Abstract
Objective
To assess body mass index (BMI) effect on cesarean risk during labor.
Study Design
The Consortium on Safe Labor collected electronic data from 228,668 deliveries. Women with singletons ≥37 weeks and known BMI at labor admission were analyzed in this cohort study. Regression analysis generated relative risks for cesarean stratifying for parity and prior cesarean while controlling for covariates
Results
Of the 124,389 women, 14.0% had cesareans. Cesareans increased with increasing BMI for nulliparas, multiparas with and without a prior cesarean. Repeat cesareans were performed in >50% of laboring women with a BMI >40kg/m2. The risk for cesarean increased as BMI increased for all subgroups, p<0.001. The risk for cesarean increased by 5%, 2%, and 5% for nulliparas, multiparas with and without a prior cesarean, respectively, for each 1kg/m2 rise in BMI.
Conclusion
Admission BMI is significantly associated with delivery route in term laboring women. Parity and prior cesarean are other important predictors.
Keywords: Cesarean delivery, body mass index, obesity
Introduction
Amidst an epidemic of obesity in the United States, obesity among pregnant women has risen dramatically. The increased perinatal morbidity associated with maternal obesity such as birth defects, preeclampsia, gestational diabetes, stillbirth, abnormal fetal growth, and cesarean deliveries has caught the attention of obstetricians-gynecologists.(1) Long-term adverse outcomes of maternal obesity, including childhood and adolescent obesity for their offspring, are becoming well-known.(2,3) Another critical issue in obstetrics is the rising cesarean rate, estimated at 31.8% in 2007.(4) In addition to the known short-term complications such as infectious morbidity and thromboembolic events, cesarean deliveries are associated with long-term complications such as abnormal placentations and hysterectomies.(5,6)
Labor management as well as cesarean delivery in the obese gravida presents many clinical challenges. The relationship between body mass index (BMI) and cesarean delivery is well-established with some studies showing a direct linear relationship between the two.(7-10) However, prior studies have not independently evaluated the associations between parity, prior cesarean, BMI, and delivery route. The objective of this study is to characterize the role of BMI at labor admission on cesarean delivery via regression analysis using data from the Consortium on Safe Labor database.
Materials and Methods
This is an analysis of data from the Consortium on Safe Labor. The primary goal of the NICHD-sponsored Consortium on Safe Labor was to establish a comprehensive database from multiple sites and characterize labor and delivery in a contemporary group of women experiencing current obstetrical clinical practices. The complete database contained 228,668 deliveries between 2002 and 2008 acquired from electronic obstetrical databases. Twelve clinical centers from 19 distinct hospitals across 9 American College of Obstetricians and Gynecologists (ACOG) districts participated in the Consortium on Safe Labor. The majority (87%) of births occurred between 2005 and 2007. All births at 23 weeks or later were included in the database. Participating institutions extracted detailed information from their electronic medical records on maternal demographic characteristics, medical history, reproductive and prenatal history, labor and delivery summary, postpartum, and newborn information. An in-house obstetrician was available 24 hours per day at 11 of the 12 participating sites. The Institutional Review Boards of all participating institutions, the NICHD, and the Data Coordinating Center (EMMES Corporation) approved this project.
For the current cohort study, the inclusion criteria were live-born cephalic singletons at ≥ 37 0/7 weeks gestation with induced or spontaneous labor, defined as those who had a vaginal delivery or those who had at least two cervical examinations documented in the obstetrical database. As such, the intent was to exclude patients with a prelabor cesarean delivery. In addition, we included only cases where the maternal height and weight at the time of labor admission were available so as to calculate BMI in kg/m2 for each patient. Further, after applying the eligibility criteria noted above, about 6% of women contributed more than one delivery to the database. To avoid intra-person correlation, we selected the first delivery captured in the study irrespective of women's parity. The primary outcome was delivery route (i.e., cesarean or vaginal delivery). Independent variables considered in the statistical analyses and adjusted for in the regression analyses included maternal age, race, gestational age, parity, short stature (height <1.50m), prior cesarean delivery, pre-gestational or gestational diabetes, cervical dilation on admission (in centimeters), and induction of labor. These independent variables were selected not only because they were available at the time of admission to labor and delivery but also because they have been shown to be associated with delivery route. The data for maternal age, gestational age, and cervical dilation on admission were analyzed as continuous variables, while the other variables were analyzed as categorical except for BMI at admission. The latter was analyzed both continuously and in categories grouped by WHO criteria (normal <25.0 kg/m2, overweight 25.0-29.9 kg/m2, obese Class I 30.0-34.9 kg/m2, obese Class II 35.0-39.9 kg/m2, and obese Class III ≥ 40 kg/m2).(11) Because parity and prior cesarean are known determinants of cesarean delivery, the relationship between BMI at admission and cesarean was also examined within the three subgroups defined by these factors: nulliparas, multiparas with a prior cesarean, and multiparas without a prior cesarean. There were 6,025 multiparas in the eligible cohort wherein prior cesarean status was not noted in the electronic medical record. Because the proportion of these cases that had a cesarean (3.5%) was very similar to the proportion that had a cesarean in multiparas without a prior cesarean (4.9%), it was assumed the lack of a comment in the electronic medical record for these cases corresponded to no prior cesarean and therefore this group with missing prior cesarean status was analyzed with those without a prior cesarean.
Analyses included descriptive and univariate statistics (Chi-square and the non-parametric Wilcoxon Rank Sum test) for describing the relationship between the independent variables and delivery route. The Cochran-Armitage trend test was used to assess the linear trend relationship in the proportion with cesarean delivery by BMI category according to WHO criteria. Modified Poisson regression methodology (with robust error variance) estimated the unadjusted and adjusted relative risk (RR) and 95% confidence intervals (CI) of a cesarean delivery (12). Predicted means generated from the overall Poisson regression multivariate model, with BMI at admission as a continuous variable, were used to calculate predicted probabilities of a cesarean delivery for each delivery. After rounding the BMI to the closest integer, the predicted probabilities were averaged for each BMI at admission value between the range of 21 kg/m2 and 50 kg/m2, which represents the 1st and 99th percentiles of the data. A locally weighted scatterplot smoothing regression method was applied to the average predicted probabilities to generate a smoothed line to visually display the relationship between BMI at admission and probability of a cesarean section. Statistical analysis was performed using Statistical Analysis Software (Version 9.2., SAS Institute Inc., Cary, NC).
Results
The entire Consortium on Safe Labor database consisted of 228,668 deliveries. After exclusions (18% prelabor cesareans, 4% multiple gestations, 14% deliveries <37 weeks, 21% missing BMI data, or a combination of these factors), 132,165 met the eligibility criteria for the current study. After removing 7,776 deliveries of multiple pregnancies from the same mother and retaining the first delivery, 124,389 patients remained in the analysis dataset, of which 17,434 (14.0%) had a cesarean delivery performed during labor. Table 1 describes the demographic data of the current study, grouped by delivery route and stratified by parity and prior Cesarean status. Maternal age ≥ 35 years, short stature, black or hispanic race, nulliparity, less dilated cervices on labor admission, diabetes, and induced labor were more common in cesarean compared to vaginal deliveries. Only 4.3% of the total group had a prior cesarean, and of these, 63% delivered vaginally. The intrapartum cesareans amongst the 12 different participating sites ranged from 9.2-26.5%.
Table 1. Demographics and Other Characteristics of the Populationa.
| Characteristic | Total | Parity group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nulliparas | Prior cesarean | No prior cesareanb | |||||||
| Mode of delivery | Mode of delivery | Mode of delivery | Mode of delivery | ||||||
| Cesarean | Vaginal | Cesarean | Vaginal | Cesarean | Vaginal | Cesarean | Vaginal | ||
| Total n(%) | 17,434 (14.0) | 106,955 (86.0) | 12,500 (21.8) | 44,730 (78.2) | 1,977 (37.4) | 3,311 (62.4) | 2,957 (4.8) | 58,914 (95.2) | |
| Maternal age (years)c | <35 | 14,596 (83.7) | 93,673 (87.6) | 10,884 (87.1) | 41,791 (93.4) | 1,574 (79.6) | 2,607 (78.7) | 2,138 (72.3) | 49,275 (83.6) |
| ≥35 | 2,829 (16.2) | 13,188 (12.3) | 1,612 (12.9) | 2,896 (6.5) | 402 (20.3) | 700 (21.1) | 815 (27.6) | 9,592 (16.3) | |
| Mean (SD) | 27.6 (6.5) | 27.1 (6.0) | 26.5 (6.4) | 24.6 (5.8) | 29.4 (5. 8) | 29.8 (5.5) | 30.6 (6.0) | 28.8 (5.4) | |
| Maternal height (meters) | <1.5 | 747 (4.3) | 2,215 (2.1) | 519 (4.2) | 862 (1.9) | 106 (5.4) | 101 (3.1) | 122 (4.1) | 1252 (2.1) |
| ≥1.5 | 16,687 (95.7) | 104,740 (97.9) | 11,981 (95.8) | 43,868 (98.1) | 1,871 (94.6) | 3,210 (96.9) | 2,835 (95.9) | 57,662 (97.9) | |
| Mean (SD) | 1.62 (0.07) | 1.64 (0.07) | 1.62 (0.07) | 1.64 (0.07) | 1.62 (0.08) | 1.63 (0.07) | 1.62 (0.07) | 1.64 (0.07) | |
| Maternal Race | Black | 4,457 (25.6) | 20,931 (19.6) | 2,969 (23.8) | 8,651 (19.3) | 503 (25.4) | 746 (22.5) | 985 (22.5) | 11,534 (19.6) |
| Hispanic | 3,557 (20.4) | 19,102 (17.9) | 2,386 (19.1) | 7,525 (16.8) | 473 (23.9) | 614 (18.5) | 698 (23.6) | 10,963 (18.6) | |
| White | 7,443 (42.7) | 56,784 (53.1) | 5,669 (45.4) | 23,524 (52.6) | 771 (39.0) | 1,655 (50.0) | 1003 (33.9) | 31,605 (53.6) | |
| Other | 1977 (11.3) | 10,138 (9.5) | 1,476 (11.8) | 5,030 (11.8) | 230 (11.6) | 296 (8.9) | 271 (9.2) | 4,812 (8.2) | |
| Parity | Nulliparas | 12,500 (71.7) | 44,730 (41.8) | 12,500 (100.0) | 44,730 (100.0) | 0 | 0 | 0 | 0 |
| Multiparas | 4,934 (28.3) | 62,225 (58.2) | 0 | 0 | 1,977 (100.0) | 3,311 (100.0) | 2,957 (100.0) | 58,914 (100.0) | |
| BMI Category (kg/m2) | <25.0 | 1,327 (7.6) | 16,903 (15.8) | 1,011(8.1) | 8,102 (18.1) | 130 (6.6) | 393 (11.9) | 186 (6.3) | 8,408 (14.3) |
| 25.0-29.9 | 5,579 (32.0) | 44,005 (41.1) | 4,172 (33.4) | 19,381 (43.3) | 616 (31.2) | 1,275 (38.5) | 791 (26.8) | 23,349 (39.6) | |
| 30.0-34.9 | 5,157 (29.6) | 28,084 (26.3) | 3,676 (29.4) | 10,998 (24.6) | 583 (29.5) | 920 (27.8) | 898 (30.4) | 16,166 (27.4) | |
| 35.0-39.9 | 2,941 (16.9) | 11,496 (10.7) | 1,994 (16.0) | 4,051 (9.1) | 363(18.4) | 468 (14.1) | 584 (19.7) | 6,977 (11.8) | |
| >40.0 | 2430 (13.9) | 6,467 (6.0) | 1,647 (13.2) | 2,198 (4.9) | 285 (14.4) | 255 (7.7) | 498 (16.8) | 4,014 (6.8) | |
| Mean (SD) | 32.9 (6.9) | 30.2 (5.7) | 32.6 (6.8) | 29.6 (5.4) | 33.2 (6.9) | 31.1 (5.9) | 33.8 (7.0) | 30.5 (5.8) | |
| Gestational age (weeks) | Mean (SD) | 39.5 (1.2) | 39.2 (1.1) | 39.6 (1.2) | 39.4 (1.1) | 39.0 (1.2) | 39.2 (1.1) | 39.3 (1.2) | 39.1 (1.1) |
| Dilation at admission (cm)d | Mean (SD) | 2.2 (1.7) | 3.5 (2.2) | 2.0 (1. 7) | 3.1 (2.1) | 2.3 (1.8) | 3.9 (2.3) | 2.7 (1.8) | 3.8 (2.1) |
| Prior Cesarean | 1,977 (11.3) | 3,311 (3.1) | 0 | 0 | 1,977 (100.0) | 3,311 (100.0) | 0 | 0 | |
| Diabetes | 1,102 (6.3) | 3,988 (3.7) | 718 (5.7) | 1,288 (2.9) | 116 (5.9) | 172 (5.2) | 268 (9.1) | 2,528 (4.3) | |
| Induction | 10,261 (58.9) | 4,4109 (41.2) | 7,833 (62.7) | 18,688 (41.8) | 665 (33.6) | 868 (26.2) | 1,763 (59.6) | 24,553 (41.7) | |
All comparisons between delivery route (cesarean vs. vaginal) were significant (p<0.01) in each group (total, nulliparas, prior cesarean, no prior cesarean except for age category (< or ≥ 35 years) and diabetes in the prior cesarean group (p>0.05)
Includes cases with unknown prior Cesarean status
103 maternal ages were missing
22,332 cervical dilations were missing.
BMI body mass index; SD standard deviation
The mean BMI (kg/m2) of the patients delivered via cesarean was greater than those delivered vaginally (32.9±6.9 vs. 30.2±5.7). Nulliparas (32.6±6.8 vs. 29.6±5.4), multiparas with a prior cesarean (33.2±6.9 vs. 31.1±5.9), and multiparas without a prior cesarean (33.8±7.0 vs. 30.5±5.8) delivered by cesarean had a greater mean BMI (kg/m2) at labor admission compared to those delivered vaginally and each of these comparisons were statistically significant (p<0.001; Wilcoxon Rank Sum test). As shown in Table 2, the cesarean delivery percentage increased with BMI category in all subgroups, and was highest in multiparas with a prior cesarean. In these deliveries, the cesarean deliveries increased from 24.9% in those with BMI < 25.0 kg/m2 to 52.8% in women > 40 kg/m2 (Class III obesity) at labor admission. Multiparas without a prior cesarean had the lowest cesarean deliveries, 2.2% in those with BMI at admission < 25.0 kg/m2 which increased to 11.0% in women with a BMI > 40 kg/m2 at labor admission. Cesarean deliveries for nulliparas fell in between the other two subgroups, with cesarean delivery rates ranging from 11.1% in those with BMI at admission < 25.0 kg/m2 to 42.8% in those with BMI > 40 kg/m2.
Table 2. Percent of women delivered by cesarean, by BMI category, stratified by parity and prior cesarean delivery status.
| Total | Nulliparas | Multiparas and Prior Cesarean | Multiparas WithoutPrior Cesareana | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total deliveries | % Cesarean | Total deliveries | % Cesarean | Total deliveries | % Cesarean | Total deliveries | % Cesarean | ||
| Total | 124,389 | 14.0 | 57,230 | 21.8 | 5,288 | 37.4 | 61,871 | 4.8 | |
| BMI Categoryb | <25.0 | 18,230 | 7.3 | 9,113 | 11.1 | 523 | 24.9 | 8,594 | 2.2 |
| 25.0-29.9 | 49,584 | 11.3 | 23,553 | 17.7 | 1,891 | 32.6 | 24,140 | 3.3 | |
| 30.0-34.9 | 33,241 | 15.5 | 14,674 | 25.1 | 1,503 | 38.8 | 17,064 | 5.3 | |
| 35.0-39.9 | 14,437 | 20.4 | 6,045 | 33.0 | 831 | 43.7 | 7,561 | 7.7 | |
| ≥40.0 | 8,897 | 27.3 | 3,845 | 42.8 | 540 | 52.8 | 4,512 | 11.0 | |
Includes cases with unknown prior Cesarean status.
P< 0.0001 based on Cochran-Armitage Trend Test, overall and within parity and prior Cesarean groups.
BMI body mass index
After adjusting for potential confounding factors (site, maternal age, maternal height, maternal race, pre-gestational or gestational diabetes, cervical dilation at admission, and induction), the regression analyses presented in Table 3 confirmed the same trends observed in Table 2. In these regression analyses, BMI at admission was considered a continuous as well as a grouped variable based on the WHO criteria. Additionally, analyses compared all group categories to the lowest BMI category as a reference, < 25.0 kg/m2. In the adjusted analysis, the risk for cesarean delivery increased as BMI increased for all subgroups (p<0.001; Table 3). The increase in risk was similar in nulliparas and multiparas without prior cesarean, with the risk over 3 times greater in those with a BMI > 40 kg/m2 compared with the <25 kg/m2 reference group. There was approximately a doubling in risk for those with a prior cesarean. When evaluating BMI as a continuum, we found that the risk for cesarean increased by 4% for the total group, 5% for nulliparas, 2% for multiparas with a prior cesarean, and 5% for multiparas without a prior cesarean for each 1 kg/m2 increase in BMI. Increasing by one category in the BMI categories was associated with increases in risk for all groups (31% total group, 32% for nulliparas, 13% for multiparas with a prior cesarean, and 33% for multiparas without a prior cesarean). For the data in Table 3, we repeated the analysis for the multiparas without a prior cesarean subgroup omitting the 6,025 multiparas in the eligible cohort wherein prior cesarean status was not noted in the electronic medical record. The results of this regression analysis were still statistically significant and with nearly identical relative risks compared to the results of the original regression analysis in which those women were classified as delivering vaginally.
Table 3. Unadjusted and Adjusted Relative Risk for the Probability of Cesarean Delivery.
| Unadjusted analysisa | Adjusted analysisa,b | |||||
|---|---|---|---|---|---|---|
| Relative Risk | Lower 95th% CI | Upper 95th% CI | Relative Risk | Lower95th %CI | Upper 95th% CI | |
| Total (kg/m2) | N=124,389 | N=101,961 | ||||
| BMI continuous (per 1 kg/m2) | 1.05 | 1.05 | 1.06 | 1.04 | 1.04 | 1.05 |
| BMI categories (per 1 stepc) | 1.37 | 1.35 | 1.38 | 1.31 | 1.29 | 1.32 |
| <25 reference | 1.0 | 1.0 | ||||
| 25.0-29.9 | 1.55 | 1.46 | 1.64 | 1.47 | 1.38 | 1.56 |
| 30.0-34.9 | 2.13 | 2.01 | 2.26 | 1.96 | 1.84 | 2.09 |
| 35.0-39.9 | 2.80 | 2.63 | 2.97 | 2.50 | 2.34 | 2.67 |
| > 40 | 3.75 | 3.53 | 3.99 | 3.06 | 2.86 | 3.29 |
| Nulliparas (kg/m2) | N=57,230 | N=47,427 | ||||
| BMI continuous (per 1 kg/m2) | 1.05 | 1.05 | 1.06 | 1.05 | 1.04 | 1.05 |
| BMI categories (per 1 stepc) | 1.38 | 1.36 | 1.39 | 1.32 | 1.30 | 1.34 |
| <25.0 reference | 1.0 | 1.0 | ||||
| 25.0-29.9 | 1.60 | 1.50 | 1.70 | 1.50 | 1.40 | 1.61 |
| 30.0-34.9 | 2.26 | 2.12 | 2.41 | 2.02 | 1.88 | 2.17 |
| 35.0-39.9 | 2.97 | 2.78 | 3.18 | 2.61 | 2.42 | 2.82 |
| > 40 | 3.86 | 3.60 | 4.14 | 3.19 | 2.95 | 3.45 |
| Prior Cesarean (kg/m2) | N=5,288 | N=4,122 | ||||
| BMI continuous (per 1 kg/m2) | 1.03 | 1.02 | 1.03 | 1.02 | 1.02 | 1.03 |
| BMI categories (per 1 stepc) | 1.18 | 1.15 | 1.22 | 1.13 | 1.10 | 1.17 |
| <25.0 reference | 1.0 | 1.0 | ||||
| 25.0-29.9 | 1.31 | 1.11 | 1.54 | 1.25 | 1.06 | 1.47 |
| 30.0-34.9 | 1.56 | 1.33 | 1.83 | 1.51 | 1.28 | 1.77 |
| 35.0-39.9 | 1.76 | 1.49 | 2.08 | 1.48 | 1.24 | 1.76 |
| > 40 | 2.12 | 1.79 | 2.51 | 1.82 | 1.52 | 2.19 |
| Multiparas w/o prior Cesareand (kg/m2) | N=61,871 | N=50,412 | ||||
| BMI continuous (per 1 kg/m2) | 1.07 | 1.06 | 1.07 | 1.05 | 1.04 | 1.05 |
| BMI categories (per 1 stepc) | 1.51 | 1.46 | 1.55 | 1.33 | 1.28 | 1.38 |
| <25.0 reference | 1.0 | 1.0 | ||||
| 25.0-29.9 | 1.51 | 1.29 | 1.77 | 1.34 | 1.12 | 1.59 |
| 30.0-34.9 | 2.43 | 2.08 | 2.84 | 1.84 | 1.54 | 2.19 |
| 35.0-39.9 | 3.57 | 3.03 | 4.20 | 2.51 | 2.08 | 3.02 |
| > 40 | 5.10 | 4.33 | 6.01 | 3.04 | 2.51 | 3.70 |
All p values < 0.001 when comparing each BMI level to the < 25.0 kg/m2 reference and when BMI considered continuous or as a grouped-category, with the exception in the Prior Cesarean and BMI 25.0-29.9 kg/m2 subgroup compared with the <25.0 kg/m2 reference where p=0.001 for unadjusted and p=0.007 for adjusted analysis.
Adjusted for site, maternal age, maternal height, maternal race, pre-gestational or gestational diabetes, cervical dilation at admission, and induction.
Per 1 step increase in BMI category (e.g., from < 25.0 to 25.0-29.9 kg/m2)
Includes cases with unknown prior cesarean status.
BMI body mass index
CI confidence interval
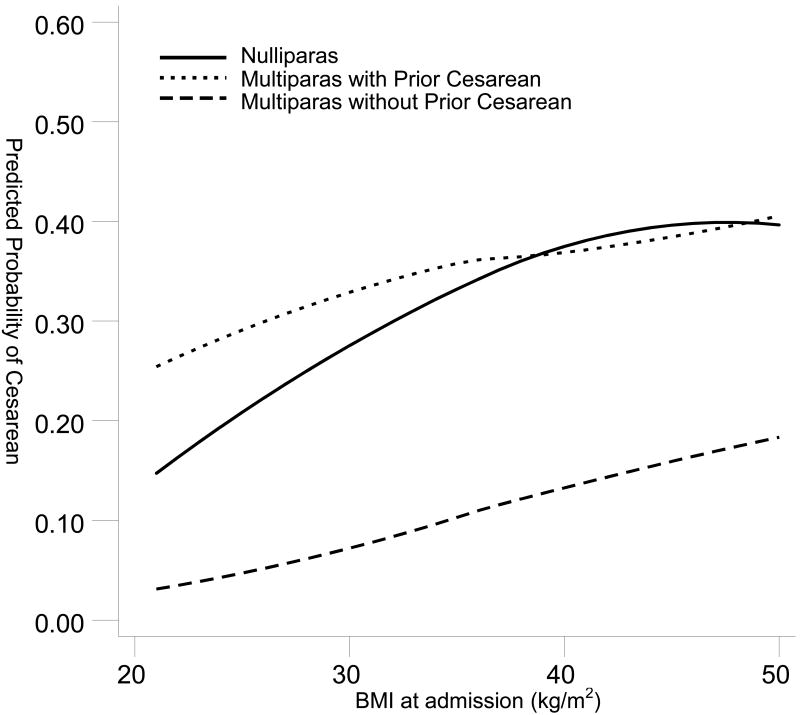
When examining the predicted probability of cesarean as BMI increased, there was a linear and rapid rise for nulliparas which was always greater than the less steep curve for the multiparas without a prior cesarean (Figure 1). The change in probability as BMI increased was not as marked for multiparas with a prior cesarean and it crossed the nulliparas' line at approximately 38 kg/m2.
Figure 1.
Predicted Probability of Cesarean with BMI as a Continuum Stratified by Parity and Prior Cesarean Status.
Comment
Increasing BMI is associated with an increased risk of perinatal complications, including cesarean delivery. As demonstrated in this multi-center study of electronic obstetrical databases, cesarean deliveries were more likely to occur in laboring patients with greater BMIs at labor admission. In addition, these findings were consistent across strata based on parity and prior cesarean status, although the magnitude of the effects differed among the subgroups. The relative effect was most pronounced for multiparas without a prior cesarean and Class III obesity, with 11% having a cesarean, compared to only 2% in those with BMI < 25 kg/m2. Furthermore, in a regression analysis, the risk for cesarean was 3.1 among women with Class III obesity after controlling for important variables such as diabetes, induction, and cervical dilation on admission. We also found that cesareans increased significantly across the different classes of obesity and this was a significant effect in the nulliparas as evidenced by non-overlapping confidence intervals (Table 3). The group with a prior cesarean had the smallest number of patients (n=5,288) and also had an attenuated increase in cesarean risk as BMI increased. This is likely due to the already high number of cesareans performed in the patients with a normal BMI (25%) in this group. We also determined that the risk for cesarean increased by 2-5% for each unit rise in BMI.
Although other investigators have reported on the increased risk for cesarean deliveries with increasing BMI (13-17) with unadjusted risks as high as 3.6, our study is unique in several ways. First, previous studies did not stratify by both parity and prior cesarean status. Lynch et al stratified cases by gravidity and found that primigravidas and multigravidas with a BMI >35 kg/m2 were 2.3 and 2.4 times more likely, respectively, to have a cesarean in labor compared to those with a normal BMI, respectively, but they did not account for those having a vaginal trial of labor after cesarean.(10) A large population based study which included pre-labor and intrapartum cesarean deliveries found a significant association between obesity and cesarean (odds ratio 3.2).(18) In their multivariable analysis, the odds ratio a repeat cesarean was 3.1 (p<0.001) for the obese group, but this represented only 1.4% of the total population.(18) Barau et al found a linear trend between BMI and cesarean with an OR of 3.6 for the 40-44.9 kg/m2 BMI group, but they included non-laboring patients as well (elective cesareans) and did not control for inductions and prior cesareans.(7) Although studies have similar findings with respect to BMI and cesarean risk, parity and prior cesarean status are important to stratify as the risks are different amongst the groups as determined by regression analysis in this study. Second, most studies have grouped patients with a BMI > 30 kg/m2 into one category.(8,9,13,17-19) Given the large number of obese patients in our database we were able to give detailed risks of cesarean deliveries within the different obesity classes. This is important given the linear relationship between BMI and cesarean and the BMI population trends in the United States. Finally, the number of subjects in this analysis exceeds all other studies on the risk of cesarean with increasing BMI.(7-10, 13, 17-20)
Unlike other studies regarding BMI and pregnancy, we chose to use the maternal weight on admission rather than the pre-pregnancy weight to calculate BMI because this variable would have more of an immediate impact on the performance of cesarean delivery as it takes into account weight gain during pregnancy. The one study that directly compared third trimester pregnancy weight to pre-pregnancy weight reported that the odds for cesarean increased more when BMI was calculated with a third trimester pregnancy weight (7.8% vs. 7.0% for each unit rise in BMI).(21) In addition, maternal weight on admission was more complete across all sites in the Consortium on Safe Labor database. It was the practice of the majority of the participating sites to calculate admission BMI based on patient self-report while other sites used the weight recorded from the most recent prenatal visit. We acknowledge the potential for inaccuracy with this practice, however, we were able to compare prepregnancy and labor admission weight for 83% of our cohort (n=103,062) and found that the mean weight gain during the pregnancy was 14.5kg. Furthermore, 98.4% (n=101,376) gained weight, 2.2% lost weight (n=22,658), and 0.3% (n=355) stayed the same as the prepregnancy weight. The differences in the prepregnancy and admission weight support that the admission BMI reflects a change from the prepregnancy weight. Although weighing patients at a labor admission is not routinely performed, this practice could provide more accurate data and impact the study of perinatal outcomes.
Although the mean BMI of the cohort was 30.5 kg/m2, this does not necessarily suggest that our patients were “obese” as defined by WHO criteria. As temporary weight gains are expected in pregnancy, crossing into an obese BMI category during a pregnancy does not necessarily increase long-term morbidity risk assuming the weight gained during pregnancy was eventually lost.(22) Given that the mean height of our population was 1.6m, a normal pre-pregnancy weight would be ≤65 kg. If a 65 kg woman gained the maximal recommended weight during pregnancy (35 lbs or 16 kg) (23), then the BMI at admission would be expected to be 31.6 kg/m2 or Class I obesity by WHO definitions. As such, a BMI in the obese range for a term gravida may be “normal” for pregnancy. However, the impact of this information is important since we determined that crossing into a greater BMI category increased the risk for cesarean by 30% for all categories except for those with a prior cesarean, which demonstrated only a 14% increase in risk for an increase in BMI category (Table 3). This highlights the difficulty of defining and characterizing obesity during pregnancy. We propose that BMI at admission, which also incorporates weight gain during pregnancy, rather than pre-pregnancy BMI, may more accurately predict cesarean risk. This hypothesis requires further testing, but is based on other studies and opinions that suggest that obese patients are more likely to require a cesarean because of a greater fetal size, a soft tissue obstruction to labor, poor uterine contractility, or a care-giver bias.(7,18-20,24) Although an explanation for this association is lacking, it is important to determine the underlying causes or mediators for cesarean deliveries as BMI increases. We did not specifically examine cesarean indications in this study and realize that the study design cannot determine whether or not the cesarean itself was indicated.
Since the goal was to provide information regarding cesarean risk based on the initial physical exam at admission and prior to delivery, we did not include variables such as the length of labor or infant weight. The multi-center approach allows for the information to be generalized to practices across the United States, but especially so for those who manage the labor of obese gravidas. Other studies have also addressed the decreasing success rate of vaginal birth after cesarean with increasing BMI (25-28). Our results parallel these findings where the vaginal birth after cesarean rate was only 47.2% with a BMI > 40.0 kg/m2.
Our findings support what is becoming widely-recognized and accepted clinical dictum—obesity increases the risk of cesarean delivery in an incremental and linear way. Furthermore, other important factors for cesarean delivery are parity and prior cesarean deliveries. As the obesity epidemic continues, this information will assist clinicians in counseling patients about the risk for cesareans. Although cesareans increased with increasing BMI and surpassed 50% in multiparas with a prior cesarean and Class III obesity, the findings do not suggest that labor should be abandoned in the obese gravida. Although there is a linear relationship with BMI and cesarean rate (7.3% for normal, 11.3% for overweight, 15.5% for obese Class I, 20.4% for obese Class II, and 27.3% for obese Class III categories, respectively), still 3 out of 4 morbidly obese (BMI >40 kg/m2) women who labored delivered vaginally in our study. Future research should identify the relative contribution of factors (i.e. fetal size, labor progress, patient counseling, and obstetrical decision-making) that might explain why obesity increases cesarean deliveries. Then the most important mechanisms can be targeted for further study and intervention. Given the increased risk of post-operative infectious morbidity and wound healing problems in obese patients, further research should also address whether the risk of peripartum complications in obese gravidas who labor exceeds the risk in obese gravidas who undergo cesarean delivery prior to labor.
Acknowledgments
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through a contract (Contract No. HHSN267200603425C).
Footnotes
Presented in part as a poster at the Annual Meeting for the Society of Maternal Fetal Medicine in Chicago, Illinois on February 3-6, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reece EA. Perspectives on obesity, pregnancy and birth outcomes in the United States: The scope of the problem. Am J Obstet Gynecol. 2008;198:23–27. doi: 10.1016/j.ajog.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 2.Boney C, Vohr BR, Verma A, Tucker R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker R. Predicting preschooler obesity at birth. The role of maternal obesity in early pregnancy. Pediatrics. 2004;114:29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2007. Nat Vital Stat Rep. 2009;57(12) [PubMed] [Google Scholar]
- 5.Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–32. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 6.Makoha FW, Felimban HM, Fathuddien MA, Roomi F, Ghabra T. Multiple cesarean section morbidity. Int J Gynecol Obstet. 2004;87:227–232. doi: 10.1016/j.ijgo.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Barau G, Robillard P, Hulsey T, et al. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. BJOG. 2006;113:1173–1177. doi: 10.1111/j.1471-0528.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol. 2004;191:969–74. doi: 10.1016/j.ajog.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar RK, Cooley SM, Donnelly JC, et al. The incidence and impact of increased body mass index on maternal and fetal morbidity in the low-risk primigravid population. J Mat Fetal Neonat Med. 2007;20:879–883. doi: 10.1080/14767050701713090. [DOI] [PubMed] [Google Scholar]
- 10.Lynch C, Sexton D, Hession M, Morrison J. Obesity and mode of delivery in primigravid and multigravid women. Am J Perinatol. 2008;25:163–167. doi: 10.1055/s-2008-1061496. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. World Health Organization Technical Report. Vol. 894. 2000. Obesity: preventing and managing a global epidemic; pp. i–xii.pp. 1–253. [PubMed] [Google Scholar]
- 12.Zho G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Seligman LC, Duncan BB, Branchtein L, et al. Obesity and gestational weight gain: cesarean delivery and labor complications. Rev Saúde Pública. 2006;40:457–65. doi: 10.1590/s0034-89102006000300014. [DOI] [PubMed] [Google Scholar]
- 14.Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstet Gynecol. 1998;91:97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- 15.Crane SS, Wojtowycz MA, Dye TD, Aubry RH, Artal R. Association between pre-pregnancy obesity and the risk of cesarean delivery. Obstet Gynecol. 1997;89:213–6. doi: 10.1016/S0029-7844(96)00449-8. [DOI] [PubMed] [Google Scholar]
- 16.Dietz PM, Callaghan WM, Morrow B, Cogswell ME. Population-based assessment of the risk of primary cesarean delivery due to excess prepregnancy weight among nulliparous women delivering term infants. Matern Child Health J. 2005;9:237–244. doi: 10.1007/s10995-005-0003-9. [DOI] [PubMed] [Google Scholar]
- 17.Vahratian A, Zhang J, Troendle JF, Savitz D, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstet Gynecol. 2004;104:943–951. doi: 10.1097/01.AOG.0000142713.53197.91. [DOI] [PubMed] [Google Scholar]
- 18.Sheiner E, Levy A, Menes TS, Silverberg D, Katz M, Mazor M. Maternal obesity as an independent risk factor for caesarean delivery. Paediatr Perinat Epidemiol. 2004;18:196–201. doi: 10.1111/j.1365-3016.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 19.Young TK, Woodmansee B. Factors that are associated with cesarean delivery in a large private practice: The importance of prepregnancy body mass index and weight gain. Am J Obstet Gynecol. 2002;187:312–320. doi: 10.1067/mob.2002.126200. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate – a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 21.Brost BC, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The preterm prediction study: Association of cesarean delivery with increases in maternal weight and body mass index. Am J Obstet Gynecol. 1997;177:333–41. doi: 10.1016/s0002-9378(97)70195-9. [DOI] [PubMed] [Google Scholar]
- 22.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine Consensus Report. Weight Gain During Pregnancy: Reexamining the Guidelines. May 28, 2009. [Google Scholar]
- 24.Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114:343–348. doi: 10.1111/j.1471-0528.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- 25.Edwards RK, Harnsberger S, Johnson IM, et al. Deciding on route of delivery for obese women with a prior cesarean delivery. Am J Obstet Gynecol. 2003;189:385–390. doi: 10.1067/s0002-9378(03)00710-5. [DOI] [PubMed] [Google Scholar]
- 26.Durnwald CP, Ehrenberg HM, Mercer BM. The impact of maternal obesity and weight gain on vaginal birth after cesarean section success. Am J Obstet Gynecol. 2004;191:954–957. doi: 10.1016/j.ajog.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 27.Goodall PT, Ahn JT, Chapa JB, Hibbard JU. Obesity as a risk factor for failed trial of labor in patients with previous cesarean delivery. Am J Obstet Gynecol. 2005;192:1423–1426. doi: 10.1016/j.ajog.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 28.Hibbard JU, Gilbert S, Landon MB, et al. Trial of labor or repeat cesarean delivery in women with morbid obesity and previous cesarean delivery. Obstet Gynecol. 2006;108:125–33. doi: 10.1097/01.AOG.0000223871.69852.31. [DOI] [PubMed] [Google Scholar]