Abstract
The mechanisms through which LH-RH antagonists suppress gonadotroph functions and LH-RH receptor (LH-RH-R) production are incompletely understood. To elucidate these mechanisms, we investigated the effects of Cetrorelix on the mRNA expression of pituitary LH-RH-R and luteinizing hormone (LH) secretion in three experimental systems with different pituitary LH-RH environments. Ovariectomy induced 3.61-fold and 6.34-fold increases in the mRNA expression of pituitary LH-RH-R in rats after 11 and 21 days, respectively. After (5 h) a single injection of 100 μg Cetrorelix, no significant decrease occurred in the mRNA levels of pituitary LH-RH-R in ovariectomized (OVX) rats with high pituitary exposure to LH-RH, but there was a significant 23.2% reduction in cycling rats with normal hypophysial LH-RH environment. Prolonged treatment for 10 days with a Cetrorelix depot formulation releasing 100 μg/day decreased the concentration of mRNA for pituitary LH-RH-R by 72.6% in OVX rats, but only by 32.9% in normal rats. The decline in serum LH was 98.7% in OVX rats and 63.2% in normal rats, resulting in a minimal 0.1–0.2 ng/ml LH concentration in both groups. A continuous exposure of pituitary cells to 100 nM Cetrorelix in the superfusion system, which is devoid of LH-RH, did not cause any significant changes in LH-RH-R mRNA level. These studies demonstrate that prolonged exposure to Cetrorelix in vivo, but not in vitro, down-regulates the mRNA expression of the pituitary receptors for LH-RH. Our findings indicate that LH-RH antagonists exert their inhibitory effects on the gene expression of pituitary LH-RH-R by counteracting the stimulatory effect of endogenous LH-RH.
The hypothalamic decapeptide LH-RH plays a pivotal role in regulating luteinizing hormone (LH) and follicle-stimulating hormone secretion through the activation of high-affinity G protein-coupled receptors on the plasma membrane of pituitary gonadotrophs (1–3). The responsiveness of the gonadotrophs to LH-RH varies under different conditions, depending on the hormonal milieu (4, 5), and seems to be correlated at least in part with the number of LH-RH receptors (6, 7). The regulation of the number and the responsiveness of LH-RH-Rs is complex, being influenced by gonadal steroids, inhibin, and gonadotropins, as well as by its own ligand, LH-RH (5, 8–10). An intermittent administration of native LH-RH or LH-RH agonists in vivo and in vitro, mimicking the natural pulsatile release of this neuropeptide from the hypothalamus, stimulates the synthesis and the release of gonadotropins and leads to an increase in the number of its receptors (11). However, continuous administration of LH-RH and its agonists, especially at high concentration, causes a down-regulation of receptors and desensitization of gonadotrophs (11–13). Thus, administration of depot preparations of LH-RH agonists results in the suppression of the secretion of gonadotropins and sex hormones which provides the basis for clinical applications of this class of compounds (1, 2, 14).
In the past 25 years, more than 3,000 analogs of LH-RH have been synthesized (1, 2). Agonistic analogs, such as Decapeptyl, Leuprolide, Zoladex, and Buserelin, have important clinical applications in gynecology and oncology (1, 2). Potent antagonists of LH-RH, such as Cetrorelix, Ganirelix, and Abarelix, have also been developed and are now available for clinical use (1, 2, 15).
Antagonists of LH-RH produce a competitive blockade of LH-RH receptors and cause an immediate inhibition of the release of gonadotropins and sex steroids, reducing the time of the onset of therapeutic effects, as compared with the agonists (1, 2, 16). LH-RH antagonists such as Cetrorelix already have major uses in gynecology, especially for in vitro fertilization (17–23) and oncology, and seem to have various advantages over the agonists (1, 2, 16).
Recent studies show that the administration of the LH-RH antagonist Cetrorelix to rats also produces down-regulation of pituitary LH-RH-Rs and a decrease in the LH-RH-R mRNA levels (16, 24, 25,). These effects seem to be similar to those produced by the agonists (16, 25, 26). In addition, Cetrorelix can inhibit growth of various experimental tumors and suppress the proliferation of prostatic, ovarian, breast, and endometrial cancer cell lines by direct effects mediated through specific LH-RH-Rs on the tumor cells (16, 27). The inhibition of tumor growth can be correlated with the decrease in mRNA levels for these receptors, suggesting a direct effect of LH-RH antagonists on the mRNA expression of tumor cells (16, 28). Similar effects of Cetrorelix on the gene expression of LH-RH-Rs in tumors and in the normal pituitary raise the question whether LH-RH antagonists could influence the gene expression of LH-RH-R in the pituitary directly, as in tumors, or whether different mechanisms mediate the effects of LH-RH antagonists in normal and tumor tissues.
Because Cetrorelix (developed in our laboratory; ref. 29) and other LH-RH antagonists are being introduced into the clinical practice, the elucidation of the mechanisms by which LH-RH antagonists down-regulate the LH-RH-R number and mRNA levels of pituitary and tumor cells is important (1, 2). This study was designed to investigate these mechanisms. To determine whether LH-RH antagonists exert a direct effect on the receptor mRNA expression of pituitary gonadotrophs, or act indirectly by preventing the stimulatory effect of LH-RH, Cetrorelix was tested in three experimental systems with different LH-RH inputs to the pituitary. Thus, we evaluated this antagonist in ovariectomized (OVX) rats with high pituitary LH-RH levels, in regularly cycling rats with normal hypophysial LH-RH concentration, and in the superfused rat-pituitary cell system, which completely lacks hypothalamic LH-RH. Both short-term and chronic effects of Cetrorelix on the mRNA expression of pituitary LH-RH receptors and on the LH secretion were studied in vivo.
Materials and Methods
Peptides.
The LH-RH antagonist [Ac-d-Nal(2)1, d-Phe(4Cl)2, d-Pal(3)3, d-Cit6, d-Ala10]LH-RH [Cetrorelix; Nal(2) is 3-(2-naphthyl)alanine, Pal(3) is 3-(3-pyridyl)alanine, and Cit is citrulline], originally synthesized in our laboratory by solid phase methods (ref. 29), was made by Asta Medica (Frankfurt/Main, Germany) as Cetrorelix acetate (D20761; ref. 15). For injection, Cetrorelix acetate was dissolved in distilled water containing 5% mannitol. Cetrorelix pamoate depot (D20762) was also made by Asta Medica. The formulation contained Cetrorelix peptide-base and pamoic acid in a molar ratio of 2:1, respectively, plus mannitol. For the injection, the depot formulation was suspended in distilled water at a final concentration of 15 mg/ml Cetrorelix pamoate in 5% mannitol. Aliquots of this suspension (3 mg/0.2 ml) were injected i.m., with an estimated daily release of about 100 μg of Cetrorelix for 30 days. For in vitro experiments, Cetrorelix and the synthetic LH-RH decapeptide were dissolved in Medium 199 (Sigma).
Animals.
Adult female Sprague–Dawley rats (Charles River Breeding Laboratories) were used in the experiments. Animals were allowed standard rat diet and tap water ad libitum and were maintained under controlled conditions (12-h light/12-h dark schedule at 24°C). Some rats were ovariectomized under isoflurane anesthesia and used for in vivo and in vitro experiments 11 days and 21 days after ovariectomy, respectively. Other rats were checked for estrous cycle by taking daily vaginal smears; animals showing three consecutive 4-day cycles were used for in vivo experiments.
Experimental Procedures
In Vivo Experiments.
Normal and OVX rats weighing 250–275 g were used for short-term (5 h) and long-term (10 days) experiments.
In the short-term experiments, groups of five to six OVX rats and five to six normal rats received one s.c. injection of 100 μg of Cetrorelix acetate. Control rats received vehicle injections only. Blood samples of 500–800 μl were taken 5 h after treatment from the jugular vein under isoflurane anesthesia. Serum was separated by centrifugation and stored at −20°C until assayed for LH. Immediately after blood samples were collected, rats were killed by decapitation. Anterior pituitaries were removed, homogenized in Tri Reagent (Sigma), and stored at −70°C until used for LH-RH-R mRNA determinations.
In long-term experiments, two groups of OVX rats and two groups of normal rats, consisting of eight animals each, were injected i.m. with either 3 mg of Cetrorelix pamoate depot or the vehicle. The estimated daily release of Cetrorelix peptide base was approximately 100 μg/day for 30 days. Blood samples of 500–800 μl were taken from the jugular vein on days 0, 1, 4, 7, and 10 of the treatment. Serum was obtained and stored as described above. After the last samples were collected on day 10, the rats were killed by decapitation and the anterior pituitaries were removed. Three of the eight pituitaries from each group were homogenized in Tri Reagent and stored at −70°C for LH-RH-R mRNA determinations. The other five pituitaries were homogenized in 0.1 M hydrochloric acid, centrifuged, and the supernatants were stored at −20°C until assayed for LH.
In Vitro Experiments in Superfusion System.
The superfused rat pituitary cell system was used for in vitro experiments (30). Briefly, rats were killed, and pituitaries were removed, cut into small pieces, and incubated with collagenase (type I, 0.5%, Worthington) for 50 min in a metabolic shaker. The cells were then dispersed, resuspended in 2 ml of tissue culture Medium 199, transferred into the superfusion chambers, and allowed to sediment simultaneously with 0.8 ml of Sephadex-G-10 (Amersham Pharmacia) that had been equilibrated with medium. Anterior pituitaries from two normal female or two OVX rats were used in each chamber of the system. After a recovery period of 5 h, 1-ml fractions of the effluent media were collected every 3 min or 10 min, and the LH concentration of the fractions was determined by an RIA. To check the inhibitory action of Cetrorelix on the stimulatory effect of native LH-RH, pituitary cells were perfused continuously with 20 nM Cetrorelix for 2 h and exposed to 3-min pulses of 1 nM LH-RH at 30-min intervals before, during, and after the perfusion with Cetrorelix. To investigate the effect of Cetrorelix on the mRNA expression of LH-RH-Rs, the cells were exposed to 100 nM Cetrorelix continuously for 10 h. The RNA content of the cells was then extracted, RNA was isolated, and the mRNA level of the LH-RH-R was determined by reverse transcription–PCR (RT-PCR). Control cells were perfused with medium. Each experiment was repeated twice.
RNA Extraction.
Total RNA from pituitary glands of intact and OVX female rats was extracted by using the Tri Reagent (Sigma) protocol, an improvement on the single-step method reported by Chomczynski and Sacchi (31). Anterior pituitaries from individual rats were placed into 1 ml of Tri Reagent and homogenized for 5 min. The extraction procedure was then continued by the addition of 1-bromo-3-chloropropane before precipitation with isopropyl alcohol. Total RNA of dispersed pituitary cells maintained in superfusion system was extracted as described by Rekasi et al. (32).
Semiquantitative RT-PCR.
One microgram of total RNA was reverse transcribed and then amplified by using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer). The RT reaction was performed in a final volume of 20 μl containing 2.5 μM random hexamers, 1 mM each of dNTPs, 1× PCR buffer, 5 mM MgCl2, 1 unit/μl RNase inhibitor, and 2.5 units/μl murine leukemia virus reverse transcriptase. One-fourth (5 μl) of the RT reaction was used for each PCR amplification with primer sets that simultaneously amplify a 441-bp and a 542-bp product for rat LH-RH-R and rat β-actin, respectively. Primers for rat LH-RH-R (rLH-RH-R) and rat β-actin were used as reported: LH-RH-R (33) sense, 5′-CTT GAA GCC CGT CCT TGG AGA AAT-3′; antisense, 5′-GCG ATC CAG GCT AAT CAC CAC CAT-3′; and β-actin (34) sense, 5′-GTC ACC CAC ACT GTG CCC ATC T-3′; antisense, 5′-ACA GAG TAC TTG CGC TCA GGA G-3′. The PCR reaction included 1× PCR buffer, 2 mM MgCl2, 1 μM of each primer, and 2.5 units/100 μl AmpliTaq DNA polymerase in a 25 μl volume. The PCR amplification was performed in a GeneAmp PCR System 2400 (Perkin-Elmer) with the following cycle profile: initial denaturation at 95°C for 3 min, followed by 24 or 25 (rLH-RH-R) and 19 or 20 (β-actin) cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 45 sec, for the in vivo and in vitro experiments, respectively. After the last cycle, there was a final extension for 7 min at 72°C. The number of cycles was determined in preliminary experiments to be within the exponential range of PCR amplification. PCR products were electrophoresed on a 1.5% agarose gel, stained with 0.5 μg/ml ethidium bromide, visualized under UV light, and followed by scanning and quantification of the gel (GDS 7500 Gel Documentation System, Ultraviolet Products, San Gabriel, CA and GS-700 Imaging Densitometer, Bio-Rad). The levels of r-LH-RH-R mRNA products were related to rat β-actin mRNA values and expressed as a percentage of the vehicle-treated controls.
RIA.
Rat LH was determined by RIA with materials provided by A. F. Parlow [National Institute of Diabetes and Digestive and Kidney Diseases' National Hormone and Pituitary Program, Torrance, CA; rLH-RP-3 (AFP-7187B), rLH-I-10 (AFP 11536B), and anti-rLH-RIA-11 (AFP C697071P)].
Analysis of Data.
Results are expressed as mean ± SEM. Statistical analysis of data was performed by using the computer software sigmastat (Jandel, San Rafael, CA). In vivo results were subjected to one-way ANOVA, followed by a Bonferroni t test. P < 0.05 was accepted as a statistically significant difference. The superfusion data were analyzed with a computer program developed in our institute (30). Using this program, we analyzed the peaks and calculated the amount of hormone secreted above the baseline.
Results
Time-Course Response of Gonadotrophs to Ovariectomy: mRNA Expression of LH-RH-Rs and LH Secretion.
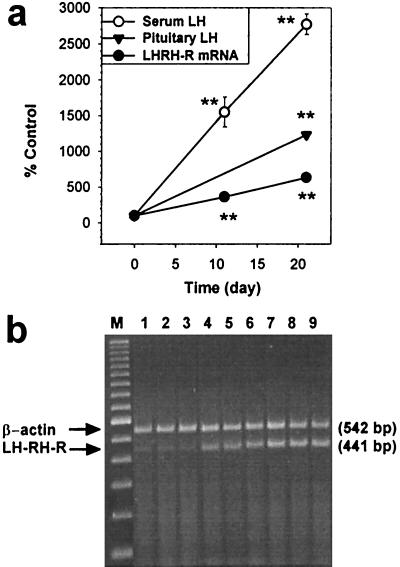
Castration of female rats induced gradual increases in pituitary LH-RH-R mRNA levels and LH secretion. LH-RH-R mRNA levels rose 3.61-fold in 11 days and 6.34-fold after 21 days compared with normal controls (P < 0.01; Fig. 1a). Still greater increases were observed in the serum and pituitary LH concentrations. Serum LH was elevated to 15.5-fold on day 11, and 27.7-fold 21 days after castration (P < 0.01 vs. normal values). The total pituitary LH concentration augmented 12.3-fold 21 days after ovariectomy (P < 0.01 vs. normal; Fig. 1a). Fig. 1b shows RT-PCR products of pituitary mRNA for LH-RH-R from OVX and normal rats.
Figure 1.
(a) The time-course of the effects of ovariectomy in normal rats on the pituitary LH-RH-R mRNA expression, serum LH, and pituitary LH concentration. (b) RT-PCR products of the pituitary LH-RH-R mRNA and β-actin mRNA, separated by agarose gel electrophoresis and stained with ethidium bromide. PCR products were the expected size of 441 bp (LH-RH-R) and 542 bp (β-actin). Lane M, 100-bp DNA molecular weight marker; lanes 1, 2, and 3, normal rats; lanes 4, 5, and 6, OVX rats after 11 days; lanes 7, 8, and 9, OVX rats after 21 days. **, P < 0.01 vs. initial values.
Short-Term Effects of Cetrorelix.
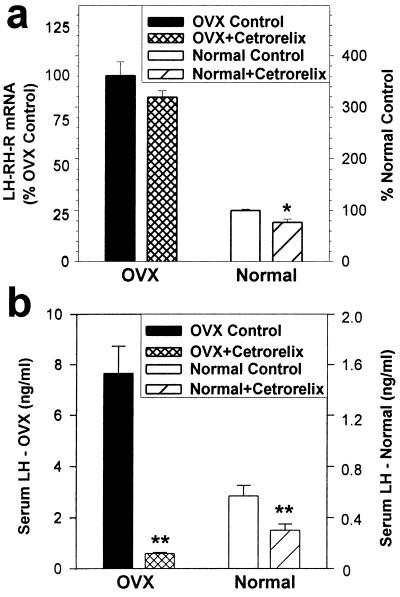
A single injection of 100 μg of LH-RH antagonist Cetrorelix 11 days after ovariectomy (when the LH-RH-R mRNA level of pituitaries was elevated to 361% of the initial level) caused a small and not significant 11.6% decrease in the elevated LH-RH-R mRNA level 5 h after injection (Fig. 2a). At that time, serum LH was decreased by 92.3% and suppressed to the normal level (P < 0.01 vs. OVX control; Fig. 2b).
Figure 2.
Effects of a single dose of 100 μg of Cetrorelix on the pituitary LH-RH-R mRNA level and the serum LH concentration in OVX and normal rats as measured 5 h after administration. (a) LH-RH-R mRNA level, % of corresponding controls. (b) Serum LH concentration. *, P < 0.05; **, P < 0.01 vs. corresponding controls.
In normal rats, a single injection of 100 μg of LH-RH antagonist Cetrorelix resulted in a significant 23.2% reduction in the pituitary LH-RH-R mRNA level 5 h after the injection (P < 0.05 vs. control) and decreased the serum LH concentration by 47.4% (P < 0.01 vs. control; Fig. 2 a and b, respectively).
Long-Term Effects of Cetrorelix.
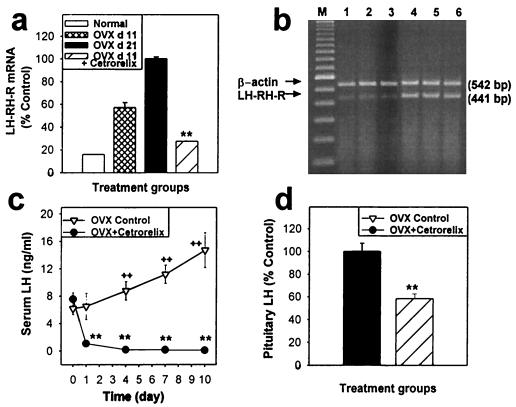
In OVX rats, the LH-RH-R mRNA level of the pituitary rose by 273% (from 361% to 634%) between postoperative days 11 and 21 (Fig. 1a). Administration of a single injection of 3 mg of Cetrorelix pamoate depot on day 11, releasing about 100 μg of Cetrorelix per day for 10 days, prevented the rise in the LH-RH-R mRNA level induced by ovariectomy, and decreased the LH-RH-R mRNA level to less than half of the pretreatment level on day 11 of ovariectomy (Fig. 3a). On day 21, LH-RH-R mRNA levels were suppressed by 72.6% (P < 0.01) in the group treated continuously with Cetrorelix for 10 days, as compared with untreated controls (Fig. 3 a and b).
Figure 3.
Pituitary LH-RH-R mRNA expression and serum and pituitary LH in OVX rats during or after the treatment for 10 days with a depot formulation of Cetrorelix pamoate. (a) LH-RH-R mRNA level in normal rats, in OVX controls 11 and 21 days after ovariectomy, and in OVX rats treated with Cetrorelix for 10 days. (b) RT-PCR products of the pituitary LH-RH-R mRNA and β-actin mRNA, after separation by agarose gel electrophoresis and staining with ethidium bromide. Lane M, 100-bp DNA molecular weight marker; lanes 1, 2, and 3, Cetrorelix-treated; lanes 4, 5, and 6, untreated control. (c) Time course of serum LH measured on days 0, 1, 4, 7, and 10 of the Cetrorelix treatment. (d) Total pituitary LH content after the treatment (% untreated control). **, P < 0.01 vs. control; ++, P < 0.01 vs. pretreatment levels on day 0.
In untreated OVX rats, a gradual increase in serum LH concentration was observed between 11 and 21 days postoperatively (which corresponds to days 1 and 10 of the Cetrorelix treatment; Fig. 3c). In that time period, the serum LH increased gradually by 35.3%, 51.5%, and 127%, on days 4, 7, and 10, respectively (P > 0.05, P < 0.01, and P < 0.01, respectively, vs. day 0 of the treatment period). Administration of Cetrorelix in depot form prevented the OVX-induced LH elevation and suppressed the serum LH level to 1.3% of the OVX control value after 10 days (P < 0.01). A major reduction in serum LH (as much as 82.5%) was already found on the first day (P < 0.01; Fig. 3c). Chronic application of Cetrorelix for 10 days also significantly decreased the pituitary LH content by 41.6% (P < 0.01 vs. OVX control; Fig. 3d).
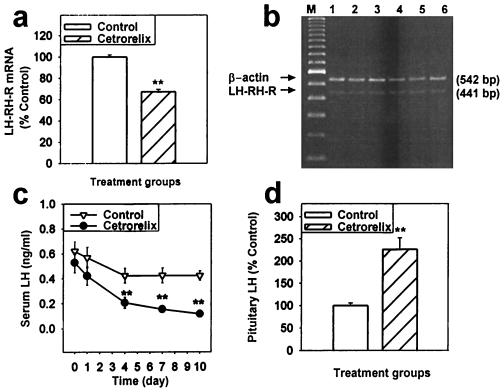
Long-term treatment of normal female rats with the pamoate depot form of Cetrorelix for 10 days resulted in a 32.9% suppression of the pituitary LH-RH-R mRNA level (P < 0.01 vs. control; Fig. 4 a and b). Serum LH concentrations were gradually decreased during the treatment with Cetrorelix. A significant 51.2% decrease in the serum LH was observed on day 4, with levels falling to 63.6% on day 7, and 72.2% on day 10 (P < 0.01 vs. controls; Fig. 4c). After the treatment with Cetrorelix, the LH content of the pituitary was increased by 126% (P < 0.01 vs. control; Fig. 4d).
Figure 4.
Chronic effects of Cetrorelix in normal rats during or after the treatment for 10 days with a depot formulation of Cetrorelix pamoate, releasing 100 μg/day, on pituitary LH-RH-R mRNA expression and serum and pituitary LH. (a) LH-RH-R mRNA level after the treatment, % of control. (b) RT-PCR products of the pituitary LH-RH-R mRNA and β-actin mRNA, after separation by agarose gel electrophoresis and staining with ethidium bromide. Lane M, 100-bp DNA molecular weight marker; lanes 1, 2, and 3, Cetrorelix-treated; lanes 4, 5, and 6, untreated control. (c) Time course of serum LH measured on days 0, 1, 4, 7, and 10 of the Cetrorelix treatment. (d) Total pituitary LH after the treatment (% of untreated control). **, P < 0.01 vs. control.
In Vitro Effect of Cetrorelix.
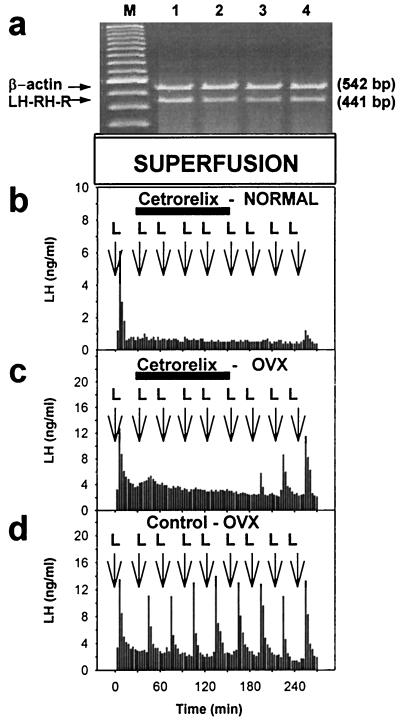
A continuous exposure of pituitary cells from normal or OVX rats to 100 nM Cetrorelix for 10 h did not cause any significant changes in the mRNA expression of the LH-RH-R, as compared with corresponding untreated controls. The LH-RH-R mRNA values in the cells obtained from OVX rats were 107 ± 0.53% and in cells from normal rats 101 ± 0.56% of the corresponding control values (Fig. 5a). Nevertheless, a continuous perfusion with 20 nM Cetrorelix for 2 h prevented the LH responses of pituitary cells to 1 nM LH-RH pulses administered at 30-min intervals during and after the Cetrorelix treatment. LH responses to LH-RH challenges of pituitary cells from OVX rats showed a recovery earlier than those from normal rats (Fig. 5 b, c, and d).
Figure 5.
In vitro effects of Cetrorelix on LH-RH-R expression and LH response in normal and OVX rat pituitary cells in the superfusion system. (a) LH-RH-R mRNA and β-actin mRNA expression of normal pituitary cells perfused continuously with 100 nM Cetrorelix for 10 h. RT-PCR products are shown after separation by agarose gel electrophoresis and staining with ethidium bromide. Lane M, 100-bp DNA molecular weight marker; lanes 1 and 2, Cetrorelix-treated; lanes 3 and 4, untreated control cells. (b) LH responses of normal rat pituitary cells to 1 nM LH-RH pulses (L) for 3 min at 30-min intervals before, during, and after a continuous superfusion with 20 nM Cetrorelix for 2 h (horizontal bar). (c) LH responses of pituitary cells from OVX rats to 1 nM LH-RH pulses (L) for 3 min at 30-min intervals before, during, and after a continuous superfusion with 20 nM Cetrorelix for 2 h (horizontal bar). (d) LH responses of control cells from OVX rats to 1 nM LH-RH pulses for 3 min at 30-min intervals (L).
Discussion
The events that follow the binding of LH-RH and its analogs to pituitary gonadotrophs and lead to changes in the number of LH-RH-R, are not fully elucidated (1, 2, 7). Much evidence indicates that the receptor number is regulated at the pretranslational level by LH-RH-R gene expression (26, 35), but data on the posttranscriptional regulation by recycling, modification, or degradation have also been reported (12).
A dramatic increase in the mRNA expression of pituitary LH-RH-R can be induced by ovariectomy (12). It is well known that the elimination of the negative feedback of ovarian steroid hormones on the hypothalamus by ovariectomy causes an increase in the pulsatile LH-RH release from hypothalamic neurons and a marked elevation in the LH-RH concentration in the pituitary portal vessels (36). Accordingly, it was reported that endogenous LH-RH stimulates the synthesis and release of gonadotropins, and increases the number and mRNA expression of its own receptor (35). In keeping with these results, we also found that ovariectomy of rats greatly increased the LH secretion and the LH-RH-R mRNA level in the pituitary. The stimulatory influence of the increased endogenous LH-RH secretion in OVX rats is exerted primarily on the LH secretion and secondarily on the LH production and LH-RH-R mRNA expression.
Chronic treatment of OVX and normal rats for 10 days with Cetrorelix significantly reduced the mRNA level for pituitary LH-RH-R. The degree of reduction in mRNA levels seems to be correlated with the hypophysial LH-RH levels in the treated rats. Thus, in OVX rats that have high LH-RH concentration in the pituitary portal vessels, we observed a significantly higher reduction in receptor mRNA levels by Cetrorelix than in normal rats (73% vs. 33%). Moreover, in the superfusion system, in which pituitary cells are devoid of LH-RH, Cetrorelix did not influence the mRNA expression of LH-RH-Rs. Our results demonstrate that the down-regulation of the mRNA expression of pituitary LH-RH-Rs by Cetrorelix requires the presence of LH-RH. This finding indicates that the down-regulatory effect of Cetrorelix results from a competitive inhibition of the stimulatory effect of endogenous LH-RH. This view is supported by the results of the in vitro experiments. Had the suppression of LH-RH-R mRNA expression by Cetrorelix resulted from a direct influence on the receptor gene expression of pituitary cells, the greatest inhibition of the receptor mRNA expression would have been found in vitro, because Cetrorelix does not have to compete with the stimulatory effect of LH-RH in the superfusion system. Consequently, the recent concept that LH-RH antagonists might directly inhibit the LH-RH-R gene expression in tumors by activating the receptor (16, 27) is not applicable to normal pituitary gonadotrophs.
The inhibitory effect of chronic treatment with Cetrorelix on LH-RH-R mRNA expression and receptor LH-RH concentration was also demonstrated in our previous studies (24, 25). In the initial study, we found that the suppression of LH secretion by Cetrorelix was strongly correlated with the reduction in LH-RH-R concentration (24). In the present work, a more intensive and more rapid suppression of the LH secretion than of the receptor mRNA levels was observed in OVX rats 5 h after the administration of Cetrorelix. Cetrorelix caused a 92% decline in the LH secretion and suppressed serum LH to the normal level. At that time, no significant change in the mRNA level for LH-RH-R could be seen. The LH secretory response of gonadotrophs to the inhibitory influence of Cetrorelix is thus more rapid than the effect on the LH-RH-R mRNA production. This finding also suggests that the inhibitory effect of Cetrorelix, on both the LH secretion and the LH-RH-R mRNA expression, results from the lack of LH-RH stimulation. A discrepancy in the down-regulation of LH-RH-R concentration and mRNA expression was also found by others (12) by using LH-RH agonists. Taken together, our results and those of others indicate that other mechanisms, such as degradation and/or subcellular translocation of the membrane receptors (24), are also involved in the down-regulation of the LH-RH-R.
Interestingly, in our experiments, a single injection of a high dose of Cetrorelix into OVX rats induced a small (12%) inhibition of the LH-RH-R mRNA expression, whereas in normal rats there was a significant 23% suppression. However, this 12% decrease in the markedly elevated LH-RH-R mRNA level in OVX rats (361% of normal) represents a greater net decrease (43% of normal) than the 23% decline obtained in normal rats. Consequently, the decrease in mRNA levels of the pituitary LH-RH-R induced by Cetrorelix depends on the number of LH-RH-Rs that have to be saturated and on the concentration of endogenous LH-RH that has to be counteracted to compensate for its stimulatory effect. A single dose of 100 μg of Cetrorelix might not have been sufficient to saturate the markedly increased LH-RH-R of OVX rats and to counteract the increased level of endogenous LH-RH, and therefore no significant decrease in the receptor mRNA level occurred 5 h after the injection. However, a continuous exposure of the pituitary LH-RH-R to relatively low doses of Cetrorelix (100 μg/day) caused a marked inhibition of LH-RH-R mRNA expression in OVX rats. This observation is in accord with our earlier findings that prolonged administration of low doses of potent LH-RH antagonists can produce the same inhibition of stimulated LH secretion as a single administration of a high dose (37). These phenomena result from the advantageous binding properties of LH-RH antagonists, such as high affinity and prolonged occupancy of the LH-RH receptor (29). Because of the high binding affinity of these peptides, the LH-RH-Rs might be gradually saturated by prolonged low-dose treatment, becoming effective in time. This view is supported by the observations of normal men that low LH-RH antagonist doses, which were ineffective in the first week, suppressed gonadotropins effectively during long-term treatment (38).
In normal rats, both the chronic administration and the single injection of a high dose of Cetrorelix caused a similar 23–33% decrease in mRNA levels of LH-RH-R. The inhibition observed at 5 h after a single high-dose injection of Cetrorelix was only slightly increased by the chronic treatment for 10 days. These results suggest that 100 μg of Cetrorelix can saturate the LH-RH-Rs in normal rats and counteract the stimulatory effect of low levels of endogenous LH-RH on the mRNA expression of its own receptor.
In contrast to the marked reduction in LH secretion and LH-RH-R mRNA levels in OVX rats after chronic treatment with Cetrorelix, only a moderate decrease in the pituitary LH content was found; in normal rats an elevation in the pituitary LH content could be seen. These findings can be explained as the acute suppression of LH release from the pituitary, which can partially compensate for the inhibition of the LH synthesis, resulting in a moderate decrease, or even an elevation, in the pituitary LH concentration. Similar observations were reported in our earlier study (39), using another potent LH-RH antagonist in female rats. These results also indicate that LH-RH has a secondary role in the regulation of gonadotropin synthesis relative to release.
In conclusion, this study reveals that LH-RH antagonists, such as Cetrorelix, suppress the mRNA expression of the pituitary LH-RH-Rs by counteracting the stimulatory effect of endogenous LH-RH.
Acknowledgments
The work described in this article was supported by the Medical Research Service of the Veterans Affairs Department and a grant from Asta Medica, Frankfurt/Main, Germany, to Tulane University (all to A.V.S.). Tulane University holds patents on LH-RH antagonist Cetrorelix cited in this paper, and A.V.S. is a coinventor on that patent.
Abbreviations
- LH
luteinizing hormone
- LH-RH
luteinizing hormone-releasing hormone
- LH-RH-R
LH-RH receptor
- OVX
ovariectomized
- RT-PCR
reverse transcription–PCR
- rLH-RH-R
rat LH-RH-R
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031582398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031582398
References
- 1.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E, Bast R C, Kufe D E, Morton D L, Weichselbaum R R, editors. Baltimore: Williams and Wilkins; 2000. pp. 715–729. [Google Scholar]
- 2.Schally A V. Peptides. 1999;20:1247–1262. doi: 10.1016/s0196-9781(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 3.Conn P M. Endocr Rev. 1986;7:3–10. doi: 10.1210/edrv-7-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Clayton R N. J Endocrinol. 1989;120:11–19. doi: 10.1677/joe.0.1200011. [DOI] [PubMed] [Google Scholar]
- 5.Clayton R N, Catt K J. Endocr Rev. 1981;2:186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- 6.Conn P M, Crawley W F. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser U B, Jakubowiak A, Steinberger A, Chin W W. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 8.Savoy-Moore R T, Schwartz N B, Duncan J A, Marshall J C. Science. 1980;209:942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- 9.Marian J, Cooper R L, Conn P M. Mol Pharmacol. 1981;19:399–405. [PubMed] [Google Scholar]
- 10.Gregg D W, Schwall R H, Nett T M. Biol Reprod. 1991;44:725–732. doi: 10.1095/biolreprod44.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Loumaye E, Catt K J. Science. 1982;215:983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- 12.Mason D R, Arora K K, Mertz L M, Catt K J. Endocrinology. 1994;135:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- 13.Katt J A, Duncan J A, Herbon L, Barkan A, Marshall J C. Endocrinology. 1985;116:2113–2115. doi: 10.1210/endo-116-5-2113. [DOI] [PubMed] [Google Scholar]
- 14.Emons G, Schally A V. Hum Reprod. 1994;9:1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- 15.Reissmann T, Engel J, Kutscher K, Bernd M, Hilgard P, Peukert M, Szelenyi I, Reichert S, Gonzales-Barcena D, Nieschiag E, et al. Drugs of the Future. 1994;19:228–237. [Google Scholar]
- 16.Schally A V, Halmos G, Rekasi Z, Arencibia J M. In: Clinic: Infertility and Reproductive Medicine Clinics of North America-January 2001. Devroey P, editor. Vol. 12. Philadelphia: Saunders; 2001. pp. 17–44. [Google Scholar]
- 17.Diedrich K, Diedrich C, Santos E, Zoll C, Al-Hasani S, Reissmann T, Krebs D, Klingmuller D. Hum Reprod. 1994;9:788–791. doi: 10.1093/oxfordjournals.humrep.a138597. [DOI] [PubMed] [Google Scholar]
- 18.Felberbaum R E, Germer U, Ludwig M, Riethmüller-Winzen H, Heise S, Buttge I, Bauer O, Reissmann T, Engel J, Diedrich K. Hum Reprod. 1998;13:1660–1668. doi: 10.1093/humrep/13.6.1660. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Barcena D, Banuelos-Alvarez R, Perez-Ochoa E, Cardenas-Cornejo I, Comaru-Schally A M, Schally A V, Engel J, Reissmann T, Riethmuller-Winzen H. Hum Reprod. 1997;12:2028–2035. doi: 10.1093/humrep/12.9.2028. [DOI] [PubMed] [Google Scholar]
- 20.Oliveness F, Fanchin R, Bouchard P. Hum Reprod. 1995;10:1382–1386. [PubMed] [Google Scholar]
- 21.Reissmann T, Felberbaum R, Diedrich K, Engel J, Comaru-Schally A M, Schally A V. Hum Reprod. 1995;10:1974–1981. doi: 10.1093/oxfordjournals.humrep.a136219. [DOI] [PubMed] [Google Scholar]
- 22.Reissmann T, Schally A V, Bouchard P, Riethmuller H, Engel J. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 23.Schally A V. Gynecol Endocrinol. 1999;13:401–409. doi: 10.3109/09513599909167587. [DOI] [PubMed] [Google Scholar]
- 24.Halmos G, Schally A V, Pinski J. Proc Natl Acad Sci USA. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinski J, Lamharzi N, Halmos G, Groot K, Jungwirth A, Vadillo-Buenfil M, Kakar S S, Schally A V. Endocrinology. 1996;137:3430–3436. doi: 10.1210/endo.137.8.8754771. [DOI] [PubMed] [Google Scholar]
- 26.Wu J C, Sealfon S C, Miller W L. Endocrinology. 1994;134:1846–1850. doi: 10.1210/endo.134.4.8137751. [DOI] [PubMed] [Google Scholar]
- 27.Halmos G, Schally A V, Kahan Zs. Int J Oncol. 2000;17:367–373. doi: 10.3892/ijo.17.2.367. [DOI] [PubMed] [Google Scholar]
- 28.Lamharzi N, Halmos G, Jungwirth A, Schally A V. Int J Oncol. 1998;13:429–435. doi: 10.3892/ijo.13.3.429. [DOI] [PubMed] [Google Scholar]
- 29.Bajusz S, Csernus V J, Janaky T, Bokser L, Fekete M, Schally A V. Int J Pept Protein Res. 1988;32:425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 30.Csernus V J, Schally A V. In: Neuroendocrine Research Methods. Greenstein B D, editor. London: Harwood; 1991. pp. 71–109. [Google Scholar]
- 31.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Rekasi, Z., Schally, A. V., Plonowski, A., Czompoly, T., Csernus, B. & Varga, J. L. (2001) Prostate, in press. [DOI] [PubMed]
- 33.Kaiser U B, Zhao D, Cardona G R, Chin W W. Biochem Biophys Res Commun. 1992;189:1645–1652. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, Takizawa T, Funaba M, Fujimura H, Murata E, Torii K. Anal Biochem. 1997;244:172–174. doi: 10.1006/abio.1996.9890. [DOI] [PubMed] [Google Scholar]
- 35.Lerrant Y, Kottler M L, Bergametti F, Moumni M, Blumberg-Tick J, Counis R. Endocrinology. 1995;136:2803–2808. doi: 10.1210/endo.136.7.7789305. [DOI] [PubMed] [Google Scholar]
- 36.Levine J E. In: Encyclopedia of Reproduction. Knobil E, Neill J D, editors. San Diego: Academic; 1999. pp. 478–482. [Google Scholar]
- 37.Csernus V, Schally A V. Proc Natl Acad Sci USA. 1992;89:5759–5763. doi: 10.1073/pnas.89.13.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behre H M, Kliesch S, Pühse G, Reissmann T, Nieschlag E. J Clin Endocrinol Metab. 1997;82:1403–1408. doi: 10.1210/jcem.82.5.3959. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs M, Mezo I, Seprodi J, Csernus V, Teplan I, Flerko B. Peptides (Tarrytown, NY) 1989;10:925–931. doi: 10.1016/0196-9781(89)90170-8. [DOI] [PubMed] [Google Scholar]