Abstract
Telesensing, or probing of the environment by the release of chemical messengers, plays a central role in the sexual programs of microbial organisms. Sex pheromones secreted by mating cells are sensed by potential partner cells and mediate cell-to-cell contact and the subsequent exchange of genetic material. Although the mechanisms used by bacterial and fungal species to promote genetic exchange are distinct, recent studies have uncovered surprising parallels between pheromone signaling in these species. In addition, it is now apparent that pheromone signaling not only controls sexual reproduction and genetic exchange but can also activate expression of potential virulence factors in diverse opportunistic pathogens.
INTRODUCTION
There is increasing awareness of the importance of telesensing in microbial systems, and excellent examples of this phenomenon are provided by pheromone signaling during sexual reproduction in fungi and during conjugation in bacteria. Telesensing refers to the secretion of molecular messengers that are then used to sample changes in the milieu (1). This is perhaps best exemplified by quorum sensing, in which microbial behavior is moderated in response to changes in population density (2). The classical quorum sensing paradigm typically involves a multicellular population composed of a single genotype. Many microbes, however, use related mechanisms of environmental sampling to determine the presence of a different cell type, that of compatible mating partners for genetic exchange.
In this article we discuss two opportunistic pathogens that, at first sight, appear to undergo very different mechanisms of pheromone signaling and genetic exchange. The first is Candida albicans, a hemiascomycete yeast that diverged from the model yeast Saccharomyces cerevisiae around 700 million years ago (3). Candida species are the fourth leading cause of nosocomial bloodstream infections in the United States, with C. albicans the principal species responsible (4). There is a high mortality rate associated with these types of infections, with death occurring in up to 50% of bloodstream cases. The second opportunistic pathogen is Enterococcus faecalis, a gram-positive bacterium that is an important cause of bacteremia, endocarditis, surgical wound infections, and urinary tract infections. Like C. albicans, E. faecalis is a common component of the microbiota inhabiting the human gastrointestinal tract but can also cause life-threatening nosocomial infections (5).
Here, we examine recent evidence that indicates that these two opportunistic pathogens show analogous modes of pheromone signaling for regulating genetic exchange. Furthermore, we discuss how paracrine and autocrine modes of pheromone signaling are implicated in pathogenesis, in addition to and distinct from their function in sexual biology. Finally, the observation that convergent evolution of these pathways has occurred in such diverse species suggests that similar links between pheromone signaling and pathogenesis are likely to be uncovered in other microbial systems.
Sexual reproduction in Candida albicans—an imperfect fungus no more?
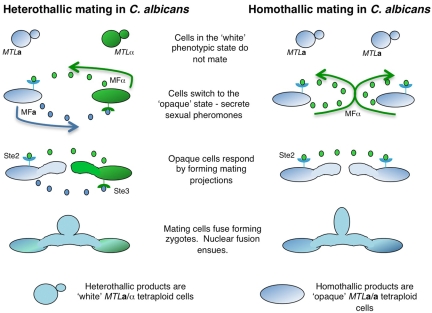
Candida species were originally defined as asexual (imperfect) yeasts that have the ability to form true hyphae or pseudohyphae. However, it is now realized that several Candida species have the ability to undergo mating and a sexual, or parasexual, reproductive cycle. Many of these species are opportunistic human pathogens, with the most prominent species being C. albicans. Long thought to be an asexual diploid yeast, C. albicans revealed a cryptic mating cycle (6–8) that shows many adaptations compared to that in the model hemiascomycete Saccharomyces cerevisiae (9). In particular, mating in C. albicans is regulated by a unique phenotypic switch in which cells have to transition from the regular “white” phenotypic state to the alternative “opaque” state to become mating competent (10). This switch is regulated by transcription factors from the MTL (mating-type-like) locus; a heterodimer of a1 (encoded by the MTLa locus) and α2 (encoded by the MTLα locus) represses switching to opaque. As a direct result of this mechanism, only a or α cells are able to form opaque cells (10). It is speculated that this epigenetic switch evolved to restrict C. albicans mating to specific niches in the human host (11). Opaque a and α cells secrete and respond to sexual pheromones in a manner similar to that established for S. cerevisiae. A paracrine mechanism of pheromone signaling occurs, in which a-type cells secrete a-pheromone which is recognized by the Ste3 receptor on α cells, while, conversely, α-type cells secrete α-pheromone which is recognized by the Ste2 receptor on a cells (Fig. 1) (12, 13). Pheromone signaling activates a mitogen-activated protein kinase (MAPK) cascade leading to arrest in G1 of the cell cycle and the formation of polarized mating projections that subsequently undergo cell and nuclear fusion. The products of C. albicans mating are tetraploid a/α cells that do not undergo a conventional meiosis but can reduce their ploidy back to diploid via a parasexual program of concerted chromosome loss (14, 15).
FIG 1 .
Heterothallic and homothallic mating in C. albicans. Heterothallic (a-α) mating occurs after a and α white cells switch to the mating-competent opaque state. Opaque cells then secrete and respond to sexual pheromones by undergoing polarized growth (formation of conjugation tubes) and cell fusion to form a zygote. Subsequent nuclear fusion results in a tetraploid a/α strain that is locked in the white state due to repression of the opaque form by the a1/α2 complex. In homothallic mating (a-a mating), a cells must again switch to the opaque state to initiate sexual differentiation. If the Bar1 protease is absent (see text for details), a cells can secrete significant quantities of α-pheromone (in addition to the canonical a-pheromone), which activates the mating pathway via an autocrine loop. Cells undergo polarized growth culminating in zygote and tetraploid formation. As the products from self-mating are a-type cells, these can still exist in the opaque state or switch back to the white state.
Conventional heterothallic mating between a and α cells is now well established, but a novel program of same-sex mating was also recently discovered in C. albicans (16, 17). This mechanism involves autocrine pheromone signaling in unisexual populations of a cells; a cells can secrete α-pheromone (in addition to a-pheromone), which then binds the Ste2 receptor and autoactivates the mating response, leading to a-a cell fusion (Fig. 1). Normally, the accumulation of α-pheromone is prevented by a cells secreting an aspartyl protease, Bar1, that degrades α-pheromone, but in the absence of Bar1, efficient autocrine signaling and self-mating can occur (see discussion below) (16). Same-sex mating (primary homothallism) has similarly been detected in other fungal pathogens, notably species of Cryptococcus, indicating that this mode of sexual reproduction may be favored by diverse pathogens (18, 19). Furthermore, an infectious outbreak by Cryptococcus gattii that initiated on Vancouver Island, Canada, has been found to be the product of a same-sex mating event (18). Thus, same-sex mating or related modes of homothallism may be important mechanisms for generating diversity and increasing virulence within populations of pathogenic fungi (20, 21).
Conjugation in Enterococcus faecalis.
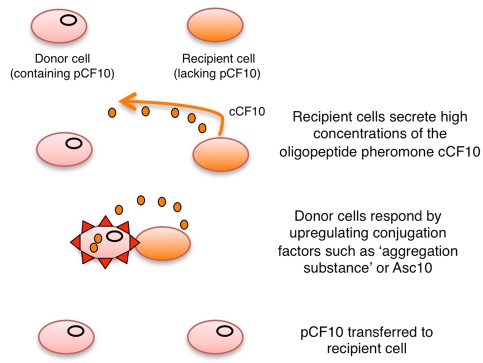
The gram-positive bacterium Enterococcus faecalis, like C. albicans, is a common component of the gastrointestinal flora. E. faecalis is also an important opportunistic pathogen, capable of causing a variety of infections including enterococcal endocarditis, urinary tract infections, and surgical wound infections (5). Many isolates are resistant to antibiotics and can acquire and transfer resistance to other strains. In common with other bacteria true sexual reproduction does not occur, but conjugative plasmids can mediate the exchange of genetic material between a donor cell and a recipient cell. In the case of the conjugative plasmid pCF10, the mechanism of plasmid transfer has been elucidated in detail and involves paracrine pheromone signaling between the two cell types (22). Recipient cells secrete a chromosomally encoded sex pheromone, cCF10, which resembles the C. albicans sex pheromones in that it is a short peptide formed by proteolytic processing of a precursor protein (23). Donor cells (i.e., those already containing pCF10) are able to internalize the pheromone and respond by upregulating the expression of the plasmid-encoded “aggregation substance,” Asc10 (22, 24), as well as additional transfer gene products. Asc10 is a cell surface molecule that promotes adherence between donor and recipient cells, thereby increasing the efficiency of cell-to-cell conjugation (Fig. 2).
FIG 2 .
Conjugative transfer in Enterococcus faecalis. The transfer of plasmid pCF10 from donor cells to recipient cells is mediated by pheromone signaling between the two cell types. Recipient cells secrete the chromosomally encoded peptide cCF10, which is internalized by donor cells and induces the expression of genes involved in the conjugative process. In particular, cells express Asc10, or “aggregation substance,” which mediates a stable interaction between the donor and recipient cells. Subsequent transfer of the pCF10 plasmid from donor cells to recipient cells occurs.
In addition to paracrine pheromone signaling between donor and recipient cells, an additional control mechanism is necessary to prevent autoactivation of the pheromone response in plasmid-carrying cells. Since the pheromone cCF10 is encoded by the chromosome, donor cells must be able to prevent self-induction due to autocrine signaling. This is achieved by two plasmid-encoded polypeptides. The first, PrgY, is a membrane protein that is thought to sequester or degrade the cCF10 pheromone as it is released from the donor cell, while the second, iCF10, is a small peptide inhibitor of cCF10 signaling. Interestingly, both the inhibitor and the pheromone peptides bind to the same site on the pheromone receptor, PrgX, but induce distinct structural changes at its C terminus. The peptides act to either stabilize (iCF10) or destabilize (cCF10) the tetrameric, DNA binding form of the PrgX repressor (24, 25), thereby controlling transcription of the conjugative transfer genes. Both PrgY and iCF10 activities are necessary to prevent aberrant initiation of the conjugative response in donor cells in the absence of recipient cells. High concentrations of cCF10 from recipient cell populations, however, can overcome these intrinsic controls and activate expression of Asc10 and associated conjugative factors. Thus, a delicate balance exists between inhibition of the conjugative pathway by PrgY/iCF10 and activation of the pathway by cCF10. It is this balance that can be disrupted during infection of the host, as explained below.
Analogous signaling mechanisms in C. albicans and E. faecalis.
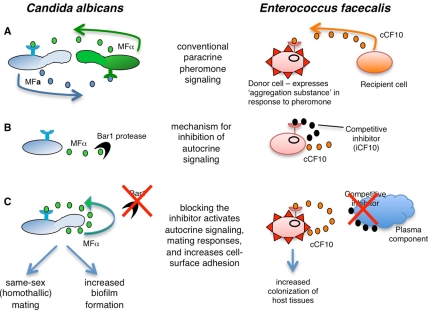
It is apparent that paracrine signaling between two different cell types regulates conventional mating processes in both C. albicans and E. faecalis. In C. albicans, paracrine communication between a and α cells drives heterothallic mating, while in E. faecalis, pheromone secretion by recipient cells activates expression of conjugation factors in donor cells. However, studies have now revealed that both species can also autoactivate the mating pathway via autocrine pheromone signaling. In C. albicans, a cells secrete both a- and α-pheromone; α-pheromone is normally degraded by the Bar1 protease, but in the absence of this protease cells activate self-mating and undergo a-a conjugation and tetraploid formation (Fig. 1 and 3) (16). Similarly, in E. faecalis, donor cells secrete low concentrations of cCF10 pheromone but normally do not undergo self-mating due to the presence of the PrgY and iCF10 inhibitors of pheromone function. In the absence of these inhibitors, autocrine pheromone signaling leads to the expression of conjugative transfer factors, particularly Asc10, which results in clumping or autoaggregation of donor cells (1, 22). Thus, in both C. albicans and E. faecalis autocrine signaling is normally restricted, albeit by different mechanisms, to prevent pheromone signaling. The loss of these inhibitory activities, however, results in efficient autocrine signaling and upregulation of the respective mating programs. In the case of C. albicans, this leads to same-sex mating, but in both species these autocrine programs are now also implicated as having important mating-independent functions.
FIG 3 .
Analogous pheromone-signaling pathways in C. albicans and E. faecalis. (A) Paracrine signaling between two cell types regulates conventional mating responses in C. albicans and E. faecalis. In C. albicans, opaque a and α cells secrete sex-specific pheromones to induce mating in the opposite cell type, while in E. faecalis, cells lacking the pCF10 plasmid secrete a pheromone to attract potential donor cells. (B) In both species, a mechanism exists to prevent autocrine pheromone signaling. In C. albicans, the Bar1 protease degrades α-pheromone produced by opaque a cells, while in E. faecalis, the activities of two proteins are necessary to block autocrine signaling. The first protein is iCF10, which is a short peptide that is a competitive inhibitor of signaling, and the second is PrgY (not shown), which acts to sequester or degrade the cCF10 pheromone (22). To simplify the figure, the peptides are shown competing for binding to the outside of the enterococcal responder cell, but the biologically relevant target of the competition between iCF10 and cCF10 is PrgX, the cytoplasmic transcription factor and master regulator (24). (C) Loss of pheromone inhibitors leads to efficient autocrine signaling in both species. At present, in vivo regulators of C. albicans Bar1 activity have yet to be identified, although it is thought that certain niches in the mammalian host can inhibit Bar1 and activate self-mating (16). In addition, C. albicans white cells may use autocrine signaling to promote biofilm formation (see text for details). In E. faecalis, a component of blood plasma can sequester/degrade the pheromone inhibitor, activating expression of the conjugation system. The result of autocrine signaling is same-sex mating and/or biofilm formation by C. albicans strains, while E. faecalis exhibits increased colonization of host tissues.
Mating biology—more than a sex addiction.
The roles of pheromone signaling in directing mating in C. albicans and E. faecalis are well established, but there is now substantial evidence that these same pathways can be directly utilized for pathogenesis. This was first demonstrated for E. faecalis, where expression of aggregation substance (Asc10) in pCF10-carrying cells was detected during infection of the host (26) and was found to be dependent on the pheromone-sensing machinery (27). The mechanism appears to involve sequestration or degradation of the iCF10 inhibitor of pheromone signaling by albumin/lipid complexes in the bloodstream (1, 22). Removal of iCF10 results in autocrine pheromone signaling and expression of aggregation substance even in the absence of recipient cells (Fig. 3). In support of this mechanism, a strain containing a mutation in the structural gene for cCF10 pheromone still expressed Asc10 when treated with exogenously supplied pheromone but no longer produced Asc10 when incubated with blood plasma (28). High levels of aggregation substance promote colonization of heart valves and increase resistance to phagocytic killing, making the presence of pCF10 a virulence factor for endocarditis (28).
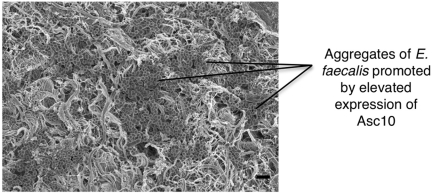
While biofilm formation by E. faecalis can occur in the absence of pCF10 or other pheromone-responsive plasmids (29, 30), several lines of evidence support the role of Asc10 in promoting bacterial attachment to host tissues. Asc10 expression appears to enhance the formation of localized microcolonies during the first few hours of biofilm development (Fig. 4), while strains lacking Asc10 require much longer time periods for comparable microcolony development (29). The Asc10-mediated adhesive interactions between bacterial cells that lead to aggregates in planktonic cultures likely contribute to the rapid formation of larger microcolonies in biofilms. Thus, the ability of pCF10-carrying cells to use the pheromone-sensing system to trigger expression of Asc10 in the mammalian bloodstream enhances their ability to colonize host surfaces, and similar processes may occur on catheters or other indwelling medical devices.
FIG 4 .
Biofilm formation by Asc10-expressing E. faecalis cells on the surface of an explanted porcine heart valve. Sections of explanted porcine heart valves were incubated for 4 h with bacteria, the unattached bacteria were rinsed away, and tissue sections were fixed and examined by high-resolution field emission scanning electron microscopy as described by Erlandsen et al. (29). The presence of large aggregates of attached bacteria on the valve surface is clearly evident, indicative of biofilm development. Comparative studies of biofilm development of Asc10-expressing strains on both heart tissue and abiotic surfaces suggest that Asc10 is not essential for biofilm development but accelerates the formation of large aggregates of bacteria as shown in this image. These types of biofilms likely contribute to the pathogenesis of endocarditis and other infections, as well as serving as a potential niche for the transfer of antibiotic resistance genes by conjugation. Accelerating voltage, 3 kV. The bar represents 2 µm. The image was prepared by Carol Wells and Olivia Chuang-Smith, University of Minnesota.
Sequence comparisons of enterococcal pheromone-responsive plasmids have revealed the presence of a conserved pheromone-inducible aggregation factor (31). It was concluded that telesensing-based control of cell aggregation probably evolved prior to pheromone control of plasmid transfer. In addition, recent molecular and structural comparisons of the PrgX/iCF10 module with peptide/protein modules regulating virulence in bacilli (32) suggest that functional interactions between PrgX and iCF10 preceded those of PrgX and cCF10. Thus, in the evolution of the pCF10 telesensing system, a classical quorum sensing mechanism first emerged whereby the iCF10 peptide was used to monitor the population density of a single cell type. This subsequently served as an evolutionary scaffold for a more complex system utilizing two opposing peptide signals (cCF10 and iCF10) enabling donor cells to monitor both the growth environment and the population density of a second cell type, that of potential conjugative recipients.
In the case of C. albicans, efficient autocrine signaling in opaque a cells can similarly be induced upon loss of the pheromone inhibitor. As discussed above, strains lacking the Bar1 protease accumulate α-pheromone, leading to self-mating of a cells. It is possible that Bar1 is inhibited or sequestered in certain niches in the host, similar to iCF10 during E. faecalis infections, but environmental conditions that inhibit Bar1 have yet to be identified (Fig. 3) (16). In addition to pheromone signaling in opaque cells, it now appears that pheromone-signaling pathways also operate in white cells of C. albicans. Originally thought to be the “ugly sister” of opaque cells and excluded from the reproductive program, white cells have been shown to mount their own unique response to pheromone. While white cells do not undergo the formation of conjugation tubes or cell-cell fusion, these cells are capable of responding to pheromone by upregulating genes involved in cohesion (33, 34). The net result of this response is increased adhesion to synthetic surfaces and enhanced biofilm formation. It is possible that the duality in the response to pheromone—conventional mating in opaque cells and adhesion/biofilm formation by white cells—originally evolved to promote mating in the host. Pheromones secreted by opaque cells may induce white cells to adhere to the surfaces of tissues and thereby stabilize pheromone gradients for opaque cells to locate one another and mate (35).
Curiously, recent evidence suggests that white cells of C. albicans may also undergo autocrine pheromone signaling analogous to that observed in opaque cells. Experiments analyzing biofilm formation by white a cells showed that a significant reduction in biofilm thickness occurred when the STE2 gene (encoding the α-pheromone receptor) was deleted, even when no exogenous pheromone was added to the system (34). Thus, apparently pure populations of white a cells can utilize a component of the mating apparatus to promote biofilm formation. The mechanism by which Ste2 promotes adhesion and biofilm formation in white cells has yet to be established, but given that efficient autocrine signaling can occur in opaque cells, a similar mechanism operating in white cells seems plausible. Taken together, these studies reveal that autocrine pheromone signaling can play important functions related to pathogenesis in both C. albicans and E. faecalis, and these mechanisms are independent of their function in promoting genetic exchange.
CONCLUSIONS
It is now apparent that convergent evolution has, on occasion, led to common strategies for promoting disease in otherwise unrelated microbial species (36). A novel example of this phenomenon is now provided by pheromone signaling in the fungus Candida albicans and the bacterium Enterococcus faecalis. These two diverse opportunistic pathogens exhibit analogous mechanisms of both paracrine and autocrine pheromone signaling. While paracrine pheromone signaling traditionally regulates the exchange of genetic material, autocrine signaling can promote the attachment of microbial cells to each other, to host cells, and to synthetic surfaces. Thus, even though the precise mechanisms of pheromone signaling are distinct between these two pathogens, regulatory circuits that result in very similar outcomes have evolved.
It should be noted that mating and biofilm formation have previously been shown to be closely related processes in other microbial species. For example, in Escherichia coli, expression of conjugative pili can enhance cell-cell and cell surface interactions that increase biofilm formation (37, 38). Similarly, parallels have been made between cell surface factors that promote cell-cell contact during S. cerevisiae mating and those promoting biofilm formation (39). There is therefore a growing consensus that a close relationship exists between factors that promote cell-cell contact for mating and those that enhance biofilm formation. In the case of the two pathogens discussed here, aggregation substance (Asc10) is the primary component responsible for adhesion by E. faecalis, while in C. albicans, multiple cell surface proteins are now implicated in biofilm formation in white cells responding to pheromone (33).
Finally, it is striking how relatively subtle changes in regulatory mechanisms can reprogram biological pathways for novel, yet complementary, functions. In both C. albicans and E. faecalis, pheromone signaling is normally repressed to prevent futile activation of the mating/conjugation pathway. However, these pathways can be activated and utilized for processes unrelated to the transfer of genetic material. Clearly, this type of functional duplicity is beneficial for microbes, which is presumably one reason why it has evolved independently in unrelated species. These conclusions also have ramifications for species that are potentially asexual relatives of C. albicans (40), as it is possible that some of these organisms have retained their sexual machinery for functions other than mating and recombination. It will therefore prove revealing to determine whether other microbes have adapted their mating machinery for novel purposes, with particular emphasis on the role of these processes in opportunistic pathogens that colonize and infect the human host.
ACKNOWLEDGMENTS
We thank Marlowe Tessmer and Kevin Alby for reading of the manuscript and useful discussions; we also thank Olivia Chuang-Smith and Carol Wells for preparing the sample shown in Fig. 4.
Work in the Bennett laboratory is supported by the NIH (R21AI081560 and R01AI081704), and research in the Dunny laboratory is supported by NIH grants 2R01GM049530 and 2R01AI058134. R.J.B. also holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Citation Bennett, R. J., and G. M. Dunny. 2010. Analogous telesensing pathways regulate mating and virulence in two opportunistic human pathogens. mBio 1(4):e00181-10. doi:10.1128/mBio.00181-10.
REFERENCES
- 1. Roux A., Payne S. M., Gilmore M. S. 2009. Microbial telesensing: probing the environment for friends, foes, and food. Cell Host Microbe 6:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waters C. M., Bassler B. L. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319–346 [DOI] [PubMed] [Google Scholar]
- 3. Hedges S. B., Blair J. E., Venturi M. L., Shoe J. L. 2004. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wisplinghoff H., Bischoff T., Tallent S. M., Seifert H., Wenzel R. P., Edmond M. B. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 5. Sava I. G., Heikens E., Huebner J. 2010. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 16:533–540 [DOI] [PubMed] [Google Scholar]
- 6. Hull C. M., Johnson A. D. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271–1275 [DOI] [PubMed] [Google Scholar]
- 7. Hull C. M., Raisner R. M., Johnson A. D. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310 [DOI] [PubMed] [Google Scholar]
- 8. Magee B. B., Magee P. T. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310–313 [DOI] [PubMed] [Google Scholar]
- 9. Bennett R. J., Johnson A. D. 2005. Mating in Candida albicans and the search for a sexual cycle. Annu. Rev. Microbiol. 59:233–255 [DOI] [PubMed] [Google Scholar]
- 10. Miller M. G., Johnson A. D. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 11. Johnson A. 2003. The biology of mating in Candida albicans. Nat. Rev. Microbiol. 1:106–116 [DOI] [PubMed] [Google Scholar]
- 12. Bennett R. J., Miller M. G., Chua P. R., Maxon M. E., Johnson A. D. 2005. Nuclear fusion occurs during mating in Candida albicans and is dependent on the KAR3 gene. Mol. Microbiol. 55:1046–1059 [DOI] [PubMed] [Google Scholar]
- 13. Soll D. R. 2004. Mating-type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioessays 26:10–20 [DOI] [PubMed] [Google Scholar]
- 14. Bennett R. J., Johnson A. D. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forche A., Alby K., Schaefer D., Johnson A. D., Berman J., Bennett R. J. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 6:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alby K., Schaefer D., Bennett R. J. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heitman J. 2009. Microbial genetics: love the one you’re with. Nature 460:807–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fraser J. A., Giles S. S., Wenink E. C., Geunes-Boyer S. G., Wright J. R., Diezmann S., Allen A., Stajich J. E., Dietrich F. S., Perfect J. R., Heitman J. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364 [DOI] [PubMed] [Google Scholar]
- 19. Lin X., Hull C. M., Heitman J. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017–1021 [DOI] [PubMed] [Google Scholar]
- 20. Nielsen K., Heitman J. 2007. Sex and virulence of human pathogenic fungi. Adv. Genet. 57:143–173 [DOI] [PubMed] [Google Scholar]
- 21. Lee S. C., Ni M., Li W., Shertz C., Heitman J. 2010. The evolution of sex: a perspective from the fungal kingdom. Microbiol. Mol. Biol. Rev. 74:298–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dunny G. M.2007. The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution. Philos Trans. R. Soc. Lond. B Biol. Sci. 362:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clewell D. B., An F. Y., Flannagan S. E., Antiporta M., Dunny G. M. 2000. Enterococcal sex pheromone precursors are part of signal sequences for surface lipoproteins. Mol. Microbiol. 35:246–247 [DOI] [PubMed] [Google Scholar]
- 24. Kozlowicz B. K., Shi K., Gu Z. Y., Ohlendorf D. H., Earhart C. A., Dunny G. M. 2006. Molecular basis for control of conjugation by bacterial pheromone and inhibitor peptides. Mol. Microbiol. 62:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi K., Brown C. K., Gu Z. Y., Kozlowicz B. K., Dunny G. M., Ohlendorf D. H., Earhart C. A. 2005. Structure of peptide sex pheromone receptor PrgX and PrgX/pheromone complexes and regulation of conjugation in Enterococcus faecalis. Proc. Natl. Acad. Sci. U. S. A. 102:18596–18601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCormick J. K., Hirt H., Waters C. M., Tripp T. J., Dunny G. M., Schlievert P. M. 2001. Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect. Immun. 69:3305–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirt H., Schlievert P. M., Dunny G. M. 2002. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect. Immun. 70:716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandler J. R., Hirt H., Dunny G. M. 2005. A paracrine peptide sex pheromone also acts as an autocrine signal to induce plasmid transfer and virulence factor expression in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:15617–15622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erlandsen S. L., Kristich C. J., Dunny G. M., Wells C. L. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J. Histochem. Cytochem. 52:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kristich C. J., Li Y. H., Cvitkovitch D. G., Dunny G. M. 2004. Esp-independent biofilm formation by Enterococcus faecalis. J. Bacteriol. 186:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirt H., Manias D. A., Bryan E. M., Klein J. R., Marklund J. K., Staddon J. H., Paustian M. L., Kapur V., Dunny G. M. 2005. Characterization of the pheromone response of the Enterococcus faecalis conjugative plasmid pCF10: complete sequence and comparative analysis of the transcriptional and phenotypic responses of pCF10-containing cells to pheromone induction. J. Bacteriol. 187:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Declerck N., Bouillaut L., Chaix D., Rugani N., Slamti L., Hoh F., Lereclus D., Arold S. T. 2007. Structure of PlcR: insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc. Natl. Acad. Sci. U. S. A. 104:18490–18495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahni N., Yi S., Daniels K. J., Srikantha T., Pujol C., Soll D. R. 2009. Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog. 5:e1000601 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yi S., Sahni N., Pujol C., Daniels K. J., Srikantha T., Soll D. R. 2009. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol. Microbiol. 71:925–947 [DOI] [PubMed] [Google Scholar]
- 35. Daniels K. J., Srikantha T., Lockhart S. R., Pujol C., Soll D. R. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 25:2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bliska J. B., Casadevall A. 2009. Intracellular pathogenic bacteria and fungi—a case of convergent evolution?.Nat. Rev. Microbiol. 7:165–171 [DOI] [PubMed] [Google Scholar]
- 37. Ghigo J. M. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445 [DOI] [PubMed] [Google Scholar]
- 38. Reisner A., Haagensen J. A., Schembri M. A., Zechner E. L., Molin S. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933–946 [DOI] [PubMed] [Google Scholar]
- 39. Nobile C. J., Schneider H. A., Nett J. E., Sheppard D. C., Filler S. G., Andes D. R., Mitchell A. P. 2008. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butler G., Rasmussen M. D., Lin M. F., Santos M. A., Sakthikumar S., Munro C. A., Rheinbay E., Grabherr M., Forche A., Reedy J. L., Agrafioti I., Arnaud M. B., Bates S., Brown A. J., Brunke S., Costanzo M. C., Fitzpatrick D. A., de Groot P. W., Harris D., Hoyer L. L., Hube B., Klis F. M., Kodira C., Lennard N., Logue M. E., Martin R., Neiman A. M., Nikolaou E., Quail M. A., Quinn J., Santos M. C., Schmitzberger F. F., Sherlock G., Shah P., Silverstein K. A., Skrzypek M. S., Soll D., Staggs R., Stansfield I., Stumpf M. P., Sudbery P. E., Srikantha T., Zeng Q., Berman J., Berriman M., Heitman J., Gow N. A., Lorenz M. C., Birren B. W., Kellis M., Cuomo C. A. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]