Abstract
Regulatory T cells (Tregs) support pregnancy maintenance by suppressing placental inflammation, while diminished Treg function may accompany reproductive failure. Experimental FIV infection frequently results in vertical transmission and increased pregnancy failure in the cat. The mechanism of reproductive compromise is unknown. We hypothesized that FIV infection alters endometrial Treg population dynamics and function, potentiating vertical transmission and reproductive failure. RNA collected from early and late gestation reproductive tissue and fetuses from FIV infected and control cats was probed for expression of FIV gag and Treg markers CD25, FOXP3, and CTLA4, using real time reverse-transcriptase (RT)-PCR. Frequent placental and fetal infection and reproductive failure were detected at early and late pregnancy. Expression of FOXP3 and CTLA4 was higher in early gestation tissues from control cats. FIV infection significantly reduced expression of FOXP3 and CTLA4 at early, but not late pregnancy. At late pregnancy, CTLA4 was expressed to higher levels in infected tissues. The number of tissues with decreased co-expression of FOXP3 and CTLA4 was significant in infected cats at early pregnancy. No significant changes in CD25 expression occurred between FIV-infected and control animals at early or late pregnancy. Differences in Treg marker expression were not significant between viable and non-viable pregnancies in infected cats. The detection of Treg markers in feline tissues provides the first evidence of feline endometrial Tregs and suggests that such cells diminish as pregnancy progresses. These cells may be depleted or rendered less functional by viral infection, but understanding their role in pregnancy requires further study.
Keywords: FIV, Tregs, CD25, FOXP3, CTLA-4
INTRODUCTION
Tregs, characterized by the expression of the alpha chain of interleukin 2 receptor (CD25), forkhead transcription factor (FOXP3) and cytotoxic T lymphocyte antigen 4 (CTLA4), are immunosuppressive, maintaining immune system homeostasis and tolerance to self antigens. They play a major role in the suppression of graft rejection and innate mucosal immunity [1]. Tregs are important to pregnancy maintenance. In both the mouse model and in humans, decidual Tregs allow maternal tolerance of the semi-allogeneic fetus [2, 3], while fetal Tregs allow fetal tolerance of maternal alloantigens [4]. In human decidua, Tregs comprise approximately 10% of CD4+ T cells during early pregnancy [5] and 14% of CD4+ T cells at term [6]. Successful pregnancy is associated with greater numbers of activated Tregs in both the periphery and deciduum [7–9]. During normal human pregnancy, CD4+CD25highCTLA4 Treg cells increase, but these cells decrease to pre-pregnancy levels if pregnancy fails [8].
Feline Tregs are characterized most definitively by co-expression of CD4, CD25, and FOXP3 [10]. Like human and murine Tregs, feline Tregs comprise 5–10% of the total CD4+ T cells in the blood and 20–30% of lymph node CD4+ T cells and express many of the same phenotypic and functional characteristics [11]. These cells support productive replication of FIV. Joshi et al. [12] found that CD4+CD25+ T cells express higher levels of CXCR4 and CD134 than CD4+CD25− T cells, and FIV entry positively correlates with levels of CXCR4 expression. In addition, CD4+CD25+ T cells constitutively express transcriptional activators which bind the FIV long terminal repeat (LTR), enhancing viral replication. Expression of co-stimulatory molecules B7.1, B7.2, and CTLA4 on blood or lymph node Tregs is upregulated in FIV-infected animals [11].
Mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) is the leading cause of pediatric AIDS worldwide [13]. Adverse pregnancy outcome, including increased levels of miscarriage, stillbirth, pre-term delivery, and low birth weight was significantly increased in HIV-infected women from the U.S., Europe, Asia, and Africa [14–18]. Others report no difference in reproductive outcome between HIV seropositive and seronegative women [19]. Mechanisms by which HIV may compromise pregnancy are not understood.
FIV naturally produces a syndrome culminating in feline AIDS and terminal susceptibility to opportunistic infections and cancer, similar to progression to AIDS in HIV-infected humans. Transplacental transmission of FIV occurs readily during experimental infection with some FIV isolates, producing frequent reproductive failure [20–23]. Thus, the FIV-infected cat provides a useful small animal model for HIV pathogenesis and MTCT.
We previously reported very high rates of MTCT and frequent reproductive failure in FIV-infected queens at both early and late pregnancy [22, 23]. Moreover, successful pregnancy was accompanied by higher levels of CD134 and CXCR4 expression [24], suggesting that cells bearing these markers, such as Tregs, may be important to pregnancy maintenance. The data indicate that FIV infection results in enhanced inflammation in the feline placenta. The role of endometrial Tregs in pregnancy maintenance in the cat is completely unknown. We hypothesized that FIV infection may alter population dynamics and function of endometrial Tregs, abrogating their immunosuppressive activity and predisposing fetal infection and/or reproductive failure. Thus, our purpose was to examine tissue specimens spanning the maternal-fetal interface for expression of Treg markers to determine whether FIV infection alters expression of these markers and to assess whether fetal viability can be correlated with marker expression.
MATERIALS AND METHODS
Animals and virus
Two groups of twenty, reproductively mature, specific pathogen-free (SPF) animals, less than 12 months of age, obtained from a commercial cattery, were used to evaluate early and late pregnancy. Ten queens from each study group were inoculated intravenously with a feline plasma pool containing approximately 1.3 × 104 copies/ml of FIV-B-2542 [22, 23], originally provided by Dr. Edward A. Hoover [21]. The other ten queens in each group were used as controls. All procedures involving the cats were performed with approval of the Mississippi State University Institutional Animal Care and Use Committee (protocol no. 05-046).
Tissue collection
Following confirmation of FIV infection using PCR and serology, the cats were allowed to breed naturally with SPF males. Pregnancies were confirmed by ultrasonography and terminated at week 3–4 (early) or week 8 (late). The time of FIV inoculation to delivery ranged from approximately 9.5 to 13.5 months (mean 11.14 months) for the early gestation study and 4.7 to 14.1 months (mean 9.5 months) for the late gestation study. Kittens from both FIV-infected and control queens were delivered by cesarean section. The placentas and underlying endometrium were collected from the intact fetal membranes, labeled by the number of the queen, and given an alphabetical designation. Fetuses and placental/endometrial tissues were collected, snap frozen in liquid nitrogen or formalin fixed, then stored at −80°C [22].
RNA isolation
RNA was isolated from early and late term tissues using TRIzol Reagent (Invitrogen, Corp. Carlsbad, CA) as described previously [24]. RNA concentrations were determined using a NanoDrop 1000 (Thermo Scientific, Waltham, MA) and frozen at −80°C.
Primer and probe sets
Primer/probe sets targeting CTLA4 and CD25 were designed using the method described previously [24]. Primer/probe sets (Table 1) for FOXP3 [10], β-actin [24], FIV gag [22], CTLA4, and CD25 were obtained commercially (MWG-BIOTECH, Inc., High Point, NC).
Table 1.
Sequences of primers and probes used in real time RT-PCR
| Receptor | Primer | Sequence (5′→3′) |
|---|---|---|
| Gag | Sense | GTATGATCGTACTCATCCTCCTGAT |
| Anti-sense | TCTACATTGCATTCTGGCTGGT | |
| Probe | AGACCACTGCCCTACTTCACTGCCG | |
| β-actin | Sense | GACTACCTCATGAAGATCCTCACG |
| Anti-sense | CCTTGATGTCACGCACAATTTCC | |
| Probe | ACAGTTTCACCACCACCGCCGAGC | |
| FOXP3 | Sense | GCCTGCCACCTGGAATCAAC |
| Anti-sense | GTGTGCTGGGGCTTGGGA | |
| Probe | CAGTGCTGGCTCCCTGGACACCCA | |
| CD25 | Sense | CCACGTGACAGAACTGTGTG |
| Anti-sense | GTGCCCGTCTTGTATGTGAG | |
| Probe | CCGCCAGATATCCAACACGCC | |
| CTLA4 | Sense | TCTCCAAAGGGATGCATGT |
| Anti-sense | CCCATATTCACACACGAAGC | |
| Probe | CTGGCCAGCACCACTGCAGG |
Target probe labels: 5′ FAM (6-carboxyfluorescein), 3′ TAMRA (6-carboxytetramethylrhodamine).
β-actin labels: 5′ HEX (hexachloro-6-carboxyfluorescein), 3′ TAMRA.
Real time reverse-transcriptase (RT)-PCR
Relative gene expression was determined using TaqMan real time RT-PCR targeting FOXP3, CTLA4, CD25, FIV gag, and the housekeeping gene β-actin, according to established parameters [22, 24, 25]. For every RNA sample, parallel reactions were run in duplicate. These data were adjusted for ease of interpretation by subtracting the normalized Ct values from the negative endpoint, 50; thus, higher adjusted Ct values indicate higher levels of expression [22, 24, 25]. For assurance that the integrity of RNA isolated from frozen early and late gestation tissues was equivalent, mean Ct values for β-actin, obtained by amplifying multiple replicas from 35 early placental samples and 31 late placental samples, were compared. β-actin mean Ct values for the two sets of tissues differed by 0.25 Ct.
Immunofluorescence Detection of FOXP3
Frozen, OCT-embedded tissues were cryosectioned to 4 μ thickness and placed onto poly-L-lysine-coated microscope slides. Tissues were fixed in 4% paraformaldehyde at 4°C for 30 min, washed in cold PBS (pH 7.2), and treated with 0.1% Triton X-100 in PBS. Sections were blocked by treatment with purified cat IgG (0.1 mg/μl; AbD Serotec, Oxford, UK) for 2h at 37°C in a humidified chamber, washed well, and treated with rabbit polyclonal antibody to FOXP3 (AbCam, Cambridge, MA) for 1h at 37°C. Following repeated washes, sections were incubated 1h at 37°C in the dark with goat anti-rabbit IgG (H+L) conjugated with fluorescein isothiocyanate (Millipore, Billerica, MA), followed by staining for 15 min with DAPI. Sections were coverslipped with Vectashield (Vector Laboratories, Inc., Burlingame, CA), then viewed with confocal microscopy using a Zeiss Axiovert 200 M Inverted Research Microscope (Carl Zeiss MicroImaging, Thornwood, NY) Negative controls included both universal negative control antibody (DAKO Corp., Carpinteria, CA) or no primary antibody.
Statistical analysis
Statistical comparisons of mean Ct values between groups were done using single-factor ANOVA followed by Wilcoxon-Mann-Whitney U test. Chi square analysis was used to compare observed and expected frequencies of FOXP3 and CTLA4 co-expression patterns in individual tissue samples
RESULTS
Pregnancy outcome
At early gestation, the rate of fetal non-viability was 22.2% (6/27) in infected queens and 4.7% (2/43) in control animals. Two infected queens did not conceive as a result of pyometra or ovarian cysts; another developed right horn pyometra but developed three fetuses in the left horn. Two control cats did not become pregnant for unknown reasons. At late gestation, fetal non-viability was 60% (15/25 fetuses) in FIV-infected queens and 3.2% (1/31) in control queens. Two infected queens did not conceive after repeated attempts at breeding (Table 2) [22, 23]. Fetal non-viability at both stages of pregnancy included resorptions and developmentally-arrested fetuses.
Table 2.
Pregnancy outcome of the study groups
| Early Pregnancy | |||||
|---|---|---|---|---|---|
| Queen Number | FIV Status | Viable Kittens | Arrested Fetuses | Fetal Resorptions | Samples Tested* (Viable/Non-Viable) |
| 6108 | − | 4 | 0 | 0 | 2/0 |
| 7284 | − | 4 | 0 | 0 | 2/0 |
| 8059 | − | 6 | 0 | 0 | 2/0 |
| 2779 | − | 7 | 1 | 0 | 2/1 |
| 3550 | − | 4 | 0 | 0 | 2/0 |
| 9276 | − | 5 | 0 | 1 | 2/1 |
| 8291 | − | 4 | 0 | 0 | 2/0 |
| 0373 | − | 7 | 0 | 0 | 2/0 |
| 6062 | + | 3 | 0 | 0 | 2/0 |
| 5111 | + | 2 | 1 | 0 | 2/1 |
| 0866 | + | 5 | 1 | 0 | 1/1 |
| 1126 | + | 4 | 0 | 0 | 2/0 |
| 0326 | + | 1 | 0 | 0 | 1/0 |
| 8035 | + | 1 | 1 | 2 | 1/3 |
| 1893 | + | 5 | 1 | 0 | 2/1 |
| Late Pregnancy | |||||
| Queen Number | FIV Status | Viable Kittens | Arrested Fetuses | Fetal Resorptions | Samples Tested* (Viable/Non-Viable) |
| 9522 | − | 3 | 0 | 0 | 2/0 |
| 9581 | − | 2 | 1 | 0 | 2/0 |
| 9746 | − | 6 | 0 | 0 | 2/0 |
| 9801 | − | 6 | 0 | 0 | 2/0 |
| 13634 | − | 3 | 0 | 0 | 1/0 |
| 13668 | − | 2 | 0 | 0 | 2/0 |
| 13671 | − | 4 | 0 | 0 | 2/0 |
| 9674 | + | 1 | 0 | 0 | 1/0 |
| 9730 | + | 2 | 1 | 2 | 2/0 |
| 9745 | + | 2 | 0 | 4 | 2/4 |
| 9806 | + | 3 | 0 | 0 | 1/0 |
| 9809 | + | 1 | 0 | 0 | 1/0 |
| 9810 | + | 1 | 0 | 0 | 1/0 |
| 9813 | + | 1 | 0 | 2 | 1/0 |
| 13226 | + | 0 | 5 | 0 | 1/5 |
RNA was tested using real time RT-PCR
Viable fetuses = live, normal offspring at delivery.
Arrested fetuses = non-viable, abnormally-small offspring at delivery
Fetal resorptions = resorbing tissues located within abnormally-small gestational sacs
Virus detection in early and late term placental and fetal samples
Real time RT-PCR was used to confirm the presence of viral RNA in placentas and fetuses collected from FIV-infected queens at early (Figure 1a) and late (Figure 1b) pregnancy. Limits of detection were reported [23]. At early gestation, all placentas (17/17; 100%) and 12/14 fetuses (85.7%) tested were positive for viral RNA. No accompanying fetal tissue could be collected from placentas 5111 C, 8035 D, and 1893 R because fetuses were resorbed (Figure 1a). Fetus 8035 C (a non-viable fetus) had the second highest level of viral RNA, based on Ct value, while no viral RNA was detected in littermates 8035 A (a viable fetus) and B (a non-viable fetus), showing variability even within a single litter. The detection of vertical transmission of FIV at this early stage of gestation was a novel finding.
Figure 1.
TaqMan real time RT-PCR analysis of FIV gag gene expression in placentas and corresponding fetuses from early (week 3–4) pregnancy and placentas from late (week 8) pregnancy in FIV-infected cats. (a) Viral mRNA was amplified from all placental samples (14 of 14 samples). FIV gag was detected in 12 of 14 fetal samples. The asterisks indicate that no fetal tissue was collected from placenta 5111 C, 8035 D, and 1893 R due to resorption. (b) Viral RNA was detected in 10 of 17 placental samples from the late term FIV-infected queens. Negative placental samples 6108 A, 9276 A, 9522 A, and 9746 A were obtained from uninoculated, control queens. Bars (adjusted mean Ct) represent mean Ct values subtracted from a negative endpoint (50).
Viral RNA expression was detected in most of the placental samples (10/17) obtained from the late term FIV-infected queens (Figure 1b). Viral RNA was not detected in one sample from queens 9746 and 9730 and four samples from queen 13226. Although we were unable to detect viral RNA from all samples, provirus was detected in 21/22 (95.5%) kittens and 14/15 (93.3%) placentas tested using standard PCR targeting a 293 bp region of FIV gag gene followed by Southern blotting [22].
Expression of Treg markers in feline endometrium at early and late gestation
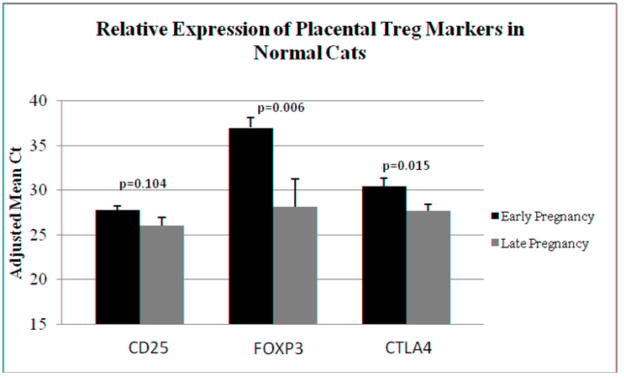
Tregs can be identified by the expression of CD25, FOXP3, and CTLA4. We analyzed the expression of these markers in early and late term control endometrial tissues to look for evidence of normal Treg dynamics as gestation progresses. Evaluating tissues from viable pregnancies only, the higher level of expression of two of three markers occurred at early pregnancy. While the slightly lower level of CD25 expression at late pregnancy did not reach the level of significance (p=0.104), FOXP3 (p=0.006) and CTLA4 (p=0.015) expression was significantly lower at late pregnancy (Figure 2).
Figure 2.
TaqMan real time RT-PCR analysis of endometrial expression of Treg markers CD25, FOXP3, and CTLA4 in early gestation control (n=18) versus late gestation control (n=13) samples. Bars (adjusted mean Ct) represent mean Ct values subtracted from a negative endpoint (50), and error bars represent standard errors of the mean. P values < 0.05 were considered significant.
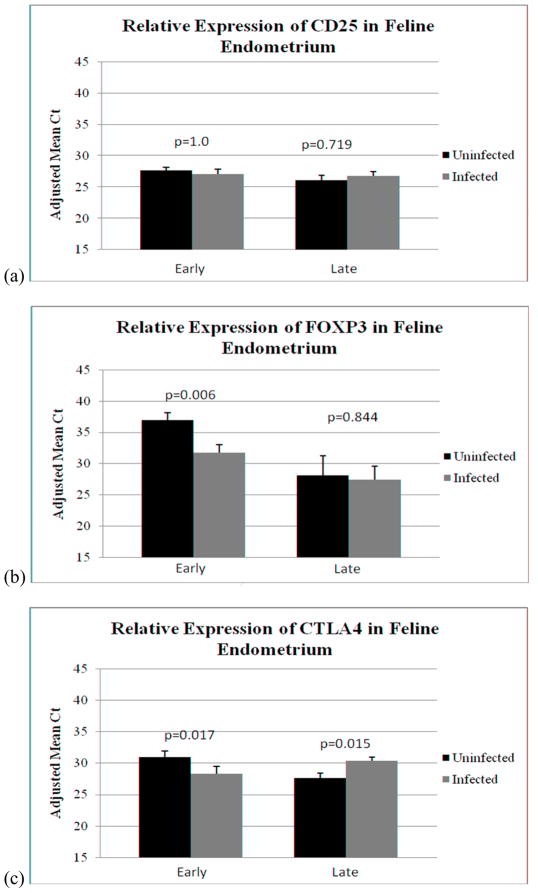
The effect of FIV infection on Treg marker expression at early and late gestation was determined (Figure 3). In this comparison, all samples (including those from viable and non-viable offspring), were included. There was no difference in CD25 expression between control and infected tissues at either early (p=1.0) or late (p=0.719) gestation (Figure 3a). At early gestation, FOXP3 expression was higher in control than infected tissues (p=0.006), but FOXP3 did not differ between control and infected cats at late pregnancy (p=0.844) (Figure 3b). CTLA4 expression also was higher in control than infected tissues at early gestation (p=0.017). Interestingly, expression of this marker was significantly elevated in infected placentas at late pregnancy (p=0.015) (Figure 3c).
Figure 3.
TaqMan real time RT-PCR analysis of expression of Treg markers CD25, FOXP3, and CTLA4 in control and infected reproductive tissues at early and late pregnancy. Samples were evaluated as follows: infected (n=17) versus control (n=18) at early pregnancy; and infected (n=18) versus control (n=13) at late pregnancy. Bars (adjusted mean Ct) represent mean Ct values subtracted from a negative endpoint (50), bracketed by standard errors of the mean. P values < 0.05 were considered significant. (a) CD25 (b) FOXP3 (c) CTLA4.
Co-expression of all three markers was detected in all individual samples evaluated except two; we failed to amplify FOXP3 in two FIV-positive samples obtained from late pregnancy. To determine whether there was a correlation in expression of Treg markers, we evaluated levels of expression of FOXP3 and CTLA4 in individual samples from both early and late pregnancy. CD25 was not included in this analysis because levels of this marker were essentially stable. The mean Ct value for each marker was calculated from the control samples and subtracted from individual Ct values for that marker. Increased or decreased levels of expression compared to mean Ct values for the control samples were determined (Table 3). There was no correlation in expression of the two markers in control tissues at early pregnancy. Both markers were decreased, increased, or showed no correlation in 33%, 22%, and 44% of samples, respectively, while at late gestation in control samples marker expression was 15%, 22%, and 62% of samples, respectively. Treg marker co-expression was significant in early pregnancy tissues from FIV-infected cats (p=0.005); both markers were decreased in 65% of samples, increased in 0% of samples, or showed no correlation in 35% of samples. At late gestation in infected cats differences in expression patterns did not reach significance (p=0.068); corresponding values were 6% decreased, 44% increased, and 50% no correlation. These data indicate that co-expression of FOXP3 and CTLA4 was impacted by FIV infection at early gestation, with infection resulting in decreased expression of Treg activation markers at this stage of pregnancy.
Table 3.
Correlation of FOXP3 and CTLA4 co-expression in individual tissues
| Early Pregnancy | |||||
|---|---|---|---|---|---|
| Tissue No. (FIV neg) | FOXP3 | CTLA4 | Tissue No. (FIV pos) | FOXP3 | CTLA4 |
| 6108 A | ↓ | ↓ | 6062 A | ↓ | ↓ |
| 6108 B | ↓ | ↑ | 6062 B | ↑ | ↓ |
| 7284 A | ↓ | ↑ | 5111 A | ↓ | ↓ |
| 7284 B | ↓ | ↓ | 5111 B | ↓ | ↓ |
| 8059 A | ↓ | ↓ | 5111 C (NV) | ↓ | ↓ |
| 8059 B | ↑ | ↓ | 0866 A | ↓ | ↓ |
| 2779 A | ↑ | ↑ | 0866 B | ↓ | ↓ |
| 2779 B | ↑ | ↑ | 1126 A | ↓ | ↓ |
| 2779 E (NV) | ↓ | ↓ | 1126 B | ↓ | ↑ |
| 3550 A | ↑ | ↓ | 0326 A | ↓ | ↓ |
| 3550 C | ↑ | ↓ | 8035 A | ↑ | ↓ |
| 9276 A | ↓ | ↑ | 8035 B (NV) | ↓ | ↑ |
| 9276 B | ↓ | ↓ | 8035 C (NV) | ↓ | ↓ |
| 9276 F (NV) | ↑ | ↑ | 8035 D (NV) | ↓ | ↓ |
| 8291 A | ↑ | ↑ | 1893 A | ↓ | ↓ |
| 8291 B | ↓ | ↑ | 1893 B | ↓ | ↑ |
| 0373 A | ↓ | ↓ | 1893 C (NV) | ↓ | ↑ |
| 0373 B | ↓ | ↑ | |||
| Both markers decreased: 6/18 (33%) | Both markers decreased: 11/17 (65%) | ||||
| Both markers increased: 4/18 (22%) | Both markers increased: 0/17 (0%) | ||||
| No correlation: 8/18 (44%) | No correlation: 6/17 (35%) | ||||
| Chi2= 1.333; P= 0.513 | Chi2= 10.727; P= 0.005 | ||||
| Late Pregnancy | |||||
| Tissue No. (FIV neg) | FOXP3 | CTLA4 | Tissue No. (FIV pos) | FOXP3 | CTLA4 |
| 9522 A | ↓ | ↓ | 9674 A | ↓ | ↑ |
| 9522 B | ↑ | ↑ | 9730 B | ↑ | ↑ |
| 9581 A | ↑ | ↓ | 9745 B | ↑ | ↑ |
| 9581 B | ↓ | ↓ | 9745 A (NV) | ↑ | ↑ |
| 9746 A | ↑ | ↓ | 9745 C (NV) | ↑ | ↑ |
| 9746 B | ↑ | ↓ | 9745 E (NV) | ↓ | ↑ |
| 9801 A | ↑ | ↑ | 9745 F (NV) | ↓ | ↑ |
| 9801 B | ↓ | ↑ | 9806 C | ↓ | ↑ |
| 13634 A | ↓ | ↑ | 9809 A | ↑ | ↑ |
| 13668 A | ↓ | ↑ | 9810 A | ↓ | ↑ |
| 13668 B | ↑ | ↑ | 9813 A | ↑ | ↓ |
| 13671 A | ↓ | ↑ | 13226 A (NV) | ↓ | ↑ |
| 13671 B | ↓ | ↑ | 13226 B (NV) | ↑ | ↑ |
| 13226 C (NV) | ↓ | ↓ | |||
| 13226 D (NV) | ↓ | ↑ | |||
| 13226 E (NV) | ↑ | ↑ | |||
| Both markers decreased: 2/13 (15%) | Both markers decreased: 1/16 (6%) | ||||
| Both markers increased: 3/13 (22%) | Both markers increased: 7/16 (44%) | ||||
| No correlation: 8/13 (62%) | No correlation: 8/16 (50%) | ||||
| Chi2=4.749; P =0.093 | Chi2=5.366; P= 0.068 | ||||
Tissue numbers = queen number followed by alphabetical designation of fetus (NV) = non-viable fetus
Increased (↑) or decreased (↓) expression is relative to mean Ct values for tissue expression of each marker obtained from control (FIV neg) cats.
Treg marker expression in viable versus non-viable pregnancies was compared in early and late term infected tissue (Figure 4). No significant difference in the expression of CD25 was found between viable and non-viable pregnancies in infected cats in either early (p=0.34) or late term tissues (p=0.34) (Figure 4a). Likewise, the expression of FOXP3 did not differ significantly between viable and non-viable pregnancies in infected cats at both early (p=0.39) and late pregnancy (p=0.74) (Figure 4b). Expression of CTLA4 was very similar between viable and non-viable pregnancies at early (p=0.84) and late (p=0.69) gestation (Figure 4c).
Figure 4.
Relative expression of CD25, FOXP3, and CTLA4 in early and late term infected reproductive tissues producing viable versus non-viable fetuses. The samples were evaluated as follows: infected cats producing viable offspring at early pregnancy (n=11) versus infected cats producing non-viable offspring at early pregnancy (n=5); and infected cats producing viable offspring at late pregnancy (n=9) versus infected cats producing non-viable offspring at late pregnancy (n=9). Bars (adjusted mean Ct) represent mean Ct values subtracted from a negative endpoint (50), bracketed by standard errors of the mean. P values < 0.05 were considered significant. (a) CD25, (b) FOXP3, (c) CTLA4.
Identification of FOXP3-expressing cells by Immunofluorescence
FOXP3-expressing cells were detected at the maternal-fetal interface following labeling with the primary polyclonal antibody to FOXP3 (Figure 5). Two negative control reactions (no primary antibody or universal negative control antibody) did not fluoresce, validating the specificity of the primary antibody for FOXP3-expressing cells. Dual labeling with CD4-specific antibody could not be done due to non-specific reactivity of numerous anti-CD4 monoclonal and polyclonal antibodies to feline reproductive tissue despite many modifications to antibody concentration and blocking procedures.
Figure 5.
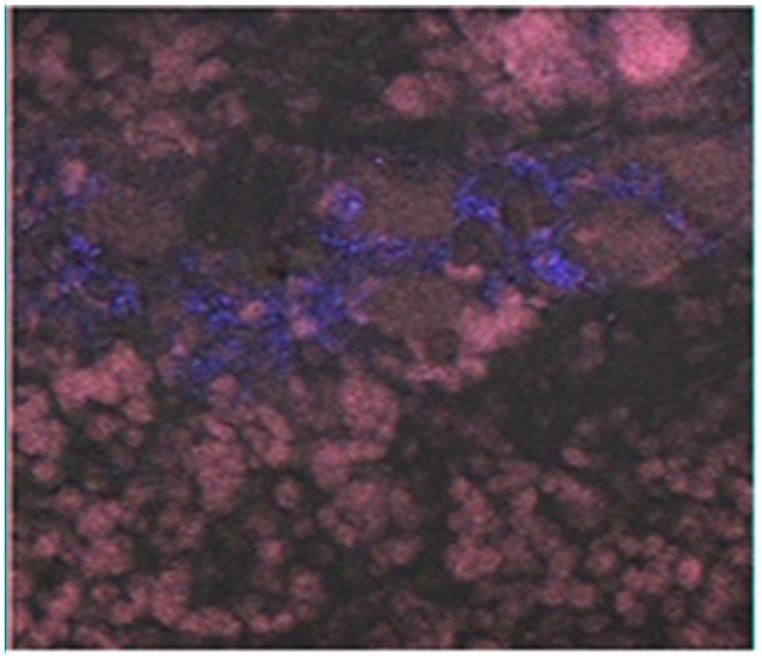
Immunofluorescence labeling of FOXP3 at the maternal-fetal interface of tissue from a representative control queen. FOXP3 (green) was detected using polyclonal rabbit anti-FOXP3 antiserum followed by goat anti-rabbit IgG (H+L) fluorescein conjugate. Cells were counterstained with DAPI (blue). Negative controls included universal negative control antibody or no primary antibody, resulting in no fluorescent labeling (data not shown). Cells were viewed by confocal laser scanning microscopy using a 40 X oil immersion objective.
DISCUSSION
MTCT occurred efficiently in chronically-infected queens by week 3–4 gestation, resulting in frequent fetal non-viability [23]. The detection of viral RNA in non-viable fetus 8035 C but not its littermates 8035A (viable) and 8035B (non-viable) suggests that fetal death may not be a direct result of fetal infection. Such findings allow a role for FIV-induced placental immunopathology in pregnancy failure. Efficient MTCT occurred in chronically-infected queens that delivered at late gestation [22]. Fetal non-viability in infected queens, including resorbed and arrested fetuses was 60%, compared to 3.2% in control queens. Enhanced reproductive failure in FIV-infected cats was consistent with reports by others using the same or different FIV isolate [21].
Feline Tregs are routinely characterized by the co-expression of CD4, CD25, FOXP3, and CTLA4. However, CD25 expression is not restricted to Tregs. Activated T cells, such as CD8+ T cells (Woo et al. 2001) and B cells express CD25 after antigenic stimulation (Lowenthal et al. 1985). FOXP3 is a transcriptional activator that plays a major role in the regulation of Treg function and differentiation (Zheng and Rudensky 2007). It is expressed by naturally occurring Treg cells and can be upregulated upon activation and differentiation of CD4+CD25− T cells to adaptive Treg cells. In the cat, FOXP3 was also detected in small amounts in CD4+CD25−, CD8+, and CD21+ lymphocytes (Lankford et al. 2008), but it was expressed much more abundantly in CD4+CD25+ T cells. Sustained expression of FOXP3 is necessary to program cells for Treg function (Gavin et al. 2007). CTLA4 is an activation marker expressed by adaptive Treg cells and naturally occurring Tregs. CTLA4 outcompetes CD28 for B7 binding (Tompkins and Tompkins 2008) and acts as a negative regulator of T cell activation by preventing cell proliferation, cell cycle progression, and IL-2 production (Krummel and Allison 1996).
We first examined Treg marker expression in reproductive tissues of normal, control cats producing viable offspring at early and late gestation to determine whether Treg populations change over the course of pregnancy. The expression of CD25 at early gestation did not significantly differ from that of late gestation. However, significantly higher levels of expression of FOXP3 and CTLA4 were found at early pregnancy. The data suggest that cell populations expressing these Treg markers may be normally diminished or less activated as pregnancy progresses. A decrease in number or function of Tregs may allow increased inflammation late in gestation, which is a normal precursor to parturition [26]. These data are supported by Shima et al [27] who reported that depletion of CD4+CD25+ T cells at early pregnancy by treatment with anti-CD25 monoclonal antibody caused implantation failure or increased resorption rates in allogeneic, but not syngeneic mice, while depletion of these cells at later stages had no adverse effect on pregnancy.
FIV infection altered the expression of Treg markers. At early gestation, there was no difference in the expression of CD25 between control and infected tissues. However, FIV infection resulted in significantly decreased expression of FOXP3 and CTLA4. Our comparison of FOXP3 and CTLA4 co-expression (Table 3) in individual samples clearly reveals that viral infection was accompanied by diminished expression of both activation markers at early, but not late pregnancy. While Treg dynamics have not yet been examined, our data suggest that at early pregnancy Tregs may be depleted or rendered less functional by viral infection, resulting in impaired immunosuppressive potential which may predispose the early fetal loss that we report [23]. However, it is possible that changes in Treg function were a postmortem effect, rather than the cause of fetal demise. The presence of viral RNA in early tissues reinforces a potential role for viral infection in diminished Treg function, yet identification of the target cell population has not been done. At late gestation, expression of CD25 and FOXP3 did not differ between control and infected placentas. A possible explanation for this finding may be that natural diminution of Treg activation at late pregnancy (Figure 2) may render these cells less vulnerable to the impact of viral infection. In contrast, FIV infection caused more CTLA4 to be expressed at this time period.
Discordant expression of Treg markers is not unusual. The Treg phenotype is diverse, with subsets expressing various cell surface markers and cytokines. CD25 is not reliably expressed on all regulatory T cells. CD25 can be down-regulated following activation in vivo, and potently suppressive CD4+CD25− regulatory cells were demonstrated in diabetes and transplant models [28, 29]. Boasso, et al [30] found increased levels of FOXP3 and CTLA4 in the CD25− T cell population in spleens and lymph nodes of viremic, SIV-infected macaques. Although constitutively expressed on Tregs, CTLA4 is also expressed on other T cell populations 24–48 hours after activation [31, 32]. Perhaps expression of this marker late in infection in FIV-infected cats was attributable to activation of non-regulatory T cells, coinciding with increased inflammation.
Finally, Treg marker expression was not definitively correlated with pregnancy outcome, as significant differences in levels of Treg marker expression were not apparent between placentas from viable and non-viable pregnancies (p>0.05). This result may have been attributable to the small number of samples from non-viable pregnancies available to perform this analysis.
An obvious limitation of this study is that Treg populations were not directly enumerated nor was RNA extracted from these precise populations for gene expression analyses. Rather, RNA was isolated from random specimens containing unknown quantities of Tregs. Because even subtle changes in gene expression may be significant in terms of function, dilution of RNA from irrelevant cell populations may have masked important information. In future studies, viral RNA will be isolated from laser capture microdissected Tregs for gene expression analyses.
An additional limitation of this report is that we evaluated target gene mRNA only, which may be transiently expressed. Given that gene expression may be regulated at the translational level, as well as the transcriptional level, we may have obtained an incomplete picture of expression of one or more genes of interest. Thus, in future studies we plan to evaluate Treg marker proteins in endometrium using immunofluorescence or immunohistochemistry. We demonstrated the ability to detect FOXP3-expressing cells at the maternal-fetal interface using immunofluorescence microscopy in the present report.
The value of the present study is that it provides the first evidence for feline Tregs in reproductive tissue. It also demonstrates that FIV infection alters expression of Treg markers in these tissues, possibly leading to compromised pregnancy or an inflammatory microenvironment that predisposes vertical infection. The data provide an early glimpse into a possible mechanism for lentivirus-induced reproductive failure, clearly revealing the need for further study of these processes using the FIV-infected cat model.
Acknowledgments
We thank Dr. Edward A. Hoover, Colorado State University, for providing the infectious plasma pool containing FIV-B-2542. We are grateful to the veterinary staff at the College of Veterinary Medicine, Mississippi State University, for assistance with animal care and surgery. We thank the Life Science and Biotechnology Institute for the use of the facilities and equipment. This project was supported by the National Institute of Health, project # 2R15AI048410-02A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197(1):111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176(10):5741–8. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 3.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero 10.1126/science.1164511. Science. 2008;322(5907):1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao KH, Wu MY, Yang JH, Chen SU, Yang YS, Ho HN. Expression of the interleukin-2 receptor alpha (CD25) is selectively decreased on decidual CD4+ and CD8+ T lymphocytes in normal pregnancies. Mol Hum Reprod. 2002;8(7):667–73. doi: 10.1093/molehr/8.7.667. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136(2):373–8. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XY, Zhou YH, Wang MY, Jin LP, Yuan MM, Li DJ. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol Reprod. 2005;72(2):338–45. doi: 10.1095/biolreprod.104.034108. [DOI] [PubMed] [Google Scholar]
- 10.Lankford S, Petty C, LaVoy A, Reckling S, Tompkins W, Dean GA. Cloning of feline FOXP3 and detection of expression in CD4+CD25+ regulatory T cells. Vet Immunol Immunopathol. 2008;122(1–2):159–66. doi: 10.1016/j.vetimm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vahlenkamp TW, Tompkins MB, Tompkins WA. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J Immunol. 2004;172(8):4752–61. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 12.Joshi A, Garg H, Tompkins MB, Tompkins WA. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J Virol. 2005;79(8):4965–76. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. Key facts by region - 2008 Report on the global AIDS epidemic. 2008. [Google Scholar]
- 14.Rollins NC, Coovadia HM, Bland RM, Coutsoudis A, Bennish ML, Patel D, Newell ML. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007;44(3):321–8. doi: 10.1097/QAI.0b013e31802ea4b0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson V, Carneiro M, Bulterys M, Douglas G, Polliotti B, Slikker W., Jr HIV in pregnancy. Perinatal infections: HIV and co-infections in the placenta and therapeutic interventions- a workshop report. Placenta. 2001;22(Supplement A) doi: 10.1053/plac.2001.0641. [DOI] [PubMed] [Google Scholar]; Trophoblast Research. 15:S34–S7. [Google Scholar]
- 16.Goldstein P, Smit R, Stevens M, Sever JL. Association between HIV in pregnancy and antiretroviral therapy including protease inhibitors and low birth weight infants. Infect Dis Obstet Gynecol. 2000;8:94–8. doi: 10.1002/(SICI)1098-0997(2000)8:2<94::AID-IDOG6>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Ubaldo C, Pezzotti P, Rezza G, Branca M, Ippolito G. Association between HIV-1 infection and miscarriage: a retrospective study. DIANAIDS Collaborative Study Group. Diagnosi Iniziale Anomalie Neoplastiche AIDS. AIDS. 1998;12(9):1087–93. doi: 10.1097/00002030-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynaecol Obstet. 1995;49(2):137–43. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- 19.Massad LS, Springer G, Jacobson L, Watts H, Anastos K, Korn A, Cejtin H, Stek A, Young M, Schmidt J, Minkoff H. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS. 2004;18(2):281–6. doi: 10.1097/00002030-200401230-00018. [DOI] [PubMed] [Google Scholar]
- 20.O’Neil LL, Burkhard MJ, Hoover EA. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J Virol. 1996;70(5):2894–901. doi: 10.1128/jvi.70.5.2894-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers AB, Hoover EA. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J Infect Dis. 1998;178(4):960–7. doi: 10.1086/515692. [DOI] [PubMed] [Google Scholar]
- 22.Weaver CC, Burgess SC, Nelson PD, Wilkinson M, Ryan PL, Nail CA, Kelly-Quagliana KA, May ML, Reeves RK, Boyle CR, Coats KS. Placental immunopathology and pregnancy failure in the FIV-infected cat. Placenta. 2005;26(2–3):138–47. doi: 10.1016/j.placenta.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Boudreaux CE, Lockett NN, Chemerys DN, Clay BT, Scott VL, Willeford B, Brown T, Coats KS. Maternal Hematological and Virological Characteristics during Early Feline Immunodeficiency Virus (FIV) Infection of Cats as Predictors of Fetal Infection and Reproductive Outcome at Early Gestation. Vet Immunol Immunopathol. 2009;131(3–4):290–7. doi: 10.1016/j.vetimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott VL, Burgess SC, Shack LA, Lockett NN, Coats KS. Expression of CD134 and CXCR4 mRNA in term placentas from FIV-infected and control cats. Vet Immunol Immunopathol. 2008;123(1–2):90–6. doi: 10.1016/j.vetimm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146(12):3217–26. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 26.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and Pregnancy Reproductive Sciences. 2009;16(2):206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 27.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. Journal of Reproductive Immunology. doi: 10.1016/j.jri.2010.02.006. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202(10):1387–97. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 30.Boasso A, Vaccari M, Hryniewicz A, Fuchs D, Nacsa J, Cecchinato V, Andersson J, Franchini G, Shearer GM, Chougnet C. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. J Virol. 2007;81(21):11593–603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tompkins MB, Tompkins WA. Lentivirus-induced immune dysregulation. Vet Immunol Immunopathol. 2008;123(1–2):45–55. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]