Abstract
Introduction
Adopting another person’s visuospatial perspective has been associated with empathy, which involves adopting the psychological perspective of another individual. Both reduced empathy and abnormal visuospatial processing have been observed in those with schizophrenia and schizophrenia-related personality traits. In the current study, we sought to explore the relationship between empathy, schizotypy, and visuospatial transformation ability.
Methods
32 subjects (16 women) performed a visuospatial perspective-taking task and a mental letter rotation task. Response times and accuracy were analysed in relation to dimensions of self-reported empathy, indexed using the Interpersonal Reactivity Index, and schizotypy, as measured by the Schizotypal Personality Questionnaire.
Results
We found that: (1) greater cognitive and affective empathy were associated with reduced negative schizotypy, and, in men, greater cognitive empathy was associated with reduced positive schizotypy; (2) improved accuracy for imagined self–other transformations in the perspective-taking task was associated with greater self-reported cognitive empathy in women and higher positive schizotypy across genders; (3) faster mental letter rotation was associated with reduced cognitive empathy and increased negative schizotypy in women.
Conclusions
Together, the findings partially support the commonalities in visuospatial transformation ability, empathy, and schizotypy, and posit an interesting link between spatial manipulations of our internal representations and interactions with the physical world.
Keywords: Empathy, Gender differences, Mental rotation, Perspective taking, Schizotypy, Spatial ability
INTRODUCTION
Navigation of both our physical and mental landscapes is thought to share common mechanisms, and there is substantial evidence for analogous manipulation of our physical and mental space. For example, Kosslyn (1973) found that the time it takes to scan between two points of a mental image of a picture is proportional to the actual distance between those two points on the image, and seminal work by Shepard and Meltzer (1971) on mental rotation found that more time needed to mentally rotate an object was linearly related to the angular disparity between the object and its prescribed or canonical orientation. Similarly, there is evidence to suggest that empathy, which involves understanding the mental state of others, is associated with imagining the visuospatial perspective of others (Mohr, Rowe, & Blanke, in press; Thakkar, Brugger, & Park, 2009). Empathy may be altered in certain psychiatric conditions (Decety & Moriguchi, 2007) that are also associated with abnormalities in visuospatial processing (Mitchell & Ropar, 2004; Morris & Mervis, 1999). Specifically, both reduced empathy (Brune, 2005; Dinn, Harris, Aycicegi, Greene, & Andover, 2002; Henry, Bailey, & Rendell, 2008; Montag, Heinz, Kunz, & Gallinat, 2007) and subtle differences in spatial attention (Bracha, Livingston, Clothier, Linington, & Karson, 1993; Brugger & Graves, 1997; Mohr, Bracha, & Brugger, 2003; Mohr, Landis, Bracha, Fathi, & Brugger, 2005; Taylor, Zach, & Brugger, 2002) and visuospatial transformation ability (Langdon & Coltheart, 1999; Mohr, Blanke, & Brugger, 2006) have been observed in schizophrenia and those high in schizophrenia-like traits. The major aim of the present study was to investigate the relationship between dimensions of self-reported empathy and schizotypy and performance on two different visuospatial transformation tasks. Secondary aims were to examine the potential role of gender in the putative relationships between visuospatial transformation ability, schizotypy, and empathy since sex differences have been reported for empathy (Hoffman, 1977), schizotypy (Raine, 1992), and visuospatial skills (Voyer, Voyer, & Bryden, 1995).
Visuospatial transformations have typically been divided into egocentric and allocentric, or object-centred, transformations (see Zacks & Michelon, 2005, for review). Egocentric mental transformation refers to imagined changes in position and orientation relative to the surrounding environment. On the other hand, allocentric mental transformation involves mentally manipulating an object relative to its own reference frame. A specific subset of egocentric mental rotation tasks, in which subjects are required to mentally transform themselves into the body of another, can be used to investigate visuospatial self–other transformations. These tasks typically require individuals to imagine taking the position of a figure on a screen and make judgements about the location of body parts (Parsons, 1987a). It has been consistently reported that response times (RTs) are longer when the position of the figure does not match the position of the subject, because he/she has to make an additional perspective transformation (e.g., Parsons, 1987a; Zacks, Rypma, Gabrieli, Tversky, & Glover, 1999). Allocentric manipulations are often measured with mental rotation tasks, which require mentally rotating a stimulus into a particular orientation in order to make a forced-choice response. RTs for mental rotation correspond to RTs for physical rotation and increase as the required degree of mental rotation increases (Shepard & Metzler, 1971). There is evidence that egocentric and allocentric mental transformations are dissociable at the level of both behaviour and brain (see Zacks & Michelon, 2005, for review).
Anomalous visuospatial experiences have been noted in individuals who score high in self-report measures of schizotypy, which refers to the personality traits that are related to symptoms of schizophrenia and imply a latent liability for the disorder. Factor analysis reveals that schizotypal traits break down into three syndromal factors—perceptual-cognitive, interpersonal, and disorganisation, which correspond to the positive, negative, and disorganisation symptoms of schizophrenia (Raine et al., 1994). Much of the research relating schizotypy to visuospatial processes has focused on biases in spatial attention and turning preferences. Although there is a tendency for the general population to show a subtle attentional bias towards the left side of space, termed “pseudoneglect”, positive schizotypal traits have generally been associated with exaggerated leftward biases in spatial attention (Brugger & Graves, 1997; Mohr et al., 2003; Taylor et al., 2002; but see Liouta, Smith, & Mohr, 2008, for exception). Moreover, a preference towards leftward turning has been noted in psychotic patients (Bracha et al., 1993) and healthy individuals who score high on positive syndrome of schizotypy (Mohr et al., 2003, 2005). Pseudoneglect is explained by right hemispheric dominance in spatial attention (Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990), and evidence from the animal literature suggests that organisms preferentially turn in the direction away from the hemisphere with increased dopamine activity (Glick, Jerussi, & Fleisher, 1976; Yamamoto & Freed, 1982). Given the purportedly crucial role of dopamine in schizophrenia and schizotypal thought (see Tost, Alam, & Meyer-Lindenberg, 2010, for review), more pronounced leftward bias of spatial attention and turning in schizotypy has been interpreted as reflecting right-lateralised hyperdopaminergia (Bracha, 1989). Along with differences in spatial attention biases, schizotypy has also been associated with impairments in imagined spatial transformations of one’s body in space (Mohr et al., 2006) and in imagined changes in visuospatial perspective (Langdon & Coltheart, 1999). However, Langdon and Coltheart (1999) found that although individuals high in schizotypy were impaired in the ability to judge the spatial relationship between items in an array when they were instructed to imagine changing their visuospatial perspective, they were better at judging the spatial relationship among items than low schizotypal individuals when instructed to imagine rotating the array to their perspective. It has yet to be investigated whether the association between positive schizotypy and imagined spatial manipulations is restricted to these egocentric transformations, or also includes object-centred transformations.
Trait empathy has also been linked to spatial attention and transformations (Mohr et al., in press; Thakkar et al., 2009). In the most general sense, empathy refers to processes of interpreting and reacting to the experiences of others, and many researchers agree that empathy is a multifaceted construct that involves both cognitive and emotional components (Preston & de Waal, 2002) and requires maintenance of a self–other distinction (de Vignemont & Singer, 2006; Eisenberg & Fabes, 1990; Lamm, Batson, & Decety, 2007). Cognitive empathy refers to a controlled process by which an individual projects himself or herself into the place of another. It is closely akin to the construct of “theory of mind”, attribution of mental states to oneself and others. On the other hand, affective empathy commonly refers to the more automatic emotional response to the experience of others, which can motivate concern and subsequent helping behaviour. Although a sense of shared interpersonal space has been thought to be a prerequisite for empathy (Gallese, 2003), and there is evidence for parietal cortex and the tempor-oparietal junction being involved in both empathy and visuospatial processing (Decety & Lamm, 2007; Iacoboni & Dapretto, 2006; Marshall & Fink, 2001), little work has been done to systematically investigate the role of visuospatial abilities in cognitive and affective empathy. In a previous study (Thakkar et al., 2009), we found a correlation between rightward biases in spatial attention and self-reported affective empathy. Biases in spatial attention were indexed by deviations on a line bisection task, which required participants to mark through the centre of a series of horizontally presented lines. We also found that slower imagined self–other spatial transformation (i.e., literally imagining yourself in another’s shoes) was associated with affective empathy, but only in women. In contrast, Mohr and colleagues (in press) found that higher self-reported empathy was correlated with faster RT on a mental self–other spatial transformation task, again only in women. Although significant relationships between self-reported empathy and egocentric visuospatial transformations have been reported in women, it is unknown whether empathy is also associated with allocentric mental transformations.
Trait empathy and schizotypy have both been related to spatial attention and egocentric transformations, and there is also evidence that self-reported empathy is related to schizotypal traits and schizophrenia. Lower self-reported cognitive, but not affective, empathy has been reported in individuals diagnosed with schizophrenia (Montag et al., 2007), and impaired theory of mind has been extensively documented in patients with schizophrenia (see Brune, 2005, for review). Increased negative schizotypy has been found to correlate with reduced self-reported cognitive and affective empathy and with a behavioural measure of empathy (Henry et al., 2008). Positive schizotypy, on the other hand, has been associated with greater self-reported affective empathy (Dinn et al., 2002; Henry et al., 2008), but poorer performance on a behavioural measure of empathy (Henry et al., 2008).
Gender differences have been described for empathy (e.g., Hoffman, 1977), schizotypy (e.g., Raine, 1992), and visuospatial skills (e.g., Voyer et al., 1995). Females typically score higher on self-report measures of empathy (e.g., Baron-Cohen & Wheelwright, 2004; Davis, 1983), and have been found to perform better on a task that requires subjects to infer emotion from the eye region of a stimulus face (Baron-Cohen, Jolliffe, Mortimore, & Robertson, 1997). Although there is compelling evidence that there is a biological basis for these sex differences in empathy (see Baron-Cohen, Knickmeyer, & Belmonte, 2005), it has been argued that gender differences in empathy are due to demand characteristics of the questionnaire or motivational differences (e.g., Eisenberg & Lennon, 1983; Ickes, Gesn, & Graham, 2000), rather than differences in ability. Gender differences have also been noted for visuospatial skills, with men outperforming women (see Voyer et al., 1995, for review). Performance on visuospatial tasks has been found to be related to levels of circulating and foetal environmental androgen levels in both men and women (see Hampson, 1995, for review). Finally, sex differences have also been reported for schizotypy, with women reporting more positive syndrome schizotypy and men reporting more negative syndrome schizotypy (Raine, 1992).
The aim of the current study was to examine the relationship between self-report measures of empathy, schizotypy, and speed and accuracy of egocentric and allocentric visuospatial transformations on a visuospatial perspective-taking task and mental letter rotation task, respectively. A secondary aim was to examine potential gender effects on these relationships. We predicted that: (1) women would report more empathy and positive schizotypal traits and men would report more negative schizotypal traits; (2) schizotypy would be associated with reduced self-reported empathy; (3) in the perspective-taking task, RT and error rates would be greater in the front-facing condition when an imagined self–other transformation was required; (4) in the letter rotation task, error rate and RT would increase with required degree of rotation; (5) increased speed of imagined visuospatial self–other transformations would be associated with increased self-reported empathy in women, consistent with prior findings; and (6) increased RT and error rate on the perspective-taking task would be associated with increased schizotypy, consistent with prior findings. Since we have no prior data to speak to the relationship between schizotypy and empathy and allocentric mental rotation, we did not form any explicit predictions. We also explored whether correlations between performance on spatial transformations, empathy, and schizotypy would differ across genders, given reported gender differences in visuospatial skills (Voyer et al., 1995), empathy (Hoffman, 1977), and schizotypy (Raine, 1992).
METHODS
Participants
Thirty-two healthy subjects (16 females) were recruited from the Psychology Subject Pool at Vanderbilt University. Participants were screened for a history of mental illness in themselves or family members, drug use, head injury, and left-handedness according to the Edinburgh Handedness Inventory. Subjects had a mean age of 21.5 (SD=3.4 years), and a mean Edinburgh score of +71.3 (SD=30.2). Neither age, t(30)=0.15, p=.88, nor handedness, t(30)=−0.76, p=.46, differed significantly between males and females. The study protocol was approved by the Vanderbilt University Institutional Review Board, and written informed consent was obtained from all subjects prior to testing. Subjects fulfilled course requirements through their participation.
Interpersonal Reactivity Index
The Interpersonal Reactivity Index (IRI; Davis, 1980) is a 28-item self-report measure of empathy, consisting of four subscales: Perspective-Taking (PT), Fantasy (FS), Empathic Concern (EC), and Personal Distress (PD), and assessed using a 5-point Likert scale (0–4). The IRI was developed using a multidimensional approach and was designed to evaluate both the cognitive and affective components of empathy. The PT subscale assesses the tendency to adopt the psychological viewpoint of others, and the FS subscale assesses the tendency to transpose oneself into the experience of a fictitious character; these two scales were designed to measure the cognitive component of empathy. The EC scale assesses “other-oriented” feelings of sympathy and concern, and the PD scale measures feelings of interpersonal anxiety in response to other people’s distress. These two scales were designed to measure the affective component of empathy. Since scores on the FS and PD scales have been associated with social dysfunction, and the PD scale was found to be negatively correlated with other empathy measures (Davis, 1983), we focused on the more psychometrically validated PT and EC scores to index cognitive and affective components of empathy, respectively.
Schizotypal Personality Questionnaire
The Schizotypal Personality Questionnaire (SPQ; Raine, 1991) is a 74-item dichotomous response (yes/no) self-report measure with nine subscales that reflect the nine syndromes of schizotypal personality disorder listed in DSM-III-R (American Psychological Association, 1987). The SPQ can be subdivided into three syndromes or factors: Cognitive-Perceptual (consisting of ideas of reference, magical thinking, unusual perceptual experiences, and paranoid ideation items), Interpersonal (consisting of social anxiety, no close friends, blunted affect, and paranoid ideation items), and Disorganisation (consisting of odd behaviour and odd speech items). Positive symptoms of schizophrenia map onto the Cognitive-Perceptual factor, negative symptoms map onto the Interpersonal factor, and disorganised symptoms map onto the Disorganisation factor.
Spatial tasks
Perspective-taking task
We used stimuli similar to those used in previous studies of perspective-taking and mental self–other transformations. A photograph of an individual with his or her arms out to the side faced either towards or away from the participant and was presented in one of six different angular orientations, ranging from 67.5° to 292.5° clockwise, from the upright position, in 45° steps (Figure 1A). Different angles of presentation is common practice in studies of imagined self–other transformations and mental rotation of objects (Parsons, 1987a,b; Shepard & Metzler, 1971), and were used in order to discourage participants from memorising associations between particular stimuli and motor responses (Zacks et al., 1999). There were four possible stimulus figures, two women and two men. Either the right or the left hand was marked by a red circle (Figure 1A). Participants were asked to imagine themselves in the position of the figure on the screen and indicate whether the circled hand would be their right or left hand by pressing a key labelled “L” and “R” with the left and right index finger, respectively. Subjects were instructed to respond as quickly and accurately as possible. A left judgement was indicated by pressing a key with the index finger of their left hand and a right judgement was indicated by pressing a key with the index finger of their right hand. Stimulus presentation and response collection were controlled by Matlab (Brainard, 1997).
Figure 1.
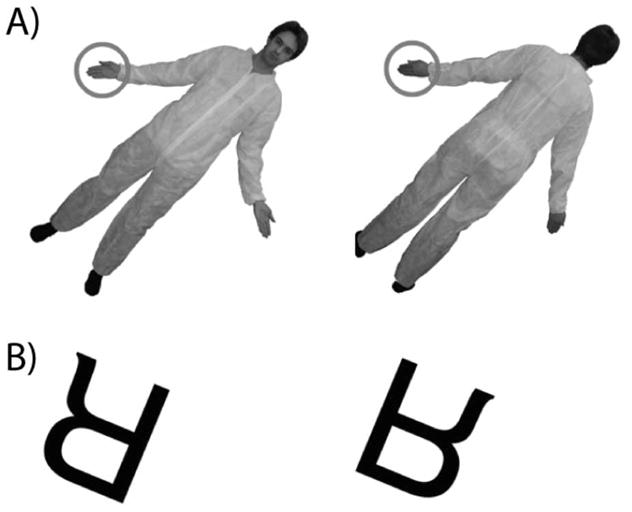
Example stimuli in the spatial transformation tasks. (A) Front- and back-facing stimuli in the perspective-taking task. (B) Normal and mirror stimuli in the letter rotation task.
Stimuli extended 12° of visual angle horizontally and vertically and were presented in the centre of the computer screen until a response was made or after a 10s time-out period. A black fixation cross was presented during the 1000 ms intertrial interval before the next trial could begin. The experiment consisted of 384 total trials, divided into four blocks, which consisted of eight repetitions of each stimulus type in a randomised order. Trials in which the subject did not respond within the 10 s time-out period were excluded from further analysis.
Letter Rotation task
One of five letters (B, K, F, R, or G) was presented in either mirror or normal orientation at one of eight different angular orientations, ranging from 22.5° to 337.5° clockwise, from the upright position, in 45° steps (Figure 1B). Participants were asked to indicate whether the letter was presented in normal or mirror orientation by pressing a key labelled “N” or “M” with their left or right index finger. Response key mappings were counterbalanced across participants so that for half of the subjects normal orientation was indicated with the left index finger and mirror orientation was indicated with the right index finger; for the other half of the subjects, response key mappings were reversed. Stimulus presentation and response collection were controlled by Matlab (Brainard, 1997).
Stimuli extended 6° of visual angle horizontally and vertically and were presented in the centre of the computer screen until a response was made or after a 10 s time-out period. A black fixation cross was presented during the 1000 ms intertrial interval before the next trial could begin. The experiment consisted of 320 total trials, divided into four blocks, which consisted of four repetitions of each stimulus type in a randomised order. Trials in which the subject did not respond within the 10 s time-out period were excluded from further analysis.
Testing procedure
For the perspective-taking and letter rotation tasks, a chinrest was used to stabilise head position. Task order was counterbalanced across subjects.
Data analysis
Separate repeated measures ANOVAs were conducted on mean RT and error rate in the perspective-taking and letter rotation tasks, with gender entered as a between-subject variable. For the perspective-taking task, perspective was entered as a within-subject variable, and for the letter rotation task, angle of rotation was entered as a within-subject variable.
The Perspective-taking RT was used to index the speed of self–other perspective transformations. As in a previous study (Thakkar et al., 2009), it was defined as the relative increase in RT for making a hand judgement for front versus back facing figures using the formula [(mean Front RT – mean Back RT)/mean Back RT], using only correct trials. Increased Perspective-taking RT indicates a relative increase in time needed to perform an imagined perspective change. Error rates in the front and back conditions were used to index the accuracy of self–other perspective transformations.
The slope of the function relating RT to angular disparity was used to index allocentric mental rotation speed in the letter rotation task. The slope of this function is interpreted to isolate the mental rotation process from other processes involved in the task (e.g., response selection). Slope of the RT function was computed separately for clockwise (202.5–337.5° angular disparity) and counterclockwise (22.5–157.5° angular disparity) rotations, and the average of the absolute value of the clockwise and counterclockwise rotation slopes was computed. Overall error rate in the letter rotation task was also calculated as an index of allocentric mental rotation ability.
Shapiro-Wilk statistics indicated that most of the self-report and behavioural variables were not normally distributed, so pairwise gender comparisons were assessed using Wilcoxon rank-sum tests. Wilcoxon signed-rank tests were used to compare the slope of the RT function and error rate on the letter rotation task and Perspective-taking RT to a hypothesised mean of 0. Spearman rank-correlation coefficients were calculated to evaluate the relationship between continuous variables, both within and across genders. All tests were two-tailed, except where otherwise noted, and the alpha level was set at .05.
RESULTS
Self-reported empathy
Means for the IRI PT and EC subscales are displayed in Table 1. No gender difference was observed on the PT subscale, Z=0.34, p=.73, but the mean IRI EC subscale score was higher for females, Z=1.93, p=.05.
TABLE 1.
Mean scores for self-reported empathy and spatial tasks, both collapsed and split by gender (mean±SD)
| Males (n=16) | Females (n=16) | All subjects | |
|---|---|---|---|
| Interpersonal Reactivity Index | |||
| Perspective-Taking | 18.1±5.6 | 19.3±4.9 | 18.7±5.2 |
| Empathic Concern* | 19.0±4.1 | 22.1±4.0 | 20.6±4.3 |
| SPQ | |||
| Cognitive-Perceptual | 4.8±5.1 | 5.1±4.1 | 4.9±4.6 |
| Interpersonal | 7.9±7.0 | 4.5±2.5 | 6.2±5.5 |
| Disorganised | 5.3±3.4 | 3.6±2.2 | 4.5±2.9 |
| Perspective-Taking task | |||
| Perspective-Taking RT | 0.16±0.17 | 0.10±0.10 | 0.13±0.14 |
| Error rate: Front-facing | 4.4±3.4 | 4.2±3.4 | 4.3±3.3 |
| Error rate: Back-facing | 2.6±2.3 | 2.6±2.5 | 2.6±2.4 |
| Letter Rotation taska | |||
| RT function slope | 0.20±0.08 | 0.22±0.05 | 0.21±0.07 |
| Overall error rate | 3.2±2.2 | 4.3±3.6 | 3.7±3.0 |
One female subject was removed because her error rate was above three standard deviations from the mean.
Gender difference, p<.05.
Self-reported schizotypy
Means for the SPQ subscales are displayed in Table 1. No gender differences were observed on either the Cognitive-Perceptual, Z=0.72, p=.47, Interpersonal, Z=1.10, p=.27, or Disorganised, Z=1.71, p=.09, sub-scales.
Visuospatial transformation tasks
Perspective-taking task
Errors
Error rates for the front- and back-facing conditions in males and females are displayed in Table 1. There was a significant effect of perspective on error rate, F(1, 30) =16.7, p=.0003, with more errors in the front-facing condition. There was no main effect of gender, nor any significant gender interactions.
RT
There was a robust effect of perspective on mean RT, F(1, 30) = 25.7, p<.0001, with slower performance in the front-facing (882 ms, SD=223 ms) versus back-facing (778 ms, SD=151 ms) condition. There was no main effect of gender, nor any significant gender interaction effects.
Mean Perspective-taking RTs for males and females are displayed in Table 1. No gender differences were observed for Perspective-taking RT, Z=0.36, p=.72, so we collapsed across gender to compare Perspective-taking RT to zero. A score of zero would indicate that no additional time was needed to make a handedness judgement when an imagined change in perspective was required. Perspective-taking RT was significantly greater than zero, Z=4.35, p<.0001.
Letter Rotation task
One female subject was excluded from these analyses because her error rate for two rotation degree conditions was well below chance performance.
Errors
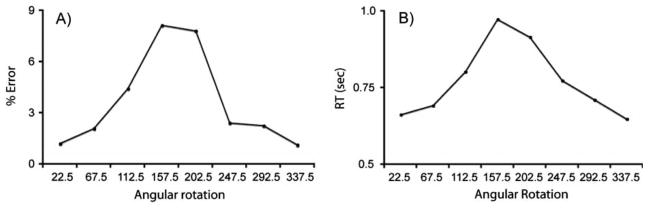
There was a significant effect of rotation angle on error rate, F(7, 203)=12.7, p<.0001, with errors increasing with greater angular disparity from the upright orientation (Figure 2A). There was no main effect of gender, nor any significant gender interactions. The overall error rates for males and females are displayed in Table 1.
Figure 2.
(A) Error rate. (B) RT as a function of angular rotation in the letter rotation task.
RT
There was a significant effect of rotation angle on RT, F(7, 203) = 100.3, p<.0001, with RTs increasing with greater angular disparity from the upright orientation (Figure 2B). There was no main effect of gender, nor any significant gender interactions.
The slope of the RT functions for males and females are displayed in Table 1. No gender differences were observed for the slope of the RT function, Z=0.91, p=.36, so we collapsed across gender to compare the slope of the RT function to zero. A score of zero would indicate that no additional time was needed to make an orientation decision with increased angle of mental rotation. The slope of the RT function was significantly greater than zero, Z=4.86, p<.0001.
Empathy, schizotypy, and spatial transformation ability correlations
Empathy and schizotypy correlations
Correlations and p-values are displayed in Table 2. Collapsed across genders, IRI PT was significantly negatively correlated with the SPQ Interpersonal and Disorganised subscales. The correlation between the SPQ Interpersonal score and IRI PT score was significant in women, but not men, and the correlation between the SPQ Disorganised score and IRI PT score was only significant when collapsed across groups. The IRI PT score was also negatively correlated with the SPQ Cognitive-Perceptual score, but only in men. Collapsed across gender, IRI EC was significantly negatively correlated with the SPQ Interpersonal subscale; this correlation only reached significance in men.
TABLE 2.
Correlations between empathy and schizotypy
| Males (n=16) |
Females (n=16) |
All subjects |
||||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| SPQ Cognitive-Perceptual | ||||||
| IRI Perspective-Taking | −.62** | .01 | .20 | .46 | −.30 | .09 |
| IRI Empathic Concern | −.32 | .22 | .01 | .98 | −.12 | .50 |
| SPQ Interpersonal | ||||||
| IRI Perspective-Taking | −.27 | .32 | −.70** | .003 | −.49** | .005 |
| IRI Empathic Concern | −.56* | .02 | −.43 | .10 | −.59** | .0004 |
| SPQ Disorganised | ||||||
| IRI Perspective-Taking | −.38 | .14 | −.41 | .11 | −.39* | .03 |
| IRI Empathic Concern | −.10 | .72 | −.03 | .91 | −.17 | .35 |
p<.05,
p<.01.
Perspective-taking correlations
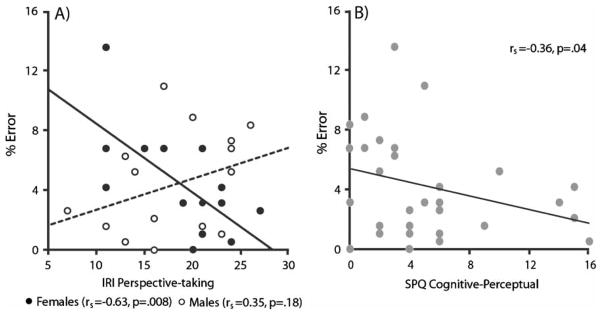
Contrary to our hypothesis, there were no significant correlations between Perspective-taking RT and the empathy subscale scores, neither collapsed across gender, nor in men or women separately. Although there was no correlation between empathy subscale scores and error rate in either the front- or back-facing conditions when genders were collapsed, in women there was a significant inverse correlation between the IRI PT score and error rate in the front-facing, rs=−.63, p=.008, but not back-facing, rs=−.41, p=.12, condition (Figure 3A). That is, in women, greater self-reported cognitive empathy was associated with greater accuracy on the perspective-taking task, but only in the condition that required an imagined change in perspective. No other significant correlations between empathy subscale scores and performance on the perspective-taking task were observed.
Figure 3.
(A) Relationship between IRI perspective-taking score and error rate in the front-facing condition of the perspective-taking task in males (empty circles, dotted line) and females (filled circles, solid line), separately. (B) Relationship between SPQ cognitive-perceptual score and error rate in the front-facing condition of the perspective-taking task, collapsed across genders.
Collapsed across groups, error rate in the front-facing, rs=−.36, p=.04, but not back-facing, rs=−.22, p=.22, condition was significantly negatively correlated with the SPQ Cognitive-Perceptual subscale (Figure 3B). That is, more accurate imagined self–other transformations in the perspective-taking task was associated with higher cognitive-perceptual abnormalities. No other significant correlations between schizotypy subscale scores and performance on the perspective-taking task were observed.
Letter Rotation correlations
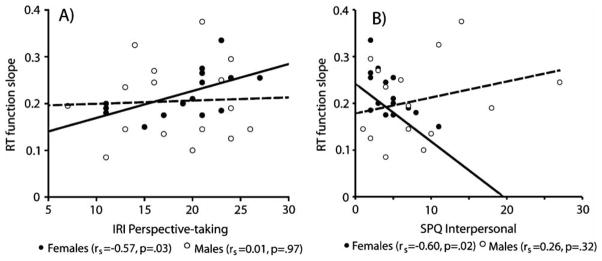
Although there were no significant correlations between the empathy subscale scores and either the slope of the RT function or overall error rate when genders were collapsed, in women, there was a significant positive correlation between the IRI PT score and slope of the RT function, rs=.57, p=.03 (Figure 4A). That is, in women, greater self-reported cognitive empathy was associated with slower speed of mental rotation. There were no other significant correlations between letter rotation performance and empathy subscale scores.
Figure 4.
(A) Relationship between IRI perspective-taking score and slope of the RT function in the letter rotation task in males (empty circles, dotted line) and females (filled circles, solid line), separately. (B) Relationship between SPQ interpersonal score and slope of RT function in the letter rotation task in males and females, separately.
No significant correlations were observed between SPQ subscale scores and either the slope of the RT function or overall error rate when genders were combined. However, in women, the slope of the RT function was negatively correlated with the SPQ Interpersonal subscale, rs=−.61, p=.02, such that faster mental rotation was associated with increased interpersonal deficits (Figure 4B). There were no other significant correlations between letter rotation performance and schizotypy subscale scores.
DISCUSSION
We examined the relationship between self-reported empathy and schizotypy and their relationship with both egocentric and allocentric visuospatial transformation ability in healthy young individuals. Like many prior reports, we found that RT and error rates increase with the degree of rotation in the letter rotation task, and performance was slower and more error prone in the perspective-taking task when an imagined self–other transformation was required. Furthermore, consistent with previous findings, self-reported cognitive and affective empathy were associated with reduced interpersonal deficits (i.e., negative schizotypy), and disorganised schizotypy was associated with reduced cognitive empathy. Both empathy and schizotypy were associated with performance on egocentric and allocentric mental rotation tasks. In women only, greater self-reported cognitive empathy was associated with more accurate performance on the perspective-taking task, but slower letter mental rotation. Like cognitive empathy, schizotypy was also related to visuospatial transformations. Collapsed across groups, more accurate performance on the perspective-taking task was associated with more cognitive-perceptual abnormalities (i.e., positive syndrome of schizotypy), and in women only, slower letter mental rotation was associated with reduced negative syndrome schizotypy. Each of these results is now discussed in turn.
Consistent with our hypothesis and a large number of previous studies on mental imagery, we found that spatial manipulation in mental space paralleled that in physical space. On the perspective-taking task, we found that participants were slower and less accurate in the front-facing condition, when an imagined spatial transformation of one’s own body was required. Similarly, in the letter rotation task we replicated findings of performance varying as a function of angular disparity; larger angles of mental rotation were accompanied by longer RTs and reduced accuracy. No gender differences were observed in performance on these visuospatial transformation tasks.
Our main goal was to understand the relationship between performance on these visuospatial transformation tasks, empathy and schizotypy. First, we predicted that schizotypy would be associated with decreased empathy, based on prior reports. Indeed, we found that both affective and cognitive components of empathy were associated with increased interpersonal aspects of schizotypy, and disorganised aspects of schizotypy were significantly associated only with cognitive empathy. We also found that positive schizotypy was associated with decreased cognitive empathy, but only in men. These findings are generally consistent with prior reports (Henry et al., 2008; Montag et al., 2007); however, we do not know the origins of these relationships. For example, is reduced empathic understanding a core problem that reduces interpersonal interactions, leading to negative syndrome? Or does the presence of negative syndrome such as avolition, anhedonia, and flat affect interfere with one’s ability or motivation to empathise with others? Moreover, is visuospatial processing a common, underlying function that links empathy and schizotypy?
Regarding the relationship between empathy and egocentric visuospatial transformation ability, we predicted that the speed of imagined visuospatial self–other transformations would be associated with decreased affective empathy in women, a finding that we previously reported (Thakkar et al., 2009). Contrary to our hypothesis, we found no relationship between the speed of imagined self–other transformations and cognitive or affective components of empathy. Instead we found that, in women, accuracy on the perspective-taking task was correlated with greater self-reported cognitive empathy, but only in the condition where an imagined change in perspective was required.
Although we did not replicate our prior finding of slower speed of imagined self–other transformations being associated with affective empathy in women (Thakkar et al, 2009), the result of the current study is not incompatible with that finding and is consistent with other extant findings. Similar to our current finding of more accurate self–other transformations being associated with cognitive empathy, Mohr and colleagues (in press) found that faster self–other transformations were associated with greater self-reported empathy, again only in women. However, the latency of imagined self–other transformations could arguably reflect different processes than the accuracy of these judgements. Whereas longer RTs reflect the duration of the mental perspective transformation, errors in the front-facing condition of this task could instead reflect failure to inhibit one’s own perspective. Recent neuropsychological and neuroimaging studies have provided evidence that inhibition of one’s own perspective and the adoption of another’s perspective rely on distinct mechanisms. Samson, Apperly, Kathirgamanathan, and Humphreys (2005) found impairments in the inhibition of visual and cognitive perspective in a patient with a right frontal lobe lesion; however, he was able to adopt another perspective as long as he did not hold a conflicting perspective. Additionally, in a fMRI experiment, Vogeley and colleagues (2001) found increased activity in right inferior frontal cortex during a mental state attribution task, but only when it was necessary for participants to inhibit their own mental states. On the other hand, based on lesion (Samson, Apperly, Chiavarino, & Humphreys, 2004) and neuroimaging studies (Ruby & Decety, 2004; Saxe & Wexler, 2005), it has been argued that the temporoparietal junction is crucial for representing another person’s belief. Thus, it is possible that, in women, greater ability to inhibit one’s own visual perspective shares a common mechanism with the ability to inhibit one’s own cognitive perspective, thereby leading to greater trait cognitive empathy. There was no significant correlation between error rate in the back-facing condition, which did not require a self–other transformation, and self-reported empathy, suggesting that the relationship between accuracy on the front-facing condition of the task and self-reported cognitive empathy is not simply a result of careless responding or inattention to task demands.
We also examined the relationship between schizotypy and egocentric visuospatial transformation ability. We predicted, first, that faster speed and increased accuracy of imagined self–other transformations would be associated with decreased positive syndrome schizotypy. Contrary to our hypothesis, we observed no significant relationship between the speed of self–other transformations and schizotypy. However, we found that greater accuracy on the perspective-taking task in the condition that required a self–other transformation was associated with increased positive syndrome schizotypy, suggesting that those individuals who tend to have anomalous cognitive and perceptual experiences are better at inhibiting their own perspective. At first glance this result seems at odds with Langdon and Coltheart’s (1999) finding that high schizotypal adults performed worse than low schizotypal adults when asked to judge the appearance of an object array after imagining they were in another perspective. However, high schizotypal adults performed better than low schizotypal adults when asked to judge the appearance of an object array when imagining that the array was rotating into their orientation. This crossover pattern was particularly apparent in those with higher cognitive-perceptual abnormality ratings. In our perspective-taking task, subjects could have either imagined themselves rotating into the position of the person on the screen, or imagined the person on the screen rotating into their position. These alternate strategies cannot be teased apart in the current data set. One potential explanation is that individuals high in schizotypy were more likely to use the latter strategy, and thus, this finding that positive schizotypy correlates positively with self–other transformation accuracy is consistent with prior findings. Enhanced egocentric manipulations may be just as problematic as an inability to imagine another person’s viewpoint, and either may lead to reduced empathic understanding. Indeed, a weakened sense of self–other distinction has been theorised to contribute to (Fenigstein & Vanable, 1992) and even define (Sass & Parnas, 2003) positive psychotic experiences.
Since, to our knowledge, no study has examined the association between allocentric visuospatial transformations and either empathy or schizotypy, we did not form any explicit hypotheses about these relationships. We found that slower mental rotation speed on the letter rotation task was associated with greater self-reported cognitive empathy, but only in women. This finding supports the hypothesis that, in women, cognitive empathy is related not only to imagined transformations of one’s body, but also to imagined transformations of objects in space. This finding is similar to our previous finding that, in women, longer time to imagine oneself in another position is related to greater self-reported affective empathy (Thakkar et al., 2009). Similarly, we also found that the interpersonal aspect of schizotypy, which exhibited a robust inverse correlation with cognitive empathy, was also correlated with the speed of allocentric mental rotation in women. That is, more interpersonal dysfunction was associated with faster mental rotation speed.
It is interesting to note that although cognitive empathy was associated with more accurate egocentric transformations in women, it was associated with slower allocentric transformations. As discussed earlier, different cognitive processes subserved by partially unique brain networks could be tapped by fast versus accurate mental transformations. It is possible that accuracy on the self–other transformation reflects inhibition of one’s own perspective, whereas RT reflects the efficiency of both egocentric and allocentric mental transformations.
One potential explanation for an association between faster allocentric mental rotation and both decreased cognitive empathy and more interpersonal dysfunction relates to the magnitude of hemispheric lateralisation. There is evidence for a right hemispheric superiority in allocentric mental rotation tasks (Corballis, 1997), and magnitude of right hemisphere specialisation on a tachistoscopic task was related to better mental rotation ability (Siegel-Hinson & McKeever, 2002). Although the right hemisphere has been more often implicated in social and emotional processes than the left hemisphere (Adolphs, 2001), there is little evidence that degree of right hemispheric specialisation predicts trait empathy or prosocial behaviour. On the contrary, reduced right compared to left hemispheric dominance for spatial attention, indexed using a line bisection task, has been found to correlate with greater self-reported affective empathy (Thakkar et al., 2009). Moreover, women, who have been found to outperform men on tests of social and emotional processing (e.g., Baron-Cohen & Wheelwright, 2004; Hall, 1978; Thayer & Johnsen, 2000), typically have less functionally lateralised brains (Voyer, 1996). Thus, one potential explanation for these findings is that reduced right hemispheric specialisation for spatial tasks is related to greater empathy and reduced interpersonal deficits.
An alternative hypothesis relates to modulation of spatial abilities by sex hormones. There is evidence for a curvilinear relationship between circulating and foetal environment androgen levels and spatial ability, such that too much or too little androgens are associated with poorer spatial abilities (Cole-Harding, Morstad, & Wilson, 1988; Gouchie & Kimura, 1991; Grimshaw, Sitarenios, & Finegan, 1995; Hampson, 1995; Resnick, Berenbaum, Gottesman, & Bouchard, 1986; Shute, Pellegrino, Hubert, & Reynolds, 1983). There is also evidence that lower oestrogen levels are associated with better spatial abilities in both women (Broverman et al., 1981; Hampson, 1990; Hampson & Kimura, 1988; Komnenich, Lane, Dickey, & Stone, 1978; Silverman & Phillips, 1993; but for exception see Gordon & Lee, 1993; Rosenberg & Park, 2002) and men (Gouchie & Kimura, 1991; Kozaki & Yasukouchi, 2008; Shute et al., 1983). Additionally, increased prenatal testosterone exposure and circulating testosterone have been linked to decreased empathy and theory of mind (Chapman et al., 2006; Harris, Rushton, Hampson, & Jackson, 1996; Knickmeyer & Baron-Cohen, 2006) and increased aggression (Berenbaum, Duck, & Bryk, 2000; Cohen-Bendahan, Buitelaar, van Goozen, Orlebeke, & Cohen-Kettenis, 2005; Hampson, Ellis, & Tenk, 2008; Harris et al., 1996). Therefore, the relationship between slower mental rotation being related to increased trait empathy and decreased interpersonal deficits in women could be mediated by levels of sex hormones; more testosterone and/or less oestrogen could lead to faster mental rotation, reduced self-reported empathy, and increased interpersonal deficits in women. Moreover, the magnitude of correlations between spatial transformation ability and empathy could vary with daily and monthly fluctuations in testosterone and oestrogen levels, which might explain sex differences in the strength of these correlations and mixed findings across studies. However, hypotheses of magnitude of hemispheric specialisation and levels of gonadal hormones are not necessarily mutually exclusive. Functional hemispheric asymmetries are typically more pronounced in men and there is evidence for a role of sex hormones in the development of functional asymmetries (see Wisniewski, 1998, for review).
Interpretation of these results must be considered in light of several methodological limitations. One important limitation of the current study is the correlational design, so the conclusions that can be based on these data are speculative. Also, we used a self-report measure of empathy, rather than a more objective test. There is evidence that women may inflate their scores when the nature of the questionnaire is obvious (Eisenberg & Lennon, 1983). Thus, higher scores could be reflective of social desirability instead of actual empathic ability. However, both empathy and social desirability are affiliative in nature and promote prosocial behaviour. Therefore, our data speak to the broader relationship between visuospatial transformation ability and affiliative behaviour. Further studies should investigate the relationship between visuospatial transformation ability and empathy by using multiple measures of empathy, including both the subjective self-report measures and experimental measures (e.g., Jackson, Meltzoff, & Decety, 2005). Additionally, the sample size is relatively small, particularly for analysing the data by gender, and thus correlations were not adjusted for multiple comparisons.
To conclude, we found that dimensions of both empathy and schizotypy were related to two types of visuospatial processes, self–other perspective transformations and allocentric mental rotation. Interestingly, these correlations were only observed in women. We hypothesise that these associations could be mediated by hemispheric asymmetry, levels of gonadal hormones, or both. Although further behavioural and neuroimaging data are needed to replicate and refine these results, these data posit an interesting link between spatial manipulations of our internal representations and our interactions with the physical world, and offer hypotheses for more rigorous future testing.
Acknowledgments
This work was supported in part by F31-MH085405-01 to KNT and NARSAD to SP. We would like to thank the individuals that permitted their photographs to be used as experimental stimuli.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. Rev. [Google Scholar]
- Baron-Cohen S, Joliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: Evidence from very high-functioning adults with autism or Asperger Syndrome. Journal of Child Psychology and Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with Asperger Syndrome or high functioning autism and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Duck SC, Bryk K. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2000;85(2):727–733. doi: 10.1210/jcem.85.2.6397. [DOI] [PubMed] [Google Scholar]
- Bracha HS. Is there a right hemi-hyper-dopaminergic psychosis? Schizophrenia Research. 1989;2(4–5):317–324. doi: 10.1016/0920-9964(89)90022-4. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Livingston RL, Clothier J, Linington BB, Karson CN. Correlation of severity of psychiatric patients’ delusions with right hemispatial inattention (left-turning behavior) American Journal of Psychiatry. 1993;150(2):330–332. doi: 10.1176/ajp.150.2.330. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Broverman DM, Vogel W, Klaiber EL, Majcher D, Shea D, Paul V. Changes in cognitive task performance across the menstrual cycle. Journal of Comparative and Physiological Psychology. 1981;95(4):646–654. doi: 10.1037/h0077796. [DOI] [PubMed] [Google Scholar]
- Brugger P, Graves RE. Right hemispatial inattention and magical ideation. European Archives of Psychiatry and Clinical Neuroscience. 1997;247(1):55–57. doi: 10.1007/BF02916254. [DOI] [PubMed] [Google Scholar]
- Brune M. “Theory of mind” in schizophrenia: A review of the literature. Schizophrenia Bulletin. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Chapman E, Baron-Cohen S, Auyeung B, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and empathy: Evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Social Neuroscience. 2006;1(2):135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Orlebeke JF, Cohen-Kettenis PT. Is there an effect of prenatal testosterone on aggression and other behavioral traits? A study comparing same-sex and opposite-sex twin girls. Hormones and Behavior. 2005;47(2):230–237. doi: 10.1016/j.yhbeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Cole-Harding S, Morstad AL, Wilson JR. Spatial abilities in members of opposite-sex twin pairs. Behavior Genetics. 1988;18:710. [Google Scholar]
- Corballis MC. Mental rotation and the right hemisphere. Brain and Language. 1997;57(1):100–121. doi: 10.1006/brln.1997.1835. [DOI] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–126. [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. BioPychoSocial Medicine. 2007;1:22–65. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vignemont F, Singer T. The empathic brain: How, when and why? Trends in Cognitive Sciences. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Aycicegi A, Greene P, Andover MS. Positive and negative schizotypy in a student sample: Neurocognitive and clinical correlates. Schizophrenia Research. 2002;56(1–2):171–185. doi: 10.1016/s0920-9964(01)00230-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Empathy: Conceptualization, measurement, and relation to prosocial behavior. Motivation and Emotion. 1990;14:131–149. [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94:100–131. [Google Scholar]
- Fenigstein A, Vanable PA. Paranoia and self-consciousness. Journal of Personality and Social Psychology. 1992;62(1):129–138. doi: 10.1037//0022-3514.62.1.129. [DOI] [PubMed] [Google Scholar]
- Gallese V. The manifold nature of interpersonal relations: The quest for a common mechanism. Philosophical Transactions of the Royal Society: Biological Sciences. 2003;358B(1431):517–528. doi: 10.1098/rstb.2002.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Jerussi TP, Fleisher LN. Turning in circles: The neuropharmacology of rotation. Life Sciences. 1976;18(9):889–896. doi: 10.1016/0024-3205(76)90405-7. [DOI] [PubMed] [Google Scholar]
- Gordon HW, Lee PA. No difference in cognitive performance between phases of the menstrual cycle. Psychoneuroendocinology. 1993;15:521–531. doi: 10.1016/0306-4530(93)90045-m. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Sitarenios G, Finegan JA. Mental rotation at 7 years: Relations with prenatal testosterone levels and spatial play experiences. Brain and Cognition. 1995;29(1):85–100. doi: 10.1006/brcg.1995.1269. [DOI] [PubMed] [Google Scholar]
- Hall JA. Gender differences in decoding nonverbal cues. Psychological Bulletin. 1978;85:845–857. [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15(2):97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hampson E. Spatial cognition in humans: Possible modulation by androgens and estrogens. Journal of Psychiatry and Neuroscience. 1995;20(5):397–404. [PMC free article] [PubMed] [Google Scholar]
- Hampson E, Ellis CL, Tenk CM. On the relation between 2D:4D and sex-dimorphic personality traits. Archives of Sexual Behavior. 2008;37(1):133–144. doi: 10.1007/s10508-007-9263-3. [DOI] [PubMed] [Google Scholar]
- Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behavioral Neuroscience. 1988;102(3):456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rushton JP, Hampson E, Jackson DN. Salivary testosterone and self-report aggressive and pro-social personality characteristics in men and women. Aggressive Behavior. 1996;22:321–331. [Google Scholar]
- Henry JD, Bailey PE, Rendell PG. Empathy, social functioning and schizotypy. Psychiatry Research. 2008;160(1):15–22. doi: 10.1016/j.psychres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Sex differences in empathy and related behaviors. Psychological Bulletin. 1977;84(4):712–722. [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Ickes W, Gesn PR, Graham T. Gender differences in empathic accuracy: Differential ability or differential motivation? Personal Relationships. 2000;7:95–109. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: A window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. Journal of Child Neurology. 2006;21(10):825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- Komnenich P, Lane DM, Dickey RP, Stone SC. Gonadal hormones and cognitive performance. Physiological Psychology. 1978;6:115–120. [Google Scholar]
- Kosslyn SM. Scanning visual images: Some structural implications. Perception and Psychophysics. 1973;14(1):90–94. [Google Scholar]
- Kozaki T, Yasukouchi A. Relationships between salivary estradiol and components of mental rotation in young men. Journal of Physiological Anthropology. 2008;27(1):19–24. doi: 10.2114/jpa2.27.19. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Langdon R, Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71(1):43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- Liouta E, Smith AD, Mohr C. Schizotypy and pseudoneglect: A critical update on theories of hemispheric asymmetries. Cognitive Neuropsychiatry. 2008;13(2):112–134. doi: 10.1080/13546800801936698. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Fink GR. Spatial cognition: Where we were and where we are. NeuroImage. 2001;14(1 Pt 2):S2–S7. doi: 10.1006/nimg.2001.0834. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Ropar D. Visuospatial abilities in autism: A review. Infant and Child Development. 2004;13:185–198. [Google Scholar]
- Mohr C, Blanke O, Brugger P. Perceptual aberrations impair mental own-body transformations. Behavioral Neuroscience. 2006;120(3):528–534. doi: 10.1037/0735-7044.120.3.528. [DOI] [PubMed] [Google Scholar]
- Mohr C, Bracha HS, Brugger P. Magical ideation modulates spatial behavior. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(2):168–174. doi: 10.1176/jnp.15.2.168. [DOI] [PubMed] [Google Scholar]
- Mohr C, Landis T, Bracha HS, Fathi M, Brugger P. Levodopa reverses gait asymmetries related to anhedonia and magical ideation. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(1):33–39. doi: 10.1007/s00406-004-0531-0. [DOI] [PubMed] [Google Scholar]
- Mohr C, Rowe AC, Blanke O. The influence of sex and empathy on putting oneself in the shoes of others. British Journal of Psychology. doi: 10.1348/000712609X457450. (in press) [DOI] [PubMed] [Google Scholar]
- Montag C, Heinz A, Kunz D, Gallinat J. Self-reported empathic abilities in schizophrenia. Schizophrenia Research. 2007;92:85–89. doi: 10.1016/j.schres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Morris CA, Mervis C. Williams Syndrome. In: Goldstein S, Reynolds C, editors. Handbook of neurodevelopmental and genetic disorders in children. New York: Guilford Press; 1999. pp. 555–590. [Google Scholar]
- Parsons LM. Imagined spatial transformation of one’s body. Journal of Experimental Psychology: General. 1987a;116(2):172–191. doi: 10.1037//0096-3445.116.2.172. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Imagined spatial transformations of one’s hands and feet. Cognitive Psychology. 1987b;19(2):178–241. doi: 10.1016/0010-0285(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25(1):1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia Bulletin. 1991;17(4):555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Raine A. Sex differences in schizotypal personality in a nonclinical population. Journal of Abnormal Psychology. 1992;101(2):361–364. doi: 10.1037//0021-843x.101.2.361. [DOI] [PubMed] [Google Scholar]
- Raine A, Reynolds C, Lencz T, Scerbo A, Triphon N, Kim D. Cognitive-perceptual, interpersonal, and disorganized features of schizotypal personality. Schizophrenia Bulletin. 1994;20(1):191–201. doi: 10.1093/schbul/20.1.191. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman I, Bouchard TJ. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Developmental Psychology. 1986;22:191–198. [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12(2):240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neu-roscience. 2004;16(6):988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience. 2004;7(5):499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: A case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–1111. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophrenia Bulletin. 2003;29(3):427–444. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Shute VJ, Pellegrino JW, Hubert L, Reynolds RW. The relationship between androgen levels and human spatial abilities. Bulletin of Psychonomic Society. 1983;21:465–468. [Google Scholar]
- Siegel-Hinson RI, McKeever WF. Hemispheric specialisation, spatial activity experience, and sex differences on tests of mental rotation ability. Laterality. 2002;7(1):59–74. doi: 10.1080/13576500143000078. [DOI] [PubMed] [Google Scholar]
- Silverman I, Phillips K. Effects of estrogen changes during the menstrual cycle on spatial performance. Ethology and Sociobiology. 1993;14:257–269. [Google Scholar]
- Taylor KI, Zach P, Brugger P. Why is magical ideation related to leftward deviation on an implicit line bisection task? Cortex. 2002;38(2):247–252. doi: 10.1016/s0010-9452(08)70653-1. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Brugger P, Park S. Exploring empathic space: Correlates of perspective transformation ability and biases in spatial attention. PLoS One. 2009;4(6):5864. doi: 10.1371/journal.pone.0005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Johnsen BH. Sex differences in judgement of facial affect: A multivariate analysis of recognition errors. Scandinavian Journal of Psychology. 2000;41:243–246. doi: 10.1111/1467-9450.00193. [DOI] [PubMed] [Google Scholar]
- Tost H, Alam T, Meyer-Lindenberg A. Dopamine and psychosis: Theory, patho-mechanisms and intermediate phenotypes. Neuroscience and Biobehavioral Reviews. 2010;34:689–700. doi: 10.1016/j.neubiorev.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Voyer D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality. 1996;1(1):51–83. doi: 10.1080/713754209. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23(5):519–547. doi: 10.1016/s0306-4530(98)00019-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Freed CR. The trained circling rat: A model for inducing unilateral caudate dopamine metabolism. Nature. 1982;298(5873):467–468. doi: 10.1038/298467a0. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Michelon P. Transformations of visuospatial images. Behavioral and Cognitive Neuroscience Reviews. 2005;4(2):96–118. doi: 10.1177/1534582305281085. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Rypma B, Gabrieli JD, Tversky B, Glover GH. Imagined transformations of bodies: An fMRI investigation. Neuropsychologia. 1999;37(9):1029–1040. doi: 10.1016/s0028-3932(99)00012-3. [DOI] [PubMed] [Google Scholar]