Abstract
Metastasis is the primary cause of death in human breast cancer. Metastasis to bone, lungs, liver, and brain involves dissemination of breast cancer cells via the bloodstream and requires adhesion within the vasculature. Blood cell adhesion within the vasculature depends on integrins, a family of transmembrane adhesion receptors, and is regulated by integrin activation. Here we show that integrin αvβ3 supports breast cancer cell attachment under blood flow conditions in an activation-dependent manner. Integrin αvβ3 was found in two distinct functional states in human breast cancer cells. The activated, but not the nonactivated, state supported tumor cell arrest during blood flow through interaction with platelets. Importantly, activated αvβ3 was expressed by freshly isolated metastatic human breast cancer cells and variants of the MDA-MB 435 human breast cancer cell line, derived from mammary fat pad tumors or distant metastases in severe combined immunodeficient mice. Expression of constitutively activated mutant αvβ3D723R, but not αvβ3WT, in MDA-MB 435 cells strongly promoted metastasis in the mouse model. Thus breast cancer cells can exhibit a platelet-interactive and metastatic phenotype that is controlled by the activation of integrin αvβ3. Consequently, alterations within tumors that lead to the aberrant control of integrin activation are expected to adversely affect the course of human breast cancer.
Complications from metastatic disease are the primary cause of death in breast cancer. Metastasis to bone, lungs, liver, and brain involves dissemination of tumor cells via the bloodstream (1). This process depends on tumor cell intravasation, adhesion to the vessel wall, extravasation, infiltration, and proliferation into target tissue. Many of these steps involve integrins, a family of transmembrane adhesion receptors composed of noncovalently linked α and β subunits (2). Integrins are known to exist in distinct activation states, which exhibit different affinities for ligand. In general, integrin activation controls cell adhesion (3). Such control is particularly important in the vasculature, where dynamic flow physically opposes cell attachment.
Integrin αvβ3 has been implicated in the pathophysiology of malignant tumors. It plays a role on endothelial cells, where it is required for tumor angiogenesis (4). In several malignancies, however, the tumor cells express αvβ3, and this expression correlates with tumor progression in melanoma, glioma, and ovarian and breast cancer (5–8). In breast cancer, αvβ3 characterizes the metastatic phenotype, as this integrin is up-regulated in invasive tumors and distant metastases (9). However, a mechanistic role of αvβ3 in breast cancer spread has yet to be established. We suggested that an interaction of circulating tumor cells with platelets represents a potential mechanism for tumor cell arrest within the vasculature (10). During blood flow, shear forces oppose cell attachment. Therefore, cells must be equipped with specific adhesive mechanisms to support cell arrest (11). Intravascular attachment of leukocytes and platelets during inflammation and thrombus formation is tightly regulated and depends on integrin activation (12–14). It is unknown whether integrin activation controls tumor cell arrest in a similar manner. Here we provide evidence that activation of integrin αvβ3 promotes breast cancer cell arrest during blood flow and controls the metastatic activity. Consequently, alterations within tumors that support integrin activation are expected to adversely affect the course of human breast cancer.
Materials and Methods
Matrix Proteins.
Bovine fibrillar collagen I (Sigma) was used as a thrombogenic matrix in blood perfusion (10). Vitronectin and fibrinogen were purified from human plasma by affinity (15) or gel filtration (16) chromatography. Human plasma fibronectin was purchased from Collaborative Biomedical Products, Bedford, MA.
Antibodies.
All antibodies were murine monoclonal IgGs except WOW-1, a recombinant Fab fragment (17). They were purified on protein A. mAb VNR1 27.1 (function blocking anti-αvβ3) (18) served to test αvβ3-mediated cell adhesion. mAb 15 (anti-β3) (18) was conjugated to saporin to select β3-negative breast cancer cells. mAbs LM609 (anti-αvβ3) (19), Tγ (anti-thyroglobulin) (control IgG), AV-8 (anti-αv), AV-10 (anti-β3) (10), 15F11 (anti-αvβ5) (20), and 12F1 (anti-α2) (21) were used to analyze integrin expression.
Cells and Cell Lines.
MDA-MB 435 human breast carcinoma cells were from J. E. Price (M. D. Anderson Cancer Center, Houston) (22). We derived variants from this cell line by injecting 5 × 105 MDA-MB 435 parental cells into the mammary fat pad of adult female C.B 17/lcrTac scid mice (Taconic Farms). After 8 weeks, tumors were removed, mice were allowed to recover, and tumors were minced and cultured. Three weeks later, mice were killed, and metastases were recovered from bone, lungs, lymph nodes, and the pleural cavity and cultured. PE02JA cells are primary metastatic human breast carcinoma cells isolated from a pleural effusion of a patient with stage IV breast cancer. All cells were grown in Eagle's minimal essential medium plus 10% FBS, pyruvate, l-glutamine, vitamins, and nonessential amino acids (BioWhittaker).
Isolation of β3 Integrin-Negative MDA-MB 435 Breast Cancer Cells.
A β3-lacking MDA-MB 435 variant was isolated by exposing the parental cell line to an anti-β3-saporin conjugate (Ab15-Sap) (18, 23). A concentration of Ab 15-Sap of 1.6 nM killed most cells within 3 days. Surviving cells were grown without toxin for 4 days and then analyzed for integrin expression by flow cytometry. Lack of β3-integrin was routinely confirmed during this study.
Transfection.
The β3-negative MDA-MB 435 cell variant was transfected with human β3WT or mutant β3D723R cDNA (24) with the use of Lipofectamine (GIBCO/BRL). Stable transfectants were selected in G418 (1.5 mg/ml for 4 weeks). Integrin expression was monitored by flow cytometry. Cell populations expressing αvβ3WT or mutant αv β3D723R at levels comparable to that of αvβ3 in parental MDA-MB 435 cells were collected by sterile FACsorting.
Flow Cytometry.
MDA-MB 435 cell variants were harvested with PBS/EDTA, and PE02JA cells were harvested with trypsin. Cells were incubated with 10 μg/ml mAb in Tris-buffered saline, 0.5% BSA (30 min on ice); washed; and stained with FITC anti-mouse IgG. To measure binding of the ligand mimetic antibody Fab WOW-1 (17), cells were incubated (30 min, 22 C°) with 10 μg/ml Fab in 137 mM NaCl, 2.7 mM KCl, 3.3 mM NaH2PO4, 3.8 mM Hepes, 1 mM MgCl2, 400 μM CaCl2, 5.5 mM glucose, and 1 mg/ml BSA, pH 7.4, with or without 250 μM MnCl2 or 2 mM RGDS peptide. Cells were washed and incubated (30 min on ice) with Alexa-Fluor 488 anti-mouse IgG (BioSource International, Camarillo, CA) and analyzed on a Becton Dickinson FACScan.
Analytical Perfusion Studies.
Breast cancer cell arrest during blood flow and interaction with platelets was measured as described (10). Briefly, tumor cells were suspended in human blood (anticoagulated with 50 nM H-d-Phe-Pro-Arg-chloro methyl ketone hydrochloride) and perfused over a collagen I matrix at a venous wall shear rate (50 s−1, 4 dynes/cm2). Adhesive events and cell interactions were visualized and recorded by fluorescence video or confocal laser microscopy (Zeiss) and quantified by image acquisition during perfusion at 50 predefined positions and computerized image analysis (metamorph; Universal Imaging, Media, PA). Tumor cells were stained with hydroethidine (Polysciences) (red fluorescence) (20 μg/ml, 30 min, 37°C), washed, and mixed with blood containing 10 μM mepacrine (green fluorescence). Blood cells, tumor cells, and platelets acquired green fluorescence and were visualized at 488/515 nm (excitation/emission). The tumor cells were identified by their unique red fluorescence at 543/590 nm. Integrin αvβ3- and αIIbβ3-dependent adhesive functions were tested with blocking anti-αvβ3 mAb VNR1 27.1 (18) or anti-αIIbβ3 mAb LJ CP8 (25) (80 μg/ml). Controls were nonfunction-blocking mAbs AV-8 (anti-αv) and AV-10 (anti-β3) (10).
Preparative Perfusion Studies to Isolate Platelet-Interactive Breast Cancer Cells.
Platelet-interactive, arrest-competent variants of the parental MDA-MB 435 breast cancer cell line were isolated during sterile perfusion in human blood on collagen I at a wall shear rate of 50 s−1. Unbound cells were removed by gentle washing with PBS. Thrombus formation was monitored by phase-contrast microscopy. The coverslips were cultured in complete Eagle's minimal essential medium as above, and decaying blood cells and platelets were removed by media changes. After 3 weeks, proliferating tumor cells from a given slide were pooled and resorted four more times to select breast cancer cells with the platelet-interactive phenotype. We generated five independently sorted MDA-MB 435 cell variants. Their abilities to undergo platelet-mediated arrest during blood flow were analyzed and quantified as above.
Haptotactic Migration Assay.
Migration of the breast cancer cell variants toward purified extracellular matrix proteins was analyzed in transwells (8-μm pore size; Costar). Filter undersides (duplicates) were coated with 10 μg/ml human vitronectin, plasma fibronectin, 20 μg/ml fibrinogen, or BSA in PBS and blocked with 5% nonfat dry milk, 0.2% Tween 20 in PBS (2 h at 22°C). Cells were starved overnight in 0.5% FBS, harvested with PBS/EDTA, washed in migration buffer (Eagle's minimal essential medium), and seeded at 6 × 104 cells per upper transwell chamber. After 14 h at 37°C, 5% CO2, filters were washed, and cells from the filter tops were removed, fixed, and stained (DiffQuick). Migrated cells were counted in 10 random optical fields per filter by two observers unaware of the conditions.
In Vivo Metastasis Assay.
To compare the metastatic potential of MDA-MB 435 breast cancer cell variants, 1 × 106 tumor cells were injected into the lateral tail vein of 6-week-old female C.B17/lcrTac scid mice (Taconic Farms) (n = 8). Forty-two days later, mice were killed, dissected, and analyzed by gross examination. The lungs were excised and fixed in Bouin's solution, and metastatic foci were counted at the lung surface under a dissecting microscope.
Results and Discussion
Metastatic Human Breast Cancer Cells Interact with Platelets and Arrest During Blood Flow.
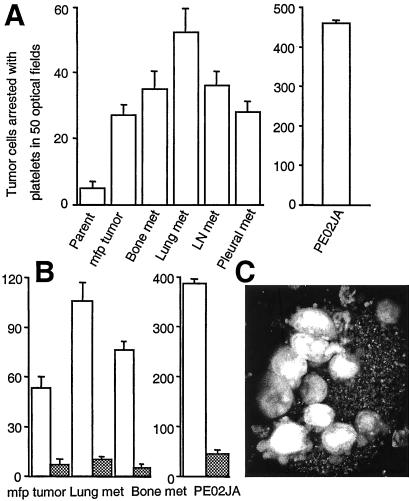
To test the hypothesis that tumor cell binding to platelets during blood flow is a critical property of metastatic tumor cells, we generated tumor- or metastasis-derived variants of the MDA-MB 435 human breast cancer cell line. Parental MDA-MB 435 cells were injected into the mammary fat pads of severe combined immunodeficient mice, and cell variants were retrieved from the resulting tumors or distant metastases to lymph nodes, lungs, bone, and the pleural cavity. These cell variants were compared for their ability to attach to activated platelets and undergo platelet-mediated arrest during blood perfusion in vitro. MDA-MB 435 parental cells largely failed to adhere or interact with platelets during blood flow. In contrast, cell variants derived from mammary fat pad tumors or distant metastases adhered and used platelet interaction for cell arrest (Fig. 1A). Importantly, primary metastatic cells isolated from a pleural effusion of a patient with advanced breast cancer exhibited a strong platelet-interactive phenotype and were incorporated into thrombi that formed at a collagen I matrix during blood perfusion. Tumor cells that bound to attached, activated platelets extended pseudopods and established shear-resistant contact with thrombi (Fig. 1C). Therefore, a platelet-interactive phenotype that promotes tumor cell arrest during blood flow correlated with a tumorigenic and metastatic phenotype in the tested human breast cancer cells.
Figure 1.
Metastatic human breast cancer cells use αvβ3 to interact with platelets and arrest during blood flow. (A) Primary metastatic human breast cancer cells (PE02JA) and MDA-MB 435 cell variants derived from mammary fat pad (mfp) tumors or metastases to bone, lungs, lymph node (LN), or the pleural cavity in mice, but not the parental MDA-MB 435 cell population at large, bind to activated platelets and thereby arrest during blood flow. Tumor cells stained with hydroethidine (red fluorescence) were suspended in human blood (containing mepacrine, green fluorescence) and perfused over a thrombogenic collagen I matrix at a venous wall shear rate (50 s−1, 4 dynes/cm2) (10). Platelets adhere to the matrix, become activated, and form thrombi, to which arrest-competent tumor cells attach (monitored by video microscopy and image acquisition at predefined positions with filter settings to discern platelet- and tumor cell-specific signals). None of the tumor cells attached directly to the matrix. Thrombus formation was not affected by tumor cell type. (B) Arrest-competent breast cancer cells use integrin αvβ3 for attachment. Tumor cells in blood were perfused and analyzed as in A in the absence (open bars) or presence (hatched bars) of function-blocking anti-αvβ3 mAb VNR1 27.1 (18). Columns represent the means of triplicate runs (±SD) with blood from the same donor. (C) Projection of confocal sections through a heteroaggregate of platelets and primary metastatic breast cancer cells, PE02JA (images acquired during perfusion). Attaching to a thrombus, tumor cells extend pseudopods for continued anchorage.
Activated αvβ3 Supports Platelet-Dependent Breast Cancer Cell Arrest During Blood Flow.
We reported that an interaction between melanoma cells and platelets during blood flow is mediated by tumor cell integrin αvβ3 and platelet integrin αIIbβ3 in the presence of connecting plasma proteins such as fibrinogen (10). To analyze whether platelet-supported arrest of tumor- or metastasis-derived human breast cancer cells depends on a similar mechanism, the cells were perfused in blood containing function-blocking anti-αvβ3 or anti-αIIbβ3 antibody. Arrest of mammary fat pad tumor- or metastasis-derived MDA-MB 435 cells and primary metastatic human breast cancer cells was strongly inhibited by anti-αvβ3 (Fig. 1B) and abolished by anti-platelet-αIIbβ3 (not shown). Similar results were obtained for all arrest-competent, platelet-interactive variants of the MDA-MB 435 cell model. Therefore, tumor cell integrin αvβ3 can mediate breast cancer cell arrest during blood flow through an interaction with platelets.
MDA-MB 435 parental cells failed to bind to platelets and arrest during blood flow, but the tumor- or metastasis-derived cell variants did bind in a platelet- and αvβ3-dependent manner. We tested whether this difference in binding patterns was caused by differences in αvβ3 expression levels. However, these differed only slightly when comparing MDA-MB 435 parental cells and their tumor- or metastasis-derived variants (Table 1). Therefore, the distinct functional activities of αvβ3 suggest that the integrin is present in a nonactivated state in the parental cell line, but in an activated state in the in vivo selected cell variants. The activation state can be defined by the platelet-interactive phenotype.
Table 1.
Integrin αvβ3 expression in the human breast cancer cell model
| MDA-MB 435 parent | MDA-MB 435 mfp tumor | MDA-MB 435 bone met | MDA-MB 435 lung met | MDA-MB 435 lymph node met | MDA-MB 435 pleural met | PE02JA | |
|---|---|---|---|---|---|---|---|
| αvβ3 | 36.87 | 40.16 | 47.23 | 40.24 | 47.24 | 41.35 | 55.53 |
| α2β1 | 28.42 | 26.37 | 22.63 | 28.54 | 20.34 | 28.70 | 235.37 |
Variants of MDA-MB 435 cells were generated by injecting the parental cell line into the mammary fat pad (mfp) of severe combined immunodeficient mice and culturing their descendants from developing tumors or distant metastases to bone, lungs, lymph node, or the pleural cavity. PE02JA cells are primary metastatic breast cancer cells from a pleural effusion of a patient with advanced breast cancer. Integrin expression levels were determined by flow cytometry with anti-αvβ3 mAb LM609 or anti-α2 mAb 12F1 and FITC-anti-mouse IgG. Values are median fluorescence intensities.
Parental MDA-MB 435 Human Breast Cancer Cells Contain a Subpopulation That Stably Expresses Activated αvβ3.
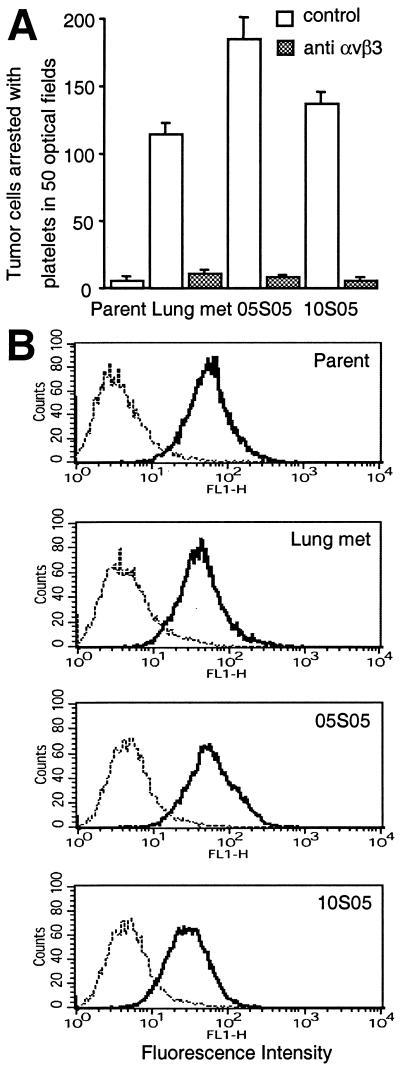
Our data are consistent with the idea that tumor cells expressing platelet-interactive αvβ3 are present in the parental MDA-MB 435 cell line at a low frequency and that these were selected in vivo during tumor growth and metastasis. The MDA-MB 435 cell line is a polyclonal cell population, but its variants derived from distant metastases in mice are oligo- or monoclonal (26). We therefore asked whether cells expressing the platelet-interactive phenotype are present in the parental MDA-MB 435 parental cell population and can be isolated in vitro based on their ability to undergo platelet-mediated arrest during blood flow. To test this possibility, parental cells were suspended in normal donor blood and perfused over a thrombogenic collagen I matrix under sterile conditions. Attached cells were expanded and resorted four times to enrich cells with a platelet-interactive phenotype. Analytical perfusion experiments, in the absence or presence of function-blocking anti-αvβ3 antibody, showed that all of five independently sorted variant cell populations expressed the platelet-interactive form of integrin αvβ3. The extent of platelet interaction was similar to that observed in the in vivo selected metastatic cell variants (Fig. 2A shows two in vitro sorted cell populations, 05S05 and 10S05). The expression levels of integrin αvβ3 were similar in the parental cell population and the in vitro selected variants (Fig. 2B). All in vitro isolated variants stably expressed the platelet-interactive phenotype over more than 15 passages in culture. This persistence of the phenotype confirms that the MDA-MB 435 parental cell line contains cells that express αvβ3 in either of two activation states, a platelet-interactive or a noninteractive state. Unless under selective pressure, as during tumor growth or metastasis, the parental MDA-MB 435 cell population conserved the ratio of cells expressing the non-platelet-interactive versus the interactive form of αvβ3. This conservation of this ratio was evident from repeated analytical blood perfusion experiments with parental MDA-MB 435 cells for more than 20 culture passages, during which the population at large maintained the non-platelet-interactive phenotype.
Figure 2.
MDA-MB 435 breast cancer cells contain an arrest-competent subset that expresses activated αvβ3. Parental MDA-MB 435 breast cancer cells were suspended in human blood and perfused as in Fig. 1, but under sterile conditions. Arrested cells were expanded and resorted four times. (A) Two independently sorted polyclonal populations (05S05 and 10S05) analyzed for their ability to undergo platelet mediated arrest during blood flow (as in Fig. 1) in the absence (open bars) or presence (hatched bars) of function-blocking anti-integrin αvβ3 mAb VNR1 27.1. Columns represent means of triplicate runs (±SD) with blood from the same donor. (B) Parental MDA-MB 435 cells (Parent) and their in vivo (Lung met) or in vitro (05S05 and 10S05) selected variants express integrin αvβ3 at similar levels. Flow cytometric analysis was carried out on cells stained with mAb LM609 (anti-αvβ3) (——) or isotype control (⋅⋅⋅⋅⋅) and FITC-anti-mouse IgG.
Integrin αvβ3 Activation Results in the Platelet-Interactive, Arrest-Competent Phenotype in MDA-MB 435 Human Breast Cancer Cells.
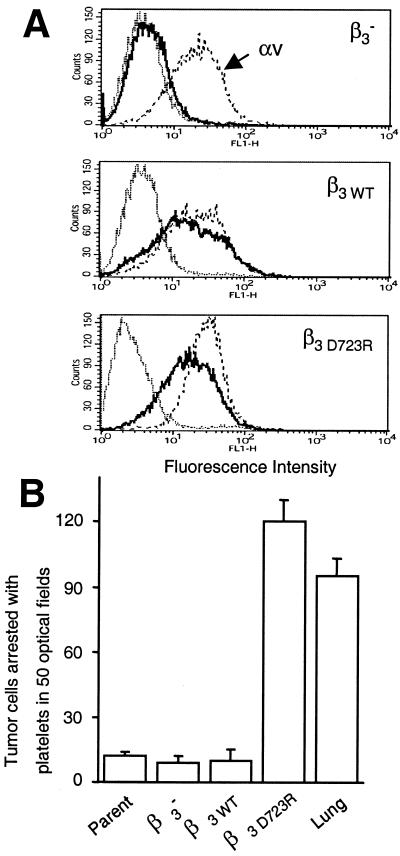
We established a correlation between the platelet-interactive and the metastatic phenotype of MDA-MB 435 breast cancer cells. We now sought to determine whether there is a causal link between these two phenomena. To test the hypothesis that the activated, platelet-interactive state of tumor cell integrin αvβ3, but not the nonactivated state, promotes hematogenous metastasis, MDA-MB 435 cells were transfected with a β3 mutant to express constitutively activated αvβ3. To accomplish this transfection, a β3-minus variant was selected from MDA-MB 435 parental cells by exposing the cells to a saporin-anti-β3 antibody conjugate that selectively killed β3-expressing cells (23). After five rounds of selection, a β3-minus population was obtained that maintained this phenotype over multiple culture passages (Fig. 3A). These cells were transfected stably with cDNA encoding full-length human β3 wild type, β3WT, or mutant β3D723R. Expression of the β3D723R mutant results in constitutively activated platelet integrin αIIbβ3 (24). It dimerizes with the αv subunit, and this dimerization results in an altered functional state of integrin αvβ3 (27). Here, stable transfectants of MDA-MB 435 β3-minus cells were generated that expressed either αvβ3WT or mutant αvβ3D723R at levels comparable to that of αvβ3 in the parental cell line (Fig. 3A). The transfectants were analyzed in vitro for their ability to arrest during blood flow. Cells expressing mutant αvβ3D723R, but not those expressing αvβ3WT or expressing no β3, were able to arrest in a platelet-dependent manner similar to that of the in vivo selected metastatic MDA-MB 435 cell variants (Fig. 3B). αvβ3 is the only β3 integrin of MDA-MB 435 cells. Therefore, the expression of mutant β3D723R resulted in functionally activated αvβ3 that supported tumor cell arrest during blood flow through interaction with platelets.
Figure 3.
Integrin αvβ3 activation renders breast cancer cells platelet-interactive and arrest-competent. A variant lacking β3 integrin expression (β3−) was selected from parental MDA-MB 435 cells by exposure to an anti-β3 saporin conjugate. β3− cells were stably transfected with the β3 wild-type gene (β3WT) or constitutively activated mutant β3D723R. (A) Flow cytometric analysis of αvβ3 expression. Cells stained with anti-αv (mAb AV-8, – – –) (10), anti-αvβ3 (mAb LM609, ——), or isotype control (⋅⋅⋅⋅⋅) and FITC-anti-mouse IgG. (B) Cells expressing constitutively activated αvβ3D723R, but not αvβ3WT, are platelet-interactive and arrest-competent. MDA-MB 435 parental cells (Parent), the β3-lacking variant (β3−), transfectants (β3WT, β3D723R), or the in vivo selected metastatic variant (Lung) were perfused in human blood, and cell arrest was analyzed as in Fig. 1. Columns represent means of triplicate runs (±SD) with blood from the same donor.
Integrin αvβ3 Activation Promotes Binding of a Ligand-Mimetic Antibody and Enhances Breast Cancer Cell Migration Toward Vitronectin.
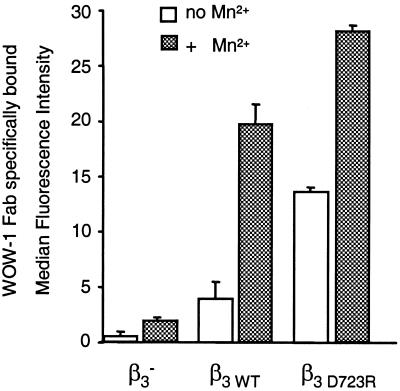
The ability of integrin αvβ3 to support breast cancer cell arrest during blood flow in one functional state, but not the other, indicates strongly that αvβ3 exists in an activated and a nonactivated or less activated state in these tumor cells. To test whether the arrest-competent state of breast cancer cell integrin αvβ3 supports other cell functions differently than the non-arrest-competent state, we analyzed binding of the ligand-mimetic antibody WOW-1. WOW-1 is a genetically engineered Fab fragment that contains a RGD sequence in the context of the adenovirus penton base protein and serves as a monovalent ligand for αv integrins (17). Importantly, WOW-1 was generated on the framework of the PAC-1 Fab, which recognizes platelet integrin αIIbβ3 in an activation-dependent manner (28). Therefore, WOW-1 specifically reports an activated state of integrin αvβ3 (17). Here we show that MDA-MB 435 breast cancer cells expressing arrest-competent αvβ3D723R bound twice as much WOW-1 as the variant expressing non-arrest-competent αvβ3WT (Fig. 4). The two cell variants expressed αvβ3 at equivalent levels (Fig. 3). In the presence of Mn2+, WOW-1 binding increased 2-fold in αvβ3D723R-expressing cells but 5-fold in αvβ3WT-expressing cells. This difference in the increase in WOW-1 binding indicates that αvβ3D723R already exists in a state of increased activation in the absence of exogenous agonist. Similar results were obtained by comparing MDA-MB 435 parental cells and the in vivo selected metastatic variant from the lung (not shown).
Figure 4.
Binding of the ligand-mimetic antibody Fab WOW-1 to functional variants of MDA-MB 435 breast cancer cells. Flow cytometric analysis of WOW-1 binding (activation-dependent anti-αvβ3 Fab) to variants of MDA-MB 435 breast cancer cells lacking β3 (β3−) or transfected with the β3 wild-type gene (β3WT) or constitutively activated mutant β3D723R. Cells were incubated with 10 μg/ml WOW-1 in the absence (open bars) or presence (hatched bars) of 250 μM MnCl2, added to activate αvβ3, and stained with Alexa Fluor 488-anti mouse IgG. Shown is specific WOW-1 binding defined as that inhibited by 2 mM RGDS peptide (means of duplicate analyses ± SD).
Integrin αvβ3-mediated cell migration on certain ligands is affected by the functional state of the receptor (29). To confirm the activated state of αvβ3 in the arrest-competent variants of our MDA-MB 435 breast cancer cell model, we analyzed cell migration toward matrix proteins. We tested vitronectin, fibronectin, and fibrinogen, which are ligands of αvβ3 and support cell adhesion through this receptor (16, 30). Integrin αvβ3 activation enhanced breast cancer cell migration toward vitronectin. Metastatic MDA-MB 435 cells from the lung and β3D723R transfectants (activated αvβ3) migrated more actively toward vitronectin than the parental cells or β3WT transfectants (nonactivated αvβ3) (Table 2). Migration toward fibronectin was also mediated by αvβ3, but was not affected by the receptor activation state. Low levels of vitronectin- and fibronectin-directed migration seen in the β3-minus variant were likely supported by integrin αvβ5 and α5β1, respectively (both receptors were expressed by all MDA-MB 435 cell variants). The tested cell variants migrated poorly toward fibrinogen.
Table 2.
Haptotactic migration of functional variants of MDA-MB 435 breast cancer cells
| Lung | Parent | β3D723R | β3WT | β3− | |
|---|---|---|---|---|---|
| VN | 473 ± 32 | 225 ± 18 | 1125 ± 85 | 478 ± 42 | 198 ± 15 |
| FN | 372 ± 42 | 441 ± 42 | 1085 ± 95 | 1205 ± 110 | 275 ± 22 |
| Fg | 33 ± 12 | 15 ± 11 | 70 ± 12 | 18 ± 6 | 6 ± 4 |
Migration toward vitronectin (VN), fibronectin (FN), or fibrinogen (Fg) was analyzed for MDA-MB 435 parental cells (parent), an in vivo selected metastatic variant (Lung), or the β3-integrin lacking variant (β3−) and its transfectants expressing either β3 wild-type (β3WT) or constitutively activated β3D723R. Cells were starved overnight in 0.5% FBS, and migration was assayed in transwell chambers (6 × 104 per well in duplicate, 14 h). Migrated cells were counted at the filter underside in 10 random optical fields per filter by two observers. Columns represent mean numbers of migrated cells per field (± SD). Data comparing the metastatic cell variant (Lung) to the parental cells and data comparing β3-lacking cells to its β3 transfectants are from independent experiments. Absolute numbers of migrated cells varied between experiments, but the ratios of migratory activities between the cell types remained constant.
Together, the activated state of integrin αvβ3 in breast cancer cells, defined here by the platelet-interactive, arrest-competent phenotype, was confirmed by increased binding of a ligand-mimetic antibody and increased support of cell migration toward vitronectin.
Integrin αvβ3 Activation Controls the Metastatic Potential in MDA-MB 435 Breast Cancer Cells.
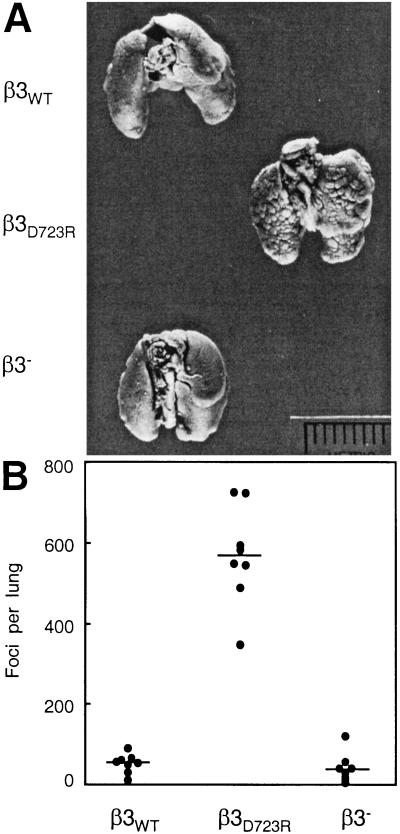
To test whether activation of tumor cell integrin αvβ3 affects the metastatic activity of breast cancer cells, MDA-MB 435 transfectants expressing either nonactivated αvβ3WT or constitutively activated mutant αvβ3D723R were injected into the circulation of severe combined immunodeficient mice. The ability of the cells to colonize the lungs was compared with that of the β3-lacking cell variant. Metastatic activity was significantly enhanced (P < 0.0001) in cells expressing mutant αvβ3D723R compared with cells expressing αvβ3WT or no β3 (Fig. 5). There was no difference between the latter two groups. Thus, in the MDA-MB 435 breast cancer cell model, expression of activated αvβ3 resulted in a platelet-interactive phenotype and strongly increased metastatic activity.
Figure 5.
Integrin αvβ3 activation controls metastatic potential in the MDA-MB 435 breast carcinoma cell model. (A) Lungs of female C.B17/lcrTac scid mice 42 days after i.v. injection of 1 × 106 tumor cells. The β3 integrin lacking cell variant and its transfectants expressing either αvβ3WT or αvβ3D723R were compared. The β3D723R-expressing variant had the platelet-interactive phenotype and showed increased metastatic activity. (B) Number of metastatic foci at the lung surface. Data points are numbers of lung surface metastases for each animal; horizontal lines are median numbers of metastases per group (n = 8). Cells expressing activated αvβ3D723R produced a significantly larger number of metastases than cells lacking β3 or expressing nonactivated αvβ3WT (P < 0.0001 by the Kruskal–Wallis test).
It is currently unknown whether an interaction between breast cancer cells and platelets within the host circulation critically affects metastatic activity. The interaction of the tumor cells with platelets during blood flow in vitro allowed us to identify a functionally activated state of tumor cell integrin αvβ3 that may promote metastasis through a combination of altered adhesive, migratory, and other cell functions. The platelet-interactive variants of the MDA-MB 435 cell model, identified by perfusion in human blood, also interacted with murine platelets when perfused in mouse blood (not shown). It is therefore possible that this mechanism promoted metastatic activity of the human breast cancer cells in the mouse model.
We showed that αvβ3 can exist in breast cancer cells in distinct functional states. The activated but not the nonactivated state supported tumor cell arrest during blood flow through interaction with platelets. We established a correlation between the expression of activated αvβ3 and the metastatic phenotype in the MDA-MB 435 human breast cancer cell model and in primary metastatic cells from a breast cancer patient. Importantly, we documented a causal relationship between the expression of activated αvβ3 and the metastatic potential in MDA-MB 435 breast cancer cells, because expression of constitutively activated mutant αvβ3D723R, but not αvβ3WT, resulted in a significant increase in metastatic activity. These results demonstrate that human breast cancer cells can exhibit a platelet-interactive and metastatic phenotype that is controlled by the activation state of tumor cell integrin αvβ3. This conclusion is consistent with a “two hit hypothesis” (31) in which αvβ3 expression is necessary but not sufficient for successful breast cancer metastasis. Rather, additional as yet undefined factor(s) that control(s) the activation state of the integrin are required for metastatic dissemination. Consequently, alterations within tumors that lead to the aberrant control of integrin activation are expected to adversely affect the course of human breast cancer.
Acknowledgments
This paper is dedicated to the memory of Faye E. Miller. We thank Dr. M. P. Kosty and T. C. Bryntesen (Scripps Clinic, La Jolla, CA) for specimens from cancer patients, Dr. J. E. Price (M. D. Anderson Cancer Center, Houston) for the MDA-MB 435 cell line, and Drs. D. A. Cheresh and J. A. Koziol (Scripps Research Institute, La Jolla, CA) for mAb LM609 and help with statistical analyses, respectively, Dorothy Markowitz for technical assistance, and Pamela Fagan and Rachel Braithwaite for secretarial help. This work was supported by grants from the National Institutes of Health (CA67988 to B.F.-H.; CA69306, HL58925 to J.W.S.; CA59692 to B.M.M.), the University of California Breast Cancer Research Program (5JB-0143 to B.F.-H.; 5JB-0033 to J.W.S.), and the U.S. Army (BC980694 to B.F.-H.). Care for the mice, surgery, and injection protocols were carried out according to institutional and National Institutes of Health guidelines. This paper is manuscript 13054-MEM of the Scripps Research Institute.
References
- 1.Price J T, Bonovich M T, Kohn E C. Crit Rev Biochem Mol Biol. 1997;32:175–253. doi: 10.3109/10409239709082573. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 4.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 5.Albelda S M, Mette S A, Elder D E, Stewart R, Damjanovich L, Herlyn M, Buck C A. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 6.Natali P G, Hamby C V, Felding-Habermann B, Liang B, Nicotra M R, Di Filippo F, Giannarelli D, Temponi M, Ferrone S. Cancer Res. 1997;57:1554–1560. [PubMed] [Google Scholar]
- 7.Gingras M C, Roussel E, Bruner J M, Branch C D, Moser R P. J Neuroimmunol. 1995;57:143–153. doi: 10.1016/0165-5728(94)00178-q. [DOI] [PubMed] [Google Scholar]
- 8.Pignatelli M, Cardillo M R, Hanby A, Stamp G W. Hum Pathol. 1992;23:1159–1166. doi: 10.1016/0046-8177(92)90034-z. [DOI] [PubMed] [Google Scholar]
- 9.Liapis H, Flath A, Kitazawa S. Diagn Mol Pathol. 1996;5:127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri Z M. J Biol Chem. 1996;271:5892–5900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 11.Konstantopoulos K, McIntire L V. J Clin Invest. 1997;100:S19–S23. [PubMed] [Google Scholar]
- 12.Diamond M S, Springer T A. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 13.Altieri D C. Thromb Haemostasis. 1999;82:781–786. [PubMed] [Google Scholar]
- 14.Savage B, Almus-Jacobs F, Ruggeri Z M. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 15.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 16.Felding-Habermann B, Ruggeri Z M, Cheresh D A. J Biol Chem. 1992;267:5070–5077. [PubMed] [Google Scholar]
- 17.Pampori N, Hato T, Stupack D G, Aidoudi S, Cheresh D A, Nemerow G R, Shattil S J. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- 18.Lam S C, Plow E F, D'Souza S E, Cheresh D A, Frelinger A L, III, Ginsberg M H. J Biol Chem. 1989;264:3742–3749. [PubMed] [Google Scholar]
- 19.Cheresh D A, Spiro R C. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 20.Stuiver I, Smith J W. Hybridoma. 1995;14:545–550. doi: 10.1089/hyb.1995.14.545. [DOI] [PubMed] [Google Scholar]
- 21.Pischel K D, Bluestein H G, Woods V L. J Clin Invest. 1988;81:505–513. doi: 10.1172/JCI113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price J E, Polyzos A, Zhang R D, Daniels L M. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 23.Dinota A, Tazzari P L, Michieli M, Visani G, Gobbi M, Bontadini A, Tassi C, Fanin R, Damiani D, Grandi M. Cancer Res. 1990;50:4291–4294. [PubMed] [Google Scholar]
- 24.Hughes P E, Diaz-Gonzalez F, Leong L, Wu C, McDonald J A, Shattil S J, Ginsberg M H. J Biol Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- 25.Niiya K, Hodson E, Bader R, Byers-Ward V, Koziol J A, Plow E F, Ruggeri Z M. Blood. 1987;70:475–483. [PubMed] [Google Scholar]
- 26.Cornetta K, Moore A, Johannessohn M, Sledge G W. Clin Exp Metastasis. 1994;12:3–12. doi: 10.1007/BF01784328. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Hughes P E, Ginsberg M H, McDonald J A. Cell Adhes Commun. 1996;4:149–158. doi: 10.3109/15419069609014219. [DOI] [PubMed] [Google Scholar]
- 28.Shattil S J, Cunningham M, Hoxie JA. Blood. 1987;70:307–315. [PubMed] [Google Scholar]
- 29.Zheng D Q, Woodard A S, Tallini G, Languino L R. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 30.Felding-Habermann B, Mueller B M, Romerdahl C A, Cheresh D A. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung R S, Xiao G H, Jin F, Lee W C, Testa J R, Knudson A G. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]