Abstract
BAX cooperates with truncated BID (tBID) and Ca2+ in permeabilizing the outer mitochondrial membrane (OMM) and releasing mitochondrial apoptogenic proteins. The mechanisms of this cooperation are still unclear. Here we show that in isolated brain mitochondria, recombinant BAX readily self-integrates/oligomerizes in the OMM but produces only a minuscule release of cytochrome c, indicating that BAX insertion/oligomerization in the OMM does not always lead to massive OMM permeabilization. Ca2+ in a mitochondrial permeability transition (mPT)-dependent and recombinant tBID in an mPT-independent manner promoted BAX insertion/oligomerization in the OMM and augmented cytochrome c release. Neither tBID nor Ca2+ induced BAX oligomerization in the solution without mitochondria, suggesting that BAX oligomerization required interaction with the organelles and followed rather than preceded BAX insertion in the OMM. Recombinant Bcl-xL failed to prevent BAX insertion/oligomerization in the OMM but strongly attenuated cytochrome c release. On the other hand, a reducing agent, dithiothreitol (DTT), inhibited BAX insertion/oligomerization augmented by tBID or Ca2+ and suppressed the BAX-mediated release of cytochrome c and Smac/DIABLO but failed to inhibit Ca2+-induced swelling. Altogether, these data suggest that in brain mitochondria, BAX insertion/oligomerization can be dissociated from OMM permeabilization and that tBID and Ca2+ stimulate BAX insertion/oligomerization and BAX-mediated OMM permeabilization by different mechanisms involving mPT induction and modulation of the SH-redox state.
Keywords: mitochondria, calcium, BAX, BID, Bcl-xL, permeability transition
1. Introduction
Apoptosis is an omnipresent form of cell death involved in various neurodevelopmental [1] as well as neuropathological processes, including age-related neurodegenerations [2-6], stroke [7-10], and secondary brain injury following mechanical brain trauma [11,12]. The release of mitochondrial apoptogenic factors, a key step in executing of apoptosis [13], occurs due to a concert action of pro-apoptotic proteins such as BID and BAX [14]. Under normal conditions, monomeric BAX and full-length BID are located in the cytosol [15]. Caspase-8 activated by apoptotic stimuli cleaves BID, producing activated (truncated) BID (tBID) [16]. In turn, tBID activates BAX either “directly” [15,17,18] or “indirectly” [19,20] leading to oligomerization of BAX, its insertion into the OMM, and OMM permeabilization culminating in the release of mitochondrial apoptogenic proteins [18,21].
In addition to tBID, elevated Ca2+ enhances the ability of BAX to integrate into the lipid membranes and permeabilize them [22-24]. Ca2+ also amplifies BAX ability to permeabilize the OMM [25], though the mechanism of such amplification is unknown. Since elevated Ca2+ induces the mitochondrial permeability transition (mPT), a phenomenon accompanied by mitochondrial depolarization and remodeling [26], it is possible that the mPT is involved in augmentation of BAX-mediated OMM permeabilization. In cerebellar granule neurons, trophic factor withdrawal in low-K+ medium resulted in the mPT that triggered BAX translocation to mitochondria and release of Cyt c [27]. In line with this, in the model of ischemia/reperfusion heart injury, inhibition of the mPT either with Ru360, an inhibitor of the mitochondrial Ca2+ uniporter [28], or with cyclosporin A (CsA), an inhibitor of the mPT [29], precluded BAX insertion in the OMM and OMM permeabilization [30]. Thus, there is evidence suggesting a synergistic relationship between the Ca2+-induced mPT and BAX in OMM permeabilization.
In the present study, we demonstrated that BAX could readily self-integrate and oligomerize in the OMM, but these events were not accompanied by massive Cyt c release. We also found that Ca2+ in an mPT-dependent and tBID in an mPT-independent manner augmented BAX insertion and oligomerization in the OMM that correlated with the increased OMM permeabilization. Moreover, we showed that the Ca2+- and tBID-stimulated BAX insertion/oligomerization depended on SH-redox state and could be inhibited by a reducing agent, dithiothreitol (DTT). DTT also attenuated BAX-mediated OMM permeabilization stimulated by Ca2+ or tBID, revealing an important role of SH-redox regulation in the release of mitochondrial apoptogenic proteins.
2. Materials and Methods
2.1 Recombinant proteins
Full-length human monomeric BAX with a tag of six histidine residues at the N-terminus was expressed in the pBAD plasmid in Escherichia coli [31]. Mouse tBID (BID cut with caspase-8 and separated from N-terminal and the caspase) was obtained from full-length BID as described previously [32]. Recombinant Bcl-xL was produced as described previously [17]. Recombinant BAX, tBID, and Bcl-xL were stored in dialysis buffer containing 25 mM HEPES-NaOH, pH 7.5, 0.2 mM dithiothreitol, 30% glycerol (v/v) at -86°C.
2.2 Isolation and purification of brain mitochondria
Mitochondria from the brains of male Sprague-Dawley rats, 200–250 g (Harlan, Indianapolis, IN, USA) were isolated in mannitol-sucrose medium according to an Institutional Animal Care and Use Committee approved protocol and purified on a discontinuous Percoll gradient as described previously [33]. Mitochondrial protein was measured by the Bradford method [34], using BSA as a standard.
2.3 Measurements of mitochondrial light scattering
Mitochondrial swelling was evaluated in the standard incubation medium at 37°C by monitoring the scattering of light directed on mitochondrial suspension under 90° to the axis of the photodetector at 525 nm in a 0.4-ml cuvette under continuous stirring using a PerkinElmer LS-55 luminescence spectrometer. The standard incubation medium used in these and other experiments contained 125 mM KCl, 10 mM HEPES, pH 7.4, 0.5 mM MgCl2, 3 mM KH2PO4, 10 μM EGTA, 0.1% bovine serum albumin (free from fatty acids), 3 mM glutamate, and 3 mM succinate.
2.4 Transmission electron microscopy
Electron microscopy of isolated brain mitochondria was performed as described previously [35]. Mitochondria were incubated in the standard 125 mM KCl-based medium at 37°C prior to fixation in 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M phosphate buffer in the same incubation medium at room temperature for 15 minutes. Transmission electron microscopy (TEM) images were taken using a Tecnai G12 BioTwin electron microscope (FEI, Hillsboro, OR) equipped with an AMT 2.6×2.6K digital CCD camera.
2.5 Alkali-resistant BAX insertion
The alkali treatment of mitochondria removes loosely attached proteins but leaves proteins inserted into the OMM [18]. We determined the alkali-resistant fraction of BAX inserted into the OMM using the earlier described method [36]. Briefly, mitochondria treated with BAX (50 or 150 nM) at 37°C for 30 minutes were pelleted at 15,800 g for 5 minutes, and supernatant was used for the Cyt c release measurements. Mitochondrial pellets were re-suspended in 0.2 ml of 0.1 M Na2CO3, pH 11.5, then incubated for 30 minutes on ice. Samples were centrifuged for 30 minutes at 100,000 g in an Optima L-100K Beckman ultracentrifuge. The pellets were solubilized using 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) or 1% [octylphenoxy] polyethoxyethanol (Nonidet P-40, Amresco, Solon, OH) and analyzed by western blotting against BAX and cytochrome oxidase subunit IV (COX IV, loading control).
2.6 Immunoblotting
The release of Cyt c and Smac/DIABLO from isolated brain mitochondria was assessed in supernatants obtained through incubation of mitochondria in the standard 125 mM KCl-based incubation medium with or without additions for 30 minutes at 37 °C. For SDS-PAGE, we used 4-12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Western blotting was performed as previously described [37]. In some experiments, alamethicin (30μg/ml) was used to produce the maximal Cyt c release. Mitochondrial cytochrome oxidase subunit IV (COX IV) was used as a loading control for the pellet samples. COX IV was detected with mouse monoclonal anti-COX IV antibody, dilution 1:5000 (Invitrogen, Carlsbad, CA). Following SDS-PAGE, proteins were transferred to Hybond™-ECL™ nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ), and blots were incubated with mouse anti-cytochrome c antibody (7H8.2C12, PharMingen, San Diego, CA) at 1:3000 dilution or with rabbit anti-Smac/DIABLO antibody (Alexis Biochemicals, San Diego, CA) at 1:1500 dilution for an hour at room temperature in 5% non-fat milk, phosphate-buffered saline, pH 7.2, and 0.15% Triton X-100. Prior to analysis of Smac/DIABLO release, the supernatants were concentrated threefold in the Microcon YM-10 filtering devices (Millipore Corporation, Bedford, MA). In the alkali-resistant BAX insertion experiments, BAX was detected by western blotting with rabbit polyclonal anti-BAX antibody (Upstate, Lake Placid, NY). Recently, it was shown that oxidation of BAX's cysteines favored formation of disulfide bridges and BAX oligomerization [38,39], so it is possible that formation of disulfide bridges might contribute to BAX oligomerization in our experiments. Correspondingly, to prevent disruption of disulfide bridges and disassembly of BAX oligomers, SDS-PAGE was performed under non-reducing conditions. Anti-BAX antibody was used at 1:2000 dilution for an hour at room temperature in 5% BSA (Jackson ImmunoResearch Laboratories, West Grove, PA), phosphate-buffered saline, pH 7.2, and 0.15% Triton X-100. Blots were developed using goat anti-rabbit or anti-mouse IgG (1:20000) coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories) and Supersignal West chemiluminescent reagents (Pierce, Rockford, IL). Molecular weight marker SeeBlue® Plus 2 Standards (5 μl), (Invitrogen, Carlsbad, CA) were used to determine the molecular weights of the bands. NIH ImageJ 1.40g software (http://rsb.info.nih.gov/ij/) was used to quantify band densities. All immunoblots are representative of at least three independent experiments.
2.7 Analytical gel-filtration
Analytical gel-filtration was carried out on a Superdex 200 HR 10/30 column using FPLC. Prior to injecting into the column, BAX (500 nM) was pre-incubated at 4°C for 24 hours in the solution containing 125 mM KCl, 10 mM HEPES, pH 7.4, and 1% CHAPS. The same solution was used to equilibrate the column. After injecting the column with 150μl sample, fractions of 0.4 ml were collected and protein was concentrated with trichloroacetic acid/acetone precipitation prior to analysis by western blotting. The column was calibrated using gel-filtration protein standards. Protein standards were Blue Dextran (2000 kDa), ferritin (440 kDa), catalase (232 kDa), albumin (66 kDa), chymotrypsinogen A (25 kDa) (all from Sigma).
2.8 Protein cross-linking
Cross-linkers were dissolved in DMSO right before the experiment. Ethylene glycol bis(succinimidyl succinate) (EGS, 0.2 mM), disuccinimidyl suberate (DSS, 0.2 mM), and bismaleimidohexane (BMH, 0.5 mM) (all from Pierce) were used. The cross-linkers were added to the standard incubation medium supplemented with 50 nM BAX for 15 minutes at 37°C. EGS and DSS were quenched by 20 mM Tris-HCl, pH 7.5, incubating with rocking for 30 minutes at room temperature. BMH was quenched by 50 mM dithiothreitol (DTT) incubating with rocking for 30 minutes at room temperature. Then, non-reducing SDS-PAGE and western blotting were performed.
2.9 Statistics
Statistical analyses of experimental data consisted of a one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test (GraphPad Prism, version 4.0, GraphPad Software, San Diego, CA). The data represent the mean ± SEM of at least three independent experiments.
3. Results
3.1 BAX insertion and oligomerization in the outer mitochondrial membrane
The release of mitochondrial apoptogenic proteins depends on BAX insertion/oligomerization in the OMM [17,18]. How Ca2+ and tBID influence BAX insertion and oligomerization in the OMM of brain mitochondria is unknown. In our study, we took advantage of isolated purified brain mitochondria as a well-defined, cell-free model system that allows direct access to the OMM and precise control of the experimental conditions. Importantly, the OMM represents a natural target for pro-apoptotic proteins like BAX and tBID and contains all necessary components involved to the release of mitochondrial apoptogenic proteins. Thus, isolated brain mitochondria represent a powerful experimental model perfectly suited for detailed analysis of BAX insertion and oligomerization in the OMM and OMM permeabilization.
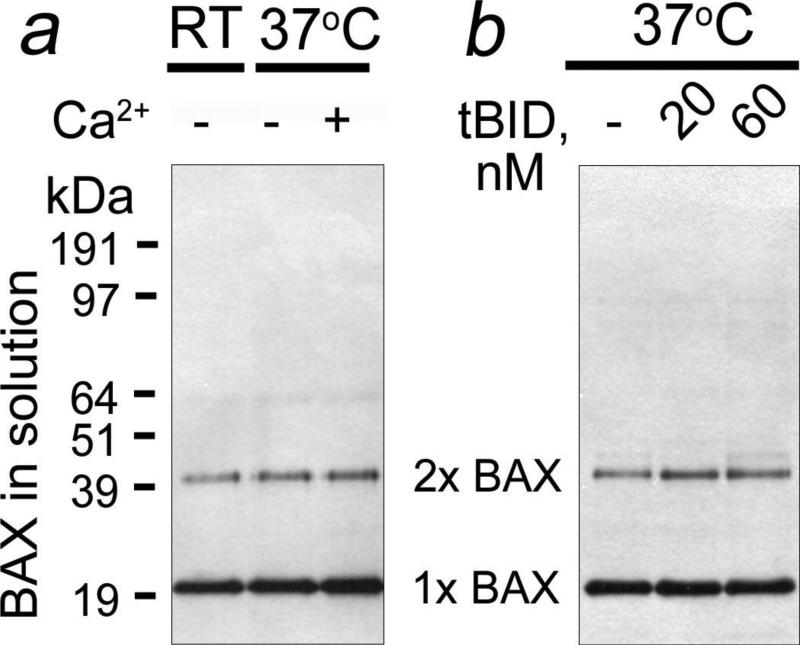
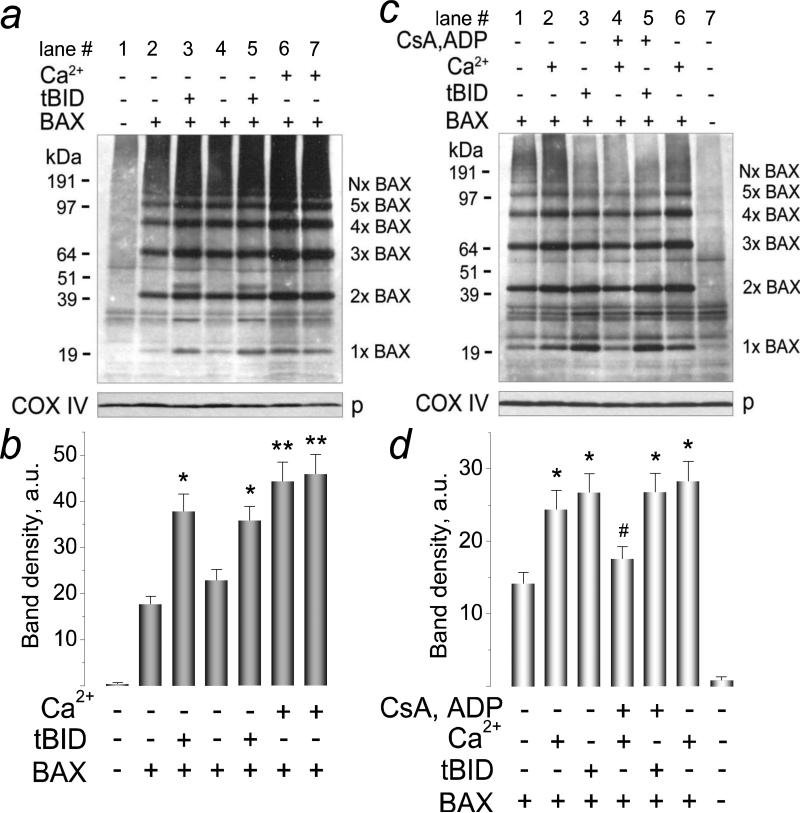
The recombinant BAX used in our study was predominantly monomeric with small amount of dimers (Fig. 1). Neither Ca2+ nor tBID triggered BAX oligomerization in the solution prior to adding mitochondria. Thus, BAX oligomerization required interaction of BAX with the OMM and, hence, most likely followed rather than preceded BAX insertion into the OMM. In the untreated mitochondria, the amount of endogenous BAX was below the detection limit of western blotting (Fig. 2a, lane 1). Incubation of mitochondria with BAX alone produced alkali-resistant BAX insertion and oligomerization in the OMM (Fig. 2a, lane 2), indicating that BAX can self-integrate and self-oligomerize in the OMM producing various BAX oligomers. Both Ca2+ and tBID significantly increased the amount of inserted/oligomerized BAX (Fig. 2a-c). In these experiments, we used previously established concentration of Ca2+ that produced distinct swelling of isolated brain mitochondria but did not cause significant Cyt c release in the standard, 125 mM KCl-based incubation medium [33]. In some western blotting experiments, the key samples were run in duplicate to demonstrate reproducibility. Figure 2b shows statistical analysis of BAX insertion based on densitometry data obtained with individual BAX bands (1×BAX-5×BAX) shown in Figure 2a. Thus, BAX could self-integrate/oligomerize in the OMM and both Ca2+ and tBID stimulated these processes. Importantly, we did not use cross-linkers in our experiments. In our hands, cross-linkers ethylene glycol bis(succinimidyl succinate) (EGS), disuccinimidyl suberate (DSS), and bismaleimidohexane (BMH, all from Pierce) triggered BAX oligomerization in the solution without mitochondria (Supplemental Fig. 1) and therefore were unacceptable. In addition, in these experiments we found that BSA-containing blocking solution was preferable for detecting BAX oligomers than non-fat milk.
Figure 1. Ca2+ and tBID failed to induce BAX oligomerization in the solution without mitochondria.
In a, 50 nM BAX was incubated in the standard mitochondrial incubation medium with or without 250μM Ca2+ for 30 minutes at room temperature (RT, 23°C) or 37°C as indicated. In b, 50 nM BAX was incubated with 20 or 60 nM tBID for 30 minutes at 37°C in the standard incubation medium. In these and other experiments with BAX, western blotting followed non-reducing SDS-PAGE.
Figure 2. Recombinant BAX self-integrated and self-oligomerized in the outer mitochondrial membrane of isolated brain mitochondria. Ca2+ and tBID stimulated BAX insertion and oligomerization. Cyclosporin A (CsA) and ADP antagonized the effect of Ca2+ but did not influence the effect of tBID.
In a, alkali-resistant BAX insertion and oligomerization with and without Ca2+ or tBID. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX, with 1.4μmol Ca2+/mg protein, or with 20 nM tBID as indicated. Following alkali treatment (30 minutes on ice in 0.1 M Na2CO3 at pH 11.5), mitochondrial membranes were solubilized with 1% CHAPS. In c, alkali-resistant BAX insertion and oligomerization with and without Ca2+ or tBID and the effect of CsA plus ADP. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX, with 1.4μmol Ca2+/mg protein, with 20 nM tBID, and with a combination of 1μM CsA and 100μM ADP (in the presence of 1μM oligomycin to prevent ADP phosphorylation) as indicated. Following alkali treatment, mitochondrial membranes were solubilized with 1% Nonidet P-40. p, pellets. In b and d, statistical analyses of BAX insertion into the OMM were performed using one-way ANOVA followed by Bonferroni's post-hoc test. The graphs show the averaged sum of 1×BAX–5×BAX band densities under corresponding experimental conditions. Here and in all other similar experiments, the densitometry of individual bands was performed with NIH ImageJ 1.40g software (http://rsb.info.nih.gov/ij/). Data are mean±SEM, N=3. In b, *p<0.05 comparing band densities with BAX versus BAX plus tBID; **p<0.01 comparing band densities with BAX versus BAX plus Ca2+. In d, *p<0.01 comparing band densities with BAX versus BAX plus tBID or BAX plus Ca2+; #p<0.05 comparing band densities with BAX plus Ca2+ versus BAX plus Ca2+ with CsA and ADP. These and all other experiments with BAX were performed without cross-linkers.
We used overnight incubation with 1% CHAPS at 4°C to solubilize mitochondrial pellets after alkali treatment (Fig. 2a). For comparison, we also used 1% Nonidet P-40 (NP-40, Amresco, Solon, OH), another non-ionic detergent, and detected the same major bands corresponding to BAX oligomers (Fig. 2c). Importantly, not all exogenous, recombinant BAX was inserted and oligomerized in the OMM. A fraction of exogenous BAX (~35-40%) remained in the incubation medium in the form of monomers and dimers (Supplemental Fig. 2). Figure 2d shows statistical analysis of BAX insertion based on densitometry data obtained with individual BAX bands (1×BAX-5×BAX) shown in Figure 2c. In the experiments with mitochondrial pellets solubilized with NP-40, we tested the hypothesis that the mPT is involved in Ca2+-stimulated BAX insertion/oligomerization in the OMM. A combination of CsA and ADP, inhibitors of the mPT [40-42], added to mitochondria prior to BAX attenuated BAX insertion and oligomerization stimulated by Ca2+ (Fig. 2d, compare lanes 2 and 6 and lane 4). On the other hand, CsA and ADP failed to attenuate tBID-stimulated BAX insertion and oligomerization (Fig. 2d, compare lanes 3 and 5), which is consistent with the insensitivity of tBID plus BAX-induced Cyt c release to mPT inhibitors [37].
3.2 Effect of different detergents on BAX quaternary structure
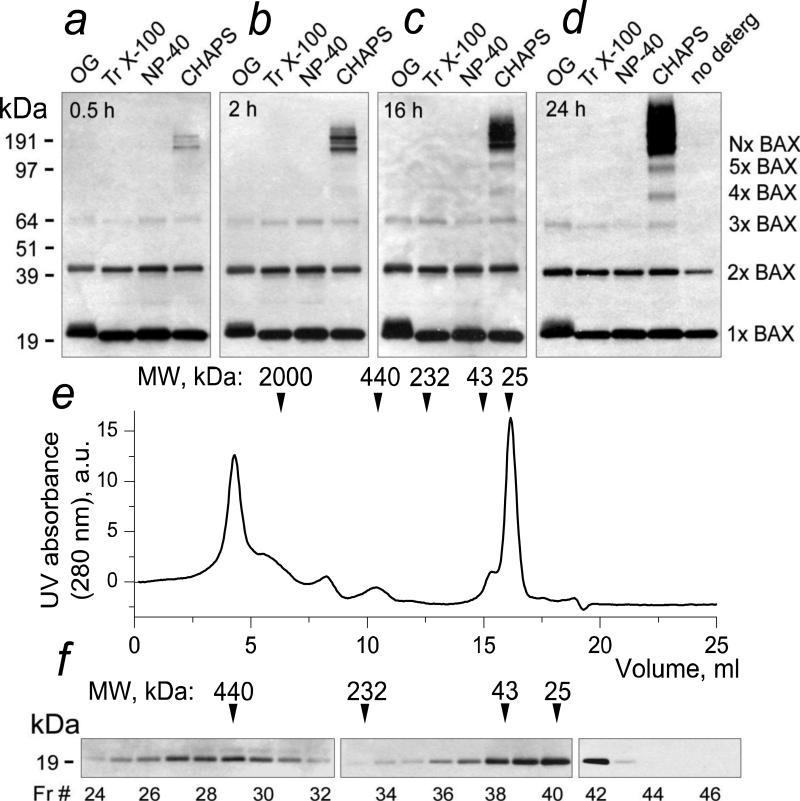
In the experiments with NP-40, the amount of large BAX oligomers (>190 kDa) was much smaller than in the experiments with CHAPS (Fig. 2a,c). This suggested that either NP-40 disassembled the large BAX oligomers (>190 kDa), or they were an artifact produced by interaction of BAX with CHAPS. We tested several non-ionic detergents to evaluate their ability to trigger BAX oligomerization. Previously, Antonsson et al. (2000) reported that octyl glucoside (OG) caused BAX oligomerization [43]. However, in our experiments we did not observe BAX oligomerization with OG. The reason for that is unclear but might be related to the difference in experimental conditions. Our experiments revealed that OG, Triton X-100 (Tr X-100), and NP-40 somewhat increased amounts of BAX dimers and produced small amount of BAX trimers but failed to trigger formation of larger BAX oligomers (Fig. 3a-d). CHAPS, on the other hand, readily oligomerized BAX, producing various forms of BAX oligomers (Fig. 3a-d). The reason other investigators did not observe BAX oligomerization in the presence of CHAPS is not clear but, possibly, this could be due to difference in experimental conditions used for western blotting. For example, in our hands 5% non-fat milk, which is also used by many investigators as blocking solution in western blotting, significantly hindered detection of BAX oligomers produced by CHAPS or by interaction of BAX with mitochondria. Interestingly, in the experiments with the experiments with CHAPS we observed an increase in the total amount of BAX immunoreactive material over time despite equal protein loading in every lane. The reason for this increase is unclear but it is possible that in these experiments monomeric BAX bands were oversaturated and this could obscure redistribution of BAX from monomeric band to the bands corresponding to BAX oligomers.
Figure 3. CHAPS but not Nonidet P-40, Triton X-100, or octyl glucoside produced oligomerization of BAX in the solution without mitochondria. Analytical gel-filtration confirmed BAX oligomerization by CHAPS.
In a-d, BAX (50 nM) was incubated for 0.5-24 hours in the incubation medium at 4°C either with 1% octyl glucoside (OG), or 1% Triton X-100, or 1% Nonidet P-40, or 1% CHAPS, or without detergent. In all cases, the amount of protein loaded on the gel was the same (100 ng/lane). In e and f, BAX (500 nM) was incubated with 1% CHAPS for 24 hours at 4°C. Larger BAX concentration was used to compensate dilution during gel-filtration. Then, BAX incubated with the detergent was passed through a Superdex-200 HR 10/30 column equilibrated with 125 mM KCl, 10 mM HEPES, pH 7.4, and 1% CHAPS. In e, a UV absorbance (280 nm) profile of the gel-filtration eluate. a.u., arbitrary units. In f, western blotting analysis of the collected fractions. Fr, fraction.
To confirm that CHAPS induced BAX oligomerization, we performed analytical gel-filtration of BAX in 1% CHAPS solution (Fig. 3e,f). In these experiments, we detected BAX in high molecular weight fractions, indicating formation of large BAX oligomers. Notably, UV absorbance measurements in the eluate revealed huge BAX aggregates with molecular weights up to several megaDa (Fig. 3e,f). Thus, both SDS-PAGE and analytical gel-filtration confirmed BAX oligomerization in the solution with CHAPS. Overall, these data suggest that in the experiments with alkali-resistant BAX insertion into the OMM, CHAPS might produce an artifact leading to formation of high-molecular weight BAX oligomers (Fig. 2a). On the other hand, these results confirmed that NP-40 did not trigger BAX oligomerization and therefore in the following experiments we used NP-40 to solubilize mitochondria.
3.3 tBID and Ca2+ increase BAX-mediated Cyt c release: role of the mPT
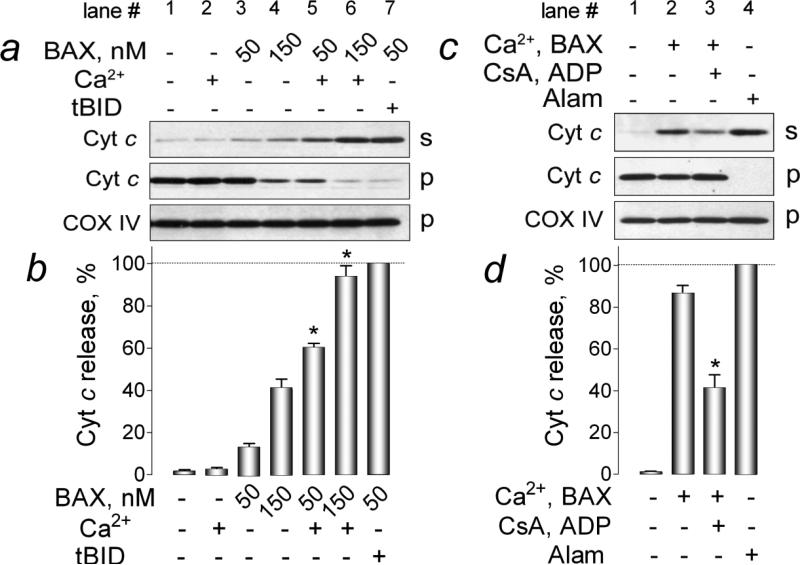
In the next experiments, we evaluated whether BAX insertion/oligomerization augmented by tBID and Ca2+ correlated with increased OMM permeabilization. We examined Cyt c release induced by BAX alone or in combination with tBID or Ca2+. Notably, in these experiments, isolated brain mitochondria retained OMM integrity and did not release Cyt c spontaneously during incubation in the standard 125 mM KCl-based medium for 30 minutes at 37°C (Fig. 4a, lane 1). BAX (50 nM) added alone produced a minuscule Cyt c release (Fig. 4a, lane 3). Larger BAX concentration (150 nM) resulted in a greater Cyt c release (Fig. 4a, lane 4) comparable with Cyt c release observed in our previous study [37]. Importantly, Ca2+ added alone to mitochondria failed to produce substantial Cyt c release (Fig. 4a, lane 2). Similar observations were reported earlier and were linked to insufficient mitochondrial swelling that was not extensive enough to rupture the OMM [33,44,45]. Nevertheless, Ca2+ significantly augmented BAX-mediated Cyt c release (Fig. 4a, lanes 5 and 6). A combination of 50 nM BAX and 20 nM tBID produced a nearly complete Cyt c release (Fig. 4a, lane 7). Pre-treatment of mitochondria with CsA plus ADP, inhibitors of the mPT [40-42], significantly diminished Cyt c release induced by a combination of BAX and Ca2+ (Fig. 4c, compare lanes 2 and 3). In these experiments, alamethicin (30μg/ml) was used as a positive control to produce maximal Cyt c release (Fig. 4c, lane 4). Thus, our data suggested mPT involvement in the Ca2+-induced stimulation of BAX-mediated OMM permeabilization. However, it remained unclear whether Ca2+ amplified membrane-permeabilizing activity of BAX, or BAX augmented Ca2+-induced mitochondrial swelling resulting in OMM damage and Cyt c release.
Figure 4. Ca2+ amplified cytochrome c release induced by recombinant monomeric BAX. Cyclosporin A and ADP, inhibitors of the mitochondrial permeability transition, attenuated the Ca2+ effect.
In a, the effect of Ca2+ and tBID on cytochrome c (Cyt c) release induced by BAX. Where indicated, 250μM Ca2+ (1.4μmol Ca2+ per mg of mitochondrial protein) or 20 nM tBID was added. Here and in other experiments, COX IV was used as a loading control. In b, densitometry analysis of western blots performed with ImageJ software. The densitometry results are shown as percentage of Cyt c release. The Cyt c release induced by 50 nM BAX plus 20 nM tBID was taken as 100%. Data are mean±SEM, *p<0.01 between Cyt c release induced by 50 or 150 nM BAX alone or in the presence of 1.4μmol Ca2+/mg protein, N=3. In c, a combination of cyclosporin A (CsA, 1μM) and ADP (100μM) (here and in other similar experiments, ADP was added in the presence of 1μM oligomycin, an inhibitor of ATP synthase, to prevent ADP phosphorylation) decreased Cyt c release induced by 50 nM BAX in the presence of 1.4μmol Ca2+/mg protein. In these experiments, alamethicin (Alam, 30μg/ml) was used to produce the maximal Cyt c release. In d, densitometry analysis performed with ImageJ software. The densitometry results are shown as percentage of Cyt c release. The Cyt c release induced by 30μg/ml alamethicin was taken as 100%. Data are mean±SEM, *p<0.01 between Cyt c release induced by BAX plus Ca2+ in the absence or in the presence of CsA and ADP, N=4. In a and c, s, supernatants; p, pellets.
3.4 BAX does not augment Ca2+-induced mitochondrial swelling
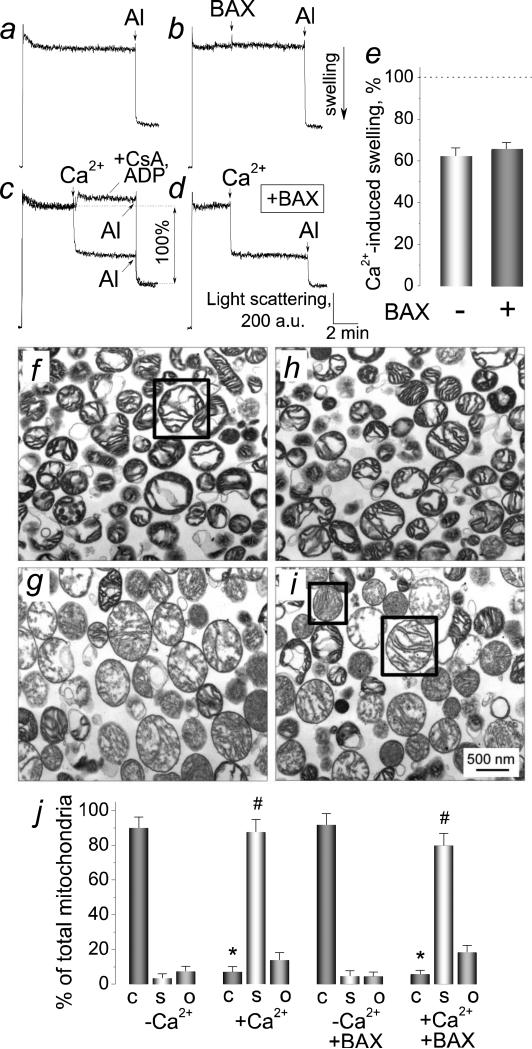
To address this question, we evaluated mitochondrial volume changes using 90° light scattering assay [46]. The untreated mitochondria did not swell spontaneously during the course of the experiment (Fig. 5a). At the end of the experiments, alamethicin (30μg/ml) was added to produce maximal swelling (Fig. 5a-d). BAX (50 nM) alone failed to induce mitochondrial swelling (Fig. 5b). On the other hand, Ca2+, an inducer of the mPT [47], produced large-amplitude mitochondrial swelling, and CsA plus ADP completely prevented this swelling (Fig. 5c). To address the question whether BAX could increase the Ca2+-induced swelling, we incubated mitochondria with BAX (50 nM) and then added Ca2+ (1.4 mol/mg protein) (Fig. 5d). To quantify our data, we measured the amplitude of mitochondrial swelling induced by Ca2+ as a percentage of maximal alamethicin-induced swelling taken as 100% (Fig. 5c). These experiments showed that BAX did not increase the Ca2+-induced mitochondrial swelling (Fig. 5e). Without BAX, Ca2+ (1.4 mol/mg protein) produced 61±5.6% of maximal swelling versus 63.2±4.9% with 50 nM BAX (mean±SEM, N=3).
Figure 5. Recombinant monomeric BAX did not cause mitochondrial swelling and failed to augment Ca2+-induced mitochondrial swelling.
In a-d, 90° light scattering traces indicative of changes in mitochondrial volume obtained with or without Ca2+ and BAX. Here, 30μg/ml alamethicin was added as indicated to cause maximal mitochondrial swelling. In c-d, 1.4μmol Ca2+/mg protein was added to mitochondria where indicated. In b, 50 nM BAX was added as indicated. In d, 50 nM BAX was added to the cuvette prior to mitochondria. In e, the extent of Ca2+-induced mitochondrial swelling with or without 50 nM BAX calculated as a percentage of maximal, alamethicin-induced swelling taken as 100% (c). In f-i, electron micrographs of isolated brain mitochondria incubated with or without Ca2+ and BAX. In f and g, mitochondria were incubated without BAX, but were treated with a vehicle (3μl of the dialysis buffer for BAX (25 mM HEPES-NaOH, pH 7.5, 0.2 mM dithiothreitol, 30% glycerol (v/v)) in 300μl of incubation medium). In h and i, mitochondria were incubated for 30 minutes at 37°C in the medium supplemented with 50 nM BAX. In g and i, the incubation medium was supplemented with 1.4μmol Ca2+/mg protein. In j, morphometric analysis of mitochondria incubated under the indicated conditions. Total mitochondrial population was categorized into three groups according to their morphology: condensed (C), swollen (S), and orthodox (O). The representative example of condensed morphology is shown in the box in panel f; swollen (small box) and orthodox (large box) morphology in panel i. *p<0.001, comparing amount of condensed mitochondria with and without Ca2+; #p<0.001, comparing amount of swollen mitochondria with and without Ca2+. Data are mean ± SEM, N=3.
Transmission electron microscopy (TEM) corroborated the results obtained with light scattering assay. Following Ca2+ application, mitochondrial matrices changed from condensed to predominantly swollen (Fig. 5f,g). BAX (50 nM) failed to affect mitochondrial morphology (Fig. 5h) and did not augment mitochondrial swelling induced by Ca2+ (Fig. 5i). In these experiments, we used the morphometric analysis described previously [35,48]. Figure 5j shows the results of morphometric analysis of mitochondria incubated with or without Ca2+ and BAX. These data indicated that BAX failed to augment the Ca2+-induced swelling. Therefore, the non-specific damage of the OMM appeared unlikely to be the mechanism of the increased Cyt c release following combined application of BAX and Ca2+.
3.5 Alkali treatment and heating are not necessary for BAX oligomerization in the OMM
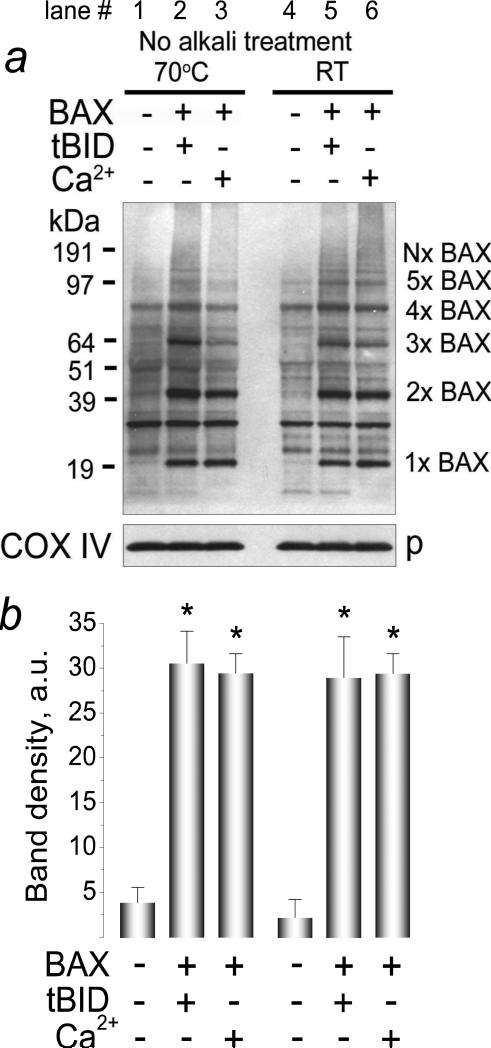
High pH or heating of BAX samples above 43-47°C could lead to BAX oligomerization [49-51]. Correspondingly, there was a possibility that BAX oligomerization in our experiments resulted from alkali treatment of mitochondria or heating samples before SDS-PAGE (15 minutes, 70°C). To rule out this possibility, we evaluated BAX oligomerization without alkali-treatment of mitochondria and heating of samples for SDS-PAGE. In these experiments, we detected the same pattern of BAX insertion/oligomerization in the OMM as we observed in our standard experiments with alkali treatment of mitochondria and heating of protein samples (Fig. 6). Interestingly, without alkali treatment, we detected a new band with molecular weight ~80 kDa in solubilized untreated mitochondria (Fig. 6, lane 1). This band was completely eliminated by alkali treatment of mitochondria (Fig. 2a, d) and therefore might represent endogenous BAX tetramers loosely attached to the OMM.
Figure 6. Alkali treatment of mitochondria and heating the samples before SDS-PAGE were not essential for detecting BAX oligomers.
The experiments were performed as described in the legend to Figure 2 but without alkali treatment of mitochondria and without heating the mitochondrial samples before SDS-PAGE. In a, mitochondria were incubated for 30 minutes at 37°C with 50 nM BAX in combination with 20 nM tBID or 1.4μmol Ca2+/mg protein as indicated. Mitochondrial pellets were solubilized with 1% Nonidet P-40. p, pellets. In b, statistical analysis of BAX insertion/oligomerization in the OMM was performed using one-way ANOVA followed by Bonferroni's post-hoc test. The graph shows the averaged sum of 1×BAX–5×BAX band densities under corresponding experimental conditions. Data are mean±SEM, N=3. In b, *p<0.01 comparing band densities with or without BAX plus tBID or BAX plus Ca2+.
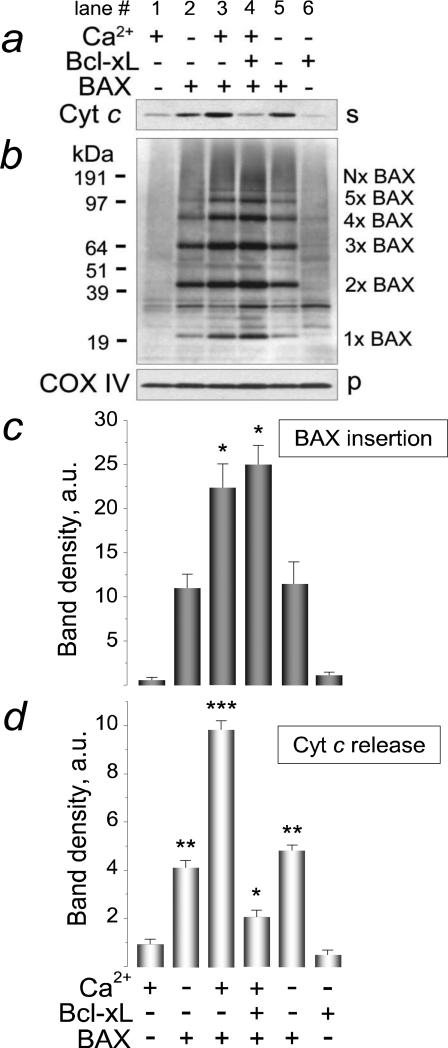
3.6 Effect of recombinant Bcl-xL on BAX insertion/oligomerization and Cyt c release
In our experiments, recombinant Bcl-xL significantly inhibited Cyt c release induced by a combination of BAX and Ca2+ (Fig. 7a, compare lanes 3 and 4). Figure 7d shows statistical analysis of the Cyt c release. Despite inhibition of Cyt c release, Bcl-xL failed to attenuate BAX insertion and oligomerization in the OMM (Fig. 7b, compare lanes 3 and 4). Figure 7c illustratesstatistical analysis of BAX insertion based on densitometry data obtained with individual BAX bands (1×BAX-5×BAX) shown in Figure 7b. Interestingly, using polyclonal anti-BAX antibody, we detected a distinct band with a molecular weight ~30 kDa (Fig. 7b, lane 6), which corresponded to molecular weight of Bcl-xL [52] and was strongly amplified after addition of exogenous Bcl-xL (Fig. 7b, compare lanes 1 and 6). It is possible that this band belonged to exogenous, recombinant Bcl-xL inserted into mitochondrial membranes in alkali-resistant manner.
Figure 7. Recombinant Bcl-xL inhibited cytochrome c release but failed to attenuate BAX insertion and oligomerization in the outer mitochondrial membrane augmented by Ca2+.
Mitochondria were treated for 30 minutes at 37°C with 150 nM BAX, 1.4μmol Ca2+/mg protein, and with 300 nM Bcl-xL as indicated. After 30 minutes of incubation, mitochondria were pelleted by centrifugation and cytochrome c (Cyt c) release was evaluated in the supernatants (a). In b, alkali-resistant BAX insertion was assessed in mitochondrial membranes solubilized with 1% Nonidet P-40. s, supernatants, p, pellets. In c, the statistical analysis of BAX insertion into the OMM was performed using one-way ANOVA followed by Bonferroni's post-hoc test. The graph shows the averaged sum of 1×BAX–5×BAX band densities under corresponding experimental conditions. Data are mean±SEM, N=3. *p<0.01 comparing band densities with BAX versus BAX plus Ca2+ or BAX plus Ca2+ and Bcl-xL. In d, the densitometry analysis of Cyt c release. Data are mean±SEM, *p<0.05, **p<0.01, ***p<0.001 versus Cyt c release in the presence of Bcl-xL alone.
3.7 Role of SH-redox state in BAX insertion/oligomerization and OMM permeabilization
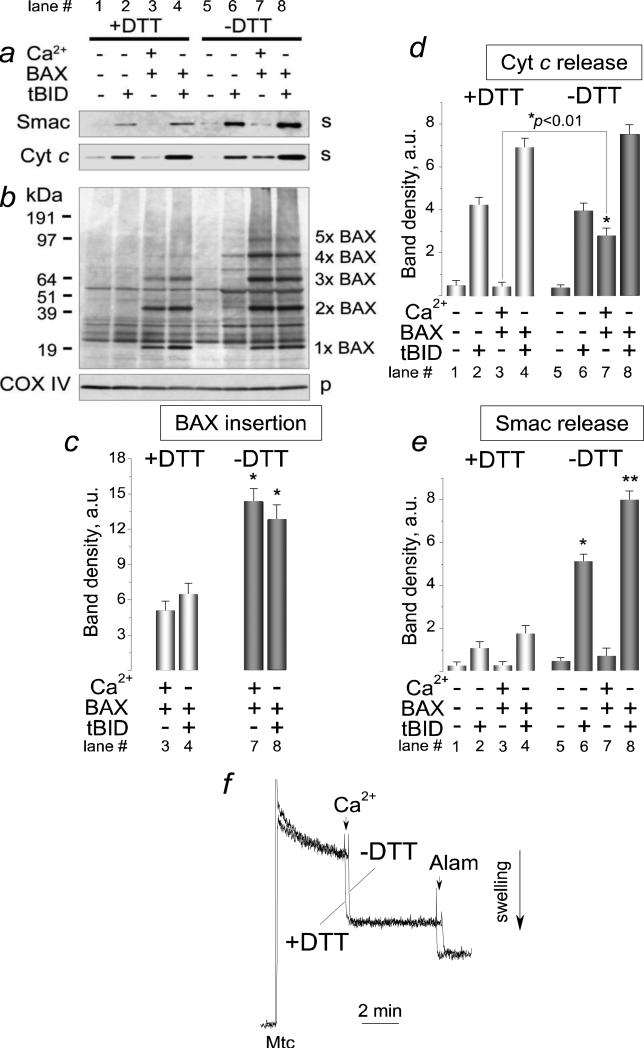
Oxidation of BAX's cysteines and formation of disulfide bridges between BAX molecules favors BAX oligomerization and OMM permeabilization [38,39]. In our experiments, a reducing agent dithiothreitol (DTT, 20 mM) dismantled BAX dimers in the solution without mitochondria (Supplemental Fig. 3). We hypothesized that tBID- and Ca2+-stimulated BAX insertion/oligomerization in the OMM and Cyt c release might depend on oxidation of SH-groups. Indeed, DTT added into the standard incubation medium significantly diminished BAX insertion/oligomerization stimulated by tBID or Ca2+ (Fig. 8b, c, compare lanes 3 and 4 versus 7 and 8). DTT also attenuated insertion/oligomerization of BAX in the absence of tBID or calcium (Supplemental Fig. 4). In addition, DTT inhibited BAX-mediated Cyt c release stimulated by Ca2+ and to a much lesser extent by tBID (Fig. 8a, compare lanes 3 and 7 versus lanes 4 and 8) but failed to inhibit Cyt c release induced by tBID alone (Fig. 8a, compare lanes 2 and 6). On the other hand, DTT strongly inhibited the release of Smac/DIABLO, another mitochondrial apoptogenic protein with twice larger molecular weight than Cyt c [53,54], induced by tBID alone or by a combination of tBID and BAX (Fig. 8a). Interestingly, a combination of Ca2+ and BAX appeared to be ineffective in the release of Smac/DIABLO. Figures 8c shows statistical analysis of BAX insertion shown in Figure 8b. Figure 8d and e show statistical analysis of densitometry data obtained with Cyt c and Smac/DIABLO bands respectively. Importantly, DTT (20 mM) failed to inhibit mitochondrial swelling induced by Ca2+ (Fig. 8f) indicating that DTT effect could not be attributed to inhibition of the mPT. Thus, these experiments revealed for the first time an important role of the SH-redox state in the regulation of BAX insertion/oligomerization and in BAX-mediated OMM permeabilization in brain mitochondria.
Figure 8. Dithiothreitol attenuated BAX insertion/oligomerization and outer mitochondrial membrane permeabilization but failed to inhibit mitochondrial swelling induced by Ca2+.
Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX, with 1.4μmol Ca2+/mg protein, and with 20 nM tBID as indicated. Where indicated, the incubation medium was supplemented with 20 mM dithiothreitol (DTT). Then, mitochondria were pelleted by centrifugation and cytochrome c (Cyt c) and Smac/DIABLO (Smac) releases were evaluated in the supernatants (a). In b, alkali-resistant BAX insertion was assessed in mitochondrial membranes solubilized with 1% Nonidet P-40. s, supernatants, p, pellets. In c, statistical analysis of BAX insertion into the OMM was performed using one-way ANOVA followed by Bonferroni's post-hoc test. The graph shows the averaged sum of 1×BAX–5×BAX band densities under corresponding experimental conditions. Data are mean±SEM, N=3. *p<0.01 comparing BAX band densities with or without 20 mM DTT. In d, the densitometry analysis of Cyt c release. In e, the densitometry analysis of Smac/DIABLO (Smac) release. Data are mean±SEM, N=3, *p<0.01, **p<0.001 comparing Smac/DIABLO release in the presence or absence of 20 mM DTT. In f, mitochondrial swelling followed with a 90° light scattering assay. Light scattering traces obtained with and without dithiothreitol (DTT, 20 mM) are overlapped for comparison. Where indicated, 1.4μmol Ca2+/mg protein, and 30μg/ml alamethicin (Alam) were added to mitochondria
4. Discussion
It has been established in early studies that the extent of Cyt c release correlates with the amount of BAX inserted in the OMM [17,18]. In addition, early studies suggested that OMM permeabilization required BAX oligomerization that occurred prior to BAX insertion into the OMM [17,18], whereas monomeric BAX neither integrated into the OMM nor released Cyt c [36]. In our study for the first time we clearly demonstrated that recombinant monomeric BAX readily self-integrated into the OMM of brain mitochondria and self-oligomerized. We found no evidence for tBID- or Ca2+-induced oligomerization of BAX in the solution prior to interaction with mitochondria. Accordingly, our results suggest that BAX most likely integrates into the OMM as a monomer and that interaction of BAX with the OMM is necessary for BAX oligomerization. Our findings are consistent with reports showing that BAX insertion into the OMM or liposomal membrane preceded the oligomerization step [55,56]. Importantly, the amount of BAX inserted into the OMM in the absence of tBID or calcium was relatively high (see Fig. 2a, lanes 2 and 4, and Fig. 2b, lane 1). On the other hand, the amount of BAX oligomers (except dimers) in the BAX preparation was below the detection limit of western blotting (Fig. 1). Therefore, the amount of BAX inserted and oligomerized in the OMM did not correspond to the amount of BAX oligomers in the BAX preparation.
In our experiments, BAX self-insertion and oligomerization in the OMM resulted in a minute release of Cyt c. Our observation echoes early findings and several recent reports indicating that BAX translocation to mitochondria does not necessarily cause massive OMM permeabilization [57-61]. Additional factors appeared to be required for unleashing the permeabilizing activity of the membrane-inserted and oligomerized BAX. Earlier, Epand et al (2002) reported that the negative curvature in membranes that is essential for OMM permeabilization was promoted by tBID [62]. Correspondingly, in our experiments the lack of massive OMM permeabilization by BAX alone could be explained by the lack of changes in the membrane curvature. In our experiments, tBID and Ca2+ augmented BAX insertion/oligomerization in the OMM and strongly amplified membrane-permeabilizing activity of BAX. The Ca2+-dependent amplification of BAX activity is of particular interest. Bearing in mind that BAX can cause Ca2+ efflux from the endoplasmic reticulum [63-66] and, hence, increase the likelihood of the Ca2+-induced mPT [67], the Ca2+-induced stimulation of BAX insertion/oligomerization in the OMM leading to enhanced OMM permeabilization might represent a feed-forward amplification loop ensuring successful, irreversible progression of the apoptotic program.
Previously, it was shown that Ca2+ stimulated BAX-mediated Cyt c release from isolated liver mitochondria [25]. However, the mechanism of this stimulation was not investigated further. In our study with isolated brain mitochondria, we demonstrated that the Ca2+-induced amplification of the BAX-mediated Cyt c release occurred parallel to augmented alkali-resistant BAX insertion/oligomerization in the OMM, and that both BAX insertion/oligomerization in the OMM and BAX-mediated Cyt c release were facilitated by mPT induction. Thus, our results suggest augmented BAX insertion/oligomerization a mechanistic link between the Ca2+-induced mPT and increased BAX-mediated Cyt c release. In contrast to Ca2+, tBID-stimulated BAX insertion, oligomerization, and Cyt c release appeared to be mPT-independent, but in this case augmented BAX insertion/oligomerization also correlated with the increased Cyt c release.
Anti-apoptotic Bcl-2, a close relative of Bcl-xL [68,69], can inhibit pro-apoptotic BAX activity by heterodimerizing with BAX [70,71] or by binding tBID and hence precluding tBID-dependent activation of BAX [56,72]. Whether Bcl-xL/BAX heterodimerization affected BAX insertion/oligomerization in the OMM or inhibited already inserted and oligomerized BAX remained unclear. In our experiments, recombinant anti-apoptotic protein Bcl-xL failed to prevent BAX insertion and oligomerization in the OMM. However, Bcl-xL strongly inhibited Cyt c release induced by a combination of BAX and Ca2+. Earlier, we showed that recombinant Bcl-xL inhibited Cyt c release induced by a combination of tBID and monomeric BAX [73]. Thus, our results support a scenario in which Bcl-xL inhibits inserted/oligomerized BAX and emphasize the fact that BAX insertion/oligomerization in the OMM could be dissociated from OMM permeabilization. How Bcl-xL restrains the inserted/oligomerized BAX from permeabilizing the OMM has yet to be determined. It seems conceivable that Bcl-xL could bind to the inserted/oligomerized BAX and physically block or disrupt the BAX pore, leading to inhibition of the BAX-mediated OMM permeabilization.
It is well established that apoptosis induced by different stimuli is often accompanied by an increase in ROS generation, and that suppression of ROS generation might protect cells against apoptosis [74-77]. Following ROS attack, critical SH-groups of different proteins might be oxidized leading to formation of intra- and inter-molecular disulfide bridges [78]. The exact role of the SH-redox state in the membrane-permeabilizing activity of BAX is not clear yet but it is possible that changes in intracellular SH-redox state could influence BAX conformation and thus stimulate BAX insertion/oligomerization in the OMM. Indeed, D'Alessio et al. (2005) demonstrated that oxidation of cysteine residues of BAX resulted in formation of disulfide bridges, causing conformational changes that favored BAX dimerization and translocation to mitochondria [38]. In our study, a reducing agent DTT inhibited tBID- and Ca2+-stimulated BAX insertion/oligomerization in the OMM, but only in the latter case DTT significantly suppressed Cyt c release. This suggests that Ca2+-stimulated BAX-mediated Cyt c release depends on oxidation of SH-groups whereas the tBID-stimulated BAX-mediated Cyt c release does not. It is conceivable that disruption of disulfide bridges between BAX molecules with DTT underlies a decrease in BAX insertion/oligomerization in the OMM affecting OMM permeability. Alternatively, DTT could antagonize the Ca2+-induced mPT [79] and hence hinder BAX-mediated Cyt c release. However, in our experiments DTT failed to inhibit mitochondrial swelling induced by Ca2+ ruling out this possibility.
The lack of correlation between diminished BAX insertion/oligomerization and virtually unchanged Cyt c release observed with tBID in the presence of DTT suggests that even small amounts of BAX inserted and oligomerized in the OMM might be sufficient for massive Cyt c release as proposed recently [80]. However, in our experiments, self-insertion and self-oligomerization of BAX in the OMM failed to induce massive Cyt c release, signifying a need for additional factors. It is also conceivable that the size of BAX pores formed with tBID remains large enough to pass Cyt c even in the presence of DTT whereas conductance of the Ca2+-activated BAX pores declines more significantly with DTT making the pores less passable for Cyt c. Our experiments with Smac/DIABLO release support this hypothesis. Smac/DIABLO is approximately twice larger than Cyt c [53,54]. While producing sizable Cyt c release, a combination of BAX and Ca2+ failed to induce Smac/DIABLO release suggesting BAX pore size a limiting factor. DTT, which failed to inhibit tBID-stimulated BAX-mediated Cyt c release, at the same time strongly decreased the release of Smac/DIABLO. It is possible that reduction of disulfides with DTT affects not only insertion and oligomerization of BAX and, correspondingly, the number of BAX pores in the OMM, but also the size of the BAX pores. Thus, in addition to the amount of BAX inserted/oligomerized in the OMM, modulation of SH-redox state might influence molecular architecture of BAX oligomers that could be critical for effective OMM permeabilization.
Overall, our results strongly suggest that BAX-mediated OMM permeabilization in brain mitochondria can be modulated by the mPT and by SH-redox state. Correspondingly, induction of the mPT, increased ROS generation, and oxidation of critical SH-groups could significantly augment BAX-mediated permeabilization of the OMM and thus promote neuronal apoptosis in various neurodegenerative diseases, stroke, and traumatic brain injury.
Supplementary Material
Supplemental Materials.
Supplemental Figure 1. Cross-linkers caused BAX oligomerization in the solution without mitochondria. Substitution of non-fat milk for BSA as a blocking solution in western blotting improved detection of BAX oligomers. In all experiments, the standard incubation medium was supplemented with 50 nM BAX and with 20 nM tBID as indicated. In a, the medium was supplemented with a vehicle (1μl DMSO in 300μl of the incubation medium) or with 0.2 mM bismaleimidohexane (BMH, Pierce). The quenching was performed with 50 mM dithiothreitol (DTT) for 30 minutes with rocking at room temperature. In b, the medium was supplemented with a vehicle (1μl DMSO in 300μl of the incubation medium) or with 0.5 mM ethylene glycol bis(succinimidyl succinate) (EGS, Pierce) or 0.5 mM disuccinimidyl suberate (DSS, Pierce), respectively. In both cases, the quenching was performed with 20 mM Tris-HCl, pH 7.5, for 30 minutes with rocking at room temperature. In a, 5% non-fat milk was used as a blocking solution in western blotting. In b, 5% BSA in PBS was used as a blocking solution.
Supplemental Figure 2. Distribution of exogenous, recombinant BAX between the OMM and the incubation medium: a fraction of non-oligomerized BAX remained in the solution. The standard incubation medium was supplemented with 50 nM BAX in a combination with 20 nM tBID or 1.4μmol Ca2+/mg protein as indicated. In a, alkali-resistant BAX insertion and oligomerization with tBID or Ca2+. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX and 20 nM tBID or 1.4μmol Ca2+/mg protein as indicated. Following alkali treatment (30 minutes on ice in 0.1 M Na2CO3 at pH 11.5), mitochondrial membranes were solubilized with 1% Nonidet P-40 and BAX was detected with western blotting. In d, BAX detected in the supernatants (Super) obtained prior to alkali treatment of mitochondria. In b and e, densitometry data obtained with individual BAX bands shown in panels a and d, respectively. In c and f, the averaged sum of densities of all BAX bands (1×BAX - 5×BAX) shown in panels a and d, respectively.
Supplemental Figure 3. Dithiothreitol disassembled BAX dimers. 50 nM BAX was incubated for 30 minutes at 37°C in the standard incubation medium supplemented with 250μM Ca2+ or 20 nM tBID with or without 20 mM dithiothreitol (DTT) as indicated. Then, non-reducing SDS-PAGE and western blotting were performed.
Supplemental Figure 4. Dithiothreitol decreased insertion and oligomerization of monomeric BAX. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX, as indicated. Where indicated, the incubation medium was supplemented with 20 mM dithiothreitol (DTT). Mitochondria were then pelleted by centrifugation and subjected to alkali treatment (30 minutes on ice in 0.1 M Na2CO3 at pH 11.5). Alkali-resistant BAX insertion was assessed by non-reducing SDS-PAGE followed by western blotting with mitochondrial membranes solubilized in 1% Nonidet P-40.
Acknowledgements
This work was supported by the NIH/NINDS R01 NS 050131, by grant from ISDH - Indiana Spinal Cord and Brain Injury Research Fund #A70-0-079212, and by grant from Ralph W. and Grace M. Showalter foundation to NB.
Abbreviations used are
- tBID
truncated BID
- mPT
mitochondrial permeability transition
- OMM
outer mitochondrial membrane
- ER
endoplasmic reticulum
- CsA
cyclosporin A
- COX IV
cytochrome oxidase subunit IV
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
- NP-40
Nonidet P-40, [octylphenoxy] polyethoxyethanol
- OG
octyl glucoside
- Tr X-100
Triton X-100
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- EGS
ethylene glycol bis(succinimidyl succinate)
- DSS
disuccinimidyl suberate
- BMH
bismaleimidohexane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 3.Deigner HP, Haberkorn U, Kinscherf R. Apoptosis modulators in the therapy of neurodegenerative diseases. Expert. Opin. Investig. Drugs. 2000;9:747–764. doi: 10.1517/13543784.9.4.747. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Ekshyyan O, Aw TY. Apoptosis: a key in neurodegenerative disorders. Curr. Neurovasc. Res. 2004;1:355–371. doi: 10.2174/1567202043362018. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Blomgren K. Mitochondrial Cell Death Control in Familial Parkinson Disease. PLoS. Biol. 2007;5:1409–1411. doi: 10.1371/journal.pbio.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 8.Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 9.Distelhorst CW, Shore GC. Bcl-2 and calcium: controversy beneath the surface. Oncogene. 2004;23:2875–2880. doi: 10.1038/sj.onc.1207519. [DOI] [PubMed] [Google Scholar]
- 10.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J. Neurotrauma. 2000;17:857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]
- 12.Plesnila N, von BL, Retiounskaia M, Engel D, Ardeshiri A, Zimmermann R, Hoffmann F, Landshamer S, Wagner E, Culmsee C. Delayed neuronal death after brain trauma involves p53-dependent inhibition of NF-kappaB transcriptional activity. Cell Death. Differ. 2007;14:1529–1541. doi: 10.1038/sj.cdd.4402159. [DOI] [PubMed] [Google Scholar]
- 13.Smith DJ, Ng H, Kluck RM, Nagley P. The mitochondrial gateway to cell death. IUBMB. Life. 2008;60:383–389. doi: 10.1002/iub.44. [DOI] [PubMed] [Google Scholar]
- 14.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death. Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 15.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 17.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 20.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 22.Epand RF, Martinou JC, Montessuit S, Epand RM. Membrane perturbations induced by the apoptotic Bax protein. Biochem. J. 2002;367:849–855. doi: 10.1042/BJ20020986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epand RF, Martinou JC, Montessuit S, Epand RM, Yip CM. Direct evidence for membrane pore formation by the apoptotic protein Bax. Biochem. Biophys. Res Commun. 2002;298:744–749. doi: 10.1016/s0006-291x(02)02544-5. [DOI] [PubMed] [Google Scholar]
- 24.Epand RF, Martinou JC, Montessuit S, Epand RM. Fatty acids enhance membrane permeabilization by pro-apoptotic Bax. Biochem. J. 2004;377:509–516. doi: 10.1042/BJ20030938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gogvadze V, Robertson JD, Zhivotovsky B, Orrenius S. Cytochrome c release occurs via Ca2+-dependent and Ca2+-independent mechanisms that are regulated by Bax. J. Biol. Chem. 2001;276:19066–19071. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell. Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 27.Precht TA, Phelps RA, Linseman DA, Butts BD, Le SS, Laessig TA, Bouchard RJ, Heidenreich KA. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death. Differ. 2005;12:255–265. doi: 10.1038/sj.cdd.4401552. [DOI] [PubMed] [Google Scholar]
- 28.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J. Biol. Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 29.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 30.Correa F, Soto V, Zazueta C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int. J. Biochem. Cell Biol. 2007;39:787–798. doi: 10.1016/j.biocel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Montessuit S, Mazzei G, Magnenat E, Antonsson B. Expression and purification of full-length human Bax alpha. Protein Expr. Purif. 1999;15:202–206. doi: 10.1006/prep.1998.1010. [DOI] [PubMed] [Google Scholar]
- 32.Kudla G, Montessuit S, Eskes R, Berrier C, Martinou JC, Ghazi A, Antonsson B. The destabilization of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved bid is inhibited by the N-terminal fragment. J. Biol. Chem. 2000;275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- 33.Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Shalbuyeva N, Brustovetsky T, Bolshakov A, Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J. Biol. Chem. 2006;281:37547–37558. doi: 10.1074/jbc.M607263200. [DOI] [PubMed] [Google Scholar]
- 36.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 2002;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brustovetsky T, Antonsson B, Jemmerson R, Dubinsky JM, Brustovetsky N. Activation of calcium-independent phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J. Neurochem. 2005;94:980–994. doi: 10.1111/j.1471-4159.2005.03248.x. [DOI] [PubMed] [Google Scholar]
- 38.D'Alessio M, De NM, Coppola S, Gualandi G, Pugliese L, Cerella C, Cristofanon S, Civitareale P, Ciriolo MR, Bergamaschi A, Magrini A, Ghibelli L. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19:1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- 39.Nie C, Tian C, Zhao L, Petit PX, Mehrpour M, Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J. Biol. Chem. 2008;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 41.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 42.Novgorodov SA, Gudz TI, Milgrom YM, Brierley GP. The permeability transition in heart mitochondria is regulated synergistically by ADP and cyclosporin A. J. Biol. Chem. 1992;267:16274–16282. [PubMed] [Google Scholar]
- 43.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- 44.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Brustovetsky T, Antonsson B, Brustovetsky N. Oligomeric BAX induces mitochondrial permeability transition and complete cytochrome c release without oxidative stress. Biochim. Biophys. Acta. 2008;1777:1409–1421. doi: 10.1016/j.bbabio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T, Brustovetsky T, Antonsson B, Brustovetsky N. Dissimilar mechanisms of cytochrome c release induced by octyl glucoside-activated BAX and by BAX activated with truncated BID. Biochim. Biophys. Acta. 2010;1797:52–62. doi: 10.1016/j.bbabio.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 48.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 49.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terrones O, Etxebarria A, Landajuela A, Landeta O, Antonsson B, Basanez G. BIM and tBID are not mechanistically equivalent when assisting BAX to permeabilize bilayer membranes. J. Biol. Chem. 2008;283:7790–7803. doi: 10.1074/jbc.M708814200. [DOI] [PubMed] [Google Scholar]
- 51.Cartron PF, Oliver L, Mayat E, Meflah K, Vallette FM. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 2004;578:41–46. doi: 10.1016/j.febslet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 52.Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 54.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 55.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Ruffolo SC, Breckenridge DG, Nguyen M, Goping IS, Gross A, Korsmeyer SJ, Li H, Yuan J, Shore GC. BID-dependent and BID-independent pathways for BAX insertion into mitochondria. Cell Death Differ. 2000;7:1101–1108. doi: 10.1038/sj.cdd.4400739. [DOI] [PubMed] [Google Scholar]
- 58.Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J. Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Upton JP, Valentijn AJ, Zhang L, Gilmore AP. The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death. Differ. 2007;14:932–942. doi: 10.1038/sj.cdd.4402092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valentijn AJ, Upton JP, Gilmore AP. Analysis of endogenous Bax complexes during apoptosis using blue native PAGE: implications for Bax activation and oligomerization. Biochem. J. 2008;412:347–357. doi: 10.1042/BJ20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epand RF, Martinou JC, Fornallaz-Mulhauser M, Hughes DW, Epand RM. The apoptotic protein tBid promotes leakage by altering membrane curvature. J. Biol. Chem. 2002;277:32632–32639. doi: 10.1074/jbc.M202396200. [DOI] [PubMed] [Google Scholar]
- 63.Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O'Neil RG, McConkey DJ. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- 64.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J. Biol. Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 65.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deniaud A, Sharaf El DO, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 68.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 69.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 70.Zha H, ime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, Huang DC, Adams JM. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS. Biol. 2008;6:e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brustovetsky N, Dubinsky JM, Antonsson B, Jemmerson R. Two pathways for tBID-induced cytochrome c release from rat brain mitochondria: BAK- versus BAX-dependence. J. Neurochem. 2003;84:196–207. doi: 10.1046/j.1471-4159.2003.01545.x. [DOI] [PubMed] [Google Scholar]
- 74.Dawson VL, Dawson TM. Free radicals and neuronal cell death. Cell Death. Differ. 1996;3:71–78. [PubMed] [Google Scholar]
- 75.Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death. Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome C release in neuronal apoptosis. Antioxid. Redox. Signal. 2003;5:589–596. doi: 10.1089/152308603770310257. [DOI] [PubMed] [Google Scholar]
- 78.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 79.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 80.Dussmann H, Rehm M, Concannon CG, Anguissola S, Wurstle M, Kacmar S, Voller P, Huber HJ, Prehn JH. Single-cell quantification of Bax activation and mathematical modelling suggest pore formation on minimal mitochondrial Bax accumulation. Cell Death. Differ. 2010;17:278–290. doi: 10.1038/cdd.2009.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials.
Supplemental Figure 1. Cross-linkers caused BAX oligomerization in the solution without mitochondria. Substitution of non-fat milk for BSA as a blocking solution in western blotting improved detection of BAX oligomers. In all experiments, the standard incubation medium was supplemented with 50 nM BAX and with 20 nM tBID as indicated. In a, the medium was supplemented with a vehicle (1μl DMSO in 300μl of the incubation medium) or with 0.2 mM bismaleimidohexane (BMH, Pierce). The quenching was performed with 50 mM dithiothreitol (DTT) for 30 minutes with rocking at room temperature. In b, the medium was supplemented with a vehicle (1μl DMSO in 300μl of the incubation medium) or with 0.5 mM ethylene glycol bis(succinimidyl succinate) (EGS, Pierce) or 0.5 mM disuccinimidyl suberate (DSS, Pierce), respectively. In both cases, the quenching was performed with 20 mM Tris-HCl, pH 7.5, for 30 minutes with rocking at room temperature. In a, 5% non-fat milk was used as a blocking solution in western blotting. In b, 5% BSA in PBS was used as a blocking solution.
Supplemental Figure 2. Distribution of exogenous, recombinant BAX between the OMM and the incubation medium: a fraction of non-oligomerized BAX remained in the solution. The standard incubation medium was supplemented with 50 nM BAX in a combination with 20 nM tBID or 1.4μmol Ca2+/mg protein as indicated. In a, alkali-resistant BAX insertion and oligomerization with tBID or Ca2+. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX and 20 nM tBID or 1.4μmol Ca2+/mg protein as indicated. Following alkali treatment (30 minutes on ice in 0.1 M Na2CO3 at pH 11.5), mitochondrial membranes were solubilized with 1% Nonidet P-40 and BAX was detected with western blotting. In d, BAX detected in the supernatants (Super) obtained prior to alkali treatment of mitochondria. In b and e, densitometry data obtained with individual BAX bands shown in panels a and d, respectively. In c and f, the averaged sum of densities of all BAX bands (1×BAX - 5×BAX) shown in panels a and d, respectively.
Supplemental Figure 3. Dithiothreitol disassembled BAX dimers. 50 nM BAX was incubated for 30 minutes at 37°C in the standard incubation medium supplemented with 250μM Ca2+ or 20 nM tBID with or without 20 mM dithiothreitol (DTT) as indicated. Then, non-reducing SDS-PAGE and western blotting were performed.
Supplemental Figure 4. Dithiothreitol decreased insertion and oligomerization of monomeric BAX. Mitochondria were treated for 30 minutes at 37°C with 50 nM BAX, as indicated. Where indicated, the incubation medium was supplemented with 20 mM dithiothreitol (DTT). Mitochondria were then pelleted by centrifugation and subjected to alkali treatment (30 minutes on ice in 0.1 M Na2CO3 at pH 11.5). Alkali-resistant BAX insertion was assessed by non-reducing SDS-PAGE followed by western blotting with mitochondrial membranes solubilized in 1% Nonidet P-40.