Abstract
Background
Large scale in vitro production of the mosquito stages of malaria parasites remains elusive, with only limited success for complete sporogonic development and only one report of development through to infective sporozoites. The initial step in this process is the production, in vitro, of ookinetes from gametocytaemic blood. Methods for isolation of these ookinetes from blood cells have been described; however, in addition to yield often being low, processing time and potential for contamination by erythrocytes remain high.
Methods
This study compares two procedures for retaining mature ookinetes from blood stage cultures, whilst removing red blood cells and other contaminants prior to further culture of the parasite. The well established method of isolation on Nycodenz cushions is compared with a novel method utilizing the innate magnetic properties of the haem pigment crystals found in the cytoplasm of ookinetes.
Results
Yield and viability of ookinetes were similar with both isolation methods. However, in our hands magnetic isolation produced a cleaner ookinete preparation much more quickly. Moreover, decreasing the flow rate through the magnetic column could further enhance the yield.
Conclusion
We recommend the enrichment of an ookinete preparation prior to further culture being performed using the magnetic properties of Plasmodium berghei ookinetes as an alternative to their density. The former technique is faster, removes more erythrocytes, but day-to-day costs are greater.
Background
Studies on ookinete stages of the malaria parasite were greatly aided by the development, over 30 years ago, of a method for the production of ookinetes in vitro following 24 h culture of gametocytaemic blood. The basic protocol for isolation of gametocytes from infected blood and subsequent ookinete development was described by Weiss & Vanderberg [1] and has since been modified and improved [2]. Experimenters have different opinions concerning the need to remove white blood cells prior to culture. This is advisable as we have shown a significant decrease in the number of ookinetes undergoing apoptosis during development, if this is performed [3]. Following culture for 20–24 h, the usual method for ookinete separation and enrichment is on density gradients. Silica gradients (Percoll) have been used since the 1980's for gametocyte concentration (e.g. [4,5]); however, Nycodenz cushions have largely replaced Percoll due to its ease of use and reduced toxicity to Plasmodium [6]. Differing cushion concentrations allow enrichment of either zygotes or ookinetes [7]. Inhibitors of DNA synthesis (such as mitomycin C, aphidicolin and Berenil [8,9]) have been successfully employed as tools to further enrich ookinete preparations by eliminating asexual stages from gametocytaemic blood. This objective has also been achieved using pyrimethamine [7]. In addition, magnetized beads coated in an antibody raised against a major ookinete surface protein, P28, have been used to isolate ookinetes from erythrocytes [10].
Ookinete enrichment is labour intensive and time consuming and is likely to dramatically reduce ookinete yield. Furthermore, complete separation to the exclusion of all other blood cells is difficult. Here we describe a novel method for purification based on the magnetic properties of the parasite itself and compare this with enrichment on a Nycodenz cushion.
Methods
Infections and blood processing
CD mice were treated with phenylhydrazine two days prior to infection by intra peritoneal inoculation with P. berghei ANKA strain, obtained from a donor mouse between the second and sixth passage from cryopreserved stock [11]. Parasitaemia was checked on day three post-infection by microscopic examination of thin blood films and the presence of exflagellating gametocytes was determined in thick blood films. Gametocytaemic blood was collected into a heparinized syringe via cardiac puncture and retained on ice prior to removal of white blood cells. This was achieved by passing the blood through a pre-equilibrated 5 ml sterile column containing 1 ml glass wool and 3 ml of Whatmann CF11 cellulose powder (Beckton & Dickenson). The blood was washed through the column with 10 mls of modified RPMI 1640 medium (9 ml RPMI [per litre: 16.4 g RPMI medium (Sigma); 2 g sodium bicarbonate; 10 ml penicillin (1000 U/ml) / streptomycin (1 mg/ml); 50 mg hypoxanthine] with 1 ml heat inactivated foetal bovine serum (Invitrogen)). The blood was collected into T25 culture flasks, leaving a final culture volume of approximately 11 ml. The majority of ookinetes had developed during 20 hours incubation at 19°C. Cultures from different mice were pooled, ookinetes counted and the culture divided equally between methods of purification.
Ookinete isolation methods
Two methods were employed for comparison of yield and efficiency of ookinete isolation from uninfected erythrocytes, namely: suspension on a Nycodenz cushion and a novel technique using a magnet to retain ookinetes.
Enrichment on a Nycodenz cushion was performed as previously described [5]. Briefly, following incubation erythrocytes were lysed with 0.34 M NH4Cl and the suspension was then layered onto a cushion of 17% Nycodenz at 20°C and spun for 30 min at 1660 g. Ookinetes were removed from the interface, washed and counted.
For magnetic isolation MidiMAC LS columns were mounted on a powerful magnet suspended from a metal plate (MidiMACS Separation system, Miltenyi Biotec) (see Figure 1). A pre-separation filter was added to the top of the column to remove cellular debris. A 21G (0.8 × 40 mm needle) was added to the base of the column to reduce the speed of flow. The column was given two washes with 2.5 ml Schneider's medium prior to addition of the ookinete culture described above. Erythrocytes were then eluted with two washes of 2.5 ml Schneider's medium and a further wash with 2.5 ml oocyst medium (per 100 ml: 23.8 mM NaHCO3, 0.367 mM hypoxanthine, 10 mg streptomycin, 10,000 U penicillin, 20 mg gentamicin, 44 nM PABA, 15 ml heat-inactivated FBS, 200 μl lipoprotein and cholesterol (Sigma), 83.48 ml Schneider's medium, pH 7.0). Ookinetes were released by removing the column from the magnetic field and washed through into a sterile collection tube with 2.5 ml of oocyst medium. Following washing, ookinetes were resuspended in 1 ml of oocyst medium and aliquots were examined in a haemocytometer. Ookinetes were counted and numbers were compared with the starting density to determine yield. In addition, an assessment was made of the number of red blood cells and the amount of debris contaminating the preparation.
Figure 1.
Equipment for isolation using the magnetic properties of ookinetes. Gametocytaemic mouse blood that has been cultured for 20 h is added to the LS column via a pre-separation filter. The speed of flow through the column is reduced by adding a needle to the base, allowing time for ookinetes to be captured in the magnetized column. Parasites are eluted with oocyst culture medium when the column is released from the magnet.
To compare the viability of ookinetes isolated using the different methods, parasites were maintained in vitro as described previously [12]. Ookinetes were seeded into 8-well slides at a density of 1 × 104 and the number of oocyst per well were counted after nine days of culture in oocyst medium in the presence of Drosophila S2 cells at 1 × 105 per well.
In an attempt to increase the yield of ookinetes from magnet isolation, the speed of flow of blood through the column was reduced by decreasing the gauge of needle used. Assessments were carried out with needle sizes of 21G (0.8 × 40 mm) (Terumo, Europe), 23G (0.6 × 25 mm) and 25G (0.5 × 16 mm) (Sherwood, UK). Yield from the magnetized columns was also compared using the syringe plunger supplied with the column, or allowing the elution to occur under gravity.
Assessment of methods
Several criteria were used to compare ookinete enrichment methods: 1) yield was calculated as the percentage of parasites recovered from each method of purification compared to the initial density of parasites, 2) observations were made of the degree of contamination with erythrocyte material after re-suspension of equal volumes of purified parasites, 3) potential to transform into oocysts and develop in culture was also compared, 4) assessments of preparation time and costs of each method were made. Finally, percentage yield when using needles of different gauge was calculated. Statistical analysis of the comparison of yield with varying needle size was performed by one-way analysis of variance (ANOVA). All other analyses were performed using the Student T test where a P value <0.05 was considered significant.
Results and Discussion
Comparison of ookinete yield using different separation techniques
Ookinete yields using the standard Nycodenz density gradient separation method were not significantly different from the novel magnet isolation using a 21G needle (Table 1, T = 0.49 p = 0.61). In our hands, only 1% of ookinetes was recovered from either separation method. However, yield is significantly improved using different gauge needles (see below).
Table 1.
Comparison of ookinete yield from two purification methods. For each purification method (Nycodenz cushion v magnet), the initial density of ookinetes is expressed per ml of blood. Final yield is expressed as a percentage ± SEM of the pre-separation density, n = 4 experiments, each using the pooled blood from two infected mice. No significant difference was observed in yield between the two methods (Student T-test, p > 0.05).
| Pre-separation | Nycodenz density gradient | Magnet |
| Density: | Yield: | Yield: |
| 3.75 ± 0.48 × 107/ml | 3.8 ± 0.56 × 105/ml | 4.1 ± 0.48 × 105/ml |
| Percentage: | Percentage: | Percentage: |
| 100% | 1.04 ± 0.17 % | 1.11 ± 0.12 % |
Comparison of ookinete purity and viability using different separation techniques
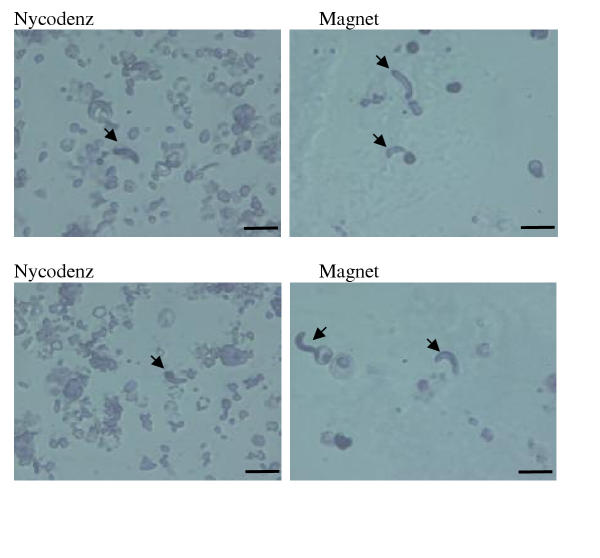
Magnetic isolation of ookinetes from infected blood proved far better for removing the majority of red blood cells and contaminating cellular debris (Figure 2). This facilitated location and quantification of ookinetes prior to further culture and reduced the potential for contaminating material to enter the oocyst culture. We regard this as a major advantage of this system if the primary purpose of ookinete preparation is as a starting point for in vitro culture of further sporogonic stages. It is interesting to note that the majority of red blood cells remaining in the preparation contained asexual stages of the parasite. Due to the presence of haemazoin, these would also be attracted to the magnet. If an ookinete preparation is required to be completely free of other parasite stages, we suggest that a technique to remove asexual stages in vivo prior to collection of gametocytes is employed [3,4] in conjunction with magnetic purification of ookinetes. Alternatively, the remaining erythrocytes could be removed by a second separation using, for example, a lectin to precipitate or immobilize these cells.
Figure 2.
Comparison of the degree of red blood cell contamination using two different purification methods. Enriched ookinete preparations were resuspended in an equal volume of oocyst medium and the degree of contamination by red blood cells and debris was assessed using an equal volume of parasite material. Preparations were viewed at a magnification of × 400. Ookinetes are indicated by an arrow. Scale bar = 10 μm. Note heavy contamination with other parasite stages/cellular debris following purification on a Nycodenz cushion.
Ookinete potential for growth in culture was assessed at day nine. Purification method did not affect the number of ookinetes transforming into oocysts and continuing growth in culture (Table 2, T = 0.12, p = 0.906). This suggests that the greatly increased length of time taken to perform the purification using Nycodenz cushions had no effect on viability.
Table 2.
Oocyst development following different separation methods. Ookinetes from Nycodenz cushion and magnet separations were cultured for nine days. The total number of oocysts per well was then counted for three wells. The experiment was replicated four times, using blood from different mice. No significant difference in oocyst numbers was observed between or within experiments (Student's T test, p = >0.05).
| Experiment | Magnet Mean no. oocysts per well ± SEM | Nycodenz Mean no. oocysts per well ± SEM |
| 1 | 788 ± 67.7 | 936 ± 52.6 |
| 2 | 872 ± 56.7 | 810 ± 64.7 |
| 3 | 773 ± 14.0 | 793 ± 25.1 |
| 4 | 843 ± 34.1 | 755 ± 54.2 |
Comparison of ookinete yield with varying needle size
As magnetic isolation was found to provide a purer ookinete preparation, attempts to optimise yield were assessed. Reduction in the flow of blood through the column by decreasing the size of the needle gauge was found to significantly increase the yield obtained (F = 17.20, p < 0.001) from 1% to 14% (Table 3). However, once the rate of flow was greatly reduced, preparation time increased and, more importantly, a large number of red blood cells were not eluted from the column and thus contamination increased considerably. The optimum needle size is thus 23G. Use of the barrel plunger did not increase yield (Table 3).
Table 3.
Ookinete yield from the magnetised column using different needle sizes. Ookinetes were separated from the remaining blood culture using the magnetized column with varying needle sizes to reduce the speed of blood flow. Ookinete yield is expressed as a percentage of the pre-separation density, calculated from pooled blood from four mice, n = 4 experiments. All three needles were used in conjunction with the accompanying column plunger, and the most effective needle in terms of yield and lack of contaminants, 23G, was also assessed without using the plunger. * = mean values with different letters are significantly different (ANOVA, p < 0.05)
| Experiment | % yield with 21G needle | % yield with 23G needle | % yield with 23G needle, no plunger | % yield with 25G needle |
| 1 | 3.73 | 9.28 | 8.89 | 21.14 |
| 2 | 4.62 | 7.40 | 7.34 | 10.46 |
| 3 | 2.79 | 6.54 | 4.90 | 16.32 |
| 4 | 5.12 | 7.97 | 7.97 | 11.41 |
|
Mean ± SEM * |
4.07 ± 0.51 a |
7.75 ± 0.57 b |
7.03 ± 0.76 b |
14.83 ± 2.46 c |
Comparison of time/cost for ookinete separation methods
One of the major advantages of the magnet separation is the speed with which ookinetes can be purified ready for further culture to oocysts. From the initial removal of the 20–24 h parasite culture from the flask, purification and counting can be completed in less than 45 min whereas this process takes 2 h 30 min if red blood cells are lysed and separated on a Nycodenz cushion.
Each magnet separation is, however, more costly than purification on Nycodenz cushions. Current costs in the UK amount to approximately £14 for purification of ookinetes obtained from two infected mice (approximately 7.5 × 105 ookinetes) compared with just over £2 for the Nycodenz cushion (Nycodenz, £1.60, ammonium chloride £0.35 and PBS £0.06). The MidiMACs column and pre-separation filter account for most of this cost (£10 and £50 respectively).
Conclusions
The main advantage of magnetic separation for routine isolation of ookinetes is its speed and simplicity. Apart from the magnet, no additional equipment is required and no skill, other than observance of routine aseptic technique, is necessary. Thus this method provides an alternative approach that other laboratories may wish to adopt. This technique has recently been used successfully to purify P. falciparum ookinetes grown in culture (JW unpublished observations).
Authors' contributions
HC performed the majority of the technique comparisons, assisted by VC, who also first drafted the manuscript and BAU who performed mouse infections. JW originally conceived of use of the magnet separation for ookinetes and participated in the design of the study. HH participated in the design and coordination of the study and drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful for financial support from the U.S. Army Medical Research and Material Command (JW, HC), the Wellcome Trust (VC) and the generous support of the Naval Medical Research Centre.
Contributor Information
Victoria Carter, Email: bia11@keele.ac.uk.
Hazel C Cable, Email: bia31@keele.ac.uk.
B Ann Underhill, Email: bia50@keele.ac.uk.
Jackie Williams, Email: Jackie.Williams@na.amed.army.mil.
Hilary Hurd, Email: h.hurd@keele.ac.uk.
References
- Weiss MM, Vanderberg JP. Studies on Plasmodium ookinetes: II. In vitro formation of Plasmodium berghei ookinetes. J Parasitol. 1977;63:932–934. [PubMed] [Google Scholar]
- Janse CJ, Mons B, Rouwenhorst RJ, Van der Klooster PF, Overdulve JP, Van der Kaay HJ. In vitro formation of ookinetes and functional maturity of Plasmodium berghei gametocytes. Parasitology. 1985;91 ( Pt 1):19–29. doi: 10.1017/s0031182000056481. [DOI] [PubMed] [Google Scholar]
- Al-Olayan EM, Williams GT, Hurd H. Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int J Parasitol. 2002;32:1133–1143. doi: 10.1016/S0020-7519(02)00087-5. [DOI] [PubMed] [Google Scholar]
- Knight A, Sinden RE. The purification of gametocytes of Plasmodium falciparum and P. yoelii nigeriensis by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1982;76:503–509. doi: 10.1016/0035-9203(82)90150-x. [DOI] [PubMed] [Google Scholar]
- Munderloh UG, Kurtti TJ. The infectivity and purification of cultured Plasmodium berghei ookinetes. J Parasitol. 1987;73:919–923. [PubMed] [Google Scholar]
- Carter EH, Suhrbier A, Beckers PJ, Sinden RE. The in vitro cultivation of P. falciparum ookinetes, and their enrichment on Nycodenz density gradients. Parasitology. 1987;95 ( Pt 1):25–30. doi: 10.1017/s0031182000057516. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Margos G, Compton H, Ku M, Lanz H, Rodriguez MH, Sinden RE. Plasmodium berghei: routine production of pure gametocytes, extracellular gametes, zygotes, and ookinetes. Exp Parasitol. 2002;101:73–76. doi: 10.1016/S0014-4894(02)00035-8. [DOI] [PubMed] [Google Scholar]
- Dearsly AL, Nicholas J, Sinden RE. Sexual development in Plasmodium berghei: the use of mitomycin C to separate infective gametocytes in vivo and ookinetes in vitro. Int J Parasitol. 1987;17:1307–1312. doi: 10.1016/0020-7519(87)90096-8. [DOI] [PubMed] [Google Scholar]
- Ono T, Ohnishi Y, Nagamune K, Kano M. Gametocytogenesis induction by Berenil in cultured Plasmodium falciparum. Exp Parasitol. 1993;77:74–78. doi: 10.1006/expr.1993.1062. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Vlachou D, Margos G, Beetsma A, Waters AP, Sinden RE, Louis C. Distinct roles for pbs21 and pbs25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J Cell Sci. 2000;113 Pt 19:3419–3426. doi: 10.1242/jcs.113.19.3419. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Infection of mosquitoes with rodent malaria. In: Crampton J M, Beard C B and Louise C, editor. The Molecular Biology of Disease Vectors: a methods manual. London, Chapman and Hall; 1997. pp. 67–91. [Google Scholar]
- Al-Olayan EM, Beetsma AL, Butcher GA, Sinden RE, Hurd H. Complete development of mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–679. doi: 10.1126/science.1067159. [DOI] [PubMed] [Google Scholar]