Abstract
Glycoprotein (GP) V is a major substrate cleaved by the protease thrombin during thrombin-induced platelet activation. Previous analysis of platelets from GP V-null mice suggested a role for GP V as a negative modulator of platelet activation by thrombin. We now report the mechanism by which thrombin activates GP V −/− platelets. We show that proteolytically inactive forms of thrombin induce robust stimulatory responses in GP V null mouse platelets, via the platelet GP Ib–IX–V complex. Because proteolytically inactive thrombin can activate wild-type mouse and human platelets after treatment with thrombin to cleave GP V, this mechanism is involved in thrombin-induced platelet aggregation. Platelet activation through GP Ib–IX depends on ADP secretion, and specific inhibitors demonstrate that the recently cloned P2Y12 ADP receptor (Gi-coupled ADP receptor) is involved in this pathway, and that the P2Y1 receptor (Gq-coupled ADP receptor) may play a less significant role. Thrombosis was generated in GP V null mice only in response to catalytically inactive thrombin, whereas thrombosis occurred in both genotypes (wild type and GP V null) in response to active thrombin. These data support a thrombin receptor function for the platelet membrane GP Ib–IX–V complex, and describe a novel thrombin signaling mechanism involving an initiating proteolytic event followed by stimulation of the GP Ib–IX via thrombin acting as a ligand, resulting in platelet activation.
Glycoprotein (GP) Ib–IX–V is a major complex on the platelet surface, second only to αΙΙbβ3. This complex consists of several subunits: GP Ibα, GP Ibβ, GP IX, and GP V in the ratio of 2:2:2:1. Absence of GP Ib–IX–V results in a severe bleeding disorder known as Bernard Soulier syndrome characterized by giant platelets and impaired von Willebrand factor (vWf) binding (1). GP Ibα is a receptor for vWf, and the GP Ib–IX–V complex is critical for platelet adhesion under arterial shear conditions (2). A role for GP Ib–IX–V in platelet activation has been proposed on the basis of observations that the signaling molecule 14–3-3ζ (3, 4) is associated with the complex, and that phosphorylation of pp72syk occurs upon vWf binding to GP Ibα (5). In fact, Zaffran et al. (6) recently showed that in heterologous Chinese hamster ovary (CHO) cells expressing both αΙΙbβ3 and GP Ib–IX, inside-out activation of αΙΙbβ3 could occur upon vWf adhesion.
The GP Ibα subunit also has a thrombin binding site on the extracellular domain that overlaps the vWf binding domain (7). Additionally, the complex has a platelet-specific thrombin substrate, GP V, that is cleaved very early during thrombin-induced platelet aggregation (8). Platelets from Bernard Soulier syndrome patients show an impaired response to thrombin (9), and antibodies that block thrombin binding to GP Ibα also partially inhibit platelet responses to thrombin (9). More recently, thrombin binding to GP Ibα has been shown to enhance platelet procoagulant activity (10). However, the physiological significance of this interaction has been unresolved because of the existence of the protease-activated receptor (PAR) family of thrombin receptors (11, 12).
To determine the contribution of GP Ib–IX–V in platelet activation by thrombin we generated a GP V −/− mouse by targeted deletion of the GP V locus (13), resulting in the expression of a mutant GP Ib–IX–V complex. Surprisingly, evaluation of platelets from GP V null mice indicated that GP V null platelets showed increased responsiveness to thrombin, and that the mice had a shorter bleeding time. Thus, it seemed that GP V was a negative modulator of platelet function. Previously, it had been shown that proteolytically inactive thrombin can potentiate the activity of suboptimal concentrations of thrombin in platelets (14). To explore the possibility that thrombin interaction with GP Ib–IX–V played a role in platelet activation, we examined the effect of proteolytically inactive thrombin on the aggregation of GP V −/− platelets. In this report, we show that proteolytically inactive thrombin can induce platelet aggregation in GP V null platelets in vitro and in vivo, and we describe a role for the GP Ib–IX–V complex as a thrombin receptor contributing to thrombin-induced platelet activation.
Methods
Generation of CHO-Expressed Thrombins.
Plasma-derived thrombin and diisopropylphospho-(DIP)-thrombin were purchased from Haematologic Technologies (Burlington, VT). Activity of plasma DIP-thrombin was between 0% and 0.03% by chromogenic assay with tosyl-Gly-Pro-Arg-4-nitroanilide (Chromozyme TH; Roche Molecular Biochemicals) as the substrate. DIP- thrombin was treated with repeated doses of diisopropyl fluorophosphate (DFP), until no chromogenic activity could be detected. Fluorimetric measurements of hirudin binding to either plasma-derived DIP-thrombin or proteolytically active thrombin were similar. CHO-expressed prothrombins [wild type (wt), S205A, R89/R93/E94, and R98A] were expressed and purified as described (15). Activation to thrombin was carried out by using the prothrombinase complex [for wt and S205A (ref. 16)], or by using Echis carinatus venom as described (ref. 15; for R89/R93/E94 and R98A). CHO-expressed wt thrombin was 70% less active compared with plasma-derived thrombin in fibrinogen clotting assays with 10 μM purified fibrinogen (Enzyme Research Laboratories, South Bend, IN). Higher concentrations of the CHO-expressed proteins were required to elicit a response in the GP V null platelets (1–2 μM) than in the plasma-derived thrombin (100–400 nM). DFP-treatment of CHO-derived proteins was carried out as described (17). Loss of proteolytic activity was determined by chromogenic assay with Chromozyme TH and S2238, a p-nitroanilide thrombin substrate (DiaPharma, West Chester, OH).
Affinity Purification of Ab 3584.
Rabbit polyclonal Ab 3584 was kindly provided by B. Steiner and S. Meyer (Hoffman-LaRoche, Basel). We first purified the human extracellular domain of GP Ibα (Glycocalicin) as described (18). Ab 3584 was then affinity purified on a glycocalicin column. The affinity-purified Ab 3584 IgG recognized human glycocalicin as assessed by Western blotting and mouse platelets by FACS analysis.
Aggregation.
Washed platelets (WP) were isolated as described previously (13). Platelets were resuspended in Tyrode–Hepes buffer and rested for 15 min before use in aggregation. Wt platelets were incubated with repeated doses (four) of 10 pM thrombin in calcium-free Tyrode-Hepes buffer with gentle mixing. Platelets were rested for at least 2 min before the addition of DIP-thrombin. Aggregation was measured as the change in transmittance obtained after the addition of agonist by using a Chronolog lumi-aggregometer. For experiments using inhibitors, aggregation was initiated after a brief incubation with inhibitors. The αΙΙbβ3 inhibitors used in this study have been described (19), and were shown to inhibit mouse αΙΙbβ3 (D. A. Law, personal communication). Whereas full aggregation varied in different platelet preparations (50–70% transmittance), in all cases DIP-thrombin-induced aggregation was equivalent to the full aggregation obtained with 10 nM thrombin.
Human platelets were isolated from freshly drawn blood anticoagulated in acid/citrate/dextrose (ACD)/prostacyclin (PGI2) containing apyrase (50 units/ml, 1 μl/10 ml of blood), and washed as described (20). Platelets were resuspended in Tyrode-Hepes buffer containing 1 mM each CaCl2 and MgCl2 at 2–2.5 × 108/ml. GP V on WP was cleaved by pretreatment with thrombin (10 pM) added four times sequentially with gentle mixing. WP were rested for 2 min and then incubated with PAR 1 antagonist [40 μM (ref. 21)] or DMSO control, and aggregation was initiated by the addition of DIP-thrombin (0.4 μM). This concentration of DIP-thrombin resulted in full aggregation.
Detection of Cleaved GP V (GP V f1) in Supernatants of wt Platelets Treated with Thrombin.
wt WP were isolated from 20 mice, and incubated with or without 50–100 pM thrombin for 30 min without stirring at 37°C. Platelets were centrifuged at 100,000 × g to remove microparticles, and the supernatant was lyophilized, reconstituted in 1·43M 2-mercaptoethanol/0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS (reducing RIPA buffer) and boiled for 5 min. Reduced samples were buffer-exchanged by dialysis into nonreducing RIPA buffer and immunoprecipitations were carried out with rabbit Ab 808 [previously shown to recognize an epitope not available in native GP V (13)], or control rabbit IgG. Samples were electrophoresed by reducing SDS/PAGE, and Western analysis was done with Ab 808.
Thrombosis Model in Mice.
This model is a modification of that described by Leon et al. (22). Briefly, mice were anesthetized and the jugular vein was exposed surgically. Retro-orbital blood (125 μl) was taken in heparin-treated tubes and was transferred into tubes containing 0.9% saline and 50 mM EDTA (125 μl) to determine the baseline platelet counts. Then, various agonists were injected into the jugular vein in a volume of 100 μl, such that the concentration noted is the final circulating concentration. After 55 s, another retro-orbital blood sample was obtained. The platelet counts in PRP obtained in duplicate from the two bleedings were used to determine the loss, if any, in platelet number. Loss in platelet counts in this model represents ongoing thrombosis. Blood volumes were assumed to be 10% of the weight of the animal. Animals were age- and weight-matched in separate experiments, and ranged in weight between 25 and 50 g.
Results
Effect of Proteolytically Inactive Thrombin on GP V-Deficient Platelets.
To evaluate whether proteolytically inactive thrombin potentiates the activity of active thrombin, we conducted aggregation experiments with GP V −/− platelets and DIP-thrombin. Surprisingly, DIP-thrombin induced platelet activation in GP V −/− platelets without the addition of any active thrombin, whereas wt platelets showed no response (Fig. 1A). Aggregation of GP V −/− platelets required approximately 10-fold more DIP-thrombin (100 nM) than untreated thrombin (10 nM), confirming the Kd values determined in recent studies (23). Platelets from wt mice pretreated with suboptimal doses (≈40–50 pM) of active thrombin (Fig. 1B) aggregated in response to inactive thrombin, but were nonresponsive without pretreatment. Western analysis showed that thrombin pretreatment hydrolyzed GP V from the platelet surface, as determined by the release of GP V f1, the thrombin hydrolytic fragment of GP V (Fig. 1B Inset). In other experiments, we also found that GP V null platelets aggregated in response to recombinant thrombin carrying a mutation [S205A (ref. 15)] that inactivates its proteolytic capacity (Fig. 1C). CHO-expressed wt thrombin inactivated by DFP also caused aggregation in GP V null platelets. Aggregation of mutant and wt platelets in response to all forms of thrombin was sensitive to inhibition by an antagonist of the αΙΙbβ3 integrin (19), indicating that the aggregation reactions involved the characteristic agonist-induced pathway (data not shown).
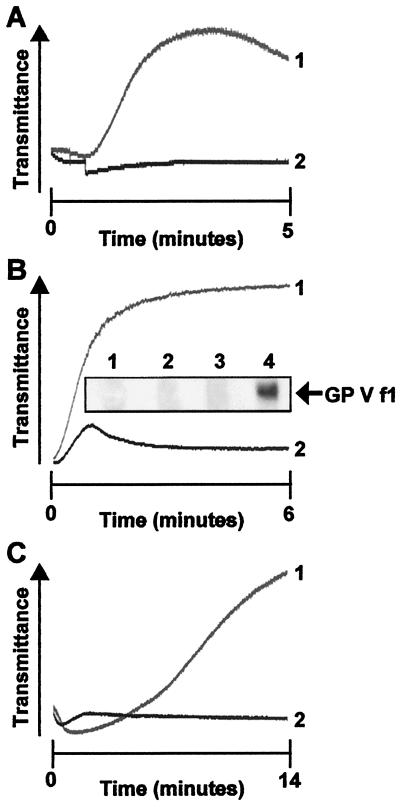
Figure 1.
DIP-thrombin-induced aggregation in washed mouse platelets. (A). Pooled WP from GP V null mice (line 1) or wt mice (line 2) were aggregated with DIP-thrombin (100 nM). The data are the average of duplicate experiments. (B). Pooled WP from wt mice (line 1) were pretreated with thrombin as described, and aggregation was initiated by the addition of DIP-thrombin (100 nM). Line 2 shows the response of wt platelets not pretreated with thrombin to DIP-thrombin with 40 pM thrombin added simultaneously. (Inset) GP V f1 is released from wt platelets treated with 50 pM thrombin. Lanes 1 and 2 are the supernatants of control platelets, and lanes 3 and 4 are the supernatants of 50 pM thrombin-treated platelets. Lanes 1 and 3 are the immunoprecipitates with control IgG, and lanes 2 and 4 are the immunoprecipitates with Ab 808. The arrow shows the position of GP V f1. (C) GPV null platelets were aggregated with S205A-thrombin (1 μM) in the absence (line 1) or presence (line 2) of a αΙΙbβ3 inhibitor. The tracings represent experiments done at least three times with pooled platelets from four mice each.
Role of GP Ib–IX in Platelet Aggregation Induced by Proteolytically Inactive Thrombin.
Ab 3584 recognizes GP Ib–IX on human and mouse platelets (13). Because GP Ibα is a candidate receptor for thrombin, we tested whether the antibody could inhibit platelet aggregation in response to proteolytically inactive thrombin. Ab 3584 (Fig. 2A) and affinity-purified Ab 3584 (which recognizes only the GP Ibα subunit; Fig. 2C; see Methods) effectively blocked aggregation of GP V-deficient platelets caused by DIP-thrombin or S205A-thrombin response (Fig. 2D), but had only a slight effect on aggregation induced by native untreated thrombin (Fig. 2B). Ab 3584 also inhibited DIP-thrombin-mediated aggregation of wt mouse platelets that had been pretreated with suboptimal doses of thrombin (data not shown). Further, Ab LJ-1B10, which inhibits thrombin binding to GP Ibα (24), inhibits the aggregation in human platelets rendered GP V deficient by thrombin pretreatment (data not shown). These data implicate thrombin binding to GP Ibα in the aggregation of platelets induced by proteolytically inactive thrombin.
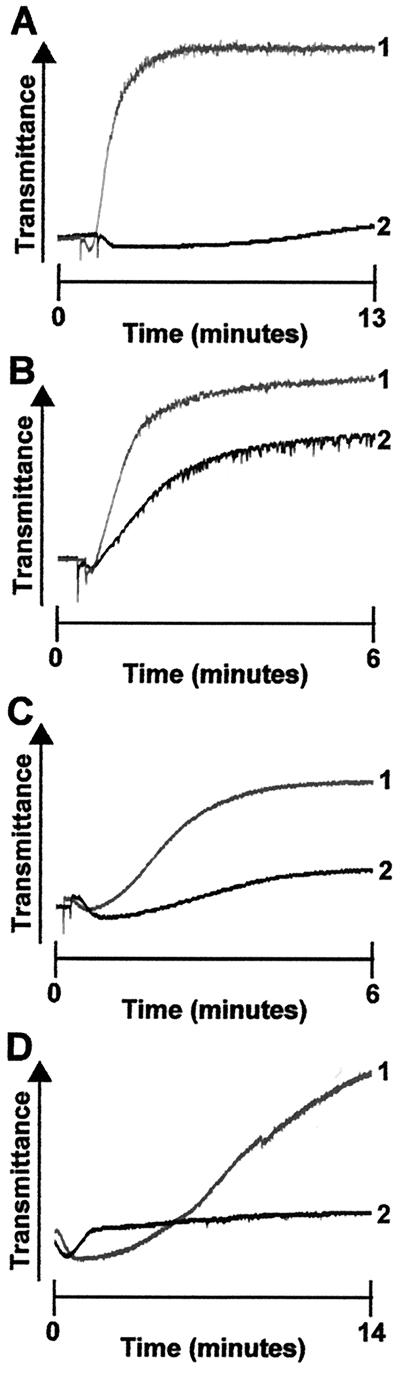
Figure 2.
DIP-thrombin-induced aggregation is inhibited by anti-GPIb antibody. WP from GP V null mice were preincubated with either anti-GPIb–IX Ab 3584 (2 μM, line 2) or control rabbit IgG (2 μM, line 1) for 10 min at room temperature. (A) Aggregation was initiated by the addition of DIP-thrombin (100 nM). (B) Aggregation was initiated by the addition of thrombin (10 nM). (C) Glycocalicin-purified Ab 3584 IgG (1.89 μM) was incubated with GP V null platelets for 10 min before the initiation of aggregation with 500 nM DIP-thrombin (line 2). Control (line 1) shows the aggregation response with 500 nM DIP-thrombin after incubation with control rabbit IgG. (D) GP V −/− WP were preincubated with either control IgG (2 μM, line 1) or Ab 3584 (2 μM, line 2) for 10 min. Aggregation was initiated by the addition of 1 μM S205A-thrombin.
Role of the Heparin Binding Exosite in DIP-Thrombin-Induced Aggregation.
The exosite II of thrombin binds heparin and may also be involved in the interaction with GP Ibα (23). We therefore examined whether aggregation induced by DIP-thrombin could be inhibited by heparin. Within the standard therapeutic dose range [0.3 unit/ml; standard range 0.2–0.7 unit/ml (ref. 25)] heparin significantly inhibited DIP-thrombin-induced platelet aggregation and completely reversed the response of both GP V null (Fig. 3A) and thrombin-pretreated wt platelets (Fig. 3C) within 5 min. In contrast, this concentration of heparin provided only marginal inhibition of aggregation caused by native thrombin applied to either GP V null (Fig. 3B) or wt platelets (Fig. 3D). Thus, the data suggest that binding between exosite II of thrombin and GP Ibα on platelets is essential for platelet activation in response to proteolytically inactive thrombin. Because heparin blocks this interaction at concentrations used therapeutically, this mechanism may also be involved in the antithrombotic activity of heparin.
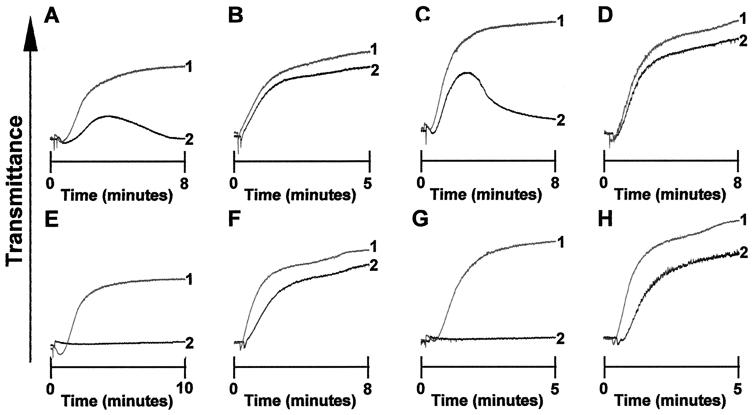
Figure 3.
Effect of various inhibitors on aggregation. Pooled WP were treated with the indicated concentrations of unfractionated heparin (0.3 unit/ml, A–D), or PGI2 (4.45 μM, E–H), and aggregation responses were measured compared with that obtained in the absence of heparin or PGI2. GP V null WP aggregated with 500 nM DIP-thrombin (A, E) or 10 nM thrombin (B, F) and thrombin-pretreated wt platelets aggregated with 500 nM DIP-thrombin (C, G) or wt platelets aggregated with 10 nM thrombin (D, H). Line 1 in each tracing represents the untreated control platelets and line 2 represents the platelets treated with inhibitor. The tracings are typical of results from experiments performed at least three times with pooled platelets from four mice each.
Signaling Pathways Mediated by GP Ib–Thrombin Interaction.
GP Ib–IX can signal in response to the binding of vWf (5, 26) and induce platelet activation, probably through 14–3–3 (27, 28), a signaling molecule constitutively associated with the cytoplasmic tail of GP Ibα that is phosphorylated at Ser-609 (29). Other studies have shown that agents such as PGI2 and prostaglandin E1(PGE1) negatively regulate GP Ib–IX signaling. The mechanism of inhibition by the adenylyl cyclase activators seems to be through the activation of the cAMP-dependent kinase and phosphorylation of the cytoplasmic domain of GP Ibα (30, 31). To determine whether the thrombin signaling function of GP Ib–IX–V is similarly regulated, we evaluated the role of the cAMP-dependent pathway in DIP-thrombin-induced signaling. Fig. 3E shows that PGI2 completely inhibited DIP-thrombin-induced shape change and aggregation of GP V −/− platelets (IC50 ≈ 70 nM). PGE1, another activator of adenylyl cyclase, also inhibited DIP-thrombin-induced aggregation in GP V −/− platelets (IC50 ≈ 300 nM, data not shown). PGI2 inhibited DIP-thrombin-induced aggregation in both mouse (Fig. 3G) and human platelets pretreated with suboptimal doses of active thrombin. In contrast, thrombin-induced aggregation was not affected significantly by PGI2 even at high concentrations (4.45 μM, Fig. 3 F and H). In fact, PGI2-treated platelets that failed to respond to DIP-thrombin were fully responsive to 10 nM α-thrombin (data not shown). Thus, the thrombin-signaling function of GP Ib–IX–V is ablated in the presence of adenylyl cyclase activators. This finding also suggests that incubation of isolated platelets with PGI2 or PGE1 could suppress this signaling pathway. In previous studies reported by Kahn et al. (12, 32), only PAR signaling was observed in the platelet response to thrombin. The inclusion of PGE1 during platelet isolation and desensitization studies described therein may have inhibited signaling through GP Ib–IX–V.
Previous studies have implicated secreted ADP in platelet aggregation by low doses of thrombin. ADP activates platelets via three distinct pathways (20): a Gq-coupled pathway mediated by the P2Y1 receptor, a Gi-coupled pathway mediated by the recently cloned P2Y12 receptor (33), and a calcium channel (P2X). We conducted platelet aggregation studies with GP V −/− platelets both in the absence and in the presence of specific antagonists to the ADP receptors P2Y12 and P2Y1. Interestingly, we found that 83 nM 2-methylthioadenosine monophosphate, a specific P2Y12 antagonist, completely inhibited platelet aggregation mediated by DIP-thrombin (Fig. 4A). However, the P2Y1 antagonist adenosine 3′,5′-bis-phosphate (2 μM) had little effect on the DIP-thrombin-induced aggregation (Fig. 4B). These data imply that the secreted ADP preferentially activates the P2Y12 receptor. Adenosine 3′,5′diphosphate did show a partial (50%) effect at 4 μM (not shown), but it seems that the Gi-coupled ADP receptor is the major contributor to the signaling pathway. Both compounds could independently inhibit ADP-mediated aggregation in mouse platelets (data not shown). These results support a role for secreted ADP in platelet activation via the thrombin-GP Ibα interaction, and specifically a role for P2Y12, and further suggest that the effects of PGI2 could be mediated by inhibition of the ADP response.
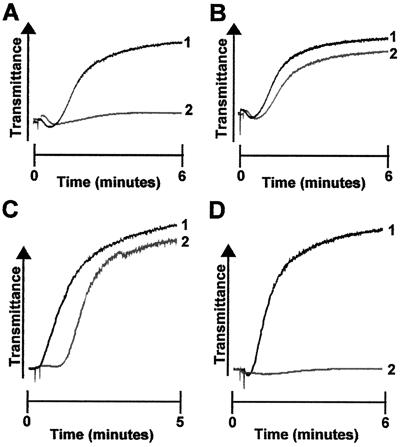
Figure 4.
Effect of ADP receptor antagonists on DIP-thrombin-induced aggregation. (A) Pooled WP from GP V null mice were aggregated with DIP-thrombin (400 nM) in the presence (line 2) or absence (line 1) of 83 nM 2-methylthioadenosine monophosphate. (B) Pooled WP from GP V null mice were aggregated with DIP-thrombin (400 nM) in the presence (line 2) or absence (line 1) of 2 μM adenosine 3′,5′-bis-phosphate. (C) Human WP were preincubated with DMSO (line 1) or PAR 1 antagonist RWJ 56110 (40 μM; line 2) and pretreated with low doses of α-thrombin as described in Methods. Aggregation was initiated by the addition of DIP-thrombin (0.4 μM). (D) Human WP were preincubated with DMSO (line 1) or PAR 1 antagonist RWJ 56110 (40 μM; line 2). Aggregation was initiated by the addition of thrombin (1 nM).
Kahn et al. (11, 12) have shown that PAR 1 activation occurs at low thrombin concentrations, whereas PAR 4 activation requires high concentrations of thrombin. Because human platelets and wt mouse platelets were pretreated with low doses of thrombin to cleave GP V and render the platelets responsive to DIP-thrombin, we evaluated the potential contribution of PAR 1 in the response to DIP-thrombin in human platelets. RWJ 56110, a PAR 1 antagonist (21), inhibited aggregation by thrombin [<1 nM (Fig. 4D)]. In contrast, RWJ 56110 had a minimal effect on the DIP-thrombin response (0.4 μM) in human platelets pretreated with thrombin to cleave GP V (Fig. 4C). Because the DIP-thrombin had no proteolytic activity in chromogenic assays, these data suggest that inhibition of PAR 1 has little or no effect on DIP-thrombin induced signaling via GP Ib–IX.
Physiological Consequences of Thrombin–GP Ib Interaction.
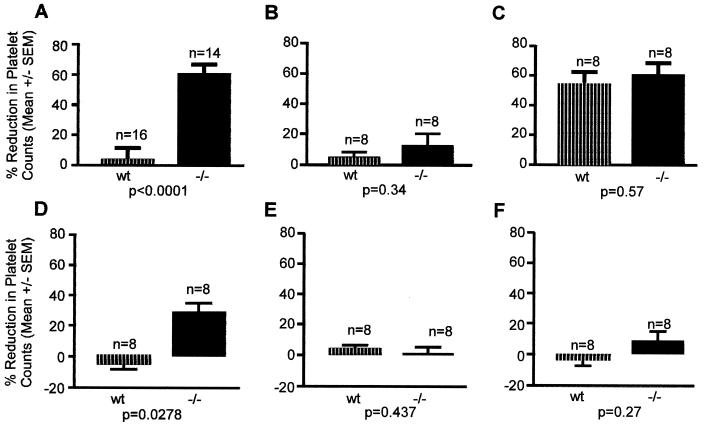
To evaluate the physiological significance of the ligand binding function of thrombin, we examined the effect of a systemic infusion of DIP-thrombin into mice. Infusion of a platelet agonist induces a decrease in platelet counts because of (ongoing) thrombosis (22). As can be seen in Fig. 5, there was significant platelet loss after the infusion of 10 nM thrombin in both GP V null and wt mice (Fig. 5C), with no statistical difference observed between the two groups. Injection of 1 nM thrombin had a marginal effect on platelet counts in either GP V null mice or wt mice (Fig. 5B). In contrast, GP V null mice showed a significant platelet loss when injected with 100 nM DIP-thrombin compared with wt mice (Fig. 5A), in which platelet loss was minimal. As additional controls, we used mutant CHO-expressed thrombins (15) in which the exosite II had been mutated (R89/R93/E94 and R98A). These exosite II mutants were inactivated by using DFP, and then injected into either GP V null or wt mice. As can be seen in Fig. 5 E and F, there was no major loss of platelets in either group. In contrast, 460 nM CHO-expressed thrombin inactivated with DFP (Fig. 5D) caused a significant platelet loss in GP V null mice, with little effect in wt mice. These results show that binding of inactive forms of thrombin to GP V-deficient platelets can occur in vivo, and that the binding results in platelet activation and thrombosis. Our data further substantiate the view that the binding site for GP Ibα is exosite II of thrombin. These results support a role for GP Ibα in thrombin-induced signaling in platelets that results in aggregation and thrombosis.
Figure 5.
Thrombosis in wt and GP V null mice. The mice were injected with 100 nM DIP-thrombin (A), 1 nM thrombin (B), 10 nM thrombin (C), 0.46 μM CHO-expressed wt DIP-thrombin (D), 0.75 μM CHO-expressed DIP-R89/R93/E94 thrombin (exosite II mutant, E) or 0.75 μM CHO-expressed DIP-R98A-thrombin (exosite II mutant, F). The number of animals used and the statistical significance is shown in each graph.
Discussion
The platelet GP Ib–IX–V complex plays a critical role in thrombus formation under conditions of high shear (34, 35). A role for GP Ib–IX–V in the inside-out activation of αΙΙbβ3 after vWf binding was shown by using a heterologous expression system (36). Recently, a role for GP Ib in platelet procoagulant activity has been proposed on the basis of observations that inhibition of thrombin binding to GP Ib inhibited annexin V binding (10) to platelets. However, a function for GP Ib–IX–V in thrombin-induced platelet activation has been unclear. In earlier work, evaluation of platelets from GP V −/− mice indicated that absence of the thrombin substrate GP V from the complex rendered the platelets more responsive to thrombin, and GP V −/− mice were found to have a shorter bleeding time (13). We now show that absence of GP V results in the ability of the platelets to signal (upon thrombin binding) to GP Ibα. In fact, the data suggest that in the absence of GP V, thrombin can function as a ligand, and that proteolytic activity is not required. Inhibitors of thrombin binding to GP Ibα ablated the aggregation response. Systemic administration of proteolytically inactive thrombin to GP V null mice results in a dramatic induction of thrombosis that does not occur in wt mice, suggesting that absence of GP V could have detrimental clinical consequences, and that this signaling pathway occurs physiologically.
It is well established that thrombin-induced platelet activation occurs via the PAR family of thrombin receptors (11). Studies also indicate that the PAR 1 cleavage occurs at thrombin concentrations, whereas PAR 4 cleavage occurs at high thrombin concentrations, and inhibition of both PAR1 and PAR4 in human platelets eliminated thrombin-induced aggregation (12). Further, thrombin-mediated aggregation seemed equivalent in GP V −/− and wt mice generated by Kahn et al. (32). However, our studies now suggest that the GP Ib–IX complex also contributes to thrombin-induced aggregation. To reconcile this apparent difference, we examined the effect of treatment conditions used by Kahn et al. on DIP-thrombin-induced aggregation. We have found that agents such as PGE1 that activate adenylyl cyclase completely inhibited the aggregation induced by proteolytically inactive thrombin, but not by native thrombin. Thus the conditions used in these studies (including PGE1 and EDTA) would inhibit platelet aggregation via GP Ib–IX, but not via the PARs. The data suggest that repression of adenylyl cyclase is critical for platelet activation through the GP Ib–IX complex. Further evidence for the importance of GP Ib–IX in thrombin-induced platelet activation is demonstrated when DIP-thrombin-induced platelet activation is carried out in the presence of a PAR 1 antagonist. Our data show that inhibition of PAR 1 has only a slight effect on the signaling response by DIP-thrombin, and this effect may be because of the inhibition of the signaling response to low doses of active thrombin used in the pretreatment. These data also suggest that inhibition of PAR 1 alone may not prevent platelet activation by thrombin, because thrombin can also signal via the GP Ib–IX complex.
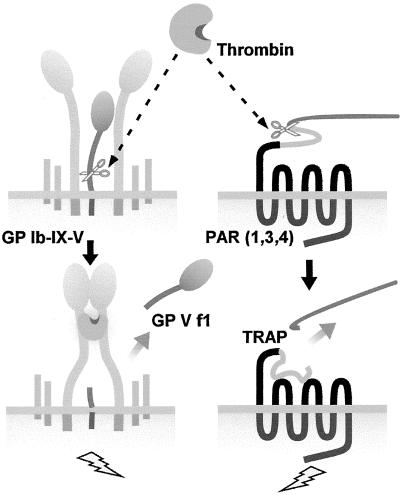
Our data support a model (Fig. 6) for thrombin-induced platelet activation that involves not only the established pathway mediated by the PARs, but also another pathway in which the presence of GP V in the GP Ib–IX–V complex inhibits the ability of thrombin to function as a receptor ligand. After the loss of GP V upon cleavage by thrombin, thrombin binding to GP Ibα results in activation of αΙΙbβ3 and consequently in aggregation. As shown here, the GP Ibα-bound thrombin need not be catalytically functional for this response to occur. Binding of thrombin to GP Ibα results in ADP secretion, which contributes to platelet activation through the P2Y12 receptor (33). The data show not only a previously unknown functional role for thrombin, but also a mechanism by which this pathway can mediate thrombosis independent of proteolytic activity. The binding of thrombin to GP Ibα occurs via the heparin binding exosite and prevents the inactivation of thrombin by antithrombin III (23). Our data indicate that this GP Ibα-bound thrombin can itself initiate additional signaling responses in platelets. The two signaling pathways may have evolved to mediate a more robust aggregation response particularly under conditions of arterial flow. Effective antithrombotic therapies targeting thrombin-induced platelet activation would thus require the inhibition of both pathways. Further, the data suggest a central role for ADP and the P2Y12 receptor in thrombin-induced signaling responses mediated by GP Ibα. The findings therefore reveal another arena for therapeutic intervention in cardiovascular disease.
Figure 6.
Thrombin-induced platelet activation. Two pathways exist on the platelet for activation by thrombin. The pathway described in this article involves the initial cleavage of GP V from the GP Ib–IX–V complex at low thrombin concentrations, resulting in a hyperresponsive platelet. Occupancy of the binding site on GP Ibα by thrombin results in a signaling response that leads to αΙΙbβ3 activation. Additionally, thrombin can cleave the PARs on platelets, and this cleavage will then also stimulate platelet aggregation.
Acknowledgments
We thank Dr. Andreas Betz for fluorimetry measurements, Dr. Zaverio Ruggeri for Ab LJ 1B10, Drs. Beat Steiner and Sylvie Meyer for Ab 3584, Dr. M. Jantzen for ADP receptor antagonists, Dr. D. Oksenberg for the PAR 1 antagonist, Dr. Debbie Law and Dr. Nigel Killeen for critical comments, Erica Thompson for technical expertise, and Rich Wong and Donna DeGuzman for help with the graphics.
Abbreviations
- GP
glycoprotein
- GP V f1
cleaved GP V
- vWf
von Willebrand factor
- PAR
protease-activated receptor
- CHO
Chinese hamster ovary
- DIP-
diisopropylphospho-
- DFP
diisopropyl fluorophosphate
- WP
washed platelets
- wt
wild type
- PGI2
prostacyclin
- PGE1
prostaglandin E1
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 1330.
References
- 1.Lopez J A, Andrews R K, Afshar-Kharghan V, Berndt M C. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 2.Ruggeri Z M, Dent J A, Saldivar E. Blood. 1999;94:172–178. [PubMed] [Google Scholar]
- 3.Calverley D C, Kavanagh T J, Roth G J. Blood. 1998;91:1295–1303. [PubMed] [Google Scholar]
- 4.Andrews R K, Harris S J, McNally T, Berndt M C. Biochemistry. 1998;37:638–647. doi: 10.1021/bi970893g. [DOI] [PubMed] [Google Scholar]
- 5.Asazuma N, Ozaki Y, Satoh K, Yatomi Y, Handa M, Fujimura Y, Miura S, Kume S. Blood. 1997;90:4789–4798. [PubMed] [Google Scholar]
- 6.Zaffran Y, Meyer S C, Negrescu E, Reddy K B, Fox J E. J Biol Chem. 2000;275:16779–16787. doi: 10.1074/jbc.275.22.16779. [DOI] [PubMed] [Google Scholar]
- 7.Andrews R K, Shen Y, Gardiner E E, Dong J F, Lopez J A, Berndt M C. Thromb Haemostasis. 1999;82:357–364. [PubMed] [Google Scholar]
- 8.Berndt M C, Phillips D R. Ann NY Acad Sci. 1981;370:87–95. doi: 10.1111/j.1749-6632.1981.tb29724.x. [DOI] [PubMed] [Google Scholar]
- 9.De Marco L, Mazzucato M, Masotti A, Fenton J W, 2n, Ruggeri Z M. J Biol Chem. 1991;266:23776–23783. [PubMed] [Google Scholar]
- 10.Dormann D, Clemetson K J, Kehrel B E. Blood. 2000;96:2469–2478. [PubMed] [Google Scholar]
- 11.Kahn M L, Zheng Y W, Huang W, Bigornia V, Zeng D, Moff S, Farese R V, Jr, Tam C, Coughlin S R. Nature (London) 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 12.Kahn M L, Nakanishi-Matsui M, Shapiro M J, Ishihara H, Coughlin S R. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramakrishnan V, Reeves P S, DeGuzman F, Deshpande U, Ministri-Madrid K, DuBridge R B, Phillips D R. Proc Natl Acad Sci USA. 1999;96:13336–13341. doi: 10.1073/pnas.96.23.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips D R. Thromb Diath Haemorrh. 1974;32:207–215. [PubMed] [Google Scholar]
- 15.Hall S W, Nagashima M, Zhao L, Morser J, Leung L L. J Biol Chem. 1999;274:25510–25516. doi: 10.1074/jbc.274.36.25510. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra O P, Nesheim M E, Mann K G. J Biol Chem. 1985;260:279–287. [PubMed] [Google Scholar]
- 17.Ramakrishnan V, Sinicropi D V, Dere R, Darbonne W C, Bechtol K B, Baker J B. J Biol Chem. 1990;265:2755–2762. [PubMed] [Google Scholar]
- 18.Vicente V, Kostel P J, Ruggeri Z M. J Biol Chem. 1988;263:18473–18479. [PubMed] [Google Scholar]
- 19.Scarborough R M, Gretler D D. J Med Chem. 2000;43:3453–3473. doi: 10.1021/jm000022w. [DOI] [PubMed] [Google Scholar]
- 20.Jantzen H M, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds E E, Conley P B. Thromb Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- 21.Andrade-Gordon P, Maryanoff B E, Derian C K, Zhang H C, Addo M F, Darrow A L, Eckardt A J, Hoekstra W J, McComsey D F, Oksenberg D, et al. Proc Natl Acad Sci USA. 1999;96:12257–12262. doi: 10.1073/pnas.96.22.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave J P, Gachet C. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Cristofaro R, De Candia E, Rutella S, Weitz J I. J Biol Chem. 2000;275:3887–3895. doi: 10.1074/jbc.275.6.3887. [DOI] [PubMed] [Google Scholar]
- 24.De Marco L, Mazzucato M, Masotti A, Ruggeri Z M. J Biol Chem. 1994;269:6478–6484. [PubMed] [Google Scholar]
- 25.Barrow R T, Healey J F, Lollar P. J Biol Chem. 1994;269:593–598. [PubMed] [Google Scholar]
- 26.Oda A, Yokoyama K, Murata M, Tokuhira M, Nakamura K, Handa M, Watanabe K, Ikeda Y. Thromb Haemost. 1995;74:736–742. [PubMed] [Google Scholar]
- 27.Du X, Harris S J, Tetaz T J, Ginsberg M H, Berndt M C. J Biol Chem. 1994;269:18287–18290. [PubMed] [Google Scholar]
- 28.Du X, Fox J E, Pei S. J Biol Chem. 1996;271:7362–7367. doi: 10.1074/jbc.271.13.7362. [DOI] [PubMed] [Google Scholar]
- 29.Bodnar R J, Gu M, Li Z, Englund G D, Du X. J Biol Chem. 1999;274:33474–33479. doi: 10.1074/jbc.274.47.33474. [DOI] [PubMed] [Google Scholar]
- 30.Fox J E, Reynolds C C, Johnson M M. J Biol Chem. 1987;262:12627–12631. [PubMed] [Google Scholar]
- 31.Fox J E, Berndt M C. J Biol Chem. 1989;264:9520–9526. [PubMed] [Google Scholar]
- 32.Kahn M L, Diacovo T G, Bainton D F, Lanza F, Trejo J, Coughlin S R. Blood. 1999;94:4112–4121. [PubMed] [Google Scholar]
- 33.Hollopeter, G., Jantzen, H.-M., Vincent, D., Li, G., England, L., Ramakrishnan, V., Yang, R.-B., Nurden, P., Nurden, A., Julius, D. J. & Conley, P. B. (2001) Nature (London), in press. [DOI] [PubMed]
- 34.Weiss H J. Thromb Haemost. 1995;74:117–122. [PubMed] [Google Scholar]
- 35.Savage B, Shattil S J, Ruggeri Z M. J Biol Chem. 1992;267:11300–11306. [PubMed] [Google Scholar]
- 36.Gu M, Xi X, Englund G D, Berndt M C, Du X. J Cell Biol. 1999;147:1085–1096. doi: 10.1083/jcb.147.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]