Abstract
Nerve agents are acetylcholinesterase inhibitors, exposure to which causes brain damage, primarily by inducing intense seizure activity. Knowledge of the brain regions that are most vulnerable to nerve agent-induced brain damage can facilitate the development of drugs targeting the protection of these regions. Both the amygdala and the hippocampus have been shown to suffer significant damage after nerve agent exposure, but the amygdala appears to be the more severely affected structure. However, damage in the amygdala has generally been compared with damage in the dorsal hippocampus, whereas there is evidence that the ventral hippocampus is significantly more susceptible to seizures than the dorsal region, and, therefore, it may also be more susceptible to nerve agent-induced neuropathology. Here, we report that after status epilepticus induced by soman administration to rats, neuronal degeneration as assessed by Fluoro-Jade C staining was more extensive in the ventral than the dorsal hippocampal subfields, 1 day after soman exposure. Seven days later, the difference between dorsal and ventral regions was not statistically significant. In the amygdala, soman-induced neurodegeneration was more severe in the posteroventral regions of the lateral, basolateral, and medial nuclei compared to the anterodorsal regions of these nuclei. The basomedial nucleus was more severely affected in the anterodorsal region, while the central nucleus was less affected than the other amygdalar nuclei. The extent of neurodegeneration in the amygdala was not significantly different from that in the ventral hippocampus. However, when compared with the whole hippocampus, the amygdala displayed more severe neurodegeneration, on both day 1 and day 7 after soman exposure. Testing the protective efficacy of drugs against nerve agent-induced brain damage should include examination of the ventral hippocampus and the posteroventral regions of the amygdala, as these areas are most vulnerable to nerve agent-induced neurodegeneration.
Keywords: hippocampus, amygdala, soman, neuronal degeneration, nerve agents, status epilepticus
1. Introduction
Nerve agents are organophosphorus acetylcholinesterase inhibitors (Bajgar, 2005; Barthold and Schier, 2005; Layish et al., 2005; López-Muñoz et al., 2008). Exposure to nerve agents can cause death, or, even when death is prevented, brain damage may occur with serious neurological and behavioral consequences (McDonough et al., 1986; Morita et al., 1995; Brown and Brix, 1998; Kassa et al., 2001; Bajgar et al., 2004; Myhrer et al., 2005; Joosen et al., 2009). In acute exposure to toxic levels of a nerve agent, brain damage is primarily due to the intense seizure activity induced by these agents (Hayward et al., 1990; McDonough et al., 1995, 2000; Shih et al., 2003; Baille et al., 2005; Myhrer et al., 2005). Identifying the brain regions that are most vulnerable to nerve agents —in terms of generating seizure activity and undergoing neuropathological alterations— along with knowledge of the biochemistry and physiology of these regions can facilitate the development of effective antidotes that will reduce brain seizures and damage after exposure.
Previous animal studies have suggested that both the amygdala and the hippocampus play a major role in the generation of seizures by nerve agents, and suffer extensive neuronal damage after nerve agent exposure (Hayward et al., 1990; Kadar et al., 1992, 1995; Baze, 1993; McDonough et al., 2000; Shih et al., 2003; Myhrer et al., 2006). The evidence available so far suggests that of the two structures the amygdala may play the predominant role (for a review see Aroniadou-Anderjaska et al., 2009). Thus, the amygdala displays the earliest and most rapid increase in extracellular glutamate after in vivo exposure of rats to the nerve agent soman (Lallement et al., 1991), while, in vitro, the rat amygdala responds to soman with prolonged seizure-like neuronal discharges at a time when the hippocampus generates only interictal-like spikes (Apland et al., 2009). In addition, in guinea pigs, the amygdala displays the most severe neuropathology of all the brain regions examined after nerve agent exposure (McDonough et al., 2000; Shih et al., 2003). However, the rodent brain sections used in in vitro electrophysiology experiments comparing the responses of the amygdala and the hippocampus to nerve agents (Apland et al., 2009), or in pathology studies after in vivo nerve agent exposure (for example, Collombet et al., 2007), contain for the most part the dorsal portion of the hippocampus (relatively anterior coronal brain sections). A number of studies have suggested that the ventral hippocampus has a significantly greater seizure susceptibility and ability to propagate seizures to other brain regions compared to the dorsal hippocampus (Racine et al., 1977; Gilbert et al., 1985; Akaike et al., 2001). As seizure susceptibility may parallel vulnerability to damage by seizurogenic insults, the ventral hippocampus may suffer more severe damage than the dorsal hippocampus after nerve agent exposure. If that is the case, then the hippocampus may actually present more severe damage than the amygdala when the ventral hippocampus is also taken into account. In the present study, we investigated 1) whether the ventral hippocampus displays more neuronal degeneration than the dorsal hippocampus after soman exposure, and 2) how neuronal degeneration in the whole hippocampus compares with that in the amygdala.
1. Methods
2.1. Animals
Experiments were performed using male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), 5–6 weeks old, weighing 150–250 g at the start of the experiments. Animals were individually housed in an environmentally controlled room (20–23°C, 12-h light/12-h dark cycle, lights on 6:00 am), with food and water available ad libitum. The animal care and use programs at the U.S. Army Medical Research Institute of Chemical Defense and the Uniformed Services University of the Health Sciences are accredited by the AAALAC International. All animal experiments were conducted following the Guide for the Care and Use of LaboratoryAnimals (Institute of Laboratory Animal Resources, National Research Council), and were approved by the Institutional Animal Care and Use Committee.
2.2. Experimental procedures
Soman (pinacoyl methylphosphonofluoridate; obtained from the U.S. Army Edgewood Chemical Biological Center, Aberdeen Proving Ground, MD, USA) was diluted in cold saline, and administered via a single subcutaneous (s.c.) injection (154 µg/kg, 1.4 × LD50). To increase survival rate, all rats were administered an intraperitoneal (i.p.) injection of HI-6 (1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxapropane dichloride; 125 mg/kg), 30 min prior to soman. HI-6 is a bispyridinium oxime that can reactivate inhibited acetylcholinesterase, peripherally (Bajgar, 2005). Within 1 min after soman exposure, all rats also received an intramuscular (i.m.) injection of atropine methyl nitrate (4 mg/kg, Sigma, St. Louis, MO) to minimize peripheral toxic effects. All animals developed status epilepticus (SE), and were injected with diazepam (10 mg/kg i.m.) 2 hours after SE onset to stop the behavioral seizures. Animals that continued to experience convulsions received s.c. injections of lactated Ringer’s solution (total of 5 ml in divided doses) late in the afternoon of the day of the experiment, and again the following morning.
Behavioral seizures were characterized according to the Racine Scale (Racine, 1972; Racine et al., 1977) with minor modifications: Stage 0, no behavioral response; Stage 1, behavioral arrest; Stage 2, oral/ facial movements, chewing, head nodding; Stage 3, unilateral/bilateral forelimb clonus without rearing, Straub tail, extended body posture; Stage 4, bilateral forelimb clonus plus rearing; Stage 5, rearing and falling; and Stage 6, tonic-clonic seizures.
2.3. Fixation & Tissue Processing
Animals surviving soman-induced SE (70.5% survival rate) were used to examine neuronal degeneration in the amygdala and hippocampus. One day or 7 days after soman exposure, rats were deeply anesthetized using pentobarbital sodium (75–100 mg/kg, i.p.) and transcardially perfused with phosphate buffered saline (PBS, 100 ml) followed by 4% paraformaldehyde (200 ml). The brains were removed and post-fixed overnight at 4° C, then transferred to a solution of 30% sucrose in PBS for 72 hours, and frozen with dry ice before storage at −80° C until sectioning. A 1-in-5 series of sections containing the rostro-caudal extent of the amygdala and the hippocampus was cut at 40 µm thickness on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus, Daigger, Vernon Hills, IL) for Nissl staining with cresyl violet. An adjacent series of sections was also mounted on slides for Fluoro-Jade C (FJC) staining.
2.4. Fluoro-Jade C Staining
FJC (Histo-Chem, Jefferson, AK) was used to identify irreversibly degenerating neurons in the amygdala and hippocampus, at 1 or 7 days after soman exposure. Mounted sections were air-dried overnight, and then immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min. The slides were then rinsed for 2 min in 70% ethanol, 2 min in deionized H20 (dH20), and incubated in 0.06% potassium permanganate solution for 10 min. After a 2-min rinse in dH2O, the slides were transferred to a 0.0001% solution of FJC dissolved in 0.1% acetic acid for 10 minutes. Following three 1-min rinses in dH20, the slides were dried on a slide warmer, cleared in xylene for at least 1 min, and coverslipped with p-Xylene-bis(N-pyridinium bromide) (DPX; Sigma).
2.5. Evaluation of Fluoro-Jade C
From an adjacent series of Nissl-stained sections, tracings were made of the amygdala and individual amygdala nuclei: basolateral nucleus (BL), lateral nucleus (La), central nucleus (Ce), medial nucleus (Me), and basomedial nucleus (BM). In addition, tracings were made of the dorsal and ventral hippocampus, as well as individual hippocampal subfields (CA1, CA3, and hilar regions), including the dentate gyrus (DG). The tracings from the Nissl-stained sections were superimposed on the FJC-stained sections. For qualitative analysis of FJC-stained sections, the following rating system —initially described by McDonough et al. (1995, 2000) and later applied for FJC-stained sections by Myhrer et al. (2005) — was used to score the extent of neuronal degeneration (neuropathology score) in each structure and substructures: 0 = no neuropathology; 1 = minimal neuropathology (1–10% of the neuronal population was FJC-stained); 2 = mild neuropathology (11–25%); 3 = moderate neuropathology (26–45%); and 4 = severe neuropathology (>45%). Qualitative assessment using this scale has been previously shown to produce results that are in agreement with quantitative assessments (Qashu et al., 2009). The scores for neurodegeneration present on FJC-stained sections were assessed considering the density of cells from Nissl-stained sections. The extent of neurodegeneration was assessed in sections between the coordinates −2.40 and −3.36 from bregma for the amygdala and dorsal hippocampus, and in sections between coordinates −5.4 and −6.36 from bregma for the ventral hippocampus. Neurodegeneration in anterior/dorsal (anterodorsal) amygdala nuclei was assessed in sections between the coordinates −2.04 and −2.4 from bregma, while neurodegeneration in posterior/ventral (posteroventral) amygdala nuclei was assessed in sections between the coordinates −3.12 and −3.48 from bregma. When the “whole amygdala” and the “whole hippocampus” were considered, neurodegeneration was assessed along the anterior to posterior extent, at 600 µm intervals. The coordinates and tracings of brain structures and substructures were based on Paxinos and Watson (2005).
2.6. Statistical Analysis
Neuropathology scores were compared between paired samples (e.g., dorsal versus ventral regions for the hippocampus, or anterior versus posterior sections for the amygdala) using the Wilcoxon test. Among more than two structures or substructures, comparisons were made using the nonparametric Friedman test, followed by the Wilcoxon test when the Friedman test revealed statistical significance among groups/regions. Differences were considered significant when p < 0.05. All statistical values are presented as median and the interquartile range (IQR, the values at the 25th and 75th percentiles). Sample sizes (n) refer to the number of rats.
3. Results
By 10 min after soman injection, all soman-exposed rats reached stage 3 of behavioral seizures and went on to develop SE. All animals continued to display characteristic behaviors of generalized seizures (stages 4, 5 and 6) for at least 2 hrs after seizure onset. Under the conditions of this study, the 24-h lethality was 29% (5 of 17). Six of the surviving animals were used to study neurodegeneration 1 day after soman exposure, and another 6 rats were studied 7 days after exposure.
3.1. Neuronal degeneration in the hippocampus
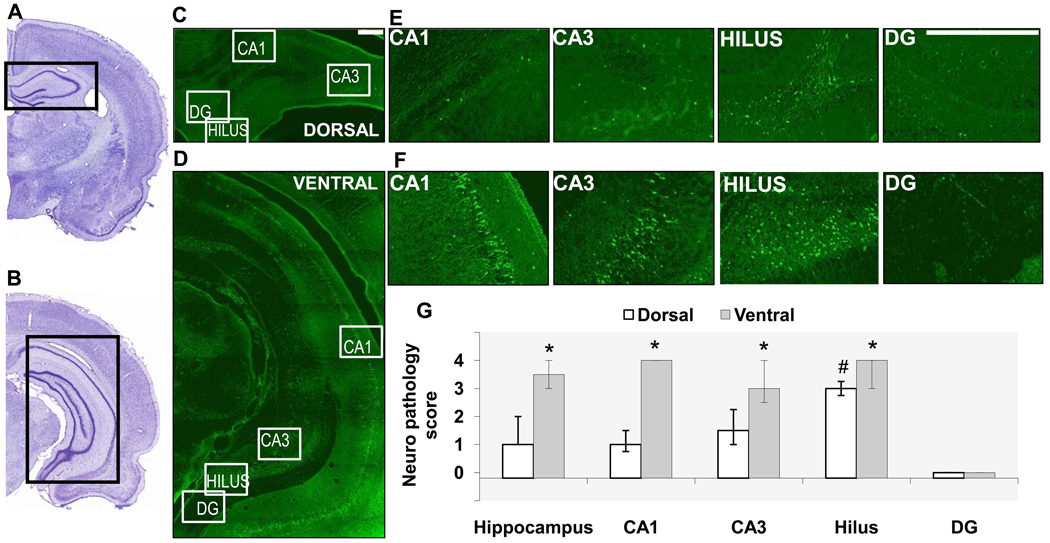
One day after soman-induced SE, the extent of neuronal degeneration in the dorsal hippocampus was minimal (median=1, IQR = 1~2), while in the ventral hippocampus it was moderate to severe (median=3.5, IQR= 3~4; p < 0.05, Fig. 1). When individual hippocampal subfields were examined, in the dorsal region, neurodegeneration was mild in the CA1 and CA3 areas, moderate in the hilus, whereas there were virtually no degenerating neurons in the granule cell layer of the DG. The moderate neurodegeneration of the hilus was significantly greater than that in the other subfields. In the ventral hippocampus, all hippocampal subfields displayed moderate to severe neurodegeneration, which was significantly greater than that of the respective subfields in the dorsal hippocampus, with the exception of the DG, where neuronal degeneration was negligible in the ventral hippocampus as well; the differences between subfields within the ventral region were not statistically significant (Fig. 1).
Fig. 1. Neuronal degeneration is greater in the ventral hippocampus compared to the dorsal hippocampus, 1 day after soman-induced SE.
A, B. Photomicrographs of panoramic Nissl-stained coronal brain sections showing the dorsal (A) and ventral (B) hippocampus. C, D, E, F. Photomicrographs of Fluoro-Jade C-stained sections showing the dorsal (C, E) and ventral (D, F) hippocampal subfields (scale bar is 300µ for both magnifications). G. Bar graph showing medians and interquartile range (IQR) of the neuropathology scores for the whole hippocampus, CA1 and CA3 subfields, hilar region, and granule cell layer of the dentate gyrus (IQR bar is not present in the CA1 area of the ventral hippocampus because there was no variability among animals in the neuropathology score). *p < 0.05 for comparisons between dorsal and ventral regions. #p < 0.05 for comparisons between the dorsal hilus and the other dorsal hippocampal subfields.
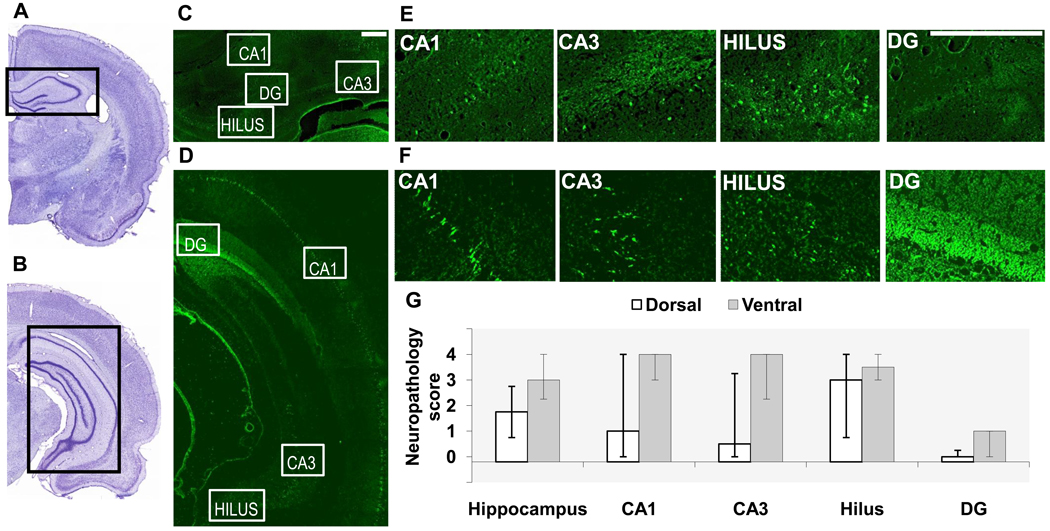
Seven days after soman-induced SE, there were still differences between the dorsal and ventral hippocampus in the extent of neuronal degeneration, but the variability among animals was high, and the differences were not statistically significant. Thus, neurodegeneration in the dorsal hippocampus was mild (median=1.75, IQR=0.75~2.75), while in the ventral hippocampus it was moderate to severe (median=3, IQR=2.25~4; p = 0.68, Fig. 2). When individual hippocampal subfields were examined, in the dorsal region, neurodegeneration was moderate in the hilus, minimal in the CA1 and CA3 areas, and absent in the DG. In the ventral hippocampus, except for the DG which had only minimal neurodegeneration, all other hippocampal subfields displayed moderate to severe neuropathology (Fig. 2).
Fig. 2. Neuronal degeneration in the dorsal and ventral hippocampus, 7 days after soman-induced SE.
A, B. Photomicrographs of panoramic Nissl-stained coronal brain sections showing the dorsal (A) and ventral (B) hippocampus. C, D, E, F. Photomicrographs of Fluoro-Jade C-stained sections showing the dorsal (C, E) and ventral (D, F) hippocampal subfields (scale bar is 300µ for both magnifications). G. Bar graph showing medians and interquartile range of the neuropathology scores for the whole hippocampus, CA1 and CA3 subfields, hilar region, and granule cell layer of the dentate gyrus (DG).
3.2. Neuronal degeneration in the amygdala
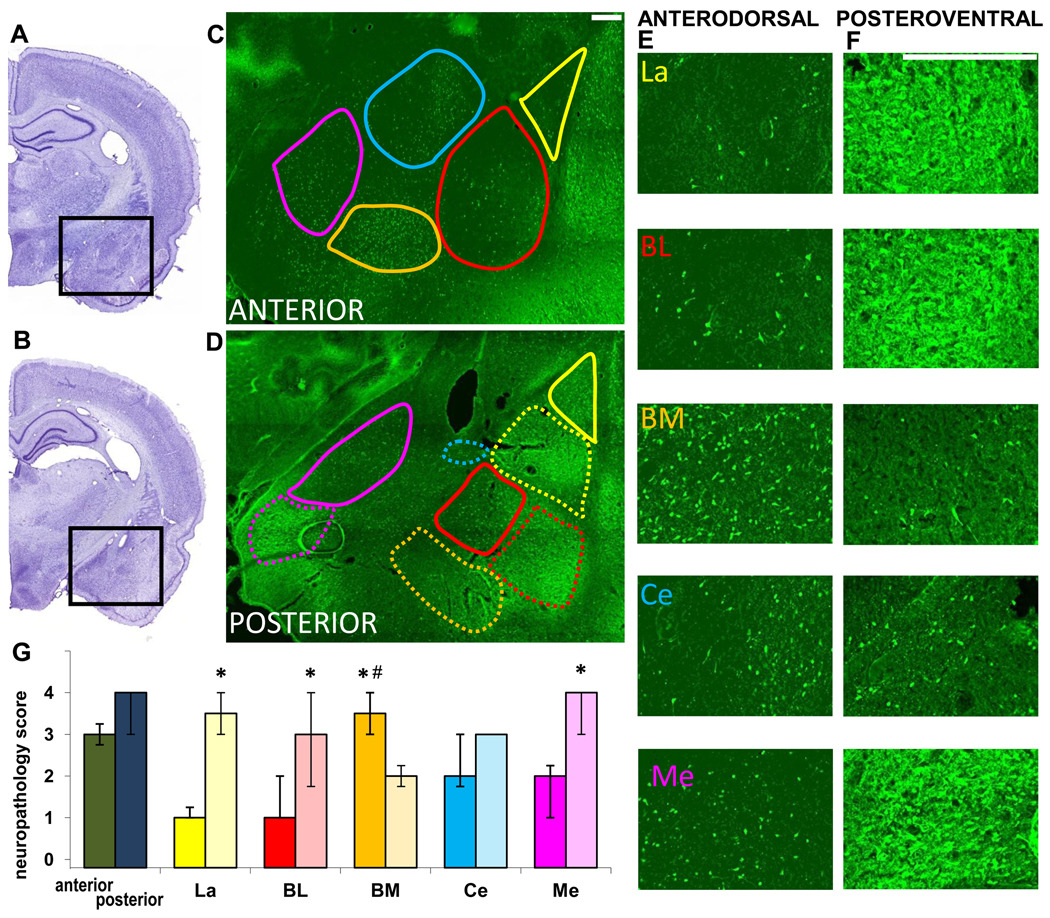
In the amygdala, 1 day after SE, the extent of neuronal degeneration was greater in the posterior sections (median=4, IQR=3~4) compared to the anterior sections (median =3, IQR=2.75~3.25; Fig. 3), but the variability among animals was high and these differences were not statistically significant. When individual nuclei were examined, neurodegeneration in the La, BL, and Me was significantly greater in the posteroventral regions compared to the same nuclei in the anterodorsal regions (Fig. 3). In contrast, the BM nucleus showed significantly greater neurodegeneration in the anterodorsal versus the posteroventral sections. Neurodegeneration in the central nucleus was not significantly different in the anterior versus the posterior sections. Among the anterodorsal nuclei, the BM displayed significantly greater neurodegeneration (moderate to severe) than the Ce and Me where neurodegeneration was moderate, as well as the La and BL where it was minimal. Among the posteroventral nuclei, neurodegeneration was moderate to severe in the La and Me, and moderate in the BL, BM, and Ce; these differences were not statistically significant (Fig. 3).
Fig. 3. Neuronal degeneration in amygdalar nuclei, 1 day after soman-induced SE.
A, B. Photomicrographs of panoramic Nissl-stained coronal brain sections showing the anterior (A) and posterior (B) amygdala. C, D. Photomicrographs of Fluoro-Jade C-stained sections of the amygdala, outlining the studied nuclei in the anterior and posterior amygdala (La-yellow; BL-red; BM-orange; Ce-blue; Me-purple). Contours are traced in solid lines for the anterior and dorsal (“anterodorsal”) subdivisions and in dashed lines for the posterior and ventral (“posteroventral”) subdivisions. E, F. Photomicrographs of Fluoro-Jade C-stained sections showing the different anterodorsal and posteroventral amygdala nuclei (scale bar is 300µ for both magnifications). G. Bar graph showing medians and interquartile range of the neuropathology scores for the anterior and posterior sections of the amygdala, and individual amygdala nuclei (solid colors for the anterodorsal and transparent colors for the posteroventral nuclei). *p < 0.05 for comparisons between the anterodorsal and posteroventral regions. #p < 0.05 for comparisons between the anterodorsal BM nucleus and the other anterodorsal nuclei.
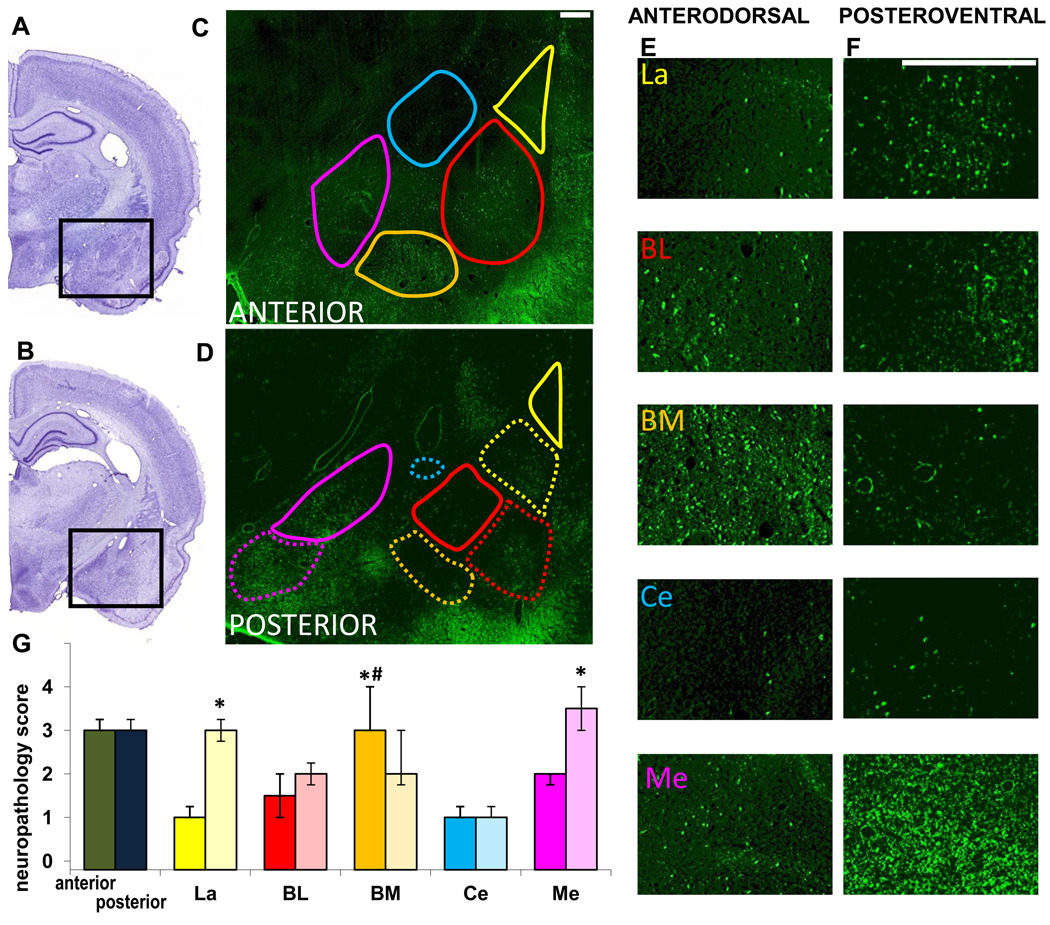
Seven days after soman-induced SE, there was no significant difference in the extent of neurodegeneration in the anterior versus the posterior sections of the amygdala (median=3, IQR=3~3.25 for both regions; Fig. 4). However, when individual nuclei were examined, the posteroventral La and Me nuclei had significantly greater neuropathology score than the anterodorsal La and Me, whereas the BM nucleus still displayed significantly greater neurodegeneration in the anterodorsal region (Fig. 4). Among the anterodorsal nuclei, the BM had moderate to severe neurodegeneration, which was significantly greater than the La, BL, Ce, and Me where neurodegeneration was relatively mild. Among the posteroventral nuclei, neurodegeneration in the Me nucleus was moderate to severe, in the La it was moderate, and in the BL, BM, and Ce it was milder (Fig. 4); these differences were not statistically significant.
Fig. 4. Neuronal degeneration in amygdalar nuclei, 7 days after soman-induced SE.
A, B. Photomicrographs of panoramic Nissl-stained coronal brain sections showing the anterior (A) and posterior (B) amygdala. C, D. Photomicrographs of Fluoro-Jade C-stained sections of the amygdala, outlining the studied nuclei in the anterior and posterior amygdala (La-yellow; BL-red; BM-orange; Ce-blue; Me-purple). Contours are traced in solid lines for the anterior and dorsal (“anterodorsal”) subdivisions and in dashed lines for the posterior and ventral (“posteroventral”) subdivisions. E, F. Photomicrographs of Fluoro-Jade C-stained sections showing the different anterodorsal and posteroventral amygdala nuclei (scale bar is 300µ for both magnifications). G. Bar graph showing medians and interquartile range of the neuropathology scores for the anterior and posterior sections of the amygdala, and individual amygdala nuclei (solid colors for the anterodorsal and transparent colors for the posteroventral). *p < 0.05 for comparisons between the anterodorsal and posteroventral regions. #p < 0.05 for comparisons between the anterodorsal BM nucleus and the other anterodorsal nuclei.
3.3. Comparisons of neuronal degeneration in the hippocampus versus the amygdala
Comparison of the neuropathology scores of the hippocampal subfields (Figs. 1G and 2G) and amygdalar nuclei (Figs. 3G and 4G) gives the following order of the different subregions, when ordered from the most severe to the least severe neuronal degeneration they displayed: On day 1 after SE, posteroventral Me ≈ ventral hilus ≈ ventral CA1 > posteroventral La ≈ anterodorsal BM > posteroventral Ce ≈ posteroventral BL ≈ ventral CA3 ≈ dorsal hilus > posteroventral BM ≈ anterodorsal Me ≈ anterodorsal Ce > dorsal CA3 > anterodorsal La ≈ anterodorsal BL ≈ dorsal CA1 > ventral DG ≈ dorsal DG; on day 7 after SE, posteroventral Me ≈ ventral CA1 ≈ ventral CA3 > ventral hilus > posterovenral La ≈ anterodorsal BM ≈ dorsal hilus > posteroventral BL ≈ posteroventral BM ≈ anterodorsal Me > anterodorsal BL > anterodorsal LA ≈ anterodorsal Ce ≈ posteroventral Ce > dorsal CA1 ≈ ventral DG > dorsal CA3 > dorsal DG.
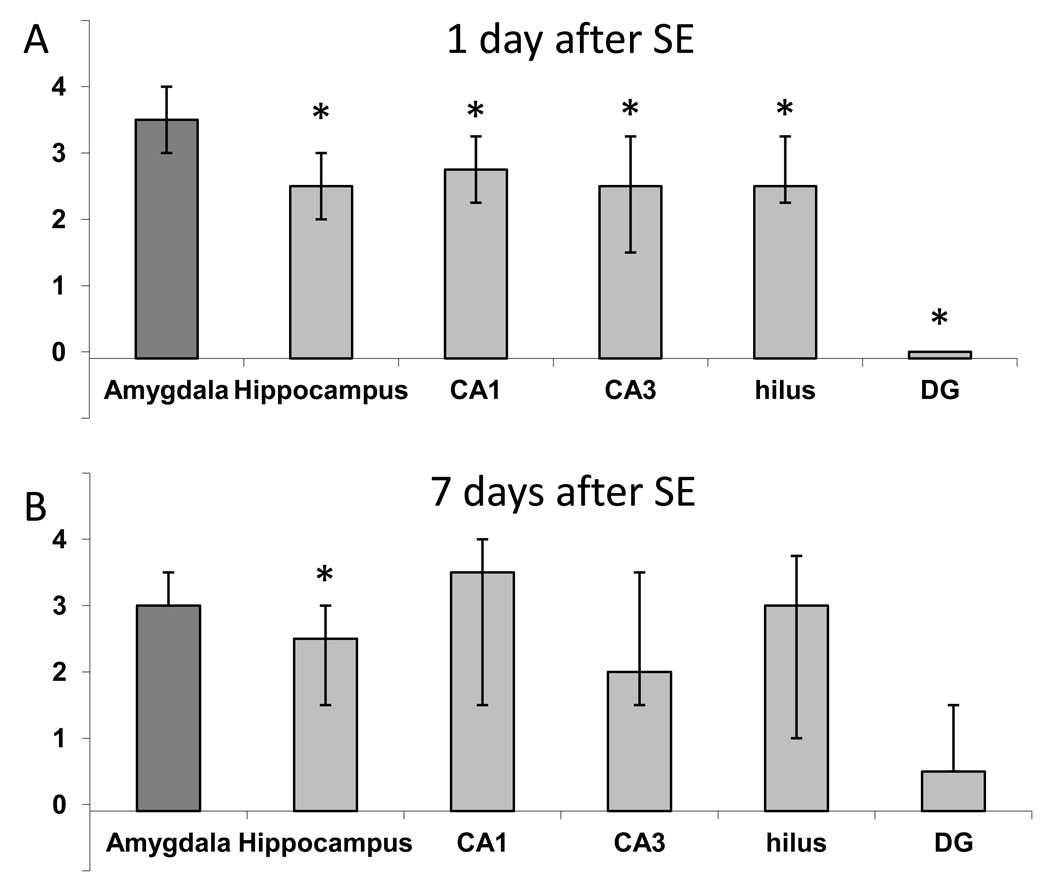
Neurodegeneration in the whole amygdala (anterodorsal to posteroventral regions) was significantly greater than that in the dorsal hippocampus, but did not differ significantly from neurodegeneration in the ventral hippocampus (compare the first set of bars on Figs. 1G and 3G for day 1 after SE, and Figs. 2G and 4G for day 7 after SE). When the whole amygdala was compared with the whole hippocampus (dorsal to ventral), the extent of neuronal degeneration in the amygdala was significantly greater than that in the hippocampus, both on day 1 and day 7 after SE (Fig. 5). Neurodegeneration in the amygdala was also significantly greater when compared with individual hippocampal subfields (CA1, CA3, hilus, and DG, throughout the dorsal to ventral extent of the hippocampus) on day 1, but not on day 7 after SE.
Fig. 5. Neuronal degeneration in the amygdala is significantly greater than that in the hippocampus, after soman-induced SE.
When the whole amygdala and the whole hippocampus were compared (average neurodegeneration score from sections along the anterodorsal to posteroventral axis), the amygdala displayed greater damage than the hippocampus, on both day 1 (A) and day 7 (B) after SE. Amygdala damage was also greater when compared with individual hippocampal subfields along the extent of the hippocampus, on day 1 but not on day 7 after SE. The bars show the medians and interquartile range. Asterisks indicating statistical significance (p < 0.05) have been placed on the bars that are statistically lower than the bar depicting amygdala neuropathology.
4. Discussion
The amygdala and the hippocampus are well known for their high propensity to generate epileptic activity, and, as seizure intensity and duration often parallel pathological damage (Hayward et al., 1990; Baze, 1993; Kadar et al., 1995; McDonough et al., 1995, 2000; Shih et al., 2003; Baille et al., 2005; Myhrer et al., 2005), these brain structures can be expected to be susceptible to damage induced by seizurogenic insults. In the present study, we found that both the hippocampus and the amygdala display significant neurodegeneration after two-hours of SE induced by soman administration. Neuronal degeneration was severe in the ventral hippocampus, and significantly greater than that in the dorsal hippocampus. In addition, the posteroventral regions of the amygdala displayed more severe neuropathology than the anterodorsal regions. Overall, when the whole hippocampus and amygdala were compared, neurodegeneration was greater in the amygdala.
4.1. Mechanisms of nerve agent-induced neurodegeneration
After nerve agent exposure, neuronal degeneration and loss are primarily caused by strong seizure activity. Thus, the extent to which seizures are pharmacologically controlled after nerve agent administration correlates well with the degree of protection against neuropathology (McDonough et al., 1995, 2000; Shih et al., 2003). Seizure activity is initiated by cholinergic mechanisms, but is sustained and reinforced by the engagement of glutamatergic mechanisms (McDonough and Shih, 1997); neuronal damage is probably caused by glutamate-mediated excitotoxicity (Lallement et al., 1992; McDonough and Shih, 1997; Solberg and Belkin, 1997).
4.2. Neuronal degeneration in the hippocampus
Prior studies have shown significant damage in the hippocampus after soman (Kadar et al., 1992) or sarin (Kadar et al., 1995) administration, but damage in the ventral versus the dorsal hippocampus has not been examined previously. There is evidence indicating that compared to the dorsal hippocampus, the ventral hippocampus clearly has a higher propensity to generate seizures and propagate them to other brain regions (Gilbert et al., 1985; Wu et al., 2005). A greater propensity for seizure generation does not necessarily imply a greater susceptibility to seizure-induced damage, as the seizure-prone structure could be endowed with mechanisms that resist pathological alterations. However, the results of the present study indicate that the ventral hippocampus is indeed more susceptible to neuronal damage than the dorsal hippocampus. Thus, on day 1 after soman-induced SE, the ventral hippocampus, as well as the individual hilar, CA1, and CA3 areas of the ventral hippocampus, displayed greater neuronal degeneration than the corresponding dorsal structures. A week later, similar differences between the dorsal and ventral regions were still present, but variability among animals was high, and the differences were not statistically significant. Substantial variability is commonly seen in this type of experiments (see for example Myhrer et al., 2005), and it may be due, in part, to the difficulty in precisely controlling the intensity and duration of SE to the same level in all animals. One of the reasons for the increased variability one week after SE compared with the first day after SE probably relates to the time factor; in addition to the variable “number of degenerating neurons,” the variable “rate of neuronal degeneration and loss” also comes into play during the elapsed time, and this latter variable can be expected to vary considerably among animals.
Interestingly, the granule cell layer of the DG was not significantly affected in either the dorsal or the ventral hippocampus, at either time point. In contrast, the hilar region appeared to be particularly susceptible to soman-induced neuronal degeneration. Resistance of the DG/granule cells to seizure-induced damage and a high susceptibility of the hilus have also been seen in response to other seizure- and SE-inducing insults (Freund et al., 1992; Choi et al., 2007). Although the reasons for that are not quite clear (see Freund et al., 1992; Bouilleret et al., 2000; Choi et al., 2007), the somatostatin-containing interneurons of the hilar region are known to be very susceptible to seizure-induced damage (Freund et al., 1992; Choi et al., 2007).
4.3. Neuronal degeneration in the amygdala
The amygdala has been shown to suffer the most extensive neuronal loss after nerve agent exposure, in guinea pigs (Shih et al., 2003), while in rats, the frequency of damage in the amygdala is exceeded only by that in the piriform cortex (McDonough et al., 1995). However, which amygdala regions are most susceptible to damage by nerve agents has not been investigated previously. Here, we found that overall (combined nuclei) neuronal degeneration in the anterior versus the posterior sections of the amygdala did not differ significantly. However, with the exception of the BM (also called accessory basal nucleus; for a review on the nomenclature/anatomy and function of the amygdala nuclei see Sah et al., 2003), which displayed more neurodegeneration in the anterodorsal part, the other major nuclei examined (La, BL, Me) were more damaged in the posteroventral than in the anterodorsal regions.
The regional pattern of neuropathology in the amygdalar nuclei seen in the present study in response to soman resembles that observed previously after kainic acid-induced SE (Tuunanen et al., 1996); this suggests that neuronal damage is caused primarily by the seizures, irrespective of the mechanisms that initiated the seizures. A major characteristic of this damage pattern is the higher vulnerability of the ventral and medial regions of the amygdala (Tuunanen et al., 1996; Pitkanen et al., 1998). Importantly, greater damage in the ventral portions of the amygdala is also seen in humans with temporal lobe epilepsy (Meyer et al., 1955).
4.4. Implications
Along with previous literature (Hayward et al., 1990; Kadar et al., 1992; Baze, 1993; Myhrer et al., 2006; McDonough et al., 2000; Shih et al., 2003), the present study indicates that the hippocampus and, particularly, the amygdala are two of the brain regions that are most susceptible to seizure-induced damage elicited by soman. Damage to the hippocampus and the amygdala can produce serious cognitive and behavioral deficits —such as memory impairment, difficulty in processing the emotional significance of sensory information, and mood disturbances— and may underlie the behavioral abnormalities seen after nerve agent exposure (McDonough et al., 1986; Morita et al., 1995; Brown and Brix, 1998; Kassa et al., 2001; Bajgar et al., 2004; Myhrer et al., 2005; Joosen et al., 2009). Furthermore, the non-uniform damage of these structures, whereby certain regions are severely damaged and others only mildly affected, may facilitate epileptogenesis and generation of spontaneous seizures (Pitkanen et al., 1998). The knowledge gained by the present study can guide the development of pharmacological treatments that can prevent brain damage after nerve agent exposure. Testing of potential treatments should take into consideration the high susceptibility of the ventral hippocampus and the posteroventral amygdala to nerve agent induced-neuropathology; therefore, the extent to which these brain regions are protected by a prospective antidote should be investigated. To target the protection of these regions further knowledge is also needed on the biochemical, physiological, or other mechanisms that render the ventral hippocampus and the posteroventral regions of the amygdala most vulnerable to nerve agent-induced neuropathology.
Acknowledgments
This work was supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award # U01 NS058162-01), and the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division, grant # 1.E0021_07_US_C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views of the authors do not purport to reflect the position or policies of the U.S. Army or the Department of Defense.
Conflict of Interest statement: The authors declare that there are no conflicts of interest.
References
- Akaike K, Tanaka S, Hideshi Tojo H, Fukumoto S, Imamura S, Takigawa M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001;900:65–71. doi: 10.1016/s0006-8993(01)02252-1. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MFM. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience. 2009;159:380–389. doi: 10.1016/j.neuroscience.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Figueiredo YH, Apland JP, Qashu F, Braga MFM. Primary brain targets of nerve agents: the role of the amygdala in comparison to the hippocampus. Neurotoxicology. 2009 doi: 10.1016/j.neuro.2009.06.011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited. Toxicology. 2005;215:1–24. doi: 10.1016/j.tox.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Bajgar J, Sevelova L, Krejcova G, Fusek J, Vachek J, Kassa J, Herink J, de Jong LP, Benschop HP. Biochemical and behavioral effects of soman vapors in low concentrations. Inhal Toxicol. 2004;16:497–507. doi: 10.1080/08958370490442430. [DOI] [PubMed] [Google Scholar]
- Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica (Hradec Kralove) 2005;48:3–21. [PubMed] [Google Scholar]
- Barthold CL, Schier JG. Organic phosphorus compounds--nerve agents. Crit Care Clin. 2005;21:673–689. doi: 10.1016/j.ccc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Baze WB. Soman-induced morphological changes: an overview in the nonhuman primate. J Appl Toxicol. 1993;13:173–177. doi: 10.1002/jat.2550130306. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000;97:47–58. doi: 10.1016/s0306-4522(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Brown MA, Brix KA. Review of health consequences from high, intermediate, and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. 1998;6:393–408. doi: 10.1002/(sici)1099-1263(199811/12)18:6<393::aid-jat528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Choi YS, Lin SL, Lee B, Kurup P, Chao HY, Naegele JR, Lombroso PJ, Obrientan K. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007;27:2999–3009. doi: 10.1523/JNEUROSCI.4913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM, Four A, Fauquette W, Burckhart MF, Masqueliez C, Bernabe´ D, Baubichon D, Lallement G. Soman poisoning induces delayed astrogliotic scarand angiogenesis in damaged mouse brain areas. NeuroToxicology. 2007;28:38–48. doi: 10.1016/j.neuro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Freund TF, Ylinen A, Miettinen R, Pitkanen A, Lahtinen H, Baimbridge KG, Riekkinen PJ. Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull. 1992;28:27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Racine RJ, Smith GK. Epileptiform burst responses in ventral vs dorsal hippocampal slices. Brain Res. 1985;361:389–391. doi: 10.1016/0006-8993(85)91309-5. [DOI] [PubMed] [Google Scholar]
- Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB. Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. Neurol Sci. 1990;98:99–106. doi: 10.1016/0022-510x(90)90185-p. [DOI] [PubMed] [Google Scholar]
- Joosen MJ, Jousma E, van den Boom TM, Kuijpers WC, Smit AB, Lucassen PJ, van Helden HPM. Long-term cognitive deficits accompanied by reduced neurogenesis after soman poisoning. NeuroToxicology. 2009;30:72–80. doi: 10.1016/j.neuro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Kadar T, Cohen G, Sahar R, Alkalai D, Shapira S. Long-term study of brain lesions following soman, in comparison to DFP and metrazol poisoning. Hum Exp Toxicol. 1992;11:517–523. doi: 10.1177/096032719201100613. [DOI] [PubMed] [Google Scholar]
- Kadar T, Shapira S, Cohen G, Sahar R, Alkalay D, Raveh L. Sarin-induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–259. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- Kassa J, Koupilova M, Herink J, Vachek J. The long-term influence of low-level sarin exposure on behavioral and neurophysiological functions in rats. Acta Medica (Hradec Kralove) 2001;44:21–27. [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Sentenac-Roumanou H, Blanchet G. Involvement of glutamatergic system of amygdala in generalized seizures induced by soman: comparison with the hippocampus. CR Acad Sci III. 1991;313:421–426. [PubMed] [Google Scholar]
- Lallement G, Denoyer M, Collet A, Pernot-Marino I, Baubichon D, Monmaur P, Blanchet G. Changes in hippocampal acetylcholine and glutamate extracellular levels during soman-induced seizures: influence of septal cholinoceptive cells. Neurosci Lett. 1992;139:104–107. doi: 10.1016/0304-3940(92)90868-8. [DOI] [PubMed] [Google Scholar]
- Layish I, Krivoy A, Rotman E, Finkelstein A, Tashma Z, Yehezkelli Y. Pharmacologic prophylaxis against nerve agent poisoning. Isr Med Assoc J. 2005;7:182–187. [PubMed] [Google Scholar]
- López-Muñoz F, Alamo C, Guerra JA, García-García P. The development of neurotoxic agents as chemical weapons during the National Socialist period in Germany. Rev Neurol. 2008;47:99–106. [PubMed] [Google Scholar]
- McDonough JH, Jr, Smith RF, Smith CD. Behavioral correlates of soman-induced neuropathology: deficits in DRL acquisition. Neurobehav Toxicol Teratol. 1986;8:179–187. [PubMed] [Google Scholar]
- McDonough JH, Jr, Dochterman W, Smith CD, Shih T-M. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;15:123–132. [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih T-M. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih T-M. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Meyer A, Beck E, Shepherd M. Unusually severe lesions in the brain following status epilepticus. J Neurol Neurosurg Psychiatry. 1995;18:24–33. doi: 10.1136/jnnp.18.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet. 1995;346:260–261. doi: 10.1016/s0140-6736(95)92170-2. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Andersen JM, Nguyen NH, Aas P. Soman-induced convulsions in rats terminated with pharmacological agents after 45 min: neuropathology and cognitive performance. Neurotoxicology. 2005;26:39–48. doi: 10.1016/j.neuro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Myhrer T, Enger S, Aas P. Efficacy of immediate and subsequent therapies against soman-induced seizures and lethality in rats. Basic Clin Pharmacol Toxicol. 2006;98:184–191. doi: 10.1111/j.1742-7843.2006.pto_268.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition. New York NY: Elsevier; 2005. [Google Scholar]
- Pitkänen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Apland JP, Braga MFM. Diazepam administration after prolonged status epilepticus reduces neurodegeneration in the amygdala but not in the hippocampus during epileptogenesis. Amino Acids. 2009 doi: 10.1007/s00726-008-0227-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by elecytrical stimulation. II. Motor seizure. Electroencaphal Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Rose PA, Burnham WM. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can J Neurol Sci. 1977;4:273–278. doi: 10.1017/s0317167100025117. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Shih T-M, Duniho SM, McDonough JH., Jr Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Solberg Y, Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. TiPS. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Holonen T, Pitkanen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Wu CP, Cheung G, Rakhshani N, Parvardeh S, Nassiri Asl M, Huang HL, Zhang L. CA3 neuronal activities of dorsal and ventral hippocampus are differentially altered in rats after prolonged post-ischemic survival. Neuroscience. 2005;130:527–539. doi: 10.1016/j.neuroscience.2004.09.041. [DOI] [PubMed] [Google Scholar]