suspended in growth factor-reduced Matrigel (Becton Dickinson) were grafted subcutaneously into 6 athymic NCr-nu/nu mice. Mice injected with non-transformed, diploid cells (D22) or LX-2 human hepatic stellate cells (n=3/group) served as negative controls. Two mice injected with L3.6pl human pancreatic cancer cells were used as positive controls. Transformed 2xSCS70 cells formed large tumors in 43–57 days (Figure 3, Supplemental Figure S6), whereas L3.6pl yielded similarly sized tumors in only 15 days. 2xSCS2F10 cells formed much smaller tumors in 83 days (Supplemental Figure S7). No tumors were detected in D22- or LX-2-injected mice 118–139 and 83 days after grafting, respectively. The lesions appeared undifferentiated, consisting of sheets of round or spindle-shaped cells with enlarged nuclei, variable numbers of prominent nucleoli, irregular nuclear membranes and a moderate amount of amphophilic cytoplasm. Mitotic figures, including atypical ones, were numerous and there were necrotic foci. The lesions infiltrated diffusely into the surrounding tissues. Most cells displayed low-to-moderate expression of both Kit and Ano1 relative to host tissues in the vicinity of the tumors or control sections. All tumors also contained numerous cells with strong Kit expression that either had bipolar, ICC-like morphology or were round or oval-shaped. Both strongly Kit+ cell types also expressed Ano1 indicating that they were not mast cells of the hosts.15 Thus, transformed ICC stem cells can form malignant, Kit- and Ano1-positive solid tumors that also contain differentiated ICC indicating they are GIST precursors.
Figure 3. Spontaneously transformed ICC stem cells transplanted into NCr-nu/nu mice give rise to malignant GIST-like tumors.
(A) Tumor formed in a 2xSCS70-transplanted mouse 43 days after grafting. Scales are in centimeters. (B) Hematoxylin-eosin. Magnification is 400x. (C) Kit immunoreactivity (red; blue: DAPI) in tumors detected with a rabbit polyclonal antibody. The third panel from left is an enlargement of the area outlined in the preceding panel. Arrowhead indicates a bipolar, ICC-like cell. Arrows show dividing ICC-like cells. (D) Secondary-antibody-only control for C and F. (E) Kit immunoreactivity detected with rat monoclonal antibody 2B8. Right panel shows donor skin tissue lacking Kit immunostaining (asterisk) in the vicinity of the tumor as control. (F) Ano1 immunoreactivity. Middle panel is an enlargement of the area outlined in the left panel. Arrowheads indicate bipolar, ICC-like cells. Right panel shows Ano1+ tumor cells infiltrating host skeletal muscle (asterisks). White and yellow scale bars are 50 μm and 12.5 μm, respectively. Note moderate, generalized Kit and Ano1 immunostaining and high Kit and Ano1 expression in ICC-like cells and in some round cells.
We investigated the dependence of ICC stem cells/GIST precursors on Kit signaling for proliferation in vitro. Under nonpermissive conditions, the proliferation of D2211B cells could be stimulated with SCF, insulin, and IGF-I, i.e., factors important for the in situ maintenance of ICC progenitors9 (Figure 4A). ACK2, a neutralizing monoclonal anti-Kit antibody and the most effective blocker of ICC survival,4,5,22 failed to inhibit their basal proliferation and treatment with an anti-SCF antibody was also ineffective. However, both ACK2 and anti-SCF partially inhibited the IGF-I-induced proliferation (Figure 4B), suggesting a role for an IGF-I-Igf1r-SCF-Kit autocrine loop.12,18,19 Strikingly, imatinib mesylate, applied at two different concentrations, neither blocked the proliferation of the D2211B cells nor had a cytocidal effect under nonpermissive conditions (Figure 4C) and also failed to prevent their exponential growth under permissive conditions although the proliferation rate was somewhat reduced by the lower dose. This reflected a cytostatic, rather than cytocidal effect since there was no proportional increase in PI+ dead cells. The cytotoxic effects of ACK2, anti-SCF and imatinib on differentiated ICC were verified in organotypic cultures of intact jejunal muscles from newborn BALB/c mice, where they all reduced the density of ICC networks as expected12,22 (Figure 4D, Supplemental Figure S8). Thus, inhibitors of SCF/Kit signaling that had been shown to deplete ICC12,22 and block GIST proliferation and survival19,23 were not cytocidal to isolated ICC stem cells and had only minor effects on their proliferation, indicating Kit independence.
Figure 4. ICC stem cells do not depend on SCF/Kit signaling for proliferation and maintenance.
(A,B) D2211B cells were maintained with 5% fetal bovine serum (FBS) but without insulin for 6–10 days under the conditions indicated. Low FBS: 0.1%. End-point counts (mean+/− SEM) of PI-excluding (live) cells determined by flow cytometry are shown. Red horizontal lines indicate starting cell numbers. ACK2 (monoclonal anti-Kit antibody): 5 μg/mL; recombinant murine (rm)-SCF (Sigma): 20 ng/mL; bovine insulin (Invitrogen): 5 μg/mL, recombinant human IGF-I (Sigma): 100 ng/mL; polyclonal anti-SCF antibodies:12 2 μg/mL. Note lack of effect of ACK2 and anti-SCF on basal proliferation and partial inhibition of IGF-I-induced growth. (C) Effects of imatinib mesylate. Solid lines, PI− cells; dashed lines, PI+ (dead) cells. Note modest cytostatic effect under permissive conditions and lack of a cytocidal effect. Imatinib may have protected the cells from the effects of inactivating the immortalizing antigen. Groups not sharing the same label were different by post-hoc multiple comparisons. (D) Kit+ ICC in jejunal muscles from newborn BALB/c mice cultured for 3 days in the absence/presence of established inhibitors of SCF/Kit signaling. Representative confocal images collected from 2–3 tissues/group are shown. Scale bars, 50 μm. Inhibiton of SCF/Kit signaling resulted in the loss of differentiated ICC; order of efficacy: ACK2>imatinib 0.2 μM> imatinib 1.0 μM≫anti-SCF.
Next, we investigated the effects of persistent activation and inhibition of SCF/Kit signaling on ICC and their precursors in vivo. We studied the gastric corpus and antrum of mice homozygous for the activating, oncogenic mutation KitK641E, a model of human familial GIST.13 Because many of these mice die from intestinal obstruction as early as 3 weeks of age, the experiments were performed between days 14–23. We also studied adult W/Wv and Sl/Sld mice, which harbor hypomorphic mutations of Kit and Kitl, respectively. Consistent with a previous report,13 KitK641E mice had marked myenteric ICC hyperplasia detected by immunostaining for both Kit and Ano1 and a paradoxical depletion of intramuscular ICC (Supplemental Figure S9A-E). The latter is a previously unrecognized manifestation in these mutants of a paradoxical loss-of-Kit-function phenotype in a subset of Kit-expressing cells.13 W/Wv and Sl/Sld mice had reduced ICC as expected4 (Supplemental Figure S9F). ICC stem cells in these tissues could be identified by previously established criteria (see above and in ref.9). Similarly to ICC, they also uniformly expressed Ano1 (Figure 5) which allowed their easy distinction from rare Kit+Ano1− mast cells with similar morphology.15 In KitK641E mice, these cells were grossly hyperplastic throughout the stomach (Figure 5C,D), and, most strikingly, in the Cd34+ perivascular connective tissue in the submucosa close to the surface of the musculature. However, they did not appear to be reduced in the W/Wv and Sl/Sld mutant mice (Figure 5E,F).
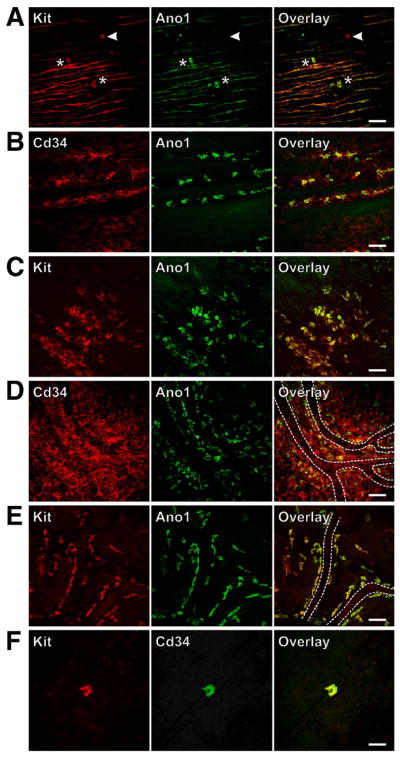
Figure 5. Gastric ICC stem cells are hyperplastic in mice with activating Kit mutation but unaffected by hypomorphic Kit.
(W/Wv) and Kitl (Sl/Sld) mutations. Representative confocal images collected from whole-mounts of 3–6 stomachs/group are shown. Scale bars, 50 μm. (A) Solitary, Kit+Ano1+ ICC stem cells (asterisks) and a rare Kit+Ano1− mast cell (arrowhead) in the circular muscle layer of the antrum in a wild-type mouse. (B) ICC stem cells in the submucosa in the vicinity of the circular muscle layer of the antrum in a wild-type mouse. (C,D) Hyperplastic ICC stem cells in the submucosa close to the surface of the circular muscle in KitK641E mutant mice. These cells were particularly abundant in the Cd34+ perivascular connective tissue (D). Capillaries are marked by dotted lines. (E) ICC stem cells along capillary walls in the antrum of a W/Wv mouse and (F) in the circular muscle of the distal antrum of an adult Sl/Sld mouse lacking intramuscular ICC.
We quantified ICC (myenteric+intramuscular; as Kit+Cd44+Cd34− cells), immature ICC (Kit+Cd44+Cd34+) and ICC stem cells (KitlowCd44+Cd34+) in the hematopoietic marker-negative fraction of gastric tunica muscularis by flow cytometry9 (Supplemental Figure S2). These experiments verified that the KitK641E mutation caused a marked increase not only in mature ICC, but also in immature ICC and ICC stem cells (Figure 6A, Supplemental Figure S10A). Relative to their age-matched controls, adult W/Wv and Sl/Sld mice had significantly reduced ICC in their stomach (Figure 6B,C, Supplemental Figure S10B,C). In marked contrast, ICC stem cells were not affected in either strain. Immature ICC were significantly reduced in Sl/Sld but not in W/Wv animals. This is consistent with our previous finding that membrane-associated SCF, an isoform lacking in Sl/Sld but not in W/Wv mice, is required for normal development of immature ICC.9 Collectively, these results indicate that ICC stem cells become hyperplastic in response to KitK641E mutation that causes GIST but do not depend on SCF/Kit signaling for maintenance.
Figure 6. ICC stem cells are hyperplastic in KitK641E mice but unaffected by hypomorphic Kit (W/Wv) and Kitl (Sl/Sld) mutations or imatinib treatment.
(A) 14–16-day-old KitK641E mice. (B) Adult W/Wv mice. (C) Adult Sl/Sld mice. +/+: age- and strain-matched, wild-type controls. Box plots show median and interquartile range; n=3–4/group. The frequency of ICC and ICC progenitors was higher in juvenile wild-type littermates of the KitK641E mice than in the adult wild-type controls for the hypomorphic mutants. (D) Effects of 28-day in vivo imatinib treatment on ICC and precursors in adult KitK641E/+ mice lacking the neomycin resistance cassette (n=3/group).
We studied the in vivo responsiveness of ICC stem cells to imatinib in KitK641E/+ mice lacking the neomycin resistance cassette by flow cytometry. These mice had a 7.1±0.2-fold increase in ICC (Supplemental Figure S11), a 2.4±0.2-fold increase in ICC stem cells and a 2.9±0.3-fold increase in immature ICC relative to adult wild-type mice (n=3/group). However, these animals did not succumb to intestinal obstruction and could be studied as adults. Imatinib administered by gavage at 5 mg/kg twice daily for 28 days significantly reduced Kit+Cd44+Cd34− ICC relative to vehicle-treated controls by 23% but did not reduce KitlowCd44+Cd34+ ICC stem cells or Kit+Cd44+Cd34+ immature ICC (Figure 6D). Thus, ICC precursors are also resistant to imatinib treatment in vivo.
Finally, we tested whether the isolated ICC stem cells proliferation could be inhibited by salinomycin, a selective inhibitor of breast cancer stem cells.24 Salinomycin (4 μM; MP Biomedicals) nearly completely inhibited the growth of D2211B cells without causing cell death (Figure 7A,B). Salinomycin appeared to promote the ICC stem cells differentiation as evidenced by the high number of multipolar, ICC-like cells (Figure 7C,D) and caused the appearance of fibroblast-like cells filled with vesicles. Submaximal doses of salinomycin (0.25 or 1 μM) combined with imatinib caused a significantly greater inhibition of cell proliferation than salinomycin alone (Figure 7E). Thus, salinomycin has the potential to control the proliferation of imatinib-resistant KitlowCd44+Cd34+ ICC stem cells and increase their sensitivity to imatinib.
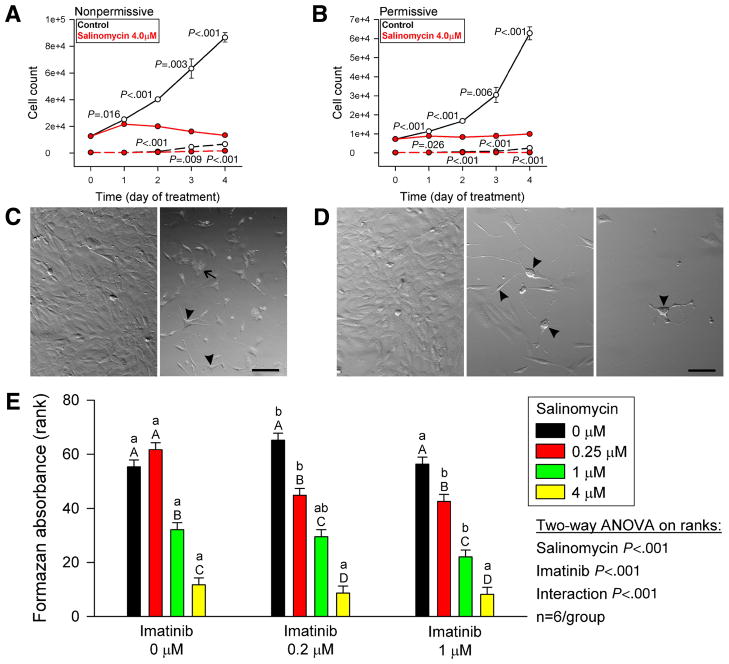
Figure 7. Salinomycin inhibits the proliferation of ICC stem cells and sensitizes them to imatinib.
(A,B) Effects of salinomycin on the proliferation of D2211B cells. Solid lines, PI− cells; dashed lines, PI+ (dead) cells. Note nearly complete inhibition. The number of PI+ dead cells was also reduced. (C) Morphology of D2211B cells cultured under nonpermissive conditions with vehicle (left panel) or salinomycin (right panel) for 4 days. Arrowhead, multipolar, ICC-like cells. Arrow, fibroblast-like cell filled with cytoplasmic vesicles. Scale bar, 25 μm. (D) Morphology of D2211B cells cultured under permissive conditions with vehicle (left panel) or salinomycin (middle and right panels) for 4 days. Note ICC-like phenotype of salinomycin-treated cells (arrowheads). Scale bar, 25 μm. (E) Dose-dependent effects of 5-day combined treatments with salinomycin and imatinib on the proliferation of D2211B cells under permissive conditions. The rank of 1 was assigned to the culture with the lowest cell number. Groups not sharing the same labels were different by post-hoc multiple comparisons. Lowercase and uppercase labels apply to groups receiving the same salinomycin and imatinib doses, respectively.
DISCUSSION
In 1998, based on the observations that GIST and ICC both express Kit and most GIST contain activating Kit mutations, Hirota et al.2 and Kindblom et al.25 proposed that GIST likely originate from the lineage of ICC. GIST and ICC also share expression of Ano1.15–17 However, the exact cell of origin of GIST has remained unclear.26 Herein we report, for the first time, that KitlowCd44+Cd34+ cells of the murine stomach previously identified as adult progenitors of ICC9 are bona fide stem cells, which also give rise to hyperplastic and neoplastic ICC underlying GIST. We also show that KitlowCd44+Cd34+ cells, unlike their differentiated progeny, do not depend on SCF/Kit signaling for survival. This characteristic is retained after the acquisition of oncogenic Kit mutation in vivo and confers inherent resistance to drugs targeting oncogenic Kit signaling including imatinib, the mainstay of treatment for metastatic GIST. In contrast, the proliferation of KitlowCd44+Cd34+ ICC stem cells could be effectively controlled by salinomycin, a drug shown to inhibit cancer stem cells selectively.24
Rare, KitlowCd44+Cd34+ cells with nondescript morphology residing mainly on the surfaces of the gastric smooth muscles can differentiate into ICC and recovery of ICC networks following prolonged growth factor deprivation depends on the survival of these cells.9 Differentiation of mature, Kit+Cd44+Cd34− ICC from these progenitors occurs via an intermediate cell type with Kit+Cd44+Cd34+ immunophenotype and ICC-like morphology ( immature ICC ). The latter cells had previously been detected and, on account of their expression of Cd34, hypothesized to be the source of GIST.26 Our present data further support a role for KitlowCd44+Cd34+ cells as adult ICC precursors. Firstly, ICC progenitors isolated from two mouse strains, including wild-type C57BL/6J mice, maintained their phenotype and capability of differentiating into ICC even after serial clonal re-derivation allowing the operational definition of these cells as ICC stem cells. Secondly, clonally derived ICC stem cells could also differentiate into Kit+Ano1+ ICC after heterotopic transplantation into nude mice and home to the stomach and incorporate into ICC networks in diabetic NOD/LtJ mice, a model of ICC injury.21 Thirdly, ICC stem cells, similarly to ICC15,17 and GIST,16 express Ano1 both in vitro and in vivo. In isolated ICC stem cells we have also detected mRNA for Pdgfra, an alternative target of oncogenic mutations in GIST20 and a marker for Kit− fibroblast-like cells.27 Thus, ICC stem cells may also give rise to a fibroblast-like component of the gastrointestinal muscles and contribute to GIST developing from oncogenic Pdgfra mutations. Future studies will determine the exact in vivo differentiation potential of these cells and their utility to replace ICC in a variety of models.
The embryonic origin of KitlowCd44+Cd34+ cells remains unclear. Preliminary results indicate that they share the expression of several markers with mesenchymal stem cells.10 The KitlowCd44+Cd34+ cells perivascular localization and the fact that they could be transplanted across MHC barriers also suggest a relationship with tissue mesenchymal stem cells.10
Our results indicate that the KitlowCd44+Cd34+ cells are also the source of hyperplastic and neoplastic ICC underlying GIST: Firstly, in mice harboring activating Kit mutation, ICC hyperplasia, a precancerous lesion,2 was accompanied by a comparable increase in KitlowCd44+Cd34+ ICC stem cells and Kit+Cd44+Cd34+ immature ICC. Secondly, spontaneously transformed ICC stem cells formed malignant, Kit+Ano1+ tumors in nude mice. These tumors, besides containing differentiated, ICC-like cells, consisted mainly of small, round cells resembling the native ICC stem cells and expressing low/moderate levels of Kit and Ano1. Epithelioid morphology is characteristic of a subset of GIST.1 Moreover, epithelioid cells with reduced Kit expression have been described in recurrent GIST in patients on prolonged imatinib treatment.28,29 Thus, transformed ICC stem cells are likely the source of human GIST and may dominate in imatinib-resistant tumors.
Differentiation, survival and function of mature ICC depend on adequate Kit signaling and stimulation from membrane-associated SCF is required for optimal effects.4,5,9,12 In contrast, proliferation of ICC stem cells can also be stimulated by secreted SCF and by insulin or IGF-I (ref.9 and present study). Moreover, some of IGF-I s effects appear to be mediated by autocrine stimulation of Kit signaling. A role for Kit activation in the isolated ICC stem cells proliferation is consistent with the hyperplasia of KitlowCd44+Cd34+ cells in KitK641E mice. The spontaneous differentiation of these cells into ICC in vitro and after heterotopic transplantation likely depends on their endogenous production of IGF-I and SCF and an IGF-I-Igf1r-SCF-Kit autocrine loop18,19 since in the gut, IGF-I and SCF are mainly provided by smooth muscle cells.12 In fact, the lack of an appropriate tissue environment may explain why most cells in the cultures and the heterotopic tumors remained undifferentiated.
While Kit activation can stimulate both the proliferation and differentiation of the ICC stem cells, all evidence presented in this paper indicates that these cells, unlike their differentiated progeny, are not dependent on SCF/Kit signaling for survival and proliferation. Firstly, their numbers were not reduced in hypomorphic Kit and Kitl mutant mice with profoundly decreased ICC. Secondly, their proliferation in vitro could not be blocked by neutralizing antibodies against Kit or SCF or by imatinib, although the same treatments inhibited mature ICC. Thirdly, imatinib also failed to reduce KitlowCd44+Cd34+ cells in mice with activating Kit mutation despite significantly reducing hyperplastic ICC. Thus, KitlowCd44+Cd34+ ICC stem cells are resistant to Kit blockade by any means. The simplest explanation for this resistance is that these cells express very little Kit on their surface.9
The ICC stem cells resistance to Kit blockade has important translational implications. Small-molecule tyrosine kinase inhibitors such as imatinib mesylate30 have become key therapeutic tools against GIST, which are highly resistant to conventional chemotherapy and radiotherapy.23 Imatinib substantially increases progression-free survival after resection of localized, primary GIST and can achieve a median survival of 57 months in patients with metastatic GIST.7 However, imatinib is rarely (≤5%) curative.1,7,8 Resistance to imatinib may develop during the treatment as a result of selection for additional point mutations.1,8 There also is evidence that some degree of imatinib resistance might be inherent in most GIST8 since (i) 10–20% of patients exhibit primary resistance;1 (ii) viable cells can be found in most patients who undergo resection during imatinib therapy28,29 and (iii) imatinib treatment must be maintained indefinitely to prevent flair-up.1,7 These observations led to the hypothesis that GIST “tumor stem cells” exist that, similarly to leukemia-initiating cells in chronic myelomonocytic leukemia,31 cannot be eradicated by Kit blockade alone.1,8 Our results indicate that since the ICC stem cells resistance to Kit blockade is retained in the presence of an activating Kit mutation, they likely represent an imatinib-resistant reservoir for GIST that explains the primary imatinib resistance inherent in these tumors.8 Interestingly, it has been reported that after prolonged imatinib therapy, primary GIST may lose their differentiated, spindle-shaped morphology and strong Kit expression and become dominated by epithelioid cells.28,29 Our results suggest that this change may reflect the selection of mutant ICC/GIST progenitors by the imatinib treatment that can only block the differentiation and survival of mature cells.
In contrast to the failure of imatinib to control the ICC stem cells, the cancer-initiating cell-targeting drug salinomycin was able to arrest their proliferation in vitro, in part by activating their differentiation program. This hypothesis is also consistent with our finding that at submaximal doses, salinomycin increased the ICC stem cells sensitivity to imatinib. Thus, salinomycin may be able to inhibit not only epithelial but also mesenchymal cancer stem cells. Future studies will determine if this drug is similarly effective at controlling GIST in vivo.
Together, our results provide critical new insight into the pathogenesis of GIST and have important therapeutic implications by providing a plausible explanation for the failure of receptor tyrosine kinase inhibitors to cure GIST. Treatments targeting Kit, however effective at killing differentiated GIST cells, will not be able to eradicate their transformed stem cells. These cells can, therefore, be considered a Kit-independent reservoir and potential cancer stem cells for GIST.
Supplementary Material
Acknowledgments
Grant support: NIH grants DK058185, RR016464 and DK041315 and The Liferaft Group.
Abbreviations
- AF
Alexa Fluor
- Ano1
anoctamin 1
- APC
allophycocyanin
- B2m
beta-2 microglobulin
- CEE
chick embryo extract
- Cy
cyanine
- FACS
fluorescence-activated cell sorting
- GIST
gastrointestinal stromal tumors
- HMC
Hoffman modulation contrast
- ICC
interstitial cells of Cajal
- IGF-I
insulin-like growth factor 1
- Igf1r
IGF-I receptor
- Insr
insulin receptor
- IFNγ
interferon-γ
- Kitl
gene/mRNA encoding stem cell factor
- MACS
immunomagnetic cell sorting
- MHC
major histocompatibility complex
- NSCPM
Neural Stem Cell Proliferation Medium
- Pdfgra
platelet-derived growth factor α
- PI
propidium iodide
- rm
recombinant murine
- PE
phycoerythrin
- RT
reverse transcriptase
- SA
streptavidin
- SCF
stem cell factor
- SmGM-2
Smooth Muscle Growth Medium-2
- tsTAg
temperature-sensitive SV40 large T antigen
Footnotes
Disclosures: Dr. Brian Rubin is a member of the Speakers Bureau and the Advisory Board of Novartis Pharmaceuticals Corp. He has also performed contract work (educational website design) and received compensation exceeding $10,000 per year. The other authors have nothing to disclose.
Author contributions:
Michael R. Bardsley: Collection of data, data analysis and interpretation, final approval of manuscript
Viktor J. Horvath: Collection of data, data analysis and interpretation, final approval of manuscript
David T. Asuzu: Collection of data, data analysis and interpretation, final approval of manuscript
Andrea Lorincz: Collection of data, data analysis and interpretation, final approval of manuscript
Doug Redelman: Collection of data, data analysis and interpretation, final approval of manuscript
Yujiro Hayashi: Collection of data, final approval of manuscript
Laura N. Popko: Collection of data, final approval of manuscript
David L. Young: Collection of data, final approval of manuscript
Gwen A. Lomberk: Provision of study material and technology, final approval of manuscript
Raul A. Urrutia: Provision of study material and technology, final approval of manuscript
Gianrico Farrugia: Data interpretation, manuscript writing
Brian P. Rubin: Provision of study material, collection of data, data analysis and interpretation, final approval of manuscript
Tamas Ordog: Conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders KM, Ordog T, Koh SD, et al. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 5.Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Streutker CJ, Huizinga JD, Driman DK, et al. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology. 2007;50:190–202. doi: 10.1111/j.1365-2559.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 7.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher JA, Rubin BP. KIT mutations in GIST. Curr Opin Genet Dev. 2007;17:3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Lorincz A, Redelman D, Horvath VJ, et al. Progenitors of interstitial cells of Cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–1093. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Rubin BP, Antonescu CR, Scott-Browne JP, et al. A knock-in mouse model of gastrointestinal stromal tumor harboring kit K641E. Cancer Res. 2005;65:6631–6639. doi: 10.1158/0008-5472.CAN-05-0891. [DOI] [PubMed] [Google Scholar]
- 14.Ordog T, Redelman D, Horvath VJ, et al. Quantitative analysis by flow cytometry of interstitial cells of Cajal, pacemakers, and mediators of neurotransmission in the gastrointestinal tract. Cytometry A. 2004;62:139–149. doi: 10.1002/cyto.a.20078. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano K, Shishido-Hara Y, Kitazawa A, et al. Expression of stem cell factor (SCF), a KIT ligand, in gastrointestinal stromal tumors (GISTs): a potential marker for tumor proliferation. Pathol Res Pract. 2008;204:799–807. doi: 10.1016/j.prp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 21.Ordog T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol. 2009;55:315–342. [PMC free article] [PubMed] [Google Scholar]
- 22.Beckett EA, Ro S, Bayguinov Y, et al. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 23.Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 24.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson TL, Sircar K, Hewlett BR, et al. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157–1163. doi: 10.1016/S0002-9440(10)64984-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iino S, Horiguchi K, Horiguchi S, et al. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels P, Debiec-Rychter M, Stul M, et al. Changing phenotype of gastrointestinal stromal tumours under imatinib mesylate treatment: a potential diagnostic pitfall. Histopathology. 2005;47:41–47. doi: 10.1111/j.1365-2559.2005.02179.x. [DOI] [PubMed] [Google Scholar]
- 29.Dudeja V, Armstrong LH, Gupta P, et al. Emergence of imatinib resistance associated with downregulation of c-kit expression in recurrent gastrointestinal stromal tumor (GIST): optimal timing of resection. J Gastrointest Surg. 2010;14:557–561. doi: 10.1007/s11605-009-1121-2. [DOI] [PubMed] [Google Scholar]
- 30.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 31.Oravecz-Wilson KI, Philips ST, Yilmaz OH, et al. Persistence of leukemia-initiating cells in a conditional knockin model of an imatinib-responsive myeloproliferative disorder. Cancer Cell. 2009;16:137–148. doi: 10.1016/j.ccr.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.