Abstract
Isotretinoin (13-cis retinoic acid) is frequently prescribed for severe acne [Peck, G. L., Olsen, T. G., Yoder, F. W., Strauss, J. S., Downing, D. T., Pandya, M., Butkus, D. & Arnaud-Battandier, J. (1979) N. Engl. J. Med. 300, 329–333] but can impair night vision [Fraunfelder, F. T., LaBraico, J. M. & Meyer, S. M. (1985) Am. J. Ophthalmol. 100, 534–537] shortly after the beginning of therapy [Shulman, S. R. (1989) Am. J. Public Health 79, 1565–1568]. As rod photoreceptors are responsible for night vision, we administered isotretinoin to rats to learn whether night blindness resulted from rod cell death or from rod functional impairment. High-dose isotretinoin was given daily for 2 months and produced systemic toxicity, but this caused no histological loss of rod photoreceptors, and rod-driven electroretinogram amplitudes were normal after prolonged dark adaptation. Additional studies showed, however, that even a single dose of isotretinoin slowed the recovery of rod signaling after exposure to an intense bleaching light, and that rhodopsin regeneration was markedly slowed. When only a single dose was given, rod function recovered to normal within several days. Rods and cones both showed slow recovery from bleach after isotretinoin in rats and in mice. HPLC analysis of ocular retinoids after isotretinoin and an intense bleach showed decreased levels of rhodopsin chromophore, 11-cis retinal, and the accumulation of the biosynthetic intermediates, 11-cis and all-trans retinyl esters. Isotretinoin was also found to protect rat photoreceptors from light-induced damage, suggesting that strategies of altering retinoid cycling may have therapeutic implications for some forms of retinal and macular degeneration.
Keywords: vitamin A, rhodopsin, rat, electroretinogram, rod photoreceptor
Visual response in vertebrate rod photoreceptors begins when light isomerizes the rhodopsin chromophore 11-cis retinal to all-trans retinal. The bleached photopigment activates the signal transduction cascade within the rod, leading to membrane hyperpolarization and thereby to retinal visual signaling. Part of the recovery mechanism from bleach requires reconversion of the chromophore to 11-cis retinal by enzymatic reactions (the vitamin A cycle) that occur within the adjacent retinal pigment epithelium (RPE) (1). Disruption of the vitamin A cycle retards restoration of night vision sensitivity. Human dietary vitamin A deficiency can cause night blindness (2), as does dietary substitution of retinoic acid in rats (3). Alternatively, night blindness can result from death and loss of rod photoreceptors in retinal degeneration (4), including some forms involving mutations in genes necessary for retinoid processing in the RPE (5). Some isotretinoin patients report impaired visual sensitivity at night and daytime glare sensitivity, suggesting photoreceptor compromise or loss (6). We have investigated the mechanism of isotretinoin-induced night blindness by evaluating ERGs, rates of rhodopsin regeneration, and retinoid processing in rats. We also evaluated the possible therapeutic benefit of isotretinoin in the rat light-damage model of retinal degeneration.
Materials and Methods
Animals.
Protocols were approved by the University of Michigan's Committee on the Use and Care of Animals. Sprague–Dawley male 7-wk-old albino rats (Charles River Breeding Laboratories) were housed in 12:12 h light/dark cycle of 5 lux fluorescent white light. Rats were fed high-fat breeding chow (Formulab; PMI Feeds, St. Louis) containing 15 units/gm vitamin A. C57BL/6 mice were born in our laboratory and housed under 50 lux 12:12 h lighting.
Isotretinoin Treatment.
Injections of 40 mg/kg isotretinoin i.p. (Hoffmann–La Roche) in 0.18 ml DMSO (Sigma) were given daily to chronic rats for 8 wk. Controls received 0.18 ml DMSO i.p. Rats were killed 2 d after final injections. Acute treatment rodents were dark-adapted overnight and given a single i.p. injection of isotretinoin 40 mg/kg in 0.18 ml DMSO under dim red light and kept in darkness for 1 h before being exposed to the bleaching light before ERG measurements.
Histology.
Rats were euthanized (sodium pentobarbital, 300 mg/kg i.p.), and eyes were removed and placed overnight at 4°C in 2% paraformaldehyde and 2.5% gluteraldehdye in 0.1 M cacodylate buffer. Eyes were postfixed in 1% osmium for 1 h and embedded in Epon. One-micrometer-thick vertical sections through the optic nerve were cut and stained with toluidine blue for light microscopy. Column cell counts of outer nuclear layer thickness and rod outer segment (ROS) length were measured every 200 μm across both hemispheres, and the numbers were averaged to obtain a measure of cellular changes across the entire retina for comparison with full-field ERGs.
ERG Recordings.
Animals were prepared in dim red light and anaesthetized with xylazine (13 mg/kg i.m. loading dose, then 0.9 mg/kg/h) and ketamine (86 mg/kg i.m. loading dose, then 5 mg/kg/h). Pupils were dilated with 0.1% atropine and 0.1% phenylephrine HCl. Body temperature was maintained near 38°C while recording ERGs from both eyes simultaneously by using gold-wire corneal loops under 1% tetracaine topical anesthesia. Gold-wire differential electrodes were placed on the sclera near the limbus. A ground wire was attached to the ear. Responses were amplified (10,000 gain, 0.1–1,000 Hz). Xenon photostrobe full-field 30-μs flashes had 0.6 logarithm (log) cd⋅s/m2 maximum intensity. Photopic cone ERGs were elicited with 0.6 log cd⋅s/m2 stimuli on a rod-suppressing 42 cd/m2 white background.
ERG Analysis After Isotretinoin Treatment.
ERGs were recorded from chronic rats at 4 and 8 wks of treatment. In acute rodents, rod recovery from bleaching light was tracked by dark-adapted ERGs by using −3.4 log cd⋅s/m2 stimuli that elicit no cone contribution (7). Cone recovery was tracked with photopic ERGs. Responses were normalized by prebleach amplitudes.
Rhodopsin Measurement.
Procedures were carried out in dim red light by using infrared goggles. Rats were dark-adapted for 12 h and euthanized. Retinas were removed (8) and placed in 0.5 ml 1% emulphogene in 1-ml Eppendorf tubes (9). The tubes were kept in the dark at 4°C for 1 h, centrifuged, and the supernatant absorbance (400–700 nm) was determined (Lambda 20 UV-Visible Spectrophotometer, Perkin–Elmer). Rhodopsin was bleached completely by 5 min exposure to bright white light and rescanned to obtain difference spectra. Spectra were zeroed at 700 nm, and the rhodopsin amount in nanomols per eye was calculated from the absorbance difference at 500 nm.
Retinal Light Damage Experiments.
Rats were acutely treated with i.p. isotretinoin and 1 h later exposed to 2,000 lux white fluorescent light for 48 h (10). ERGs were recorded 7 d later, and histology was performed immediately.
Retinoid Extraction and Analysis.
Rats were euthanized, and eyes were enucleated and frozen at −80°C (11). Eye tissue was homogenized in 0.1 M Mops, pH 6.5/10 mM NH2OH/0.2% SDS (2 ml) in a ground glass homogenizer; 2 ml ethanol was added, and retinoids were extracted into hexane (10 ml total). Extracts were evaporated under argon, dissolved in 0.3 ml hexane, and the retinoid content analyzed by using HPLC normal phase HPLC [Supelcosil LC-SI column (250 mm × 4.6 mm, 3 μm); Supelco] and mobile phase of 95:5 hexane/dioxane at 1 ml/min flow rate. Absorbance was monitored at 325 nm, and peaks were identified by comparison to standards. The retinyl ester peak was collected, saponified with 0.35 M ethanolic KOH (12), and products analyzed by HPLC as described above. 13-cis retinal, all-trans retinal and all-trans retinyl palmitate standards were purchased (Sigma). 11-cis retinal (gift from the National Eye Institute, Bethesda, MD, and Rosalie Crouch, Medical University of South Carolina, Charleston). All-trans, 11-cis, and 13-cis retinol were generated from the aldehydes by reduction with NaBH4, and all-trans, 11-cis, and 13-cis retinal oxime were generated from the aldehydes by reaction with NH2OH (13).
Statistics.
Student two-tailed t test was performed with unequal variance and sample size (14). Two-way ANOVA was performed by using unbalanced repeated measures (15).
Results
Isotretinoin Chronically Treated Rats.
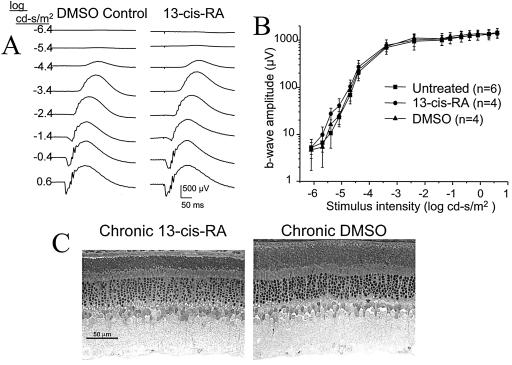
After 8 wks of daily 40 mg/kg isotretinoin i.p., rats showed systemic toxicity of weight loss, stomach distension, and diarrhea (16). The ERG waveforms remained normal (Fig. 1A), and the ERG intensity-response functions overlapped for isotretinoin-treated, DMSO control, and untreated rats (Fig. 1B). ERGs gave no evidence of impaired rod function. The dark-adapted b-wave maximum amplitude was unchanged from DMSO controls (P = 0.94) and untreated controls (P = 0.88) (Table 1). These animals were infirm after 8 wk of treatment, and one died from anesthesia during the ERG recordings before the maximum response was elicited. Retinal histology at 8 wk showed no loss of photoreceptor nuclei compared with DMSO controls (P = 0.37) (Fig. 1C; Table 2). ROS length was shorter (P = 0.02) by about 20% in the isotretinoin animals, consistent with a possible photostasis mechanism (17) (discussed below). One can conclude that chronic daily isotretinoin injections, sufficient to cause systemic toxicity, neither caused rod cell loss nor altered the ERG function after overnight dark adaptation.
Figure 1.
Chronic isotretinoin-treated rat. Rats received daily isotretinoin 40 mg/kg i.p. injections in DMSO. (A) ERG waveforms at 4 wk treatment, and (B) intensity-response function after 8 wk treatment. SE bars are shown. (C) Retinal histology after 8 wk treatment shows no significant outer nuclear layer cell loss (Table 2).
Table 1.
Chronic treatment: Dark-adapted maximum b-wave after 8 wk isotretinoin
| b-wave amplitude (μV) (stimulus 0.6 log cd⋅s/m2) | P value* vs. isotretinoin | |
|---|---|---|
| No. animals | Mean (SD) | |
| Normal (n = 6) | 1449 (293) | 0.88 |
| DMSO (n = 4) | 1503 (206) | 0.94 |
| Isotretinoin (n = 3) | 1484 (390) | — |
Student's two-tail t test across animals with both eyes averaged.
Table 2.
Histology of isotretinoin chronic treatment rats
| ONL (cell count) | ROS, μm | |
|---|---|---|
| 13-cis RA (n = six eyes) | 8.05 = 0.57 | 18.19 = 2.49 |
| DMSO (n = six eyes) | 8.49 = 0.96 | 22.05 = 2.27 |
| Student's two-tail t test | P = 0.37 | P = 0.02 |
ONL, outer nuclear layer.
Rod and Cone Recovery from Intense Bleach After Acute Treatment.
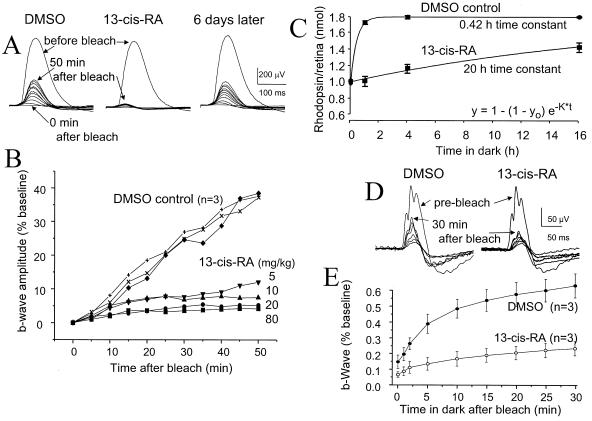
Dark-adapted rod b-wave recovery after intense bleaching light exposure was slowed markedly within 1 h of a single isotretinoin i.p. injection (Fig. 2 A and B). This effect dissipated within 6 days. The slowing of recovery correlated in graded fashion to isotretinoin doses of 5–80 mg/kg (Fig. 2B). Plotting the natural logarithm of b-wave normalized amplitude versus time linearized the recovery function (not shown), indicating that it followed an exponential course. The exponential recovery time constant (To) was slowed 15-fold by 40 mg/kg isotretinoin compared with untreated normals (P < 0.0001) and DMSO controls (P = 0.004) (Table 3). The recovery function had reverted to normal when recorded in these same animals 3–6 d later. Rat cone recovery from bleach (Fig. 2 D and E) was also slowed markedly by 40 mg/kg isotretinoin i.p., with To of 0.006 + 0.001/min (mean + SD, n = 3) compared with 0.037 + 0.016/min (n = 3) for DMSO controls (P = 0.03).
Figure 2.
Bleach recovery after acute isotretinoin in rat. (A and B) Rod dark-adapted ERG b-wave amplitude recovery in rat after 1,000-lux 25-s light exposure that bleaches 21% of rhodopsin. ERGs were recorded at bleach cessation and then every 5 min for 50 min, by using −3.4 log cd⋅s/m2 stimuli. (A) Three records from a single rat: in the DMSO “untreated” state, the b-wave amplitude recovered to 40% of prebleach amplitude by 50 min. After 40 mg/kg isotretinoin i.p. 1 h before the bleach, the b-wave recovered minimally over 50 min. b-wave bleach recovery had normalized on retesting 6 d later. (B) Graded b-wave recovery for 5–80 mg/kg isotretinoin. (C) Rhodopsin regeneration in vivo. Time points were determined from separate retinas, beginning 1 h after drug injection, with 6–12 control and isotretinoin replicates at 0, 1, and 4 h; 2 control and 4 isotretinoin replicates at 16 h. SE bars are shown. (D and E) Cone photopic ERG b-wave recovery in rat after a 1,000-lux 30-min bleach is slowed by a single isotretinoin 40 mg/kg i.p. injection. Cone photopic b-waves. SE bars are shown.
Table 3.
Time constant of rat rod b-wave recovery from bleach after acute isotretinoin (stimulus −3.4 log cd⋅s/m2)
| Exponential time constant (1/min) | P value* vs. isotretinoin | |
|---|---|---|
| No. animals | Mean (SD) | |
| Untreated (n = 4) | 0.0090 (0.0030) | <0.0001 |
| DMSO (n = 4) | 0.0089 (0.0041) | 0.0004 |
| Isotretinoin (n = 7) | 0.0006 (0.0004) | — |
| Recovery 3–6 d (n = 4) | 0.0095 (0.0032) | <0.0001 |
Student's two-tail t test; both eyes of each animal averaged.
Rhodopsin Regeneration After Bleaching.
Rhodopsin regeneration was measured to learn whether delayed ERG recovery resulted from slowed photopigment formation. The regeneration time constant To after 40 mg/kg isotretinoin i.p. was 20 h in treated animals versus 0.42 h in DMSO controls, or about 50 times longer (P < 0.0001) by two-way ANOVA. Isotretinoin did not alter the bleaching rate, because both control and treated retinas reached the same level of bleach after the short 5-min 300-lux exposure during which rhodopsin regeneration is negligible (Fig. 2C).
Isotretinoin Effect on Mouse Rod and Cone Recovery from Bleach.
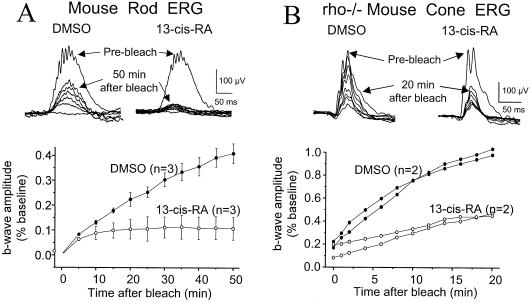
To learn whether the isotretinoin effect could be observed in other species, we injected mice (Fig. 3) and evaluated rod and cone recovery. Isotretinoin slowed rod recovery in mice to 0.000 + 0.0002/min (mean + SD), n = 3 versus 0.0106 + 0.0025/min, n = 3 for DMSO (P = 0.003). Cone ERG recovery was evaluated in Rho−/− mice (18), which do not express rhodopsin, allowing unequivocal isolation of cone responses (7). Cone b-wave recovery after bleach was slowed by isotretinoin [0.012 + 0.012/min (mean + SD), n = 2] versus DMSO control (0.125 + 0.005/min, n = 2; P = 0.007).
Figure 3.
Bleach recovery in mouse. (A) Rod dark-adapted b-waves before and after 1,000-lux 60-s bleach, by using −3.4 log cd⋅s/m2 stimuli. Isotretinoin 80 mg/kg i.p. was given 1 h before the bleach. SE bars are shown. (B) Cone photopic ERG recovery in Rho−/− mice after 5,000-lux 10-min bleach, showing 2 isotretinoin-treated and 2 DMSO control mice.
Analysis of Retinoid Processing.
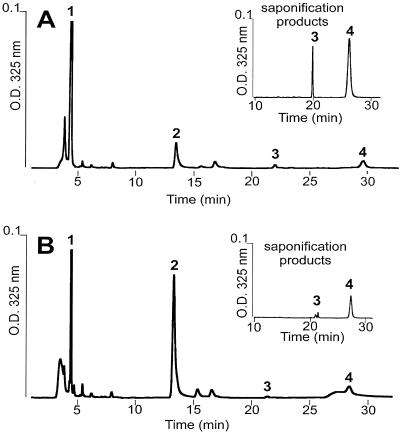
HPLC analysis of the retinoid content of the RPE and retina was used to determine the effect of isotretinoin treatment on the activity of the enzymes of the vitamin A cycle. Retinoid accumulation was assayed under various conditions. Control and acute-treatment rats were exposed to bleaching light and then dark-adapted for 2 h before retinoid extraction. Isotretinoin treatment resulted in two major changes in the HPLC chromatograms (Fig. 4 A and B): the accumulation of 11-cis retinal (measured as 11-cis retinal oxime) decreased to approximately one-fourth of control levels, and the retinyl esters increased almost 3-fold. Saponification showed that the accumulated esters consisted of all-trans and 11-cis retinyl esters in a 4:1 ratio (Fig. 4 A and B Insets). Considerable levels of isotretinoin were found in eye tissues by formaldehyde extraction conditions (19), but isotretinoin treatment alone, without light exposure followed by a recovery period in the dark, did not show any retinoid changes (data not shown). The findings are consistent with isotretinoin inhibition of the retinoid isomerase (isomerohydrolase) activity that is thought to convert and hydrolyze all-trans retinyl esters to 11-cis retinol (20, 21). In addition, evidence for inhibition of 11-cis retinol dehydrogenase (RDH) activity, which converts 11-cis retinol to 11-cis retinal, comes from the accumulation of 11-cis-retinyl esters. Gene disruption studies have shown that loss of RDH activity results in accumulation of 11-cis retinyl esters rather than 11-cis retinol (11). Significant accumulation of 11-cis retinol is limited by product inhibition of the retinoid isomerase (22). The small increase in 11-cis retinol seen after isotretinoin treatment was confirmed by using another method favorable to extraction of retinols and esters (data not shown) (23).
Figure 4.
HPLC retinoid analysis. Normal phase of retinoids extracted with hexane from four eyes each (A) isotretinoin and (B) untreated. (A and B Insets) The retinyl ester fraction (2.5–5.0 min) was collected, dried under argon, saponified, and analyzed as above. Peaks: (1) retinyl esters; (2) 11-cis retinal oxime syn; (3) 11-cis retinol; (4) all-trans retinol.
Rescue from Light-Induced Photoreceptor Damage.
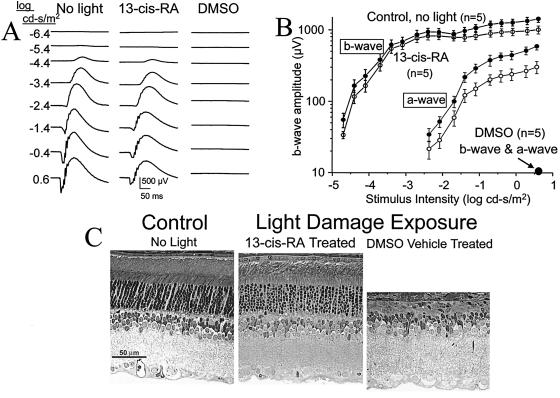
The light-damaged albino rat is a model of photoreceptor degeneration induced by exposure to moderate light levels for a number of hours (24, 25). There is increasing evidence that rhodopsin is the chromophore that mediates light damage (26, 27). Because isotretinoin slowed rhodopsin recovery after a bleaching light exposure, we explored the possibility that isotretinoin might convey light damage protection. Rats were given 40 mg/kg isotretinoin i.p. 1 h before starting 48-h 2,000-lux continuous light exposure. As the mean elimination half-life of isotretinoin is 10–20 h for human (28) but is unknown for rat, we injected the rats again at 24 h, halfway through the light exposure. Isotretinoin protection was evident by ERG functional assay (Fig. 5 A and B) and by histologic counts of photoreceptor nuclei remaining 1 week later (Fig. 5C). Compared with no-light controls, threshold sensitivity of the b-wave was shifted <0.2 log unit (50 μV criterion responses) in the isotretinoin treated eyes versus >5 log unit for DMSO treated animals; a-wave threshold (50 μV criterion responses) was shifted only one-third log unit for the treated eyes versus >2.5 log unit for the DMSO animals. a-wave and b-wave amplitudes in DMSO animals did not reach 50 μV levels even for the brightest stimulus. Retinal histology (Fig. 5C), performed 7 d later, showed substantial preservation of photoreceptor nuclei in isotretinoin-treated animals compared with DMSO controls (Table 4, P < 0.0001).
Figure 5.
Isotretinoin rescue from photoreceptor light damage in SD rat. (A and B) ERG a- and b-waves. Responses of DMSO control rats were <10 μV for both the a- and b-waves. SE bars are shown. (C) Histology of control and treated rats.
Table 4.
Histology 7 days after 48-h light damage exposure
| No. animals | ONL (cell count) | P value* |
|---|---|---|
| No light (n = 3) | 9.02 (SD = 0.36) | |
| Isotretinoin (n = 5) | 7.27 (SD = 0.91) | P = 0.02 vs. control |
| DMSO (n = 5) | 0.69 (SD = 0.33) | P < 0.0001 vs. 13-cis RA |
ONL, outer nuclear layer.
Student's two-tail t test; both eyes of each animal averaged.
Discussion
Relationship to Clinical Symptoms.
Isotretinoin administration in rats caused night blindness from impaired rod function without apparent retinal damage. Photoreceptor function returned to normal within days of acute treatment, consistent with the reversal of visual symptoms and ERG function in humans after discontinuing isotretinoin (6). Even chronic treatment that caused systemic toxicity did not cause rod loss, indicating that night blindness is not the result of rod cell death. The chronically treated rats were reared in dim light and were not exposed to intense bleaching light, and consequently they showed normal rod function after prolonged overnight dark adaptation. Human isotretinoin therapy involves far smaller doses than were used in this rodent study, which may account for the few numbers of individuals who experience substantial night blindness.
Slight ROS shortening after chronic isotretinoin treatment may result from “photostasis,” as albino rat photoreceptors adjust the ROS length and rhodopsin packing density to regulate the daily quantity of photons absorbed when animals are reared in cyclic illuminances over a 2-log unit range from 3 to 400 lux (17). This autoregulation occurs primarily through ROS renewal (29, 30). The slowed regeneration in isotretinoin-treated rats results in proportionally greater levels of steady-state bleach (although fewer photons absorbed) than control animals reared in the same light, and this might result in shorter ROS.
Mechanism of Action.
Isotretinoin slowed rhodopsin regeneration and chromophore recycling within 1 hour after systemic administration by inhibition of the retinoid isomerase and 11-cis retinol dehydrogenase enzymes that are necessary for the synthesis of 11-cis retinal in the RPE. Although retinoic acid and other retinoids can act as transcription factors (31), and 13-cis retinoic acid is a potent human teratogen (32), the time course of altered function observed experimentally appears to be too rapid to implicate a transcriptional mechanism.
11-cis retinol dehydrogenase (RDH5) is a candidate for inhibition by isotretinoin, as 13-cis retinoic acid inhibits this activity in vitro (33, 34). Human Rdh5 gene mutations (35) cause fundus albipunctatus, a retinal dystrophy with markedly delayed recovery of visual sensitivity after bleaching light exposure (5, 36). Both 11-cis retinol and 11-cis-retinyl esters accumulate in mice lacking Rdh5. However, Rdh5 knockout mice display normal dark-adaptation kinetics under bleaching light exposure conditions that cause considerable functional recovery deficit in fundus albipunctatus subjects (11). Further, the Rdh5 knockout mouse showed accumulation of all-trans-retinyl esters. Thus, isotretinoin inhibition of 11-cis retinol dehydrogenase appears insufficient to explain the effects we observed in rats and mice in vivo. Although isotretinoin did not inhibit retinoid isomerase activity of frog RPE membranes in vitro (34), our finding of the accumulation of all-trans-retinyl esters and 11-cis retinol in both rat and mouse treated with 13-cis-retinoic acid indicates inhibition of both the 11-cis retinol dehydrogenase and the retinoid isomerase in vivo. This effect could be direct, at the level of the enzymes themselves, or could result from competitive binding of 13-cis retinoic acid to retinoid-binding proteins that are involved in retinoid transport within and between cells.
Light Damage Rescue.
The slowing of rhodopsin regeneration by isotretinoin protects photoreceptors from light damage and provides evidence that bleaching and turnover of rhodopsin are required to initiate damage. This is consistent with recent studies showing that the absence of rhodopsin in Rho−/− mice (18) and Rpe65−/− mice (37) confers nearly complete protection from light damage (27). Although the subsequent steps in the damage cascade are unknown, these isotretinoin studies serve to exclude several possible mechanisms. First, bleaching all of the rhodopsin in the retina and maintaining this fully bleached state by preventing rhodopsin regeneration is not sufficient to cause substantial light damage. The high-intensity damaging light and 50-fold slowing of rhodopsin regeneration result in a nearly complete bleach for the duration of exposure. However, because chromophore regeneration is the rate-limiting step in rhodopsin turnover at high-light levels (17, 38), many fewer photons are absorbed in the retina of isotretinoin-treated rats during the 48-h exposure. Second, “constitutively active” rhodopsin (39) is believed to contribute to photoreceptor degeneration in some genetic models through continuous low-level transduction signaling (40). Free opsin may activate transduction with low catalytic activity (41, 42), and this is thought to be the mechanism of bleaching adaptation (43). Slowed chromophore recycling after isotretinoin will load the ROS with opsin during the 48-h light exposure, but evidently the resulting low level of transduction activation is not sufficient for significant light damage. Third, residual transduction activation should hyperpolarize the rod (43). Further, even with the 50-fold slowing of rhodopsin regeneration by isotretinoin, residual cycling of chromophore will reconstitute some rhodopsin and activate transduction by conventional photon capture. Consequently, rods in these treated animals should remain hyperpolarized for the duration of the light exposure, but this chronic hyperpolarization apparently does not cause significant damage.
Therapeutic Implications for Genetic Photoreceptor Degeneration.
Manipulation of rhodopsin turnover may provide a useful therapeutic strategy for retinal and macular degenerations. Light accelerates retinal degeneration in several animal models that have corresponding human conditions, including transgenic mice expressing mutant opsin (44), the RCS rat (45), the rds mouse (46), the rhodopsin kinase knockout mouse (47), and the arrestin knockout mouse (48). Reducing the numbers of photoisomerization events may be beneficial in some genetic retinal diseases. A possible therapeutic strategy would be to decrease rhodopsin turnover by limiting the production of 11-cis retinal.
This strategy might be tested in the Abcr−/− mouse. The ABCR protein moves bleached retinoid across the rod disk membrane (49). In the Abcr−/− mouse, bleached chromophore couples with ethanolamine to form lipofuscin (50), which accumulate in the RPE (51), and in human, this leads to vision loss in Stargardt macular degeneration (52). As dark rearing of Abcr−/− mice prolongs rod cell survival, presumably by decreasing the retinoid turnover, a possible therapeutic strategy would be to slow production of 11-cis retinal and thereby limit isomerization events.
Acknowledgments
Laureen Kononen, Brad Nelson, and Austra Liepa assisted with these studies. This work was supported by National Institutes of Health Grants R01-EY06094, R01-EY12298, P30-EY07003, the Foundation Fighting Blindness (Hunt Valley, MD), Senior Scientific Investigator (P.A.S.) and Lew Wasserman Merit (D.A.T.) Awards from Research to Prevent Blindness, an Alcon Research Institute Award (P.A.S.), and a research gift from Thyssen Steel, North America. The 13-cis retinoic acid was a gift from Hoffmann–La Roche.
Abbreviations
- RPE
retinal pigment epithelium
- ERG
electroretinogram
- ROS
rod outer segment
- log
logarithm
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041606498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041606498
References
- 1.Crouch R K, Chader G J, Wiggert B, Pepperberg D R. Photochem Photobiol. 1996;64:613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 2.Hecht S, Mandelbaum J. Science. 1938;88:219–221. doi: 10.1126/science.88.2279.219. [DOI] [PubMed] [Google Scholar]
- 3.Dowling J E, Wald G. Proc Natl Acad Sci USA. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berson E L. Am J Ophthalmol. 1995;120:683–684. doi: 10.1016/s0002-9394(14)72223-8. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Simon A, Eriksson U, Harris E, Berson E L, Dryja T P. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 6.Weleber R G, Denman S T, Hanifin J M, Cunningham W J. Arch Ophthalmol. 1986;104:831–837. doi: 10.1001/archopht.1986.01050180065031. [DOI] [PubMed] [Google Scholar]
- 7.Toda K, Bush R A, Humphries P, Sieving P A. Visual Neurosci. 1999;16:391–398. doi: 10.1017/s0952523899162187. [DOI] [PubMed] [Google Scholar]
- 8.Fulton A B, Manning K A, Baker B N, Schukar S E, Bailey C J. Invest Ophthalmol Visual Sci. 1982;22:386–393. [PubMed] [Google Scholar]
- 9.Williams T P, Henrich S, Reiser M. Invest Ophthalmol Visual Sci. 1998;39:603–609. [PubMed] [Google Scholar]
- 10.Sugawara T, Sieving P A, Bush R A. Exp Eye Res. 2000;70:693–705. doi: 10.1006/exer.2000.0842. [DOI] [PubMed] [Google Scholar]
- 11.Driessen C A, Winkens H J, Hoffmann K, Kuhlmann L D, Janssen B P, Van Vugt A H, Van Hooser J P, Wieringa B E, Deutman A F, Palczewski K, et al. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges C D, Alvarez R A. Methods Enzymol. 1982;81:463–485. doi: 10.1016/s0076-6879(82)81065-3. [DOI] [PubMed] [Google Scholar]
- 13.Landers G M. Methods Enzymol. 1990;189:70–80. doi: 10.1016/0076-6879(90)89277-o. [DOI] [PubMed] [Google Scholar]
- 14.Sokol R R, Rohlf F J. Biometry. San Francisco: Freeman; 1969. [Google Scholar]
- 15.Glantz S A, Slinker B K. Primer of Applied Regression and Analysis of Variance. New York: McGraw–Hill; 1990. [Google Scholar]
- 16.Lambert R W, Smith R E. Invest Ophthalmol Visual Sci. 1988;29:1559–1564. [PubMed] [Google Scholar]
- 17.Penn J S, Williams T P. Exp Eye Res. 1986;43:915–928. doi: 10.1016/0014-4835(86)90070-9. [DOI] [PubMed] [Google Scholar]
- 18.Humphries M M, Rancourt D, Farrar G J, Kenna P, Hazel M, Bush R A, Sieving P A, Sheils D M, McNally N, Creighton P, et al. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Fujita Y, Noda Y, Miyata S. Vision Res. 1986;26:425–429. doi: 10.1016/0042-6989(86)90185-9. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein P S, Law W C, Rando R R. J Biol Chem. 1987;262:16848–16857. [PubMed] [Google Scholar]
- 21.Deigner P S, Law W C, Canada F J, Rando R R. Science. 1989;244:968–971. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- 22.Winston A, Rando R R. Biochemistry. 1998;37:2044–2050. doi: 10.1021/bi971908d. [DOI] [PubMed] [Google Scholar]
- 23.Okajima T L, Pepperberg D R. Exp Eye Res. 1997;65:331–340. doi: 10.1006/exer.1997.0331. [DOI] [PubMed] [Google Scholar]
- 24.Noell W K, Walker V S, Kang B S, Berman S. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- 25.Organisciak D T, Winkler B S. In: Progress in Retinal and Eye Research. Osborne N N, Chader G J, editors. Vol. 13. New York: Pergamon; 1994. pp. 1–29. [Google Scholar]
- 26.Williams T P, Howell W L. Invest Ophthalmol Visual Sci. 1983;24:285–287. [PubMed] [Google Scholar]
- 27.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond T M, Reme C E. Nat Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 28.Dowd A L, editor. PDR. Physicians' Desk Reference. Montvale, NJ: Medical Economics Company; 1993. [Google Scholar]
- 29.Schremser J L, Williams T P. Exp Eye Res. 1995;61:25–32. doi: 10.1016/s0014-4835(95)80055-7. [DOI] [PubMed] [Google Scholar]
- 30.Schremser J L, Williams T P. Exp Eye Res. 1995;61:17–23. doi: 10.1016/s0014-4835(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 31.Kelley M W, Williams R C, Turner J K, Creech-Kraft J M, Reh T A. NeuroReport. 1999;10:2389–2394. doi: 10.1097/00001756-199908020-00031. [DOI] [PubMed] [Google Scholar]
- 32.Stern R S. N Engl J Med. 1989;320:1007–1009. doi: 10.1056/NEJM198904133201510. [DOI] [PubMed] [Google Scholar]
- 33.Gamble M V, Mata N L, Tsin A T, Mertz J R, Blaner W S. Biochim Biophys Acta. 2000;1476:3–8. doi: 10.1016/s0167-4838(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 34.Law W C, Rando R R. Biochem Biophys Res Commun. 1989;161:825–829. doi: 10.1016/0006-291x(89)92674-0. [DOI] [PubMed] [Google Scholar]
- 35.Simon A, Lagercrantz J, Bajalica-Lagercrantz S, Eriksson U. Genomics. 1996;36:424–430. doi: 10.1006/geno.1996.0487. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Fernandez F, Kurz D, Bao Y, Newman S, Conway B P, Young J E, Han D P, Khani S C. Mol Vis. 1999;5:41. http://www.molvis.org/molvs/v5/p41 . ( http://www.molvis.org/molvs/v5/p41) ) [PubMed] [Google Scholar]
- 37.Redmond T M, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma J X, Crouch R K, Pfeifer K. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 38.Penn J S, Anderson R E. In: Progress in Retinal Research. Osborne N, Chader G, editors. Oxford: Pergamon; 1992. pp. pp.75–98. [Google Scholar]
- 39.Robinson P R, Cohen G B, Zhukovsky E A, Oprian D D. Neuron. 1992;9:719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- 40.Fain G L, Lisman J E. Exp Eye Res. 1993;57:335–340. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- 41.Cornwall M C, Fain G L. J Physiol (London) 1994;480:261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornwall M C, Matthews H R, Crouch R K, Fain G L. J Gen Physiol. 1995;106:543–557. doi: 10.1085/jgp.106.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kefalov V J, Carter Cornwall M, Crouch R K. J Gen Physiol. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Lam T T, Tso M O, Naash M I. Visual Neurosci. 1997;14:55–62. doi: 10.1017/s0952523800008750. [DOI] [PubMed] [Google Scholar]
- 45.Organisciak D T, Li M, Darrow R M, Farber D B. Curr Eye Res. 1999;19:188–196. doi: 10.1076/ceyr.19.2.188.5333. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal S, Hawkins R K. Vision Res. 1986;26:1177–1185. doi: 10.1016/0042-6989(86)90099-4. [DOI] [PubMed] [Google Scholar]
- 47.Chen C K, Burns M E, Spencer M, Niemi G A, Chen J, Hurley J B, Baylor D A, Simon M I. Proc Natl Acad Sci USA. 1999;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Simon M I, Matthes M T, Yasumura D, LaVail M M. Invest Ophthalmol Visual Sci. 1999;40:2978–2982. [PubMed] [Google Scholar]
- 49.Weng J, Mata N L, Azarian S M, Tzekov R T, Birch D G, Travis G H. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 50.Eldred G E. Gerontology. 1995;41:15–28. doi: 10.1159/000213722. [DOI] [PubMed] [Google Scholar]
- 51.Mata N L, Weng J, Travis G H. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. . (First Published June 13, 2000; 10.1073/pnas.130110497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allikmets R, Singh N, Sun H, Shroyer N F, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]