Abstract
We describe a methodology for studying protein kinetics using a rapid-scan technology for collecting 2D IR spectra. In conjunction with isotope labeling, 2D IR spectroscopy is able to probe the secondary structure and environment of individual residues in polypeptides and proteins. It is particularly useful for membrane and aggregate proteins. Our rapid-scan technology relies on a mid-IR pulse shaper that computer generates the pulse shapes, much like in an NMR spectrometer. With this device, data collection is faster, easier, and more accurate. We describe our 2D IR spectrometer, as well as protocols for 13C=18O isotope labeling, and then illustrate the technique with an application to the aggregation of the human islet amyloid polypeptide form type 2 diabetes.
1. Introduction
Infrared spectroscopy has been used for decades to study protein structure [1, 2], but has largely become overshadowed by the atomic-level resolution now attainable with high-resolution NMR spectroscopy and x-ray crystallography. However, a renaissance is currently underway with the combined development of 2D IR spectroscopy and isotope labeling that is leading to new insights into the structures and kinetics of proteins. These techniques are proving to be especially useful on systems that are difficult to study with NMR spectroscopy and x-ray crystallography, such as membrane-bound or aggregated proteins. Moreover, with isotope labeling, individual bonds can be spectroscopically resolved, such as the backbone carbonyl groups that create the so-called amide-I vibrational mode. Thus, quantitative conclusions can be drawn about specific structural features. Of course, isotope labeling has long been used with IR spectroscopy [3–7] but 2D IR spectroscopy provides 2D lineshapes that give information on the environment of the label and cross peaks that report on the secondary structure of the label. As an example, Fig. 1a contains a 2D IR spectrum of fibers created from aggregation of the human islet amyloid polypeptide involved in type 2 diabetes in which a single residue has been isotope labeled. It is clear from this spectrum that the information content obtained with infrared spectroscopy is now much richer than it was 20 years ago.
Fig. 1.
(a) 2D IR spectrum of the human islet amyloid polypeptide fibril with a single 13C=18O isotope label at the L16 position with diagonal and off-diagonal doublet features highlighted. (b) Pulse sequence for measurement of 2D IR spectra. The mid-IR pulse shaper generates k1 and k2 and controls the delay between them. Pulses k3 and kLO refer to the probe pulse before and after the sample, respectively, while kS is the signal.
Perhaps one of the most important capabilities of infrared spectroscopy is its time-resolution. With femtosecond pulses, structural fluctuations in the protein structure or its environment can be measured with <100 fs resolution [8–12] or if IR spectra are used as a probe of protein kinetics, then it has an intrinsic time-resolution of a few picoseconds (set by the vibrational free induction lifetime). As a result, the time-resolution is often not limited by the IR probe, but by the method of initiating the kinetics, such as in temperature-jump studies [13–15]. In a similar manner, 2D IR spectroscopy can be used to study fast protein kinetics [16–18]. However, the technology originally used to collect 2D IR spectra is not well suited for studying protein kinetics. To obtain femtosecond time-resolution with 2D IR spectroscopy, one needs to signal average each time-delay many times. Signal averaging is not a problem for samples that are very reproducible and reusable. But in systems like amyloids, which are infamous for their poor repeatability, one cannot reliably average together multiple data sets. Instead, kinetics must be monitored in real time.
To monitor protein folding kinetics, we have developed a rapid-scan method for collecting 2D IR spectra.[19] It is based on a mid-IR pulse shaper [20, 21] that allows the 2D pulse sequences to be computer generated. Our device makes data collection faster, easier, and more accurate. Moreover, it enables us to set the individual phases of the pulses so that we can phase cycle to remove scatter or work in the rotating frame as is done in NMR spectroscopy.[22] But most importantly for protein folding, it enables kinetics to be measured in real time.[23]
A generic 2D IR pulse sequence is shown in Fig. 1b. It consists of 3 excitation pulses (k1, k2, and k3) that generate a vibrational free induction decay that is emitted from the sample (kS). To measure the free induction decay, the signal is heterodyned with a local oscillator pulse (kLO). In most 2D IR instruments, each of these pulses and the signal has its own optical path, so that the spectrometer optical design is quite complicated and difficult to align. In our design, we have just two optical paths, one of which traverses the pulse shaper and the other that serves as both the 3rd excitation pulse and the local oscillator. In a previous review article we have described many of the new capabilities made possible by this technology, such as phase cycling [22]. In what follows, we describe the experimental details of our method so that others can implement this technique more easily in their own laboratories.
2. Pulse shaper
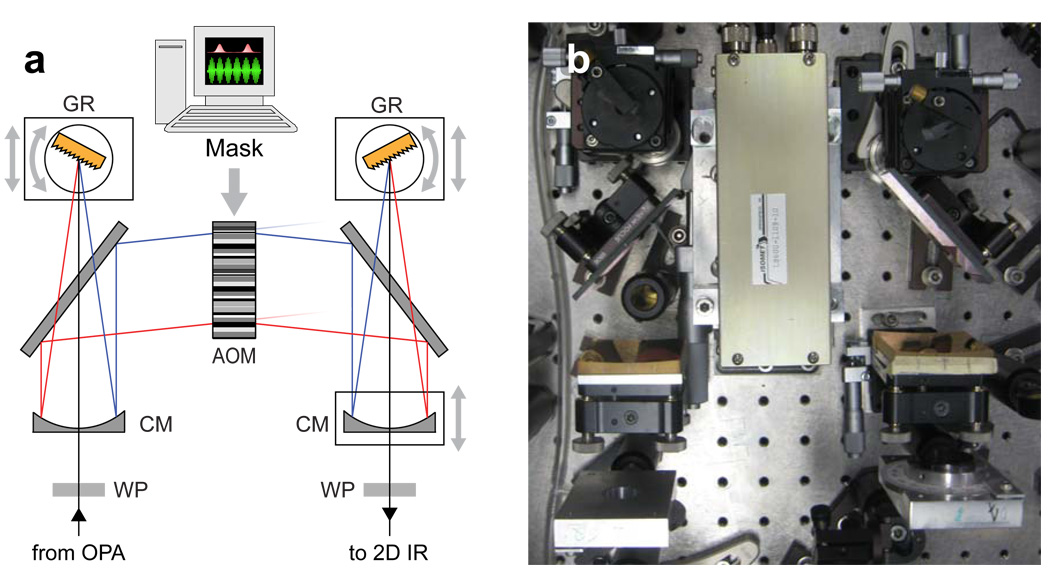
The mid-IR pulse shaper (Fig. 2) is based on a germanium acousto-optical modulator (AOM). The pulse is shaped in the frequency domain through the application of a complex-valued transfer function, or mask [24], according to Eq. 1,
| (1) |
where Ein(v), Eout(v) and M(v), are the input-pulse electric field, output-pulse electric field and mask at each frequency v. For independent modulation of different frequencies, the incoming pulse is spectrally dispersed by a grating at Littrow angle. A cylindrical mirror placed one focal length, f, from the grating is used to focus the beam to a cylindrical focus, with each frequency focused at a different position at a Ge AOM (Isomet LS600-1109-10). A piezoelectric transducer converts a 75 MHz carrier frequency waveform into an acoustic wave which propagates through the Ge crystal, perpendicular to the mid-IR pulse propagation, at a velocity of 0.55 cm/µs. Since the velocity of the mid-IR pulse through the Ge crystal is 4 orders of magnitude faster than the acoustic wave velocity, the periodic modulation of the Ge refractive index appears to the pulse as a static Bragg diffraction grating, mapping the time-dependence of the RF wave onto the spatial dimension of the AOM. The pulse is shaped by mapping the desired complex-value mask on to the RF wave [25] according to Eq. 2,
| (2) |
Fig. 2.
(a) Optical layout of mid-IR pulse-shaper. (b) Top down photograph of shaper. WP: MgF2 zero-order λ/2 waveplate, CM: cylindrical mirror (f = 12.5 cm), GR: diffraction grating (150/mm), AOM: Ge acousto-optic modulator.
The RF mask intensity should be calibrated to compensate for the nonlinear dependence of the AOM diffraction efficiency on the RF power [21]. More details about the form of M used for 2D IR measurements are given below. The diffracted beam is focused by a second cylindrical mirror onto a second grating which overlaps all the frequencies.
Further details on the mid-IR pulse shaper [20] and AOM pulse shaping [26] are given elsewhere. We recently demonstrated a modified mid-IR pulse shaper which was additionally capable of polarization shaping [27]. Polarization shaping has many benefits for multidimensional IR spectroscopy as well as coherent control applications [28]. A indirect method of shaping mid-IR pulses has also been described but suffers from poor efficiency and pulse-shape fidelity [29, 30].
2.1. Laser System and Electronics
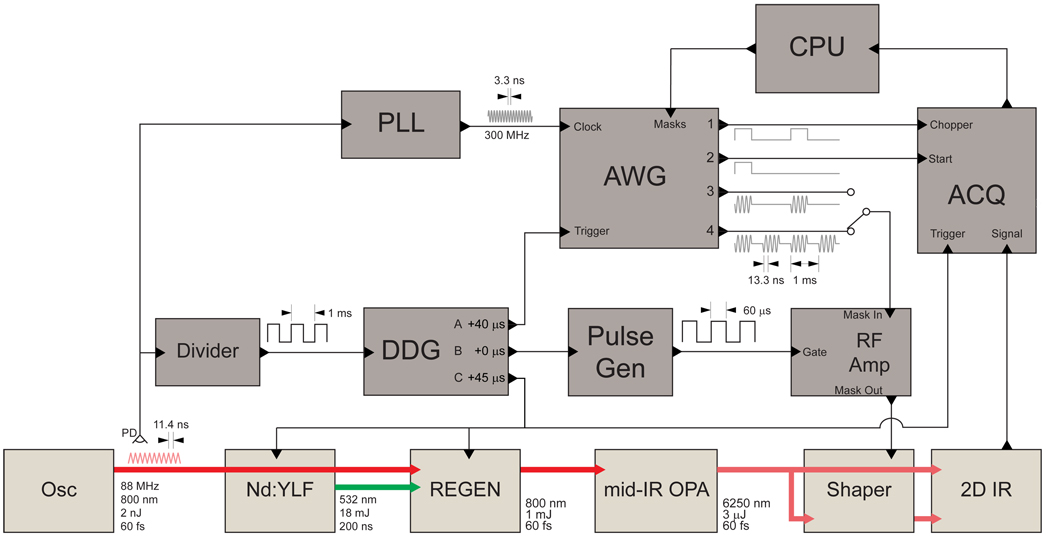
The laser system consists of a commercial regenerative amplifier (Spitfire, Spectra Physics) seeded by a home-built Ti:sapphire oscillator and an optical parametric amplifier (OPA) with difference frequency mixing. The OPA and mid-IR generation have been previously described in detail [21]. The electronics system for the laser, shaper and detection used to collect 2D IR spectra are diagrammed in Fig. 3. Since heterodyne-detected 2D IR signals are phase-sensitive [31], the setup has been optimized to minimize jitter in the relative timing of the shaper mask and the laser pulse, leading to improved phase stability of the shaped mid-IR pulses. The setup begins with a mode-locked Ti:Sapphire oscillator operating at a repetition rate of 88 MHz. A small portion of the laser output is detected with a high-speed Si photodiode and the signal is input into a divider circuit (Clark-MXR DT-505) to generate a 1 kHz TTL pulse train. The 1 kHz signal triggers five devices with three independent delays controlled by a digital delay generator (BNC 575).
Fig. 3.
(a) Schematic diagram of the laser system and electronics used for collecting 2D IR spectra with a mid-IR pulse shaper. Electrical connections are shown as thin lines and optical connections are shown thick lines. Osc: Ti:Sapphire femtosecond oscillator, YLF: Nd YLF pump laser, REGEN: regenerative amplifier, mid-IR OPA: optical parametric amplifier with difference frequency generation, Shaper: mid-IR pulse shaper (see Fig. 2), 2D IR: 2D IR spectrometer with detector (see Fig. 5), Divider: frequency divider, DDG: digital delay generator, Pulse Gen: pulse generator, RF Amp: radio frequency amplifier, ACQ: data acquisition box for array detector, PLL: phase-locked loop circuit, AWG: arbitrary waveform generator, CPU: computer, PD: high-speed silicon photodiode.
Delay A (40 µs) is used to trigger an arbitrary waveform generator (AWG). The AWG (GaGe CompuGen 4300) generates the RF masks which are sent to the AOM in order to generate the acoustic wave and shape the mid-IR pulses. An RF amplifier (Isomet) is used to amplify the RF signal before it is sent to the AOM. Delay B (0 µs) is used to trigger a pulse generator (TENMA TGP110), which generates a 50 µs long gate pulse for the RF amplifier, rising 40 µs before the mask to be amplified. Delay C (45 µs) is used to trigger the regenerative amplifier (Spectra-Physics Spitfire) and the Nd:YLF pump laser (Spectra-Physics Evolution 30) for the amplifier as well as the detection electronics (Infrared Associates IR 0128). The laser trigger is delayed relative to the AWG trigger by an amount such that the laser pulse will arrive at the AOM coincident with the mask. In order to determine the required delay, a mid-IR detector is placed near the AOM and the signal observed with an oscilloscope along with the RF mask output from the AWG. The laser delay is adjusted such that the initial rise of the detector signal coincides with the center of the mask.
2.2. Alignment
Initial shaper setup and alignment can be challenging because mid-IR beams are invisible to the eye. For this reason, the alignment is first performed using a HeNe laser. High-order harmonic diffractions from the first grating can be used to approximate the beam paths for the center and edge frequencies of the mid-IR pulse. For example, the 10th order diffraction of a HeNe laser (632.8 nm) will follow the same path of the center wavelength of a 6328 nm mid-IR pulse while the 9th and 11th order diffractions would follow paths that encompassed 99% of the frequencies in a 300 nm FWHM mid-IR pulse. Since the Ge AOM does not appreciably transmit visible wavelengths, the initial alignment is performed without the AOM in place.
Once overlapped with the mid-IR, the HeNe beam is centered on the first diffraction grating. Many orders are observed diffracting from the grating. The grating is rotated until the first-order diffraction of the mid-IR is found with a mid-IR detector placed to one side. The HeNe diffraction order which is closest to the detector is used the center frequency for aligning the shaper, while the diffraction orders on either side substitute for the low and high wavelength edges of the pulse spectrum.
The first grating angle is set to retroreflect the main HeNe beam at a 0° angle. The grating is also vertically tilted so the diffracted HeNe beam travels below the incoming beam at a small angle. The first cylindrical mirror is place centered below the incoming beam and adjusted to retroreflect and level the main diffracted beam. The distance between the concave mirror and grating is adjusted such that the three reflected diffraction orders are equidistant. The first folding mirror is then placed between the grating and cylindrical mirror to fold the collimated beam by 90°. When setup correctly, the three HeNe beams should all focus in a line which defines the Fourier plane.
The second half of the shaper is setup by mirror symmetry. The second folding and cylindrical mirrors are placed in the beam path. The distance between the two cylindrical mirrors is set by ensuring that the diffraction orders reflecting from the 2nd concave mirrors are vertically collimated. The second cylindrical mirror is vertically tilted to send the reflected beam upward at a small angle so as to not clip on the folding mirror. The second grating should be positioned at the convergence point of the HeNe beams and vertically tilted to level the diffracted beam. If the grating is set properly, the different HeNe orders should be spatially overlapped exiting the shaper.
Once the HeNe alignment is complete, the mid-IR alignment should closely follow. To check this, a mid-IR detector can be placed after the shaper centered on the HeNe beam. Small adjustments may be necessary to ensure that the mid-IR is also centered on the detector. The shaper output should be sent into a spectrometer and the shaper checked to ensure that the mid-IR pulses is not significantly clipping any of the optics and adjustments made as necessary. The horizontal spatial chirp can be check by monitoring the spectrum as a slit is scanned across the beam profile. The second grating position should be adjusted to minimize any spatial chirp. The AOM can then be placed in focal plane. It is useful to have translational control over the AOM position in both directions as well as rotation about a vertical axis through the AOM center. A mid-IR detector is placed after the shaper and the shaper triggered at half the repetition rate of the laser. Two signals should be observed on the oscilloscope, the full pulse when the shaper is off and a reduced pulse when the shaper is on because a portion of the pulse is diffracted by the AOM. The folding mirrors are adjusted to align the diffracted pulse to the detector. The two signals on the oscilloscope should now correspond to a large signal when the shaper is on and no signal when the shaper is off. A waveplate placed before the shaper is adjusted to maximize the amplitude of the diffracted pulse. The AOM diffraction efficiency is greater than 80% over 4–10 µm and greater than 65% over 3–12 µm. At 5–6 µm, we achieve an overall shaper efficiency of ~30% due to losses from the gratings and other optics. We carefully check for clipping of the beam, which will further decrease the shaper efficiency and distort the pulse spectrum.
2.3. Frequency Calibration
The grating in the shaper maps the frequencies in the pulse onto the spatial dimension of the AOM. The propagation velocity of the acoustic-wave in the AOM also maps the time dependence of the RF mask produced by the AWG onto the spatial dimension of the AOM. There therefore exists a direct mapping between the pulse frequencies and different times in the RF mask. To determine this mapping, the mid-IR pulse is shaped first into a frequency comb and second into a single narrowed peak and the resulting spectra measured.
Fig. 4a shows the amplitude masks used for frequency calibration of the shaper and Fig. 4b show the resulting spectra of the shaped pulse. Together these provide the relationship between shaper pixel and frequency. The first mask (red) is used to generate a single narrow peak near the center of the pulse. The second mask (black) is used to generate many narrow peaks at regular pixel intervals across the AOM. Because the position of the single-peak mask and the interval between the multiple peaks are known the position of all peaks can be calculated. This provides the relationship between time t and frequency v shown in Fig. 4c, which can be fit a second-order polynomial,
| (3) |
where pi are the fit parameters and fs is the sampling frequency of the AWG (300 MHz). This relationship can also be calculated based on the geometry of the shaper,
| (4) |
where t0 and v0 are the time and frequency of the single peak, c is the speed of light in air, f is the focal length of the cylindrical mirror, vac is the acoustic-wave velocity and D is the grating period. The calculated frequency-time relationship using Eq. 4 is shown in Fig. 4c. This analysis neglects the Bragg angle of the shaper, assumes that the acoustic wave velocity is constant over the AOM and other simplifications but fits the measured relationship well. Thus for most applications is likely adequate to skip the full calibration procedure and simply measure the frequency at a single time point. Eq. 4 can also be useful for determining the grating dispersion and focal length that is best for a specific application.
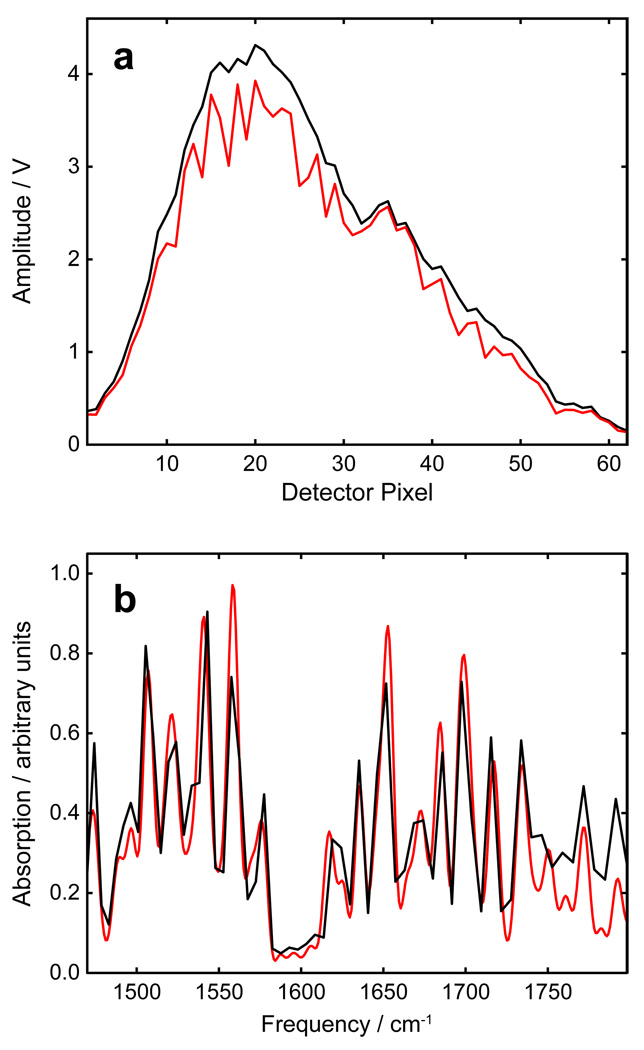
Fig. 4.
Frequency calibration of the shaper. a) Select region of the multiple peak (solid black) and single peak (dashed red) masks used for calibration. b) Selected region of the multiple peak (solid black) and single peak (dashed red) spectra of the shaped pulses produced by the corresponding masks in top panel. The full spectrum of the shaped pulse (gray fill) is shown for reference. Vertical lines indicate peak positions. c) Measured (open circles) and calculated (line) time-frequency calibration plot along with the percent error of the calculated plot (solid circles).
2.4. Dispersion Compensation
It is desirable to have dispersion-free pulses for 2D IR measurements. The spectral phase of the pulse is altered by transmission through the Ge AOM (or any material) due to the frequency-dependence of the refractive index, which can be described by a Taylor expansion [32]. The zero-order and first-order terms do not alter the temporal profile of the pulse while the affect of terms higher than third-order are typically negligible. This dispersion can be compensated by using the AOM to apply a phase mask to the pulse although compensating large amounts of dispersion requires high resolution.
For a pulse centered at 6250 nm, Ge introduces 646 fs2/mm of second-order and 2376 fs3/mm of third-order spectral phase dispersion. Our 20 mm thick Ge AOM will stretch a 60 fs FWHM input pulse to ~ 300 fs. Much of the second-order dispersion from the AOM is compensated by the MgF2 waveplates placed directly before and after the shaper. The exact dispersion of the final pulse will also depend on the initial dispersion, alignment, etc.
Initial compensation of the second-order dispersion or group velocity dispersion (GVD) in performed by translating the second grating as shown in Fig. 2 in order to optimize the SHG efficient of the output pulse, although this method introduces spatial chirp to the pulse. Alternatively, one could take advantage of existence the fact that some materials have negative GVD in the mid-IR while others have positive GVD [33]. The shaper can then be use for fine adjustment of the GVD as well as compensation of the third-order dispersion (TOD).
First, a spectrum of the shaped pulse is measured to obtain the center frequency, v0. Then GVD and TOD are removed by shaping the pulse with a frequency-dependent phase mask according to Eq. 5,
| (5) |
where v is the set of calibrated AOM frequencies determine by Eq. 4, and βGVD and βTOD are the second-order and third-order dispersion constants, respectively. The optimal β values are found by maximizing the SHG efficiency over the two-dimensional parameter space of βGVD and βTOD. As shown in Fig. 5 of Ref. [21], the SHG signal is roughly symmetric about both βGVD and βTOD, although asymmetries and local maxima may be present which necessitates a full 2D scan. The optimized dispersion-correction phase function is used in all subsequent masks, i.e., pump-probe and 2D-IR masks.
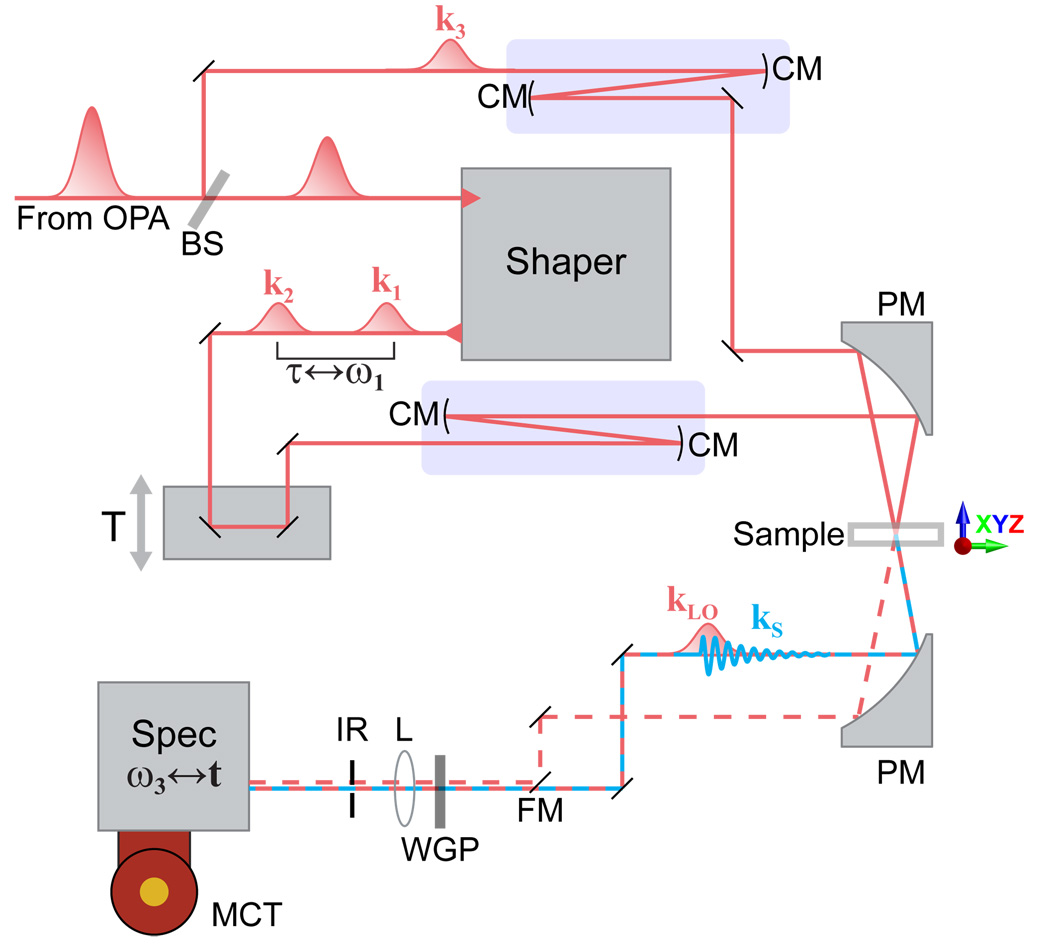
Fig. 5.
Schematic diagram of 2D IR spectrometer. BS: 5% beam splitter, FM: flipper mirror, CM: concave mirror, PM: parabolic mirror, XYZ: manual three-axis translation stage, WGP: wire grid polarizer, L: lens, IR: iris, Spec: spectrometer, MCT: mercury cadmium telluride IR detector array. Blue rectangles outline 2× beam expanding telescopes.
2.5. Masks
The AWG has four independent channels which can output a continuously looping series of masks (see Fig. 3). Two of these channels are used for generating pump-probe signals while the other two channels are used for generating 2D IR signals. In each case, there is a marker channel which is detected in parallel with the MCT array detector signal and a mask channel which is sent to the AOM. An electrical switch determines whether pump-probe or 2D IR masks are sent to the AOM.
Channel 1 is the marker channel for pump-probe measurements and is used to determine which detected probe pulses correspond to the pump being present and which correspond to the pump being absent. It therefore consists of alternating high and low levels for each laser pulse.
Channel 2 is the mask channel for pump-probe measurements and consists of a single-pulse mask, with dispersion compensation but alternating between full and zero amplitude for each laser pulse.
Channel 3 is the marker channel for 2D IR measurements and is used to determine the first pulse in the sequences of pulses. It consists of a high level synchronized with the first 2D IR mask and low level otherwise. The use of this channel is described further in Section 3.3.
Channel 4 is the mask channel for 2D IR measurements and consists of a series of masks generating double pulses (a traveling pulse and a stationary pulse) with various time delays and relative phases. Four masks are used for each time delay with different relative phases between the two pulses so as to reduce scatter and remove transient absorption background, as previously described [22]. A two-frame method can be used for low-scattering samples, which increases acquisition rates by a factor of two. An additional phase shift, proportional to the time delay, is applied to the traveling pulse to shift the 2D IR signal into a rotating frame. The double pulse masks are calculated as,
| (6) |
where vrf is the rotating frame frequency, τ is the time delays between the two pulses and ϕ1 and ϕ2are constant phase shifts for the traveling and stationary pulse, respectively. Additionally, the phase mask for dispersion correction is added to both pulses,
| (7) |
3. 2D IR
The setup and many of the procedures we detail below are identical to that of a typical mid-IR pump-probe experiment. Here we describe the 2D IR spectrometer and its alignment as well as the initial steps required to find pump-probe signal. Finally, we describe how 2D IR signals are acquired and processed.
3.1. Alignment
The 2D IR spectrometer is shown in Fig. 5. The setup is analogous to a pump-probe spectrometer except that there is a shaper in the pump path to create the double pulses necessary for 2D IR measurements. Initial alignment is performed with HeNe laser beams. Pump and probe beams each pass through 2× telescopes in order to expand the beam sizes to 13 mm (1/e2 diameter) and obtain a tighter focus at the sample position. The pump and probe beams are aligned level, parallel and vertically separated by 2 cm. The two beams are focused and spatially overlapped at the sample using a parabolic mirror. The position of the sample is controlled using a manual three-axis translation stage. The pump and probe beams are recollimated after the sample by a second matched parabolic mirror. After a short distance, the probe beam is focused onto the slit of a spectrometer, spectrally dispersed and detected on a shot-to-shot basis with a 64-element MCT linear array detector. The spectrometer has a focal length of 150 mm and uses a grating with 150 grooves/mm. The resulting spectral range on the array detector is over 300 cm−1 wide with the spacing between pixels varying across the array from 4 cm−1 at the lowest frequencies to 7 cm−1 at the highest frequency. The spectral resolution is approximately 8 cm−1 as judged by comparison with an 8 cm−1 resolution FTIR spectrum of water vapor (Fig. 7b). The pump beam is generally discarded after the sample but a flip mirror allows for detecting of the pump beam for diagnostics and calibration. The size of an iris aperture centered on the probe beam is reduced as necessary to minimize pump scatter reaching the detector.
Fig. 7.
a) Uncalibrated spectrum of probe pulse with (black) and without (red) purging. b) Comparison of water vapor absorption spectra obtained with FTIR and probe pulse.
3.2. Spatial and Temporal Overlap
The pump and probe beams must be spatially overlapped at the sample. This is done precisely using a 100 µm aperture, placed inside a sample cell in the sample position. A three-axis translation stage is used to position the aperture and maximize the probe intensity on the array detector. Translation perpendicular to the probe beam centers the aperture on the beam waist while translation parallel to the probe beam centers the aperture at the focus. The pump beam is then temporarily sent to the detector using a flip mirror and the pump beam centered on the aperture using the mirror immediately preceding the first parabolic mirror. This also requires iterative correction of the pump beam after the aperture to maintain alignment to the detector. Once spatial overlap is achieved, it is necessary to find temporal overlap of the pump and probe beams in the sample.
For the pump and probe pulses to be temporally overlapped at the sample, the lengths of the travel by each after their initial separation must be equal, accounting for delays due to transmission through optics. The temporal overlap can first by set with an accuracy of a few cm by measuring the beam paths. This is done most accurately by taking into consideration the position of the beams on each mirror and so is best done when HeNe beams are overlapped with the mid-IR beams. Transmission optics make a generally modest contribution to the travel time of the pulses due to the constant and linear terms of the dispersion Taylor expansion. For example, transmission through a 1 cm of CaF2 adds an additional 6.2 ps delay, equivalent to modest additional 1.9 mm path length. Our setup (Fig. 5) minimizes the number of transmission optics used, however the Ge AOM in the pulse shaper has a large effect due to the high refractive index of Ge in the mid-IR. Transmission through 20 mm of Ge, the thickness of our AOM, delays the mid-IR pulse by about 470 ps, equivalent to an additional 141 mm path length which must be accounted for.
Once the travel time of the pump and probe are roughly matched the precise temporal overlap can be found by scanning a delay stage while looking for a signal that depends on the time delay between pump and probe pulses. In each case detection of the signal is improved by chopping the pump beam and measuring a difference signal. This can be done physically using an optical chopper but it is more convenient to chop the pump electronically by using the AWG to alternately send zero-amplitude masks to the AOM. The probe pulses detected in the presence or absence of the pump pulse are then averaged separately and the difference signal is calculated as,
| (8) |
where Ion and Ioff are intensity on the detector in the presence and absence of the pump pulse, respectively. The chop signal from the AWG is sampled for every detected laser pulse to determine which are with and without the pump pulse. We recommend using two types of signals, each with their own advantages and disadvantages. Both signals are maximized when the pump and probe pulses have parallel polarizations.
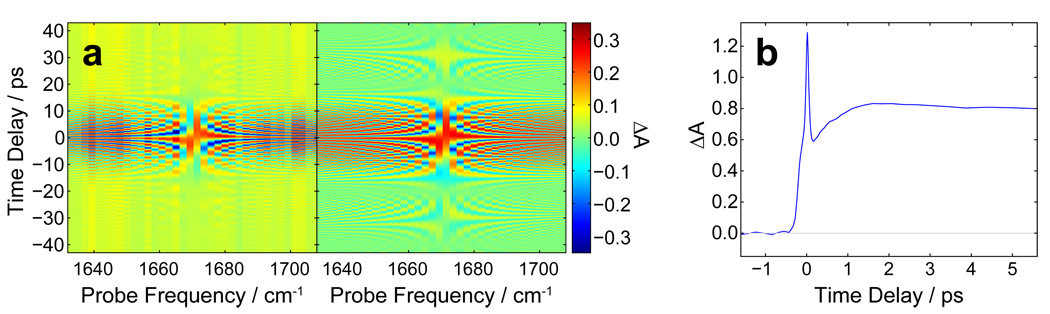
(1) Spectral interference [34] with pump scatter generated at the sample produce fringes in the probe pulse spectrum which depend on the time delay, as shown in Fig. 6 together with a simulation of the signal. In the simulation the pump and probe pulses are 50 fs (FWHM) in duration and centered at 1668 cm−1 with a relative phase which was adjusted to match experiment. Additionally a convolution parameter, also adjusted to match experiment, was used to take into account the finite spectral resolution. There is an offset of unknown origin in the experiment data which does not affect the utility of the signal for determining time zero. As both the experimental data and simulation show, the fringe period is equal to the absolute value of the reciprocal of the time delay. The fringe amplitude decreases with time delay due to the finite spectral resolution of the detection scheme. In our case, we are able to detect fringes at delays larger than ± 40 ps. Once spectral fringes are detected, the time delay can be scanned to find time zero. This method is recommended because results in strong signals at both positive and negative time delays and over a range that covers the typical uncertainty in time zero estimation by measurement. Also, since the pump scatter is produced over a wide volume angle, it requires less-stringent spatial overlap.
Fig. 6.
A) Experimental (left) and simulated (right) difference signal resulting from interference of probe pulse and scattered pump pulse. B) Pump-probe signal in Ge crystal showing coherent spike at time zero and incoherent ground-state bleach at positive time delays.
(2) Ge gives signal throughout the mid-IR region. Ge crystals give a large-amplitude and long-lived pump-probe signal that lasts for well over 100 ps as well as a strong coherent spike at time-zero [35], as shown in Fig. 6b. Because of this long-lived signal it is possible to overestimate the required probe path length by a few cm, find pump-probe signal, and then reduce the probe path to find time zero. Because the signal amplitude depends sensitively on the spatial overlap, it is Ge that should be used to optimize the spatial overlap. One disadvantage of the Ge signal is that it requires multiphoton excitation in the mid-IR and so the signal amplitude is nonlinearly dependent on the energy and duration of the pump pulse. For weak or poorly compressed pulses it may be possible to generate larger signals from a molecular sample.
Final adjustment of the spatial and temporal overlap is performed with a molecular sample. The signal is typically smaller and exists over a shorter range of time delay. Typical N-methylacetamide in D2O is used as an optimization sample used for the amide-I region.
3.3. Data Collection and Processing
Once spatial and temporal overlap is obtained and the pump-probe signal is optimized, acquisition of 2D IR spectra requires only that we send the 2D IR masks to the AOM instead of the pump-probe masks. To record a single 2D IR spectrum, a segment of N consecutive laser shots are acquired, where N is the number of masks in a 2D IR sequence. Since the start of the segment collection is not synchronized with the start of the mask sequence, the acquired data is rotated. The high-level mask in the Channel 2 AWG output is synchronized with the first mask in the 2D IR sequence. The Channel 2 output is sampled along with the mid-IR signal and can be used to rotate the data into the proper sequence. Time-domain data is process with one level of zero filling and, if desired, the signal can be apodized. The time-domain signal corresponding to τ = 0 ps is divided by 2 to remove frequency-domain baseline offsets, as commonly done in NMR spectroscopy [36]. A first- or second-order polynomial background correction is applied in the time domain, provided that the correction does not alter spectral features. Fourier transform of the time-domain trace at each pixel is taken, producing an uncalibrated 2D spectrum.
3.4. Frequency Calibration
The probe frequency axis of our 2D IR spectra is determined by the spectrometer and the MCT array detector. Calibration of the wavelength at each pixel is performed using atmospheric water vapor which has many sharp, strong absorption features from ~1350–1900 cm−1 due to the R and P rotational bands of the water bend vibration. The spectrum of the probe pulse as measured by the array detector with and without water vapor purging is shown in Fig. 7a. The logarithm of the ratioed spectra provides an absorption spectrum that can be compared with a known water vapor absorption spectrum, as in Fig. 7b. In other spectral regions, the shaper can be used to calibrate the detector. Once the shaper is calibrated as described above, a calibration amplitude mask can be applied and the shaped pulse spectrum as measured with the detector compared with the amplitude mask.
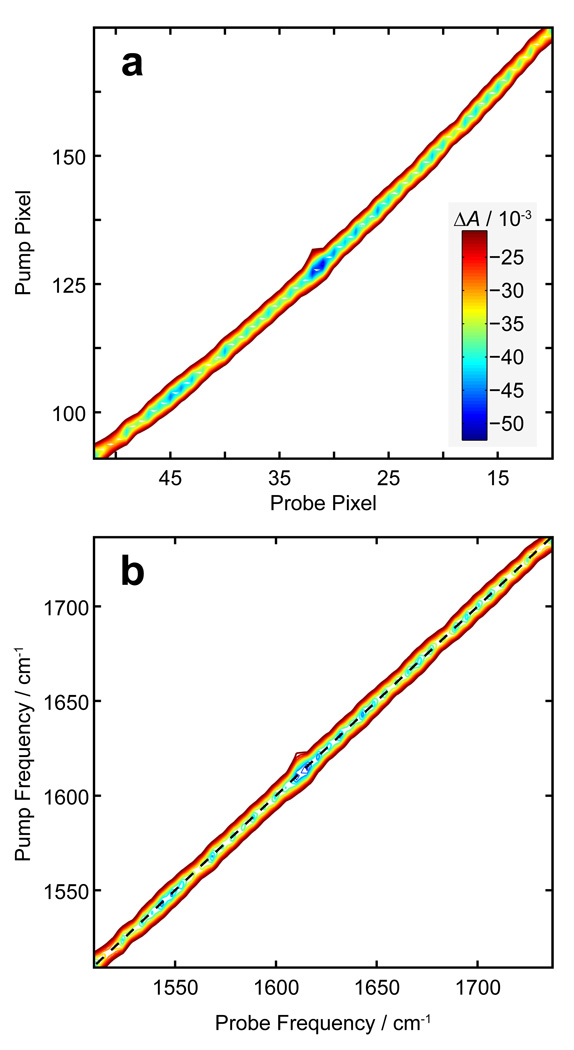
Once the array detector has been calibrated, the pump frequency can be calibrated in a number of ways. First, the pump frequency can be calculated based on the time-domain sampling parameters used. In practice this provides only a close estimate as the true pump frequencies depend slightly on the experimental conditions. Another method involves calibrating with an FTIR spectrum of the sample. However, this method provides a small number of calibration points and overlapping resonances and differing lineshapes may complicate this approach. We present here a different method which takes advantage of the collinear nature of the two pump pulses. A flip mirror placed after the sample is used to temporarily direct the pump beam through the spectrometer and onto the array detector. The spectrum of the interfering pump pulses is collected and processed in the exact same manner as a 2D IR spectra. The resulting plot shows a single strong feature which defines the frequency diagonal, as shown in before (Fig. 8a) and after (Fig. 8b) calibration. The feature in the uncalibrated plot has a slight curvature due to the nonlinear relationship between detector pixel and frequency.
Fig. 8.
a) Uncalibrated 2D frequency spectrum of double pulses used for 2D IR measurements. b) Data in panel A, after probe calibration and pump calibration has been performed.
4. Membrane Proteins and Amyloids
Having described the experimental setup we now turn to practices specific to the studies of proteins particularly membrane and amyloid peptides which require special considerations. First we describe the procedure for synthesizing isotope-labeled amino acids then the synthesis and purification of the peptides. We describe the procedure for preparing lipid vesicles suitable for 2D IR studies of membranes proteins and finally discuss measurement of 2D IR kinetics as applied to an amyloid peptide.
4.1. Isotope-Labeled Amino Acids
Isotope editing is a powerful tool for obtaining residue-specific structural information from IR spectra [37–44]. The primary purpose of isotope editing is to separate overlapping vibrational features so that the properties such as the frequency and linewidth of the individual features can be accurately determined without deconvolution. In addition, the frequency shift greatly reduces mixing of the labeled local mode with the exciton manifold of unlabeled modes, resulting in an approximately block-diagonal vibrational Hamiltonian. For hIAPP amyloid fibers, for example, where the labeled residues are aligned in-register, this allows for simple modeling of the labeled resides as a linear chain [45]. Additional structural information can be obtained due to the effect of removing the labeled residue on the unlabeled Hamiltonian [44].
Due to common use for NMR spectroscopy, most 13C-labeled amino acids are commercially available but only induce a ~40 cm−1 in the amide-I frequency. A 40 cm−1 shift is not always sufficient to resolve the isotope labeled residue, especially in peptides with large β-sheet character. This is also problematic for large peptides because the presence of natural abundance (1.1%) 13C atoms becomes significant. Arkin and coworkers pioneered the use of 13C=18O labels which induces a ~60 cm−1 shift and largely alleviates these problems [46, 6]. For example, a 300 residue protein will naturally have, on average, three 13C=16O backbone amide carbonyls but a < 1% probability of having a single natural 13C=18O carbonyl.
To site-specifically label a backbone carbonyl group in a peptide, the labels are incorporated very early in the synthesis. Free amino acids with 13C at the backbone carbonyl position are received from Cambridge Isotope Laboratories, Inc. The two carboxylic oxygen atoms are exchanged under refluxing acidic conditions similar to the method of Torres, et al. [46]:
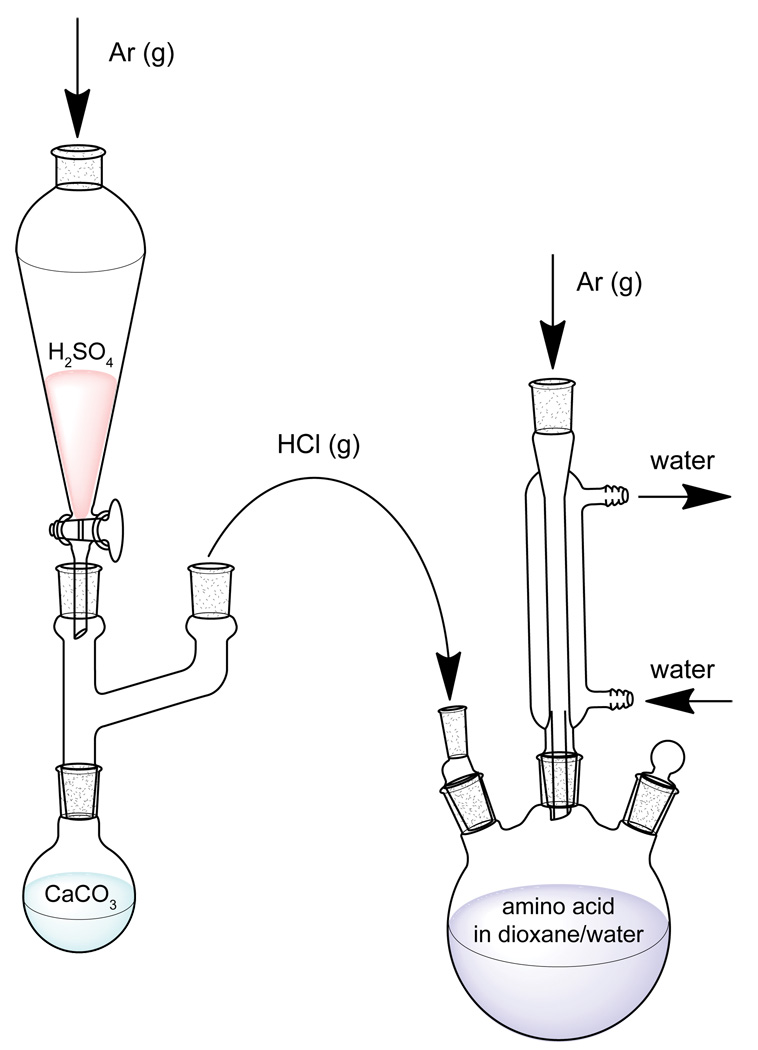
The apparatus is setup is comprised of a reaction vessel and an HCl gas generator, as shown in Fig. 9.
All glassware is flame-dried under vacuum and washed with argon three times.
13C-labeled amino acid (10 mmol) is added to the reaction vessel and dried with flame under vacuum. Steps 4–6 are performed under Argon atmosphere.
18O-enriched water (95% 18O, 1 mL) and 1,4-dioxane (10 mL) are added to the amino acid. Hydrophobic amino acids may require up to 20 mL of 1,4-dioxane. The resulting suspension is stirred and cooled with an ice bath.
Exchange occurs at mildly acidic conditions and room temperature; however, to reduce the time required for efficient 18O uptake, the reaction is performed at low pH and 100°C. Dropwise addition of concentrated anhydrous sulfuric acid onto anhydrous calcium chloride produces dry HCl gas, which flows into the dioxane/water suspension via tubing attached to a hollow glass rod. The amino acid dissolves more readily as the suspension pH decreases. Bubbling of HCl is performed for approx. 20 minutes until the pH of the suspension is 1–2 [47].
The HCl port is closed and the solution is refluxed with stirring for 3–6 hours. Exchange time depends on steric hinderance caused by the amino acid side chain [48].
The suspension is brought to room temperature and neutralized with 1 M NaOH while stirring.
The dioxane and water are removed under vacuum and collected with a small liquid nitrogen trap.
The enrichment is determined by mass spectrometry and the above procedure reiterated with fresh 18O-enriched water until the desired enrichment is achieved.
Fig. 9.
Apparatus setup for synthesis 18O exchange of amino acids. HCl gas generator (left) for reduction of dioxane/water suspension pH and reaction vessel (right).
At this point protecting groups must be added for Fmoc solid-phase peptide synthesis. Hydrophobic amino acids only require Fmoc protection, and we use the one-pot procedure reported by Cruz et al. with yields of 81–96% [49]. Alternatively, the Fmoc protecting group can be added first, followed by 18O exchange for 3–10 hours (longer for β-branched amino acids) [48]. Most amino acids require both Fmoc and side-chain protection groups for peptide synthesis. Since the Fmoc group is base labile while the side-chain protection groups are acid labile, orthogonal protection requires multiple steps and is generally impractical. For this reason, isotope labeling has been almost exclusively limited to amino acids with nonreactive side chains, specifically glycine (G), alanine (A), leucine (L), isoleucine (I), valine (V) and phenylalanine (F). The lone exception is lysine (K) which has been successfully 13C=18O labeled and incorporated into a peptide [50]. In this case the side-chain protection group was added through a multi-step synthesis via a copper intermediate as described by Wiejak [51].
A promising new method for synthesizing 13C=18O amino acids has recently been demonstrated [52]. This one-pot synthesis uses multiple-turnover activation chemistry to efficiently exchange 16O with 18O at pH ~5. The use of moderate pH allows the 13C amino acid to be purchased with all the necessary protecting groups attached and have them retained during 18O exchange. We have recently used this approach to 13C=18O label serine.
4.2. Peptide Synthesis & Purification
Synthesis of peptides is performed with standard Fmoc solid-phase chemistry. Standard commercial reagents are used with the exception of isotope-labeled amino acids. Because hIAPP has a high tendency to aggregate additional precautions are necessary. We follow the synthesis and purification methods developed by Raleigh et. al [53, 54] utilizing pseudoproline dipeptide derivatives to reduce aggregation and double couplings for β-branched residues and residues immediately following β-branched residues. Additionally, the first coupling uses a 1:1 ratio of Fmoc- and Boc-protected amino acids to cap half the resin sites and spatially separate the peptides during synthesis. The amyloid peptides were purified with reverse-phase HPLC using 0.045% HCl as an ion pairing agent.
4.3. Preparation of Lipid Vesicles
The primary concern when preparing samples of membrane proteins in lipid vesicles is the tendency of these samples to strongly scattering of the incident mid-IR beams. The scatter produces a signal which can strongly overlap with 2D IR signal and make collecting data for aggregating peptides or samples with lipid vesicles difficult. The primary source of scatter is the two pump pulses, k1 and k2. Scatter from either k1 or k2 alone can be reduced by phase cycling [22]. The scatter that remains results from the interference between scatter from both k1 and k2 and presents along the diagonal of the 2D IR spectrum as shown in Fig. 10a.
Fig. 10.
2D IR spectra of ovispirin, 13C=18O-labeled at K9, in POPC/POPG vesicles prepared (a) without and (b) with sonication. Sonication decreases the vesicles size and size distribution as well as the amount of scatter signal along the diagonal. Diagonal slices with (black) and without (red) sonication are shown in the top panel.
Scatter from lipid vesicles can be avoided by preparing small uniformly-sized unilamellar vesicles as follows. POPC and POPG lipids (Avanti Polar Lipids) are first dissolved in chloroform, combined in the desired proportions, and the solvent evaporated. The sample is reconstituted with buffer, forming a wide distribution of multilamellar lipid structures and sonicated for at least 10 min. For reducing scatter, sonication is preferred over extrusion because it produces vesicles with diameters < 100 nm [55]. The resulting vesicles appear uniform and clear to the eye and the peptide samples with these vesicles produce little or no scatter in the mid-IR, as shown in Fig. 10b.
4.4. Kinetics of Amyloid Fiber Formation
Following purification, lyophilized peptides are dissolved in 1,1,1,3,3,3-hexafluoro-2-propan(old) to a concentration of 1 mM in order to exchange labile protons and prevent aggregation. A small portion (5–10 µL) of the stock solution is transferred to a small eppendorf tube and the solvent evaporated under a light flow of nitrogen for 20 minutes producing a dried film. Aggregation is initiated by dissolution of dried peptide films in pH 7.4, 20 mM dipotassium phosphate D2O buffer. The dissolved peptide is then immediately transferred to a sample cell and place in the sample position of the 2D IR spectrometer. While monitoring the pump-probe signal, small adjustments of the sample position may be needed to locate a region with low scatter and center the sample at the beam focus. Once the pump-probe signal is optimized, the AOM masks switched to the 2D IR sequence and data acquisition is begun. Once data collection is started, a 2D IR spectrum is acquired every 500–600 ms including the time required to process and save the data. The dead time between the addition of buffer and the start of data collection is typically 2 minutes.
Shown in Fig. 11 is representative data from a kinetics measurement on the human islet amyloid peptide isotope labeled at L16. We continuously monitored the aggregation kinetics for 200 minutes. After data collection, we smoothed the data with a 10 minute running average. We usually use windows of 4 to 15 minutes depending on the signal-to-noise, which depends on peptide concentration, laser intensity fluctuations, etc. The sample shown here has a peptide concentration of only 800 µM. The two representative 2D IR spectra illustrate the change in the spectra over the course of fibril formation. At early times the peptides are largely random coil (1650 cm−1) which then converts into β-sheet fibrils (prominent absorption at 1620 cm−1). Notice that the isotope label feature also shifts frequency, which is an indication of excitonic coupling between stacked polypeptides. Thus, the isotope label measures aggregation taking place at L16. Shown in Fig. 11b are plots of the intensities of these features, which provides the kinetics. The kinetics are fit to a sigmodial function, typical of amyloid formation, that is characterized by a t50 time. We have used experiments like these to monitor residue-specific aggregation in hIAPP [43] as well as membrane-catalyze hIAPP aggregation [56].
Fig. 11.
Kinetics of hIAPP amyloid fiber formation monitored by 2D IR spectroscopy. (a) 2D IR spectrum of hIAPP 13C=18O-labeled at L16 average at 7 min. (left) and 187 min. (right) of aggregation. (b) Kinetics of diagonal features at ωpump = ωprobe = 1589 cm−1 (red), 1620 cm−1 (green) and 1659 cm−1 (blue), corresponding to the isotope label, β-sheet and random coil features, respectively.
In principle, the same kinetics can be extracted from linear IR (e.g., FTIR) spectra. However there are many advantages to using 2D IR to monitor protein folding kinetics. 2D IR signals have a fourth-power dependence on the transition dipole moment (|μ|4) while linear IR spectra have a square dependence (|μ|2). As a result, 2D IR spectra exhibit narrower linewidths and provide better spectral resolution than linear IR spectra. In particular, 13C=18O label peaks are significantly better resolved in 2D IR spectra than linear spectra [57]. Another consequence of the transition dipole dependence is that the intensity of some weak side chain absorption features which overlap with 13C=18O peaks is reduced in 2D IR spectra. Weak background peaks, like the H2O bend or lipid absorption of partially hydrated membrane samples, are similarly minimized. Another significant advantage of 2D IR spectra is the presence of cross-peaks between vibrations due to coupling [58], chemical exchange [59], or vibrational energy transfer [60]. Cross-peak features provide additional information on protein structure [61, 62, 42] including amyloid fibers [44] and permit identification of weak diagonal features. While our current kinetic studies have focused on diagonal features, there is nothing about the technique which prevents monitoring cross-peak kinetics as future studies will demonstrate.
5. Conclusions
2D IR spectroscopy is a powerful technique for studying protein folding and aggregation because of its time-resolution, applicability to a range of samples, and its residue-by-residue structural resolution (when used with isotope labeling). One difference between our method and the traditional four-wave mixing geometry, which has not been quantified, is signal-to-noise. The signal with our method may be smaller because k3 must be weak so that it doesn’t saturate the detector. However, the noise may also be smaller because we can average faster which minimizes problematic long-term drifts. The best of both methods may be combined through polarization shaping [27] which allows the use of pulse intensities comparable to four-wave mixing.
The biggest advantages of our design are that the optical layout is much simpler, the spectra are automatically properly phased and absorptive, and the acquisition rate is much faster. A commercial 2D IR spectrometer is not yet available and there are several good spectrometer designs [63–65], but with pulse shaping, the technological hurdles are greatly diminished, making the technique more easily accessible to a wider range of scientists. Moreover, the technology is making it possible to study ever more sophisticated systems and obtain structure and time-information not easily obtained with other techniques.
Acknowledgements
Support for this research was provided by the NIH (DK79895) and the NSF through a CRC grant (0832584). We are also grateful to D. P. Raleigh and I. T. Arkin for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mantsch HH, Chapman D, editors. Infrared Spectroscopy of Biomolecules. New York, NY: Wiley-Liss; 1996. [Google Scholar]
- 2.Barth A. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Halverson KJ, Sucholeiki I, Ashburn TT, P.T.L. J. Am. Chem. Soc. 1991;113:6701–6703. [Google Scholar]
- 4.Haris PI, Robillard GT, Dijk AAV, Chapman D. Biochemistry. 1992;31:6279–6284. doi: 10.1021/bi00142a016. [DOI] [PubMed] [Google Scholar]
- 5.Sonar S, Lee C-P, Coleman M, Patel N, Liu X, Marti T, Khorana HG, RajBhandary UL, Rothschild KJ. Nat. Struct. Biol. 1994;1:512–517. doi: 10.1038/nsb0894-512. [DOI] [PubMed] [Google Scholar]
- 6.Jaume T, Andreas K, Jonathan MG, Isaiah TA. Biopolymers. 2001;59:396–401. [Google Scholar]
- 7.Decatur SM. Acc. Chem. Res. 2006;39:169–175. doi: 10.1021/ar050135f. [DOI] [PubMed] [Google Scholar]
- 8.Anfinrud PA, Han C, Hochstrasser RM. P. Natl. Acad. Sci. USA. 1989;86:8387–8391. doi: 10.1073/pnas.86.21.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anfinrud PA, Han CH, Lian T, Hochstrasser RM. J. Phys. Chem. 1991;95:574–578. [Google Scholar]
- 10.Hamm P, Lauterwasser C, Zinth W. Opt. Lett. 1993;22:1943–1945. doi: 10.1364/ol.18.001943. [DOI] [PubMed] [Google Scholar]
- 11.Hill JR, Tokmakoff A, Peterson KA, Sauter B, Zimdars D, Dlott DD, Fayer MD. J. Phys. Chem. 1994;98:11213–11219. [Google Scholar]
- 12.Rella CW, Kwok A, Rector K, Hill JR, Schwettman HA, Dlott DD, Fayer MD. Phys. Rev. Lett. 1996;77:1648. doi: 10.1103/PhysRevLett.77.1648. [DOI] [PubMed] [Google Scholar]
- 13.Ballew RM, Sabelko J, Gruebele M. P. Natl. Acad. Sci. USA. 1996;93:5759–5764. doi: 10.1073/pnas.93.12.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer RB, Gai F, Woodruff WH, Gilmanshin R, Callender RH. Acc. Chem. Res. 1998;31:709–716. [Google Scholar]
- 15.Eaton WA, Muñoz V, Hagen SJ, Jas GS, Lapidus LJ, Henry ER, Hofrichter J. Annu. Rev. Biophys. Biomol. Struct. 2000;29:327–359. doi: 10.1146/annurev.biophys.29.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bredenbeck J, Helbing J, Kumita JR, Woolley GA, Hamm P. P. Natl. Acad. Sci. USA. 2005;102:2379–2384. doi: 10.1073/pnas.0406948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung HS, Ganim Z, Jones KC, Tokmakoff A. P. Natl. Acad. Sci. USA. 2007;104:14237–14242. doi: 10.1073/pnas.0700959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamm P, Helbing J, Bredenbeck J. Ann. Rev. Phys. Chem. 2007;59:291–317. doi: 10.1146/annurev.physchem.59.032607.093757. [DOI] [PubMed] [Google Scholar]
- 19.Shim S-H, Strasfeld DB, Ling YL, Zanni MT. P. Natl. Acad. Sci. USA. 2007;104:14197–14202. doi: 10.1073/pnas.0700804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim S-H, Strasfeld DB, Fulmer EC, Zanni MT. Opt. Lett. 2006;31:838–840. doi: 10.1364/ol.31.000838. [DOI] [PubMed] [Google Scholar]
- 21.Shim S-H, Strasfeld DB, Zanni MT. Opt. Express. 2006;14:13120–13130. doi: 10.1364/oe.14.013120. [DOI] [PubMed] [Google Scholar]
- 22.Shim S-H, Zanni MT. Phys. Chem. Chem. Phys. 2009;11:748–761. doi: 10.1039/b813817f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasfeld DB, Ling YL, Shim S-H, Zanni MT. J. Am. Chem. Soc. 2008;130:6698–6699. doi: 10.1021/ja801483n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner AM. Rev. Sci. Inst. 2000;71:1929–1960. [Google Scholar]
- 25.Bardeen CJ, Yakovlev VV, Wilson KR, Carpenter SD, Weber PM, Warren WS. Chem. Phys. Lett. 1997;280:151–158. [Google Scholar]
- 26.Fetterman M, Goswami D, Keusters D, Yang W, Rhee J-K, Warren W. Opt. Express. 1998;3:366–375. doi: 10.1364/oe.3.000366. [DOI] [PubMed] [Google Scholar]
- 27.Middleton CT, Strasfeld DB, Zanni MT. Opt. Express. 2009;17:14526–14533. doi: 10.1364/oe.17.014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strasfeld DB, Middleton CT, Zanni MT. New J. Phys. 2009;11:105046. doi: 10.1088/1367-2630/11/10/105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte T, Zeidler D, Proch D, Kompa KL, Motzkus M. Opt. Lett. 2002;27:131–133. doi: 10.1364/ol.27.000131. [DOI] [PubMed] [Google Scholar]
- 30.Tan H-S, Warren W. Opt. Express. 2003;11:1021–1028. doi: 10.1364/oe.11.001021. [DOI] [PubMed] [Google Scholar]
- 31.Ding F, Mukherjee P, Zanni MT. Opt. Lett. 2006;31:2918–2920. doi: 10.1364/ol.31.002918. [DOI] [PubMed] [Google Scholar]
- 32.Backus S, Durfee Iii CG, Murnane MM, Kapteyn HC. Rev. Sci. Inst. 1998;69:1207–1223. [Google Scholar]
- 33.Demirdöven N, Khalil M, Golonzka O, Tokmakoff A. Opt. Lett. 2002;27:433–435. doi: 10.1364/ol.27.000433. [DOI] [PubMed] [Google Scholar]
- 34.Lepetit L, Chériaux G, Joffre M. J. Opt. Soc. Am. B. 1995;12:2467–2474. [Google Scholar]
- 35.Roskos H, Rieck B, Seilmeier A, Kaiser W. Appl. Phys. Lett. 1988;53:2406–2408. [Google Scholar]
- 36.Cavanagh J, Fairbrother WJ, A.G.P., Rance M, Skelton NJ. Protein NMR Spectroscopy: Principles and Practice. London: Elsevier Academic Press; 2007. [Google Scholar]
- 37.Fang C, Hochstrasser RM. J. Phys. Chem. B. 2005;109:18652–18663. doi: 10.1021/jp052525p. [DOI] [PubMed] [Google Scholar]
- 38.Arkin IT. Curr. Opin. Chem. Biol. 2006;10:394–401. doi: 10.1016/j.cbpa.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee P, Kass I, Arkin IT, Zanni MT. J. Phys. Chem. B. 2006;110:24740–24749. doi: 10.1021/jp0640530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YS, Liu L, Axelsen PH, Hochstrasser RM. P. Natl. Acad. Sci. USA. 2008;105:7720–7725. doi: 10.1073/pnas.0802993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langosch D, Arkin IT. Protein Sci. 2009;18:1343–1358. doi: 10.1002/pro.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekawa H, De Poli M, Toniolo C, Ge N-H. J. Am. Chem. Soc. 2009;131:2042–2043. doi: 10.1021/ja807572f. [DOI] [PubMed] [Google Scholar]
- 43.Shim S-H, Gupta R, Ling YL, Strasfeld DB, Raleigh DP, Zanni MT. P. Natl. Acad. Sci. USA. 2009;106:6614–6619. doi: 10.1073/pnas.0805957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strasfeld DB, Ling YL, Gupta R, Raleigh DP, Zanni MT. J. Phys. Chem. B. 2009;113:15679–15691. doi: 10.1021/jp9072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hochstrasser RM, Whiteman JD. J. Chem. Phys. 1972;56:5945–5958. [Google Scholar]
- 46.Torres J, Adams PD, Arkin IT. J. Mol. Biol. 2000;300:677–685. doi: 10.1006/jmbi.2000.3885. [DOI] [PubMed] [Google Scholar]
- 47.Murphy RC, Clay KL, James AM. Method Enzymol. 1990;193:338–348. doi: 10.1016/0076-6879(90)93425-k. [DOI] [PubMed] [Google Scholar]
- 48.Marecek J, Song B, Brewer S, Belyea J, Dyer RB, Raleigh DP. Org. Lett. 2007;9:4935–4937. doi: 10.1021/ol701913p. [DOI] [PubMed] [Google Scholar]
- 49.Cruz LJ, Beteta NG, Ewenson A, Albericio F. Org. Proc. Res. Dev. 2004;8:920–924. [Google Scholar]
- 50.Woys AM, Lin Y-S, Reddy AS, Xiong W, de Pablo JJ, Skinner JL, Zanni MT. J. Am. Chem. Soc. 2010;132:2832–2838. doi: 10.1021/ja9101776. [DOI] [PubMed] [Google Scholar]
- 51.Wiejak S, Masiukiewicz E, Rzeszotarska B. Chem. Pharm. Bull. 1999;47:1489–1490. doi: 10.1248/cpb.49.1189. [DOI] [PubMed] [Google Scholar]
- 52.Seyfried MS, Lauber BS, Luedtke NW. Org. Lett. 2009;12:104–106. doi: 10.1021/ol902519g. [DOI] [PubMed] [Google Scholar]
- 53.Abedini A, Raleigh DP. Org. Lett. 2005;7:693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 54.Cao P, Meng F, Abedini A, Raleigh DP. Biochemistry. 2010;49:872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lapinski MM, Castro-Forero A, Greiner AJ, Ofoli RY, Blanchard GJ. Langmuir. 2007;23:11677–11683. doi: 10.1021/la7020963. [DOI] [PubMed] [Google Scholar]
- 56.Ling YL, Strasfeld DB, Shim S-H, Raleigh DP, Zanni MT. J. Phys. Chem. B. 2009;113:2498–2505. doi: 10.1021/jp810261x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim YS, Hochstrasser RM. J. Phys. Chem. B. 2009;113:8231–8251. doi: 10.1021/jp8113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krummel AT, Mukherjee P, Zanni MT. J. Phys. Chem. B. 2003;107:9165–9169. [Google Scholar]
- 59.Fayer MD. Ann. Rev. Phys. Chem. 2009;60:21–38. doi: 10.1146/annurev-physchem-073108-112712. [DOI] [PubMed] [Google Scholar]
- 60.Rubtsov IV. Acc. Chem. Res. 2009;42:1385–1394. doi: 10.1021/ar900008p. [DOI] [PubMed] [Google Scholar]
- 61.DeFlores LP, Ganim Z, Nicodemus RA, Tokmakoff A. J. Am. Chem. Soc. 2009;131:3385–3391. doi: 10.1021/ja8094922. [DOI] [PubMed] [Google Scholar]
- 62.Kim YS, Liu L, Axelsen PH, Hochstrasser RM. P. Natl. Acad. Sci. USA. 2009;106:17751–17756. doi: 10.1073/pnas.0909888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woutersen S, Hamm P. J. Phys.: Condens. Matter. 2002;14:R1035–R1062. [Google Scholar]
- 64.DeFlores LP, Nicodemus RA, Tokmakoff A. Opt. Lett. 2007;32:2966–2968. doi: 10.1364/ol.32.002966. [DOI] [PubMed] [Google Scholar]
- 65.Anna JM, Nee MJ, Baiz CR, McCanne R, Kubarych KJ. J. Opt. Soc. Am. B. 2010;27:382–393. [Google Scholar]