Abstract
Background
The assumption that the assessment of FEF25-75 does not provide additional information in asthmatic children with normal FEV1 % predicted has not been adequately tested.
Objective
To determine whether the measurement of the FEF25-75 % predicted offers advantages over the FEV1 % predicted and the FEV1/FVC % predicted for the evaluation of childhood asthma.
Methods and Measurements
This is a secondary analysis of data from the “Pediatric Asthma Controller Trial” and the “Characterizing the Response to a Leukotriene Receptor Antagonist and Inhaled Corticosteroid” trials. Pearson correlation coefficients, Pearson partial correlation coefficients, canonical correlations, and receiver operator characteristic (ROC) curves were constructed.
Results
Among 437 children with normal FEV1 % predicted, FEF25-75 % predicted and FEV1/FVC % predicted were (1) positively correlated with log2 methacholine PC20, (2) positively correlated with morning and evening peak expiratory flow % predicted, and (3) negatively correlated with log10 FeNO and bronchodilator responsiveness. Pearson partial correlations and canonical correlations indicated that FEF25-75 % predicted was better correlated with bronchodilator responsiveness and log2 methacholine PC20 than were the FEV1 % predicted or FEV1/FVC % predicted. In the ROC curve analysis, FEF25-75 at 65% predicted had a 90% sensitivity and a 67% specificity for detecting a 20% increase in FEV1 following albuterol inhalation.
Conclusion
FEF25-75 % predicted was well correlated with bronchodilator responsiveness in asthmatic children with normal FEV1. FEF25-75 % predicted should be evaluated in clinical studies of asthma in children, and may be of use in predicting the presence of clinically relevant reversible airflow obstruction.
Keywords: FEF25-75, bronchodilator responsiveness, asthma, FEV1/FVC, canonical correlations and ROC curves
Introduction
The guidelines of the American Thoracic Society (ATS) do not suggest that the assessment of the forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) plays a significant role in the measurement of airflow obstruction (1,2), and by inference in the clinical assessment of patients with airflow limitation. Recent studies have demonstrated that asthmatic patients may have ventilatory defects in the presence of a normal FEV1 (3, 4). There also are studies that suggest that the FEF25-75 is more sensitive as an indicator of symptomatic asthma than the FEV1 in children (5,6,7) as well as adults (8,9). Since it does not include flows high in the lung volume, the FEF25-75 is theoretically less effort-dependent than the FEV1 and is believed to be a measurement of small airway patency (10,11,12). Similarly, the FEV1/FVC has been found to correlate with symptoms and medication use in children while the FEV1 does not (13).
The current study is a secondary analysis of pulmonary function data derived from two multicenter pediatric asthma clinical trials conducted by the Childhood Asthma Research and Education (CARE) Network funded by the NHLBI, entitled “Pediatric Asthma Controller Trial (PACT)” (14) and “Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid (CLIC)” trial (15).
The objective of this study is to determine whether the measurement of the FEF25-75 in children contributes additional information about clinical variables and airway inflammation to the information obtained from the “gold standard” measurement, the forced expiratory volume in 1 second (FEV1).
Methods
We evaluated baseline lung function in relation to clinical and physiologic outcome data from two NHLBI Childhood Asthma Research and Education network studies, the Pediatric Asthma Controller (PACT) trial and the Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid (CLIC) trial. PACT (14) enrolled 413 children between the ages of 6 to 14 years with mild-to-moderate persistent asthma. There was a 2 to 4 week run-in period. The CLIC trial (15) enrolled 191 children between the ages of 6 to 17 years with mild-to-moderate persistent asthma. There was a 7 to 10 day run-in period. The CLIC trial excluded children with FEV1 below 70% predicted. Children who completed the run-in periods were potentially eligible for entry into this analysis of baseline and run-in data.
An electronic peak flow meter (AM1; Jaeger-Toennies GmbH, Hoechberg, Germany) was used. Twice-daily entries of asthma symptoms, nighttime awakenings, and rescue albuterol use were recorded in a weekly diary. At the end of the run-in period, the diary and electronic peak flow meter data were recorded. Subjects with <80% compliance in the diary documentation of peak expiratory flow (PEF) measurements, symptom scores, and albuterol use were excluded. Spirometry was performed by CARE Network-certified pulmonary function technicians with re-reading of results from all sites performed by two individuals following a standardized manual of operation in order to ensure consistency. The tests were performed using a pneumotachograph-type spirometer interfaced with a personal computer system (Jaeger-Toennies GmbH, Hoechberg, Germany). Equipment and testing procedures for the maximal expiratory flow volume maneuvers met American Thoracic Society (ATS) 1994 spirometry standards (15, 16) with techniques modified for children less than 8 years of age as described by Eigen et al (17) and Arets et al. (18). Age, gender, and ethnicity appropriate prediction equations (19) were used to calculate percent of predicted values for FEV1 and forced vital capacity (FVC). Spirometry was performed at least 4 hours after the last use of a short-acting bronchodilator. Bronchodilator responsiveness was tested by repeating spirometry after administering 4 to 8 puffs of albuterol using a valved holding chamber in a graded maximal bronchodilation test. Exhaled nitric oxide was measured using the NIOX® device (Aerocrine Inc, USA, New Providence, NJ) following recommended procedures (21).
Caucasian and African-American children ages 6-7 years were excluded from our analyses because we did not have reliable FEF25-75 predicted equations for them. Only baseline and run-in data prior to randomization were analyzed. There were 272 PACT children and 165 CLIC children with sufficient baseline data to be included in these secondary analyses. All of the correlational analyses described below were applied to the combined data set with 272 + 165 = 437 PACT and CLIC children, with an adjustment for the binary indicator of the child's enrolled study.
The baseline measurements were partitioned into two distinct sets. Set 1 consisted of the three spirometric variables (FEV1 % predicted, FEF25-75 % predicted, and FEV1/FVC % predicted – all pre-bronchodilator). Set 2 consisted of nine variables describing clinical (diary symptoms, diary rescue albuterol use, asthma control questionnaire (ACQ) (22) scores, and prior asthma hospitalization) and physiologic outcomes (morning and evening diary PEF % predicted, maximum bronchodilator (BD) response as % change in FEV1, methacholine PC20, and exhaled nitric oxide (FeNO). Methacholine PC20 values were transformed to log2 and FeNO values were transformed to log10 in order to normalize their distributions. The % predicted spirometry values that we used in the analyses are adjusted for gender, race, height, and age. All of the correlational analyses described below also are adjusted for asthma duration, age at asthma onset, atopic status, and body mass index
Pearson correlation coefficients and Pearson partial correlation coefficients were constructed between the spirometry variables and the outcome variables. The partial correlation coefficient between a spirometry variable and each of the outcome variables was adjusted for the presence of the remaining two spirometry variables. Thus, the partial correlation coefficient indicates whether a spirometry variable is correlated with an outcome variable after accounting for the other two spirometry variables.
Canonical correlations, a generalization of Pearson correlations, were also performed to determine how two sets of variables relate to one another (23). The Pearson correlation coefficient investigates the linear relationship between one spirometry variable and one outcome variable. The Pearson partial correlation coefficient demonstrates the linear relationship between one spirometric variable and one outcome variable while accounting for the other spirometric variables. There are nine outcome variables and three spirometric variables. Therefore, the Pearson correlations and the Pearson partial correlations do not provide an adequately thorough analysis and interpretation of the complex relationships that may exist between the set of spirometric variables and the set of outcome variables. Canonical correlation analysis is a multivariate statistical method. It finds the linear combinations of two sets of variables that have the largest Pearson correlation. It is a generalization of a Pearson correlation coefficient and investigates the relationships between the two sets of variables.
The basic steps of a canonical correlation analysis are as follows.
The pair of linear combinations of the two sets of variables (Set 1 and Set 2) that has the largest Pearson correlation is determined. This is called Variate Pair 1, and the correlation between the two linear combinations is the first canonical correlation.
Variate Pair 2 is the pair of linear combinations that have the largest correlation among all those linear combination pairs that are uncorrelated with Variate Pair 1, yielding the second canonical correlation.
This process is continued until the number of Variate Pairs equals the number of variables in the smaller of the two variable sets. With respect to the PACT and CLIC data sets, there were three spirometric variables in Set 1 and nine outcome variables in Set 2. Thus, a maximum of three Variate Pairs and their corresponding canonical correlations can be constructed, although not all three of the canonical correlations actually may be statistically significant. The magnitudes of the coefficients of the variables in the Variate Pairs with significant canonical correlations indicate which variables within Set 1 are strongly correlated with variables within Set 2.
All correlations were performed using the combined data from the PACT and CLIC trials. These analyses were also performed using the data from each trial separately.
Finally, receiver operator characteristic (ROC) curves were calculated for FEF25-75 % predicted and FEV1/FVC % predicted to evaluate their sensitivity and specificity in predicting bronchodilator responsiveness (24). We chose to use a pre- to post-bronchodilator increase in FEV1 of 20% as the target value for the ROC curve because a 12% response was a potential entry criterion for the CLIC trial and because a 20% increase in FEV1 represents a clinically relevant response.
The PACT and CLIC data were also analyzed independently so that those analyses could be compared with each other and with the analyses of the combined data.
Results
Table 1 shows the baseline characteristics of CLIC and PACT cohorts. The PACT children were slightly younger, had a shorter duration of asthma, higher FEV1% predicted, higher FEF25-75% predicted, lower maximum bronchodilator response, lower methacholine response and lower exhaled NO than the CLIC children.
Table 1.
Baseline Characteristics of CLIC and PACT Cohorts
| Baseline Characteristic | CLIC | PACT | P-value* comparing CLIC and PACT | CLIC and PACT Combined |
|---|---|---|---|---|
| Baseline Participants, n | 151 | 328 | –- | 479 |
| Age, years | 12.8 ± 2.8 | 10.9 ± 1.7 | <0.0001 | 11.5 ± 2.3 |
| Male gender, n (%) | 91 (60.3%) | 201 (61%) | 0.8323 | 292 (61%) |
| Caucasian, n (%) | 111 (74%) | 243 (74%) | 0.8940 | 354 (74%) |
| Hispanic or Latino, n (%) | 40 (27%) | 82 (25%) | 0.7280 | 122 (25%) |
| BMI (kg/m2) | 21.8 ± 5.6 | 21.1 ± 5.1 | 0.2008 | 21.3 ± 5.3 |
| Duration of Asthma, years | 7.6 ± 4.0 | 6.5 ± 3.3 | 0.0017 | 6.8 ± 3.6 |
| Spirometry Outcomes | ||||
| FEV1 % Predicted | 93.9 ±14.3 | 98.2 ± 12.6 | 0.0030 | 96.7 ± 13.3 |
| FEF25-75 % Predicted | 67.3 ± 22.7 | 73.8 ± 21.4 | 0.0040 | 71.4 ± 22.1 |
| FEV1/FVC % Predicted | 90.8 ± 9.6 | 93.0 ± 8.6 | 0.0511 | 92.3 ± 9.0 |
| Clinical Outcomes | ||||
| Diary symptoms (Cough and Wheeze), 1 week | 0.54 ± 0.43 | 0.49 ± 0.36 | 0.2474 | 0.51 ± 0.38 |
| Diary Rescue Albuterol Use | 0.82 ± 1.07 | 0.82 ± 1.00 | 0.9928 | 0.82 ± 1.02 |
| ACQ Score | 1.05 ± 0.76 | 1.14 ± 0.57 | 0.2485 | 1.11 ± 0.64 |
| Prior Hospitalizations, past year | 0.26 ± 0.44 | 0.24 ± 0.43 | 0.5724 | 0.25 ± 0.43 |
| Diary AM PEF % predicted | 76.7 ± 13.4 | 76.9 ± 15.0 | 0.8703 | 76.9 ± 14.4 |
| Diary PM PEF % predicted | 79.3 ± 13.4 | 78.7 ± 15.4 | 0.6855 | 78.9 ± 14.7 |
| Physiological Outcomes | ||||
| Max BD Response (% Change in FEV1) | 14.9 ± 10.3 | 9.4 ± 7.2 | <0.0001 | 11.1 ± 8.7 |
| Log2 (PC20 methacholine) | 3.3 ± 4.5 | 2.2 ± 2.8 | 0.0321 | 2.5 ± 3.4 |
| Log10 (FeNO) | 1.5 ± 0.4 | 1.4 ± 0.4 | 0.0041 | 1.4 ± 0.4 |
P-value calculated using chi-square test (for categorical variables) or student's t-test (for continuous variables).
Pearson Correlation Coefficients
The estimated Pearson correlations among the three pre-bronchodilator spirometry variables appear in Table 2. The estimated correlations for the combined PACT and CLIC data were relatively strong, although they did not exceed 0.80. Therefore, multi-collinearity was not an issue of concern in the ensuing analyses and results. Similar results were found when the PACT and CLIC data were analyzed separately (eTables 1 and 2).
Table 2.
Confidence Intervals and P-values for Testing Null Correlations Among Spirometry Variables Using Combined PACT and CLIC Baseline Data
| FEV1 % Pred | FEF25-75 % Pred | FEV1/FVC % Pred | |
|---|---|---|---|
| FEV1 % Pred | 0.69 (0.62, 0.75) p < 0.0001 |
0.58 (0.50, 0.66) p < 0.0001 |
|
| FEF25-75 % Pred | 0.69 (0.62, 0.75) p < 0.0001 |
0.79 (0.74, 0.83) p < 0.0001 |
|
| FEV1/FVC % Pred | 0.58 (0.50, 0.66) p < 0.0001 |
0.79 (0.74, 0.83) p < 0.0001 |
The Pearson correlations between the spirometric variables and the outcome variables appear in Table 3. None of the spirometric variables was significantly correlated with asthma symptoms and only FEF25-75 and FEV1/FVC demonstrated small negative correlations with rescue albuterol use. However, all variables did demonstrate significant negative correlations with the ACQ score and FEF25-75 demonstrated a small negative correlation with prior asthma hospitalization. There was a higher level of correlation between the spirometric variables and the physiologic outcomes including morning and evening PEF % predicted, BD response as % change in FEV1, methacholine PC20, and FENO. In general, FEF25-75 and FEV1/FVC % predicted were better correlated with the physiological outcome variables than was FEV1 % predicted. Indeed, the strongest estimated correlation in Table 3 was between FEF25-75 % predicted and maximum BD response, in which r = -0.56 with 95% confidence interval (-0.62, -0.49). Similar results were found when the PACT and CLIC data were analyzed separately (eTables 3 and 4).
Table 3.
Pearson Correlation and Partial Correlation Coefficients (with 95% Confidence Intervals and P-values for Testing Null Correlations) of Spirometry Variables with Clinical Variables Using Combined PACT and CLIC Baseline Data
| FEV1 % Predicted Correlations | FEV1 % Predicted Partial Correlations | FEV1 % Predicted Partial Correlations (Partialling out Clinical Variables) | FEF25-75 % Predicted Correlations | FEV25-75 % Predicted Partial Correlations | FEV1 % Predicted Partial Correlations (Partialling out Clinical Variables) | FEV/FVC % Predicted Correlations | FEV/FVC % Predicted Partial Correlations | FEV1 % Predicted Partial Correlations (Partialling out Clinical Variables) | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical Outcomes | |||||||||
| Diary Symptoms (Cough and Wheeze) | 0.07 (-0.03, 0.17) p = 0.16 |
0.02 (-0.09, 0.14) p = 0.70 |
0.01 (-0.12, 0.13) p = 0.92 |
0.01 (-0.08, 0.11) p = 0.78 |
-0.01 (-0.13, 0.10) p = 0.78 |
-0.03 (-0.15, 0.09) p = 0.64 |
-0.02 (-0.13, 0.10) p = 0.78 |
0.02 (-0.13, 0.10) p = 0.77 |
0.04 (-0.09, 0.16) p = 0.54 |
| Diary Rescue Albuterol Use | -0.09 (-0.19, 0.01) p = 0.08 |
-0.05 (-0.17, 0.06) p = 0.44 |
-0.06 (-0.18, 0.07) p = 0.38 |
-0.13 (-0.23, -0.03) p = 0.008 |
-0.07 (-0.18, 0.05) p = 0.23 |
-0.05 (-0.18, 0.07) p = 0.40 |
-0.21 (-0.32, -0.10) p = 0.0002 |
-0.05 (-0.16, 0.07) p = 0.44 |
-0.05 (-0.17, 0.08) p = 0.44 |
| ACQ Score | -0.23 (-0.32, -0.13) p < 0.0001 |
-0.19 (-0.21, 0.02) p = 0.12 |
-0.10 (-0.22, 0.02) p = 0.10 |
-0.17 (-0.27, -0.07) p = 0.001 |
0.04 (-0.07, 0.16) p = 0.49 |
0.00 (-0.12, 0.13) p = 0.98 |
-0.23 (-0.33, -0.11) p < 0.0001 |
-0.14 (-0.25, -0.02) p = 0.02 |
-0.09 (-0.21, 0.03) p = 0.15 |
| Prior Hospitaliztions | -0.11 (-0.21, 0.01) p = 0.03 |
0.03 (-0.09, 0.14) p = 0.65 |
0.03 (-0.09, 0.16) p = 0.59 |
-0.14 (-0.23, -0.04) p = 0.005 |
-0.11 (-0.22, 0.01) p = 0.07 |
-0.08 (-0.20, 0.04) p = 0.19 |
-0.02 (-0.13, 0.10) p = 0.74 |
0.07 (-0.04, 0.19) p = 0.21 |
0.06 (-0.07, 0.18) p = 0.35 |
| Physiological Outcomes | |||||||||
| Diary AM PEF % Predicted | 0.38 (0.29, 0.46) p < 0.0001 |
0.30 (0.19, 0.40) p < 0.0001 |
0.30 (0.19, 0.40) p < 0.0001 |
0.28 (0.18, 0.37) p < 0.0001 |
0.02 (-0.13, 0.10) p = 0.77 |
-0.01 (-0.12, 0.11) p = 0.89 |
0.28 (0.17, 0.39) p < 0.0001 |
0.06 (-0.06, 0.17) p = 0.34 |
0.04 (-0.07, 0.16) p = 0.47 |
| Diary PM PEF % Predicted | 0.35 (0.26, 0.44) p < 0.0001 |
0.28 (0.17, 0.38) p < 0.0001 |
0.30 (0.18, 0.40) p < 0.0001 |
0.24 (0.15, 0.33) p < 0.0001 |
0.01 (-0.12, -0.11) p = 0.89 |
0.00 (-0.13, 0.12) p = 0.97 |
0.23 (0.12, 0.33) p < 0.0001 |
0.02 (-0.10, 0.13) p = 0.79 |
0.00 (-0.12, 0.12) p = 0.99 |
| Max BD Response (% Change in FEV1) | -0.24 (-0.33, -0.14) p < 0.0001 |
0.23 (0.12, 0.33) p < 0.0001 |
0.25 (0.13, 0.36) p < 0.0001 |
-0.56 (-0.62, -0.49) p < 0.0001 |
-0.49 (-0.58, -0.40) p < 0.0001 |
-0.49 (-0.58, -0.39) p < 0.0001 |
-0.38 (-0.47, -0.27) p < 0.0001 |
0.12 (0.00, 0.23) p = 0.05 |
0.11 (-0.02, 0.23) p = 0.09 |
| log2 (PC20 methacholine) | 0.27 (0.17, 0.37) p < 0.0001 |
0.10 (-0.03, 0.22) p = 0.12 |
0.10 (-0.03, 0.22) p = 0.12 |
0.36 (0.26, 0.45) p < 0.0001 |
0.16 (0.03, 0.28) p = 0.01 |
0.15 (0.03, 0.27) p = 0.02 |
0.40 (0.30, 0.51) p < 0.0001 |
0.10 (-0.03, 0.22) p = 0.16 |
0.08 (-0.05, 0.20) p = 0.23 |
| log10(FeNO) | -0.13 (-0.23, -0.03) p = 0.01 |
0.04 (-0.07, 0.16) p = 0.47 |
0.07 (-0.05, 0.19) p = 0.27 |
-0.22 (-0.31, -0.13) p < 0.0001 |
-0.10 (-0.21, -0.02) p = 0.02 |
-0.09 (-0.21, 0.03) p = 0.15 |
-0.25 (-0.35, -0.13) p < 0.0001 |
-0.09 (-0.20, 0.03) p = 0.13 |
-0.07 (-0.19, 0.06) p = 0.30 |
Pearson Partial Correlation Coefficients
The Pearson partial correlations also appear in Table 3 (in columns that are adjacent to the Pearson correlations). The Pearson partial correlation between a spirometric variable and an outcome variable is adjusted for the presence of the other two spirometric variables. Table 3 indicates that FEF25-75 % predicted and FEV1/FVC % predicted are not correlated with diary morning and evening PEF % predicted when FEV1 % predicted is accounted for. In particular, (1) the Pearson correlation between FEF25-75 % predicted and diary morning PEF % predicted is 0.28, but the Pearson partial correlation is 0.02, and (2) the Pearson correlation between FEV1/FVC % predicted and diary morning PEF % predicted is 0.28, but the Pearson partial correlation is 0.06.
The Pearson partial correlation between FEF25-75 % predicted and maximum BD response as % change in FEV1 is not very different from the Pearson correlation −0.49 and -0.56, respectively), whereas the Pearson partial correlation between FEV1/FVC % predicted and BD response is drastically lower in magnitude than its Pearson correlation (0.12 and −0.38, respectively). This means that there is a strong correlation between the FEF25-75 % predicted and the BD response after adjustment for the presence of the other two spirometric variables, FEV1 % predicted and FEV1/FVC % predicted. The Pearson partial correlation between FEV1 % predicted and maximum BD response does not change in magnitude, but it does change in sign (0.23 and −0.24, respectively). Nevertheless, the Pearson partial correlation between FEV1 % predicted and maximum BD response is much weaker than the Pearson partial correlation between FEF25-75 % predicted and maximum BD response (0.23 and −0.49, respectively). Thus, FEF25-75 % predicted appears to be the most important of the three spirometric variables in terms of a relationship with maximum BD response as % change in FEV1.
Canonical Correlation Coefficients
The results of the canonical correlation analysis appear in Table 4. Only the first two canonical correlations are statistically significant (0.65, p < 0.0001; 0.46, p < 0.0001). For the first canonical correlation, the pair of linear combinations of the spirometric variables (Set 1) and the clinical variables (Set 2) that has the largest Pearson correlation was determined. The standardized coefficients of the canonical correlation indicate that the clinical set of variables depends most on maximum BD response as % change in FEV1 (-0.61), and, to a lesser extent, on the diary morning PEF % predicted (0.32), and the log2(PC20 methacholine)(0.41), whereas the spirometric variables depend most on FEF25-75 % predicted (0.88). For the second canonical correlation, the pair of linear combinations of the clinical variables and spirometric variables (Variate Pair 2) that has the largest Pearson correlation, while being uncorrelated with Variate Pair 1, was determined. The standardized coefficients of the second canonical correlation indicate that the clinical set of variables depends most on diary morning PEF % predicted (0.82) and the maximum BD response (0.77), whereas the spirometric variables depend most on FEF25-75 % predicted (−1.40) and FEV1 % predicted (1.25). The third canonical correlation is not significant (p = 0.23) which indicates that there are no other linear relationships between the clinical and spirometric variables (Variate Pair 3). The canonical correlation analysis confirms the importance of the relationship between the FEF25-75 % predicted and the maximum BD response as % change in FEV1. Similar results were found when the PACT and CLIC data were analyzed separately (eTables 5 and 6).
Table 4.
Canonical Correlation Analysis Between Spirometry Variables and Clinical Variables Using Combined PACT and CLIC Baseline Data
| Combined PACT and CLIC Canonical Variate Pairs | |||
|---|---|---|---|
| Standardized Coefficients | |||
| Variate Pair 1 | Variate Pair 2 | Variate Pair 3 | |
| Diary AM PEF % Predicted | 0.32 | 0.82 | -1.43 |
| Diary PM PEF % Predicted | 0.05 | -0.22 | 1.94 |
| Max BD Response (% Change in FEV1) | -0.61 | 0.77 | -0.17 |
| log2(PC20) | 0.41 | 0.20 | -0.13 |
| Diary Symptoms (Cough and Wheeze) | 0.11 | 0.32 | -0.35 |
| Diary Rescue Albuterol Use | -0.01 | -0.14 | 0.07 |
| log10(Exhaled NO) | -0.01 | 0.07 | 0.46 |
| ACQ Score | -0.17 | -0.37 | 0.21 |
| Prior Hospitalizations | 0.07 | 0.08 | -0.31 |
| FEV1 % Predicted | 0.10 | 1.25 | 0.58 |
| FEF25-75 % Predicted | 0.88 | -1.40 | 0.83 |
| FEV1/FVC % Predicted | 0.06 | 0.58 | -1.54 |
| Canonical Correlation | 0.65 p < 0.0001 |
0.46 p < 0.0001 |
0.20 p = 0.23 |
Receiver Operator Characteristic (ROC) Curves
Because there is controversy regarding which FEF25-75 % predicted value differentiates normal versus abnormal findings, a receiver operator characteristic (ROC) curve of FEF25-75 % predicted and BD response as % change in FEV1 was constructed. The inflection point indicated that the greatest sensitivity and specificity was found with a FEF25-75 % predicted of 68%. In particular, the FEF25-75 at 68% of predicted had a 94% sensitivity and a 63% specificity for detecting a 20% increase in FEV1 following albuterol inhalation (Figure 1). The area under the curve = 0.88. For a BD response = 12% increase in FEV1, the area under the curve = 0.77 with no clear inflection point. Similar results were found when the PACT and CLIC data were analyzed separately (eFigures 1). An ROC curve of FEV1/FVC % predicted and BD response also was constructed. The inflection point that the greatest sensitivity and specificity for FEV1/FVC % predicted was found at 95%. The FEV1/FVC at 95% of predicted had an 87% sensitivity and a 70% specificity for detecting a 20% increase in FEV1 following albuterol inhalation. The area under the curve = 0.83. A meaningful ROC curve could not be constructed for FEV1 % predicted which had an area under the curve = 0 0.62 and no inflection point that could be used to indicate the greatest sensitivity and specificity. Similar results were found when the PACT and CLIC data were analyzed separately (eFigure 2).
Figure 1.
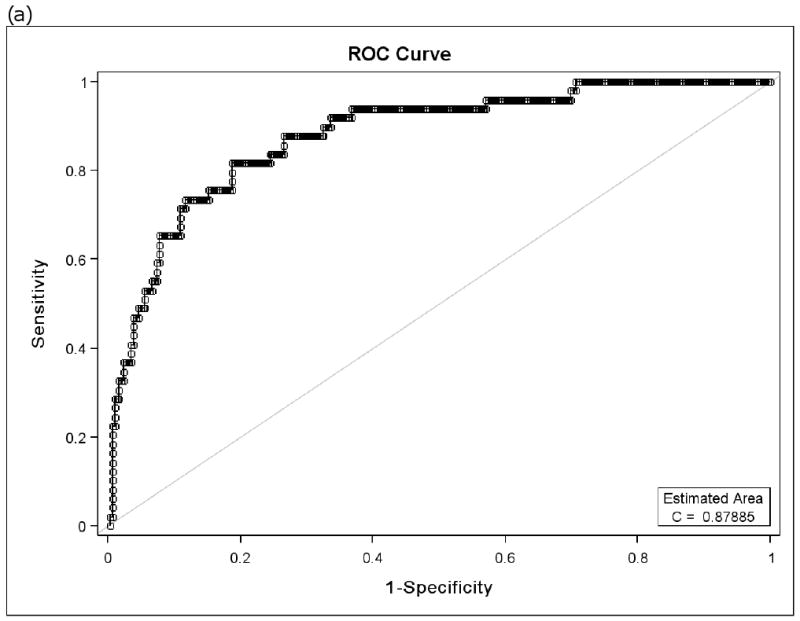
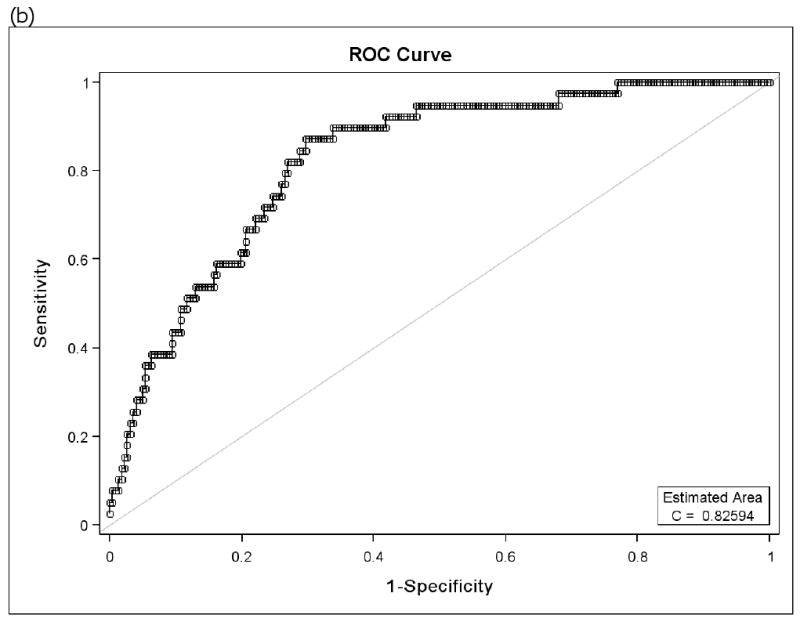
Receiver Operator Characteristic (ROC) curves of FEF25-75 % predicted for bronchodilator responsiveness (BD) as 20% change in FEV1 (a) and ROC curve of FEV1/FVC % predicted for BD responsiveness as 20% change in FEV1 (b). The inflection value for FEF25-75 is at 68% predicted and that for the FEV1/FVC is at 95% predicted. (CLIC and PACT combined)
In order to calculate the number needed to test (NNT), we selected the cut points which yield equivalent values for sensitivity and specificity. The cut point that yields equivalent estimates of sensitivity and specificity for FEF25-75 % predicted from its ROC curve is 58.2% (sensitivity = specificity = 0.82). The cut point that yields equivalent estimates of sensitivity and specificity for FEV1/FVC % predicted is 91.6% (sensitivity = specificity = 0.74). Thus, the NNT = 1/(0.82 − 0.74) = 12.5. In other words, for every 12.5 children in whom measurements of FEF25-75 % predicted and FEV1/FVC % predicted are available, 1 child will be identified who would benefit from bronchodilator based on his/her FEF25-75 % predicted measurement that was not identified based on his/her FEV1/FVC % predicted measurement. An NNT analysis was not performed using the FEV1 % predicted because its correlation with BD responsiveness was much weaker than that of both the FEF25-75 % predicted and the FEV1/FVC % predicted and ROC curves with FEV1 % predicted performed too poorly to allow a cut point to be developed for an NNT analysis.
Discussion
Asthma is characterized by inflammation of the large and small airways (25, 26). Small airway obstruction has been demonstrated in asthmatic subjects (27, 28, 29). Exhaled nitric oxide (FeNO) is a measure of airway inflammation in asthma which has been associated with small airway obstruction (30). These two features of asthma are believed to be causally related. Air trapping in asthmatic subjects in the presence of normal FEV1 has been documented (3, 4). The midflow rates measured during spirometric testing are believed to represent small airway airflow (10, 28). The measurement of the midflow rate, the FEF25-75, may be a more sensitive indicator of symptomatic childhood asthma than is the FEV1 (5, 6, 7). FEF25-75 may better reflect small airways disease than the FEV1 due to peripheral positioning of the airflow choke point in mid-to-low lung volumes and because “slow” lung units contribute gas later in the volume (by definition). The FEF25-75 % predicted has been demonstrated to be better correlated with air trapping in asthmatics than the FEV1 % predicted and the FEV1/FVC % predicted (4).
We hypothesized that the FEF25-75 % predicted would correlate with asthma symptoms, asthma medication use, bronchial hyperreactivity, albuterol-induced bronchodilation and FeNO in two groups of children with mild asthma and normal FEV1 % predicted. Although the FEF25-75 % predicted and FEV1/FVC % predicted generally performed better than the FEV1 % predicted, the striking finding was in their ability to predict response to bronchodilator administration. The Pearson partial correlations and the canonical correlation analysis of the combined CLIC and PACT data indicate that the FEF25-75 % predicted has stronger relationship with maximum BD response than does the FEV1/FVC % predicted and the FEV1 % predicted (partial correlations of −0.49, 0.12, and 0.23, respectively). Further, the estimated correlation of −0.49 is a relatively strong correlation in an analysis of biological variables in a population study.
These findings suggest that high FEF25-75 % predicted tends to be associated with normal airway patency with a limited possibility of further bronchodilation. This is in contrast to a high FEV1 % predicted in our analyses and supports others' findings of airflow obstruction in asthmatic subjects with a normal FEV1 (3, 4).
FEF25-75 has previously been found to be significantly low in children with an asthma diagnosis and a history of wheezing (31). These investigators also reported that the FEF25-75 was highly correlated with the slope of the methacholine dose-response curve and with degree of methacholine responsiveness, respectively. Our findings are also consistent with those earlier observations. In addition, a previous study by one of our authors found that the FEF25-75 was significantly lower in transient early and persistent wheezers when they were reassessed at ages 11 and 16 years (32).
A recent study in a small number of patients with mild asthma demonstrated that FeNO was correlated with closing volume which was used as a measure of small airway obstruction while FeNO was not correlated with % predicted FEV1 (30). Our results support those findings of a correlation, albeit weak, of small airway patency (FEF25-75 in our study) and FeNO in a much larger number of patients with mild asthma. However, unlike that report, but like another earlier study (33), we also found that FeNO also had a weak negative correlation with % predicted FEV1This latter finding in our subjects is at odds with the previously reported lack of a correlation of FEV1 % predicted with FeNO (34, 35, 36). However, the last of these studies in children with persistent asthma, one performed by some of the current investigators, did find a weak negative correlation of FeNO with the FEV1/FVC ratio. Measures of small airway patency were not reported in those previous studies.
The ROC curve analysis demonstrated that 62 % predicted FEF25-75 had a sensitivity of 90% and a specificity of 73% in detecting ≥20% increase in FEV1 following inhalation of albuterol. The % predicted FEF25-75 has a NNT of 7 relative to the FEV1/FVC % predicted to identify one child who would benefit from bronchodilator testing. This suggests that the FEF25-75 is moderately efficacious in identifying otherwise undiagnosed bronchodilator responsiveness. Our finding that the FEF25-75 is sensitive and specific for bronchodilator responsiveness is of interest in the context of previous reports that bronchodilator responsiveness predicts an increase in FEV1 following inhaled corticosteroid treatment (37), and is associated with several indicators of poor asthma control (38, 39). This finding may be especially useful to clinicians because this clinically significant increase in FEV1 suggests suboptimal asthma control, provided that the FVC measurements obtained during multiple spirometric studies are comparable and reproducible. It would be of interest to determine whether the FEF25-75 can serve as a surrogate for bronchodilator responsiveness.
What may render the FEF25-75 problematic in a given patient is that the variance is much higher than that of the FEV1. Thus, even though the FEF25-75 is more physiologically sensitive, it lacks specificity due to its variability; i.e. the lower limit of normal is substantively lower than for FEV1 or FEV1/FVC. Therefore, it is of limited diagnostic value in detecting an abnormality per se. However, if a patient is already known to have asthma, then the sensitivity of the FEF25-75 appears to be valuable in suggesting the likelihood that reversible bronchoconstriction is present. FEV1/FVC also performed well in predicting bronchodilator response. Ratios of flows divided by volumes, such as the FEV1/FVC, represent a rate constant and have units of 1/second. The FEV1/FVC describes the average rate constant over the first second, of the VC. It is likely that one of the reasons that the FEV1/FVC is so robust is that it averages the rate constants over the first 70%-95% of the FVC in most children with asthma. It has been suggested that the association of the FEV1/FVC with asthma symptoms and medication use in a previous study was due to the reflection of the increased dysanapsis by the FEV1/FVC which is present in asthmatics (13). In large studies which have the statistical power to detect low levels of correlation that are statistically significant, or that can compensate for the variability about a mean (SEM) to detect group differences, the FEF25-75 is of more use because the variance is compensated for by the large numbers. Under these conditions, the benefit of the increased physiologic sensitivity of the FEF25-75 remains.
The strengths of this current study include the large numbers of subjects studied and that all subjects had normal FEV1 % predicted. A weakness of this study is that we were not able to determine whether FEF25-75 predicted the future clinical course since all subjects were treated. Another weakness is that the entry criteria, and therefore the study populations of the CLIC trial and PACT were not identical. The CLIC trial excluded children with FEV1 <70% predicted and PACT excluded children with FEV1<80% predicted. A final potential weakness is that unpublished work by Dr. Ron Sorkness (personal communication) suggests a non-linear relationship between FEF25-75 and bronchodilator responsiveness. However, this is not a significant factor when the FEV1 is in the normal range as in our patient groups.
These results suggest that the FEF25-75 % predicted should be included among the spirometric data assessed in clinical trials if a 20% bronchodilator response as % change in FEV1 following albuterol inhalation is not determined. In addition, FEF25-75 % predicted is useful to clinicians who have performed spirometry in that it is correlated with bronchodilator responsiveness in children. Its sensitivity is estimated at 90% from the PACT and CLIC studies. These findings need to be verified by additional similar analyses in children. The reproducibility of these results also needs to be tested in data obtained in studies of adult patients.
Conclusion
The FEF25-75 % predicted was correlated with bronchodilator responsiveness and methacholine PC20 and was sensitive and specific for bronchodilator responsiveness. We believe that the usefulness of the FEF25-75 in asthmatic children with normal FEV1 % predicted should be reevaluated. It appears likely that FEF25-75 % predicted can supplement the FEV1 in the clinical evaluation of mild asthma in such children. In addition, this report demonstrates that the FEF25-75 % predicted is negatively correlated with FeNO in children with mild asthma and a normal FEV1 % predicted. These results suggest that FEF25-75 % predicted should be evaluated in clinical studies of asthma in children and may be of use in predicting the presence of clinically relevant reversible airflow obstruction.
Clinical Implications or Key Message.
The FEF25-75 % predicted correlates with, and is sensitive and specific for, bronchodilator responsiveness in asthmatic children with normal FEV1 % predicted. This is consistent with airway dysfunction despite the presence of a normal FEV1.
Supplementary Material
eFigure 1. Receiver Operator Characteristic (ROC) curves of FEF25-75 % predicted for bronchodilator responsiveness (BD) as 20% change in FEV1 (a) and ROC curve of FEV1/FVC % predicted for BD responsiveness as 20% change in FEV1 (b). The inflection value for FEF25-75 is at 36% predicted and that for the FEV1/FVC is at 79% predicted. (CLIC alone)
eFigure 2. Receiver Operator Characteristic (ROC) curves of FEF25-75 % predicted for bronchodilator responsiveness (BD) as 20% change in FEV1 (a) and ROC curve of FEV1/FVC % predicted for BD responsiveness as 20% change in FEV1 (b). The inflection value for FEF25-75 is at 50% predicted, while that for the FEV1/FVC cannot be determined. (PACT alone)
eTable 1. Confidence Intervals and P-values for Testing Null Correlations) Among Spirometry Variables Using CLIC Baseline Data
eTable 2. Confidence Intervals and P-values for Testing Null Correlations) Among Spirometry Variables Using PACT Baseline Data
eTable 3. Pearson Correlation and Partial Correlation Coefficients (with 95% Confidence Intervals and P-values for Testing Null Correlations) of Spirometry Variables with Clinical Variables Using CLIC Baseline Data
eTable 4. Pearson Correlation and Partial Correlation Coefficients (with 95% Confidence Intervals and P-values for Testing Null Correlations) of Spirometry Variables with Clinical Variables Using PACT Baseline Data
eTable 5. Canonical Correlation Analysis Between Spirometry Variables and Clinical Variables Using CLIC Baseline Data
eTable 6. Canonical Correlation Analysis Between Spirometry Variables and Clinical Variables Using PACT Baseline Data
Acknowledgments
Supported by grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, and 5U10HL064313 from the National Heart, Lung, and Blood Institute. This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and National Jewish (M01 RR00051).
List of Abbreviations/Acronyms
- ACQ
Asthma Control Questionnaire
- ATS
American Thoracic Society
- BD
bronchodilator
- CARE Network
Childhood Asthma Research and Education Network
- CLIC
Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid
- FEF25-75
forced expiratory flow between 25% and 75% of vital capacity
- FeNO
exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- NHLBI
National Heart, Lung and Blood Institute
- NNT
number needed to treat
- PACT
Pediatric Asthma Controller Trial
- PC20
provocative concentration that yields a 20% decrease in FEV1
- PEF
peak expiratory flow
- ROC
receiver operator characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 2.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 3.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP, 3rd, Ciambotti JM, Alford BA, Brookeman JR, Platts-Mills TA. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003;111:1205–1211. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 4.de Lange EE, Altes TA, Patrie JT, Gaare JD, Knake JJ, Mugler JP, 3rd, Platts-Mills TA. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest. 2006;130:1055–1062. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 5.Weiss ST, Tosteson TD, Segal MR, Tager IB, Redline S, Speizer FE. Effects of asthma on pulmonary function in children. A longitudinal population-based study. Am Rev Resp Dis. 1992;145:58–64. doi: 10.1164/ajrccm/145.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Lebecque P, Kiakulanda P, Coates AL. Spirometry in the asthmatic child: Is FEF25-75 a more sensitive test than FEV1/FVC? Pediatr Pulmonol. 1993;16:19–22. doi: 10.1002/ppul.1950160105. [DOI] [PubMed] [Google Scholar]
- 7.Valetta EA, Piacentini GL, Del Col G, Boner AL. FEF25-75 as a marker of airway obstruction in asthmatic children during reduced mite exposure at high altitude. J Asthma. 1997;34:127–131. doi: 10.3109/02770909709075657. [DOI] [PubMed] [Google Scholar]
- 8.Lebowitz MD, Holberg CJ, Knudson RJ, Burrows B. Longitudinal study of pulmonary function development in childhood, adolescense, and early adulthood. Development of pulmonary function. Am Rev Resp Dis. 1987;136:69–75. doi: 10.1164/ajrccm/136.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Chiang CH, Hsu K. Residual abnormalities of pulmonary function in asymptomatic young adult asthmatics with childhood-onset asthma. J Asthma. 1997;34:15–21. doi: 10.3109/02770909709071199. [DOI] [PubMed] [Google Scholar]
- 10.McFadden ER, Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52:725–737. doi: 10.1016/0002-9343(72)90078-2. [DOI] [PubMed] [Google Scholar]
- 11.Gelb AF, Zamel N. Simplified diagnosis of small-airway obstruction. N Engl J Med. 1973;288:395–398. doi: 10.1056/NEJM197302222880805. [DOI] [PubMed] [Google Scholar]
- 12.Frank R, Liu MC, Spannhake EW, Mlynarek S, Macri K, Weinmann GG. Repetitive ozone exposure of young adults. Evidence of persistent airway dysfunction. Am J Resp Crit Care Med. 2001;164:1253–1260. doi: 10.1164/ajrccm.164.7.2010043. [DOI] [PubMed] [Google Scholar]
- 13.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 14.Sorkness CA, Jr, Lemanske RF, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, Strunk RC, Szefler SJ, Zeiger RS, Bacharier LB, Bloomberg GR, Covar RA, Guilbert TW, Heldt G, Larsen G, Mellon MH, Morgan WJ, Moss MH, Spahn JD, Taussig LM, Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute Long-term comparison of three controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial (PACT) J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Jr, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM, Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society Committee of Proficiency Standards for Clinical Pulmonary Function Laboratories. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Childhood Asthma Management Program. Childhood Asthma Management Program Spirometry Manual, Version 3.0. Springfield, VA: National Technical Information Service; 1994. [Google Scholar]
- 18.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, Ambrosius WT, Tepper RS. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med. 2001;163:619–623. doi: 10.1164/ajrccm.163.3.2002054. [DOI] [PubMed] [Google Scholar]
- 19.Arets HGM, Brackel HJL, van der Ent CK. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18:655–660. doi: 10.1183/09031936.01.00204301. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society/European Respiratory Society. ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 22.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–90723. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 6th. Englewood Cliffs, NJ: Prentice Hall; 2007. [Google Scholar]
- 24.Zweig M, Campbell G. Receiver-operating Characteristics (ROC) Plots: A Fundamental Evaluation Tool in Clinical Medicine. 1993;39:561–577. [PubMed] [Google Scholar]
- 25.Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, Hogg JC. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 26.Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, Sugarbaker DJ, Doerschuk CM, Drazen JM. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998;158:565–572. doi: 10.1164/ajrccm.158.2.9705036. [DOI] [PubMed] [Google Scholar]
- 27.Woolcock AJ, Vincent NJ, Macklem PT. Frequency dependence of compliance as a test for obstruction in the small airways. J Clin Invest. 1969;48:1097–1106. doi: 10.1172/JCI106066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner EM, Liu MC, Weinmann GG, Permutt S, Bleecker ER. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis. 1990;141:584–588. doi: 10.1164/ajrccm/141.3.584. [DOI] [PubMed] [Google Scholar]
- 29.Ueda T, Niimi A, Matsumoto H, Takemura M, Hirai T, Yamaguchi M, Matsuoka H, Jinnai M, Muro S, Chin K, Mishima M. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006;118:1019–1025. doi: 10.1016/j.jaci.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia S, den Hertog H, Timmers MC, Lazeroms SP, Vignola AM, Rabe KF, Bellia V, Hiemstra PS, Sterk PJ. Small airways function and molecular markers in exhaled air in mild asthma. Thorax. 2005;60:639–644. doi: 10.1136/thx.2004.035279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ownby DR, Peterson EL, Johnson CC. Factors related to methacholine airway responsiveness in children. Am J Respir Crit Care Med. 2000;161:1578–1583. doi: 10.1164/ajrccm.161.5.9812156. [DOI] [PubMed] [Google Scholar]
- 32.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, Priest J, Nelson B, Osborne ML. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000;106:645–650. doi: 10.1067/mai.2000.109618. [DOI] [PubMed] [Google Scholar]
- 34.Baraldi E, Carra S, Dario C, Azzolin N, Ongaro R, Marcer G, Zacchello F. Effect of natural grass pollen exposure on exhaled nitric oxide in asthmatic children. Am J Respir Crit Care Med. 1999;159:262–266. doi: 10.1164/ajrccm.159.1.9804063. [DOI] [PubMed] [Google Scholar]
- 35.Langley SJ, Goldthorpe S, Custovic A, Woodcock A. Relationship among pulmonary function, bronchial reactivity, and exhaled nitric oxide in a large group of asthmatic patients. Ann Allergy Asthma Immunol. 2003;91:398–404. doi: 10.1016/S1081-1206(10)61688-2. [DOI] [PubMed] [Google Scholar]
- 36.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, Hodgdon K, Morgan W, Sorkness CA, Lemanske RF, Jr, Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112:883–892. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, Szefler SJ, Weiss ST, Childhood Asthma Management Program Research Group Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006;117:1264–1271. doi: 10.1016/j.jaci.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Jones RS. Assessment of respiratory function in the asthmatic child. Brit Med J. 1966;2:972–975. doi: 10.1136/bmj.2.5520.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, Zeiger RS, Murphy AJ, Weiss ST, Childhood Asthma Management Program Research Group Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122:921–928. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Receiver Operator Characteristic (ROC) curves of FEF25-75 % predicted for bronchodilator responsiveness (BD) as 20% change in FEV1 (a) and ROC curve of FEV1/FVC % predicted for BD responsiveness as 20% change in FEV1 (b). The inflection value for FEF25-75 is at 36% predicted and that for the FEV1/FVC is at 79% predicted. (CLIC alone)
eFigure 2. Receiver Operator Characteristic (ROC) curves of FEF25-75 % predicted for bronchodilator responsiveness (BD) as 20% change in FEV1 (a) and ROC curve of FEV1/FVC % predicted for BD responsiveness as 20% change in FEV1 (b). The inflection value for FEF25-75 is at 50% predicted, while that for the FEV1/FVC cannot be determined. (PACT alone)
eTable 1. Confidence Intervals and P-values for Testing Null Correlations) Among Spirometry Variables Using CLIC Baseline Data
eTable 2. Confidence Intervals and P-values for Testing Null Correlations) Among Spirometry Variables Using PACT Baseline Data
eTable 3. Pearson Correlation and Partial Correlation Coefficients (with 95% Confidence Intervals and P-values for Testing Null Correlations) of Spirometry Variables with Clinical Variables Using CLIC Baseline Data
eTable 4. Pearson Correlation and Partial Correlation Coefficients (with 95% Confidence Intervals and P-values for Testing Null Correlations) of Spirometry Variables with Clinical Variables Using PACT Baseline Data
eTable 5. Canonical Correlation Analysis Between Spirometry Variables and Clinical Variables Using CLIC Baseline Data
eTable 6. Canonical Correlation Analysis Between Spirometry Variables and Clinical Variables Using PACT Baseline Data


