Abstract
The incidence of excessive adiposity is increasing worldwide and is associated with numerous adverse health outcomes. We compared outcomes by body mass index (BMI) for adult patients with acute myeloid leukemia (AML) who underwent autologous (auto, n=373), related donor (RD, n=2041), or unrelated donor (URD, n=1801) allogeneic myeloablative hematopoietic cell transplantation (HCT) using marrow or peripheral blood stem cells reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1995-2004. Four weight groups by BMI (kg/m2) were defined: underweight < 18; normal 18 – 25; overweight >25 – 30; and obese > 30. Multivariable analysis referenced to the normal weight group showed an increased risk of death for underweight patients in the RD group (RR, 1.92; 95% CI, 1.28-2.89; P = 0.002) but not in the URD group. There were no other differences in outcomes among the other weight groups within the other HCT groups. Overweight and obese patients enjoyed a modest decrease in relapse incidence, though this did not translate into a survival benefit. Small numbers of patients limit the ability to better characterize the adverse outcomes seen in the underweight RD but not the underweight URD allogeneic HCT patients. Obesity alone should not be considered a barrier to HCT.
Keywords: Hematopoietic cell transplantation, obesity, outcomes, acute myeloid leukemia
INTRODUCTION
Obesity remains an increasingly prominent and challenging international health issue, particularly in the developed world(1-7). Excessive adiposity has been associated with a number of medical complications including cardiovascular disease and diabetes that could adversely impact outcomes for hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML)(6, 8, 9). AML is often optimally treated with HCT and in some cases, affords the best opportunity for long-term disease free survival. Recently, it was demonstrated that even in the intermediate-risk setting, allogeneic HCT (alloHCT) improves overall survival compared to other approaches(10). However, there has been concern that obese and overweight patients may not have equivalent outcomes when compared to those of normal weight. To date, transplant outcomes for patients with AML based on BMI have not been well-characterized.
In 2004, we published results from an observational study performed by the Center for International Blood and Marrow Transplantation Research (CIBMTR) for patients undergoing autologous HCT (autoHCT) for lymphoma(11). In that study, we showed that obese patients fared at least as well as patients with normal body mass index. The purpose of this study was to explore the impact of BMI in a different disease setting and in the context of allogeneic transplantation to understand if the previous observations regarding obesity apply.
PATIENTS AND METHODS
Data Sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) established in 2004 that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic SCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continual review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
Patients
Our study inclusion criteria included all patients with AML who received a first allogeneic marrow or peripheral stem cell graft HCT from a related donor (RD alloHCT) or an unrelated donor (URD alloHCT) or received an autoHCT between 1995 and 2004 and reported to the CIBMTR. Patients whose transplant center reported myeloablative conditioning (as determined by the transplant center) and whose disease status prior to transplant was reported as primary induction failure (PIF), first or second complete remission (CR1, CR2) or first relapse were included in this study. For the autologous cohort, recipients of purged grafts (n=55) were excluded. A total of 4,735 patients met these initial selection criteria. We further excluded 520 patients (74 autologous; 305 RD, 141 URD) from teams with inadequate follow-up or inconsistent reporting over the study period in order to reduce selection and reporting bias of patients. To ensure that the research patients were representative of all registered patients in the CIBMTR database, demographics and relapse and survival rates between research and registered patients were compared and no differences were noted.
The final study population included 1,801 and 2,041 patients who received an URDalloHCT or RDalloHCT, respectively, and 373 patients who underwent autoHCT. Patients were divided into groups based on body mass index (BMI) calculated from weight at the time of transplantation. Weight groups were defined according to consensus weight designations by the World Health Organization(12) and the National Heart Lung and Blood Institute Expert Panel(13) as follows: underweight, BMI <18 kg/m2; normal, BMI 18 to 25 kg/m2; overweight, BMI >25 to 30 kg/m2; and obese, BMI >30 kg/m2. Obese (BMI >30 to 34) and morbidly obese groups (BMI ≥ 35) were combined for all analyses after confirmation of the lack of significant outcomes differences when analyzed separately (see section on overall survival-multivariate analysis for details).
Data Collection
All missing or inconsistent data at the time of data file preparation were queried. Unavailable data from the transplant centers was treated as missing in the analysis. Cytogenetics data at the time of diagnosis or prior to transplantation were queried if not previously reported. Cytogenetic data were available for 79% of patients. Follow-up was updated for all patients in the data file. The median follow-up by transplant type and the completeness of follow-up index at 3 years(14) were 74 months and 94% for RD alloHCT, 58 months and 86% for URD alloHCT, and 85 months and 80% for autoHCT, respectively.
Study End Points
Primary end points were overall survival (OS), transplant-related mortality (TRM), relapse, and leukemia-free survival (LFS). OS was defined as time to death from any cause; surviving patients were censored at time of last follow-up. TRM was defined as death within the first 28 days of transplantation from any cause or death in continuous complete remission at any subsequent time point. Relapse was defined as the time to onset of clinical or hematologic recurrence, disease progression, or persistent disease. For relapse, patients with persistent disease were considered events at day 28. LFS was defined as survival in continuous complete remission of primary disease; disease relapse or persistence, or deaths were considered as events.
Secondary end points studied included rates of primary neutrophil and platelet engraftment, grade II-IV acute graft-versus-host disease (GVHD), and chronic GVHD. Neutrophil engraftment was defined as the time to achieve a sustained absolute neutrophil count ≥500 cells/μL for three consecutive days. Time to platelet engraftment was defined as time to achieve a platelet count of 20,000/μL, evaluable at 7 days from the last platelet transfusion.
Acute and chronic GVHD were graded by the transplant center according to standard criteria(15, 16).
Statistical Analysis
Patient-, disease-, and transplant-related factors were compared among the four BMI groups by using the Chi-squared test for categorical variables and the Kruskal-Wallis test for continuous variables. Univariate probabilities LFS and OS were estimated by using the Kaplan-Meier method(17). The log rank test was used for comparing survival curves. Probabilities of TRM, relapse, neutrophil engraftment, platelet engraftment, acute and chronic GVHD were estimated by using cumulative incidence to allow for competing risks. In the multivariate analyses we used Cox proportional hazards regression models separately for each donor type. Models were constructed to compare the outcomes among the four BMI groups, with normal BMI used as the baseline group, while adjusting for all covariates listed in the demographics tables (Table 1). A model was built for each primary outcome of interest as a dependent variable and all the relevant exposure variables as explanatory variables. A main effect term for the four BMI groups was forced into the model. The proportional hazards assumption for all the variables was examined by using time-varying covariates, but violations of this assumption were not detected. Interactions between weight groups and other significant explanatory variables were explored but none were found significant. The models were adjusted for the geographical region of the patient (US, Canada, Europe, Asia, Australia/New Zealand, Mideast/Africa, Central/South America) using a stratified Cox model to account for imbalances in the BMI groups by region. Bonferroni corrections were applied to allow adjustment for multiple comparisons between each weight group and the normal weight group. A P value <.0167 was therefore considered statistically significant, whereas the P values for inclusion in the final models of all other potentially confounding covariates were set at <.05. Comparisons of all secondary outcomes were limited to univariate comparisons.
Table 1. Characteristics of patients with age ≥18 years, who underwent a myeloablative bone marrow or peripheral blood transplantation for AML, reported to the CIBMTR between 1995 and 2004.
| Characteristics of patients | Total N (%) |
Underweight N (%) |
Normal N (%) |
Overweight N (%) |
Obese/Morbidly Obese (N %) |
P-valuec |
|---|---|---|---|---|---|---|
| Autologous | ||||||
| Number of patients | 373 | - | 169 | 119 | 85 | |
| Age, median (range), years | 46 (18-71) | - | 42 (18-71) | 50 (19-71) | 49 (18-70) | 0.001 |
| Male sex | 183 (49) | - | 74 (43) | 73 (61) | 36 (42) | 0.005 |
| Karnofsky score≥ 90 | 285 (76) | - | 128 (76) | 92 (77) | 65 (77) | 0.989 |
| Disease status at transplant | - | 0.069 | ||||
| PIF/1st Relapse | 27 ( 7) | - | 14 (8) | 9 (8) | 4 (5) | |
| CR1 | 247 (68) | - | 122 (74) | 68 (59) | 57 (67) | |
| CR2 | 92 (25) | - | 30 (18) | 38 (33) | 24 (28) | |
| Cytogenetics | - | 0.049 | ||||
| Favorable risk | 66 (18) | - | 33 (20) | 16 (13) | 17 (20) | |
| Intermediate risk | 204 (54) | - | 83 (49) | 75 (63) | 46 (55) | |
| High risk | 30 ( 8) | - | 15 (9) | 6 (5) | 9 (11) | |
| Unknowna | 73 (20) | - | 38 (22) | 22 (18) | 13 (15) | |
| Peripheral blood Graft | 308 (83) | - | 141 (83) | 95 (80) | 72 (85) | |
| Total body irradiation | 98 (97) | - | 47 (94) | 31 (100) | 20 (100) | 0.206 |
| Lung shielding for TBI | 24 ( 6) | - | 9 (5) | 8 (7) | 7 (8) | 0.663 |
| Year of Transplant | - | 0.045 | ||||
| 1995-1999 | 271 (73) | - | 130 (77) | 88 (74) | 53 (62) | |
| 2000-2004 | 102 (27) | - | 39 (23) | 31 (26) | 32 (38) | |
| Median follow-up, (range), months | 85 (3-148) | - | 87 (3-148) | 80 (8-139) | 72 (12-137) | |
| HLA-match Sibling Donor Allogeneic | ||||||
| Number of patients | 2041 | 32 | 1178 | 552 | 279 | |
| Age, median (range), years | 39 (19-67) | 36 (19-54) | 37 (18-67) | 43 (18-67) | 43 (18-64) | <0.001 |
| Male sex | 1065 (52) | 13 (41) | 603 (51) | 323 (59) | 126 (45) | 0.001 |
| Karnofsky score≥ 90 | 1398 (69) | 19 (59) | 811 (69) | 389 (70) | 179 (64) | 0.353 |
| Disease status at transplant | <0.001 | |||||
| PIF/1st Relapse | 572 (28) | 12 (38) | 322 (27) | 744 (26) | 94 (34) | |
| CR1 | 1172 (57) | 11 (34) | 693 (59) | 331 (60) | 137 (49) | |
| CR2 | 297 (15) | 9 (28) | 163 (14) | 77 (14) | 48 (17) | |
| AML Cytogenetics | 0.327 | |||||
| Favorable risk | 223 (11) | 3 (9) | 132 (11) | 56 (10) | 32 (11) | |
| Intermediate risk | 1078 (53) | 15 (47) | 614 (52) | 290 (53) | 159 (57) | |
| High risk | 275 (13) | 8 (25) | 158 (13) | 79 (14) | 30 (11) | |
| Unknowna | 465 (23) | 6 (19) | 274 (23) | 127 (23) | 58 (21) | |
| Year of Transplant | 0.124 | |||||
| 1995-1999 | 1300 (64) | 17 (53) | 769 (65) | 350 (63) | 164 (59) | |
| 2000-2004 | 741 (36) | 15 (47) | 409 (35) | 202 (37) | 115 (41) | |
| Bone marrow graft | 1004 (49) | 12 (38) | 605 (51) | 262 (47) | 125 (45) | |
| Total body irradiation | 873 (98) | 12 (100) | 522 (98) | 231 (98) | 108 (97) | 0.851 |
| Lung shielding for TBI | 506 (25) | 9 (28) | 328 (28) | 115 (21) | 54 (19) | 0.001 |
| GVHD Prophylaxis | 0.084 | |||||
| T-cell depletion | 105 (5) | -- | 56 ( 5) | 31 ( 6) | 18 ( 6) | |
| CNI + MTX ± other | 1555 (76) | 24 (75) | 904 (77) | 427 (77) | 200 (72) | |
| Other | 381 (19) | 8 (25) | 218 (18) | 94 (17) | 61 (22) | |
| Median follow-up (range), months | 74 (2-152) | 83 (24-142) | 74 (2-152) | 81 (3-149) | 73 (3-152) | |
| Unrelated Donor Allogeneic | ||||||
| Number of patients | 1801 | 33 | 864 | 529 | 375 | |
| Age, median (range), years | 40 (18-70) | 26 (18-52) | 37 (18-65) | 42 (18-70) | 42 (19-68) | <0.001 |
| Male sex | 954 (53) | 14 (42) | 430 (50) | 320 (60) | 190 (51) | <0.001 |
| Karnofsky score ≥ 90 | 1102 (61) | 14 (42) | 520 (60) | 339 (64) | 229 (61) | 0.008 |
| Disease status at transplant | <0.001 | |||||
| PIF/1st Relapse | 729 (41) | 14 (42) | 368 (43) | 421 (40) | 133 (36) | |
| CR1 | 549 (30) | 14 (42) | 268 (31) | 172 (33) | 95 (25) | |
| CR2 | 523 (29) | 5 (15) | 228 (26) | 143 (27) | 147 (39) | |
| Cytogenetics at diagnosis | 0.020 | |||||
| Favorable risk | 191 (11) | 3 (9) | 88 (10) | 40 (8) | 60 (16) | |
| Intermediate risk | 897 (50) | 17 (51) | 438 (51) | 275 (52) | 167 (47) | |
| Unfavorable risk | 378 (21) | 9 (27) | 188 (22) | 111 (21) | 70 (19) | |
| Unknowna | 335 (19) | 4 (12) | 150 (17) | 103 (19) | 78 (21) | |
| Year of Transplant | 0.074 | |||||
| 1995-1999 | 747 (41) | 16 (48) | 383 (44) | 205 (39) | 143 (38) | |
| 2000-2004 | 1054 (59) | 17 (52) | 481 (56) | 324 (61) | 232 (62) | |
| Bone marrow Graft | 1295 (72) | 25(76) | 629 (73) | 383 (72) | 258 (69) | |
| TBI Conditioning | 150 ( 8) | 7 (21) | 83 (10) | 45 ( 9) | 15 ( 4) | 0.040 |
| Lung shielding for TBI | 150 ( 8) | 7 (21) | 83 (10) | 45 ( 9) | 15 ( 4) | <0.001 |
| GVHD Prophylaxis | 0.247 | |||||
| T-cell depletion | 227 (13) | 3 ( 9) | 101 (12) | 62 (12) | 61 (16) | |
| CNI +MTX ± other | 1359 (75) | 30 (91) | 664 (77) | 400 (76) | 265 (71) | |
| Other | 215 (12) | - | 99 (11) | 67 (12) | 49 (13) | |
| HLA Match statusb | <0.001 | |||||
| Well matched | 699 (39) | 6 (18) | 314 (36) | 202 (38) | 177 (47) | |
| Partially matched | 643 (36) | 14(42) | 315 (36) | 193 (36) | 121 (32) | |
| Mismatched | 297 (16) | 5 (15) | 143 (17) | 86 (16) | 63 (17) | |
| Missing HLA data | 162 ( 9) | 8 (24) | 92 (11) | 48 ( 9) | 14 ( 4) | |
| Median follow-up, (range), months | 58 (3-149) | 57 (31-119) | 60 (4-149) | 54 (4-146) | 51 (3-136) |
Abbreviations: HLA= human leukocyte antigen; GVHD=graft versus host disease; CNI = calcineurin inhibitors, MTX = methotrexate, PIF=primary induction failure; CR1=first complete remission; CR2=second complete remission.
Unknown cytogenetics includes patients not tested for cytogenetics or, have insufficient cytogenetic information to categorize or have non evaluable metaphases.
HLA Match status: Well matched was defined as no known disparity at HLA A,B,C,DRB1, partially matched as one locus known or likely disparity with their donors and mismatched as ≥2 locus disparity.
Chi-square p-value was calculated for categorical values
RESULTS
Patient Characteristics
Patients included in this analysis were age 18 years or older, with AML in first or second complete remission (CR), in first relapse, or with primary induction failure after initial therapy who underwent HCT between 1995-2004, inclusive. A total of 4,215 patients were evaluated in this study. Comparisons of patient-, disease-, and transplant-related characteristics among the weight groups are listed in Table 1. Because of low numbers of patients in the underweight arm for those undergoing autoHCT (n=5), this group was omitted from analysis. With respect to the key risk characteristics of age, Karnofsky performance status (KPS) < 90, disease status at transplant, cytogenetic risk group, and, for unrelated allogeneic transplants, donor matching, no differences among the normal weight, overweight, and obese groups were observed. For the underweight group, there were some differences compared to the normal weight group for the RD alloHCT group (more primary induction failures [PIF] and first relapses: 38% vs 28%, respectively) and for the URD alloHCT group (median age: 26 vs 40; KPS <90: 58 vs 32%; and well-matched donor(18): 18 vs 39%, respectively). Table 2 summarizes the rates of neutrophil and platelet engraftment according to BMI group and transplant type. Hematopoietic recovery was similar among all BMI groups.
Table 2. Univariate probabilities of patients ≥ 18 years of age who received a myeloablative bone marrow or peripheral blood transplantation for AML, from a related donor, reported to the CIBMTR between 1995 and 2004.
| Outcome event | Total N (eval) |
Underweight | Normal | Overweight | Obese/morbidly obese |
P-valuea |
|---|---|---|---|---|---|---|
| Autologous | ||||||
| Transplant-related mortalityb | 364 | |||||
| @ 1 year | - | 5 (2-9)% | 4 (1-9) % | 5 (1-11) % | 0.973 | |
| @ 3 years | - | 6 (3-10) % | 6 (3-11) % | 6 (2-13) % | 0.998 | |
| Relapsec | 364 | |||||
| @ 1 year | - | 36 (29-44) % | 45 (36-54) % | 38 (28-49)% | 0.341 | |
| @ 3 years | - | 46 (38-54) % | 53 (44-62) % | 47 (36-58)% | 0.506 | |
| Neutrophil Recoveryc | 365 | |||||
| @ 60 days | - | 96 (92-99) % | 98 (95-100)% | 95 (90-99) % | 0.417 | |
| Platelet engraftmentc | 357 | |||||
| @ 100 days | - | 78 (72-84) % | 83 (75-89) % | 86 (77-92) % | 0.345 | |
| Related Donor Allogeneic | ||||||
| Transplant-related mortalityb | 2008 | |||||
| @ 1 year | 29 (15-46) % | 17 (15 -19) % | 21 (18-25) % | 25 (16-31 )% | 0.007 | |
| @ 3 years | 29 (15-46) % | 21 (18-23) % | 25 (22-29) % | 30 (24-35) % | 0.010 | |
| Relapsec | 2008 | |||||
| @ 1 year | 39 (23-56) % | 24 (21-26) % | 22 (18-25) % | 26 (21-31) % | 0.214 | |
| @ 3 years | 42 (25-59) % | 30 (27-32) % | 27 (23-31) % | 31(26-37) % | 0.288 | |
| Neutrophil engraftmentc | 2026 | |||||
| @ 60 days | 94 (83-99) % | 96 (95-97) % | 95 (94-97) % | 96 (93-98) % | 0.916 | |
| Platelet engraftmentc | 1962 | |||||
| @ 100 days | 72 (55-86) % | 86 (84-88) % | 82 (79-86) % | 79 (74-84) % | 0.011 | |
| Chronic GVHDb | ||||||
| @ 1 year | 19 (7-34) % | 36 (34-39) % | 36 (32-40) % | 32 (27-38) % | 0.053 | |
| Acute GVHDb | ||||||
| Grades 2-4 @100 days | 1953 | 16 (6-31) % | 29 (27-32) % | 34 (30-38) % | 36 (30-42) % | 0.007 |
| Unrelated Donor Allogeneic | ||||||
| Transplant-related mortalityb | 1779 | |||||
| @ 1 year | 22 (10-38) % | 32 (28-35) % | 35 (31-39) % | 43 (38-48) % | 0.001 | |
| @ 3 years | 28 (14-45) % | 36 (32-39) % | 40 (35-44) % | 46 (41-51) % | 0.003 | |
| Relapseb | 1779 | |||||
| @ 1 year | 31 (17-48) % | 31 (28-34) % | 26 (23-30) % | 22 (18-27) % | 0.010 | |
| @ 3 years | 44 (27-61) % | 36 (33-39) % | 31 (27-35) % | 25 (21-29) % | <0.001 | |
| Neutrophil engraftmentb | 1797 | |||||
| @ 60 days | 97 (85-100)% | 91 (89-93) % | 91 (88-93) % | 91 (88-94) % | 0.520 | |
| Platelet engraftmentb | 1768 | |||||
| @ 100 days | 67 (50-82) % | 69 (66-72) % | 69 (65-73) % | 65 (61-70) % | 0.668 | |
| Chronic GVHDb | 1787 | |||||
| @ 1 year | 38 (22-55) % | 32 (29-36) % | 36 (32-41) % | 34 (29-38) % | 0.502 | |
| Acute GVHDb | 1773 | |||||
| Grades 2-4 @100 days | 48 (31-66) % | 44 (41-47) % | 46 (42-50) % | 50 (45-55) % | 0.228 |
Abbreviations: GVHD= graft vs. host disease
Point-wise p-value unless otherwise noted.
Probabilities of relapse, treatment-related mortality, engraftment and GVHD were calculated using the cumulative incidence.
Overall Survival-Univariate Analysis
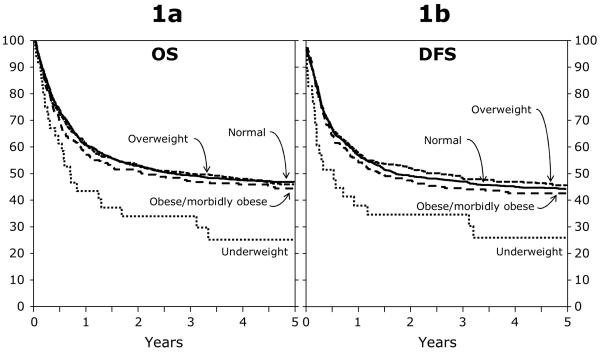
Figure 1 shows Kaplan-Meier estimates of OS by transplant type and weight group. For the RD alloHCT group, probabilities of OS in the univariate analysis were similar between the normal (63% [95% CI, 60%-66%]), and overweight (60% [95% CI, 56%-64%]) groups, slightly worse in the obese group (52% [95% CI, 47%-58%]), and markedly worse in the underweight group (38% [95% CI, 22%-55%]) at 1 year. Corresponding OS probabilities at 5 years were 47% (95% CI, 44%-50%), 44% (95% CI, 40%-49%), 37% (95% CI, 31%-43%), and 19% (95%CI, 6%-35%), respectively. For the URD alloHCT and the autoHCT groups, there were no statistically significant differences among the weight groups.
Figure 1.
a. Adjusted probability of overall survival among BMI groups for patients (≥ 18 years) after an RD alloHCT for AML between 1995 and 2004.
1b. Adjusted probability of disease free survival among BMI groups for patients (≥ 18 years) after an RD alloHCT for AML between 1995 and 2004
Overall Survival-Multivariate Analysis
In multivariate analysis (Table 3) in the RD alloHCT setting, with normal weight patients as the reference, the underweight group had a higher risk of mortality (RR, 1.92; 95% CI, 1.28-2.89; P = .002); there were no differences among the normal, overweight, and obese groups. The morbidly obese group (BMI ≥ 35) was analyzed separately for OS versus the normal weight group: RD group n=118, RR=1.05 (0.81-1.35), p=0.733; URD group: n=170, RR=1.11 (0.91-1.35), p=0.317. Other factors associated with higher risks of mortality were age >50 years, KPS <90%, and disease stage worse than first remission at transplantation; CSA +/− other, T cell depletion for GVHD prophylaxis (CSA/MTX as the reference group); high risk cytogenetics (normal cytogenetics as reference); and use of TBI. For the URD alloHCT and autoHCT groups, there were no differences in overall survival among the weight groups.
Table 3. Multivariable analysis of AML patients ≥18 years of age who received a bone marrow or peripheral blood transplant between 1995 and 2004, reported to the CIBMTR.
| HCT Type | Normal | Underweight | Overweight | Obese | Overall P-value |
|---|---|---|---|---|---|
| Autologous | n=164 | n=5 | n=112 | n=81 | |
| Death | -- | 0.98 (0.70-1.38) | 0.89 (0.61-1.29) | ||
| (p=0.925) | (p=0.532) | Poveral=0. 809 | |||
| Treatment failure | -- | 0.98 (0.70-1.38) | 0.12 (0.78-1.59) | ||
| (p=0.919) | (p=0.542) | Poverall=0.768 | |||
| Relapse | -- | 1.11 (0.76-1.62) | 1.19 (0.81-1.75) | ||
| (p=0.574) | (p=0.363) | Poverall=0.649 | |||
| TRM | -- | 0.79 (0.33-1.91) | 0.97 (0.37-2.52) | ||
| (p=0.606) | (p=0.953) | Poverall=0.861 | |||
|
| |||||
| Related Allogeneic | n=1176 | n=32 | n=553 | n=275 | |
| Death | 1.92 (1.28-2.89) | 1.05 (0.91-1.21) | 1.16 (0.97-1.38) | ||
| (p=0.002) | (p=0.532) | (p=0.109) | Poverall=0.008 | ||
| Treatment failure | 2.07 (1.36-3.13) | 0.97 (0.84-1.12) | 1.09 (0.91-1.31) | ||
| (p= <0.001) | (p= 0.720) | (p=0.330) | Poverall=0.005 | ||
| Relapse | 2.06 (1.20-3.54) | 0.87 (0.71-1.05) | 0.96 (0.75-1.23) | ||
| (p=0.009) | (p=0.146) | (p=0.757) | Poverall=0.020 | ||
| TRM | 2.23 (1.17-4.25) | 1.12 (0.90-1.38) | 1.27 (0.97-1.66) | ||
| (p=0.014) | (p=0.304) | (p=0.081) | Poverall=0.040 | ||
|
| |||||
| Unrelated Allogeneic | n=846 | n=31 | n=523 | n=368 | |
| Death | 0.86 (0.56-1.33) | 0.96 (0.84-1.09) | 1.04 (0.89-1.21) | ||
| (p=0.496) | (p=0.502) | (p=0.621) | Poverall=0.683 | ||
| Treatment failure | 0.91 (0.60-1.38) | 0.93 (0.82-1.06) | 0.99 (0.86-1.15) | ||
| (p=0.652) | (p=0.284) | (p=0.931) | Poverall= 0.716 | ||
| Relapse | 1.04 (0.60-1.78) | 0.82 (0.68-0.99) | 0.76 (0.60-0.96) | ||
| (p=0.893) | (p=0.044) | (p=0.022) | Poverall= 0.059 | ||
| TRM | 0.85 (0.44-1.66) | 1.03 (0.86-1.24) | 1.16 (0.96-1.41) | ||
| (p=0.635) | (p=0.712) | (p=0.129) | Poverall=0.439 | ||
Abbreviations: -- = not done due to insufficient number of pts; treatment failure = death or recurrence of disease; TRM = treatment related mortality
Transplant-Related Mortality
Point wise probabilities of TRM are summarized in Table 2. In multivariable analysis in the RD alloHCT setting, the underweight group experienced a relative risk (RR) of TRM of 2.23 (95% CI: 1.17-4.25; P =0.014) compared to the normal weight group. There were no differences among the other weight groups. Other significant variables increasing the risk of TRM were age >40, KPS <90, GVHD prophylaxis with CSA +/− other or T cell depletion, and a disease status of primary induction failure at transplant. A favorable factor was year of transplant between 2000 and 2004 (versus 1995 to1999). In the URD alloHCT and autoHCT settings, there were no differences among the BMI groups.
Relapse
Table 2 summarized the univariate probabilities of leukemia relapse by BMI group at each transplant type. In multivariate analysis, in the RD alloHCT setting, the underweight group had a higher risk of relapse compared to the normal weight group, with a RR of 2.06 (95% CI, 1.20-3.54, P = 0.009). There were no differences in the other weight groups. Interestingly, similar to the previous study of autoHCT for lymphoma, the relative risk of relapse was reduced for the URD alloHCT overweight (RR 0.82, 95% CI 0.68-0.99, P = 0.044) and obese (RR 0.76, 95% CI 0.0.60-0.96, P = 0.022) groups, though this difference did not translate into a survival benefit. There were no differences among the weight groups in the autoHCT group (underweight was excluded, n=5).
Leukemia-Free Survival
In multivariate analysis, LFS was worse in the RD alloHCT setting for the underweight group (RR 2.07, 95% CI 1.36-3.13, P = <0.001). Otherwise, no other differences were observed for any other groups in any other setting.
Acute and Chronic Graft-versus-Host Disease (GVHD)
The rates of acute and chronic GVHD by transplant type are summarized in Table 2. No statistically significant differences were observed among the weight groups in either allo HCT setting for either type of GVHD.
DISCUSSION
In this contemporary, retrospective, large study in AML patients, we demonstrated that obesity as defined by BMI at time of transplantation does not correlate with worse survival outcomes but that underweight recipients of RD allo HCT have shorter survival compared to patients within the normal BMI range. Similar to our previous study in patients with lymphoma, the current study demonstrates that obesity does not appear to represent a significant barrier to successful HCT for AML.
The impact of obesity on transplant outcomes remains controversial. The HCT-specific comorbidity index developed by Sorror et al included obesity (BMI >35kg/m2) as one of the components to predict non-relapse mortality at 2 years(19). This study included 708 patients in the training set who underwent allogeneic HCT for several indications; of these, 2% were obese. The data in this analysis predate the collection of HCT-CI-specific information initiated by CIBMTR in 2007 so no direct comparison is possible. However, transplant-related mortality in our study was not significantly higher in obese AML patients compared to normal weight patients, regardless of the donor type.
In the previous lymphoma study, we observed poorer outcomes in one of the underweight groups. Interestingly, poorer survival outcomes were observed in underweight patients in the RD allo HCT group but not the URD alloHCT group. Because of the small numbers of patients in the RD alloHCT group, there is some imbalance compared to the other weight groups with respect to disease status at time of transplantation with disproportionately more PIF/relapse and CR2 patients, though it is not clear how much this finding accounts for the difference in leukemia-free and overall survival. Such an imbalance of disease status was not seen in the underweight URD alloHCT group. It is noteworthy that the underweight RD alloHCT group had a similar KPS (P = 0.353) and cytogenetic risk (P = 0.327) compared to the other weight groups; these important factors do not appear to account for the difference in survival. It may also be that the higher risk of the URD alloHCT procedure masks important but less obvious risks associated with being underweight whereas in the related donor HCT setting, such risks become manifest. Small numbers of patients and lack of available data pertinent to nutritional status such as serum albumin or TPN use limit the ability to better characterize this observation in underweight patients. Moreover, the analysis does not account for unknown biological factors not included in the model that may be influencing outcomes in the underweight RD allo HCT group.
An important limitation of this study is that any conditioning regimen dose adjustments for overweight and obesity used by the various transplant centers could not be assessed from the CIBMTR data. Since chemotherapy dosing in the conditioning regimen may be based on actual weight or adjusted ideal body weight, clinical outcomes may have been confounded by whether dose adjustments were made for patients with high BMI. There is currently no accepted standard conditioning regimen dose adjustment schema based on weight and various methodologies are used, as was ascertained by Grigg and colleagues(20). A small study of AML patients undergoing autoHCT without dose adjustment has previously suggested that some adjustment may be beneficial, as the lack of conditioning regimen dose adjustment in that study resulted in unacceptable treatment-related mortality(21).
Similar to our previous study in lymphoma, the current study demonstrates that obesity does not appear to represent a significant barrier to successful HCT in AML. This conclusion must be tempered, however, with the acknowledgment that the patients who received myeloablative HCT were likely selected by their transplant centers, and were deemed to be “fit” to withstand the rigors of HCT. The limitations of pre-transplant co-morbidity data within the CIBMTR database preclude an assessment of this issue. Thus, the caveat is that it appears that overweight and obese patients have similar outcomes to normal weight patients when they otherwise appear to be eligible HCT candidates. Obesity alone, however, should not preclude HCT when appropriate for the treatment of AML.
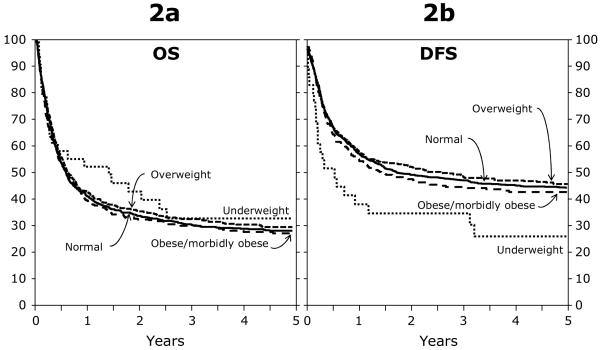
Figure 2.
a. Adjusted probability of overall survival among BMI groups for patients (≥ 18 years) after an URD alloHCT for AML between 1995 and 2004.
2b. Adjusted probability of disease free survival among BMI groups for patients (≥ 18 years) after an URD alloHCT for AML between 1995 and 2004.
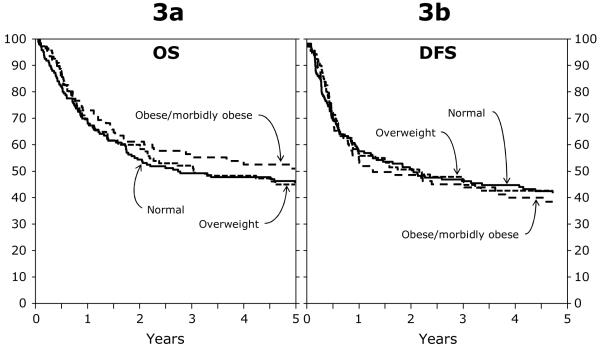
Figure 3.
a. Adjusted probability of overall survival among BMI groups for patients (≥ 18 years) after an autologous HCT for AML between 1995 and 2004.
3b. Adjusted probability of disease free survival among BMI groups for patients (≥ 18 years) after an autologous HCT for AML between 1995 and 2004.
Acknowledgments
SUPPORT
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Al Rashdan IR, Nesef YA. Prevalance of Overweight, Obesity, and Metabolic Syndrome Among Adult Kuwaitis: Results From Community-based National Survey. Angiology. 2009 doi: 10.1177/0003319709333226. [DOI] [PubMed] [Google Scholar]
- 2.Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008;9(Suppl 1):14–21. doi: 10.1111/j.1467-789X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Kosti RI, Panagiotakos DB. The epidemic of obesity in children and adolescents in the world. Cent Eur J Public Health. 2006;14:151–159. doi: 10.21101/cejph.a3398. [DOI] [PubMed] [Google Scholar]
- 4.Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 5.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 7.Oguz A, Temizhan A, Abaci A, et al. Obesity and abdominal obesity; an alarming challenge for cardio-metabolic risk in Turkish adults. Anadolu Kardiyol Derg. 2008;8:401–406. [PubMed] [Google Scholar]
- 8.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 9.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro WH, Loberiza FR, Jr., Bajorunaite R, et al. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:541–551. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 13.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 14.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigg A, Harun MH, Szer J. Variability in determination of body weight used for dosing busulphan and cyclophosphamide in adult patients: results of an international survey. Leuk Lymphoma. 1997;25:487–491. doi: 10.3109/10428199709039036. [DOI] [PubMed] [Google Scholar]
- 21.Meloni G, Proia A, Capria S, et al. Obesity and autologous stem cell transplantation in acute myeloid leukemia. Bone Marrow Transplant. 2001;28:365–367. doi: 10.1038/sj.bmt.1703145. [DOI] [PubMed] [Google Scholar]