FIG 3 .
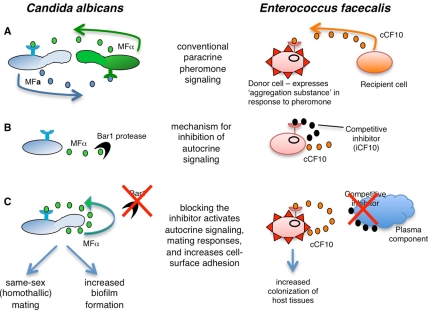
Analogous pheromone-signaling pathways in C. albicans and E. faecalis. (A) Paracrine signaling between two cell types regulates conventional mating responses in C. albicans and E. faecalis. In C. albicans, opaque a and α cells secrete sex-specific pheromones to induce mating in the opposite cell type, while in E. faecalis, cells lacking the pCF10 plasmid secrete a pheromone to attract potential donor cells. (B) In both species, a mechanism exists to prevent autocrine pheromone signaling. In C. albicans, the Bar1 protease degrades α-pheromone produced by opaque a cells, while in E. faecalis, the activities of two proteins are necessary to block autocrine signaling. The first protein is iCF10, which is a short peptide that is a competitive inhibitor of signaling, and the second is PrgY (not shown), which acts to sequester or degrade the cCF10 pheromone (22). To simplify the figure, the peptides are shown competing for binding to the outside of the enterococcal responder cell, but the biologically relevant target of the competition between iCF10 and cCF10 is PrgX, the cytoplasmic transcription factor and master regulator (24). (C) Loss of pheromone inhibitors leads to efficient autocrine signaling in both species. At present, in vivo regulators of C. albicans Bar1 activity have yet to be identified, although it is thought that certain niches in the mammalian host can inhibit Bar1 and activate self-mating (16). In addition, C. albicans white cells may use autocrine signaling to promote biofilm formation (see text for details). In E. faecalis, a component of blood plasma can sequester/degrade the pheromone inhibitor, activating expression of the conjugation system. The result of autocrine signaling is same-sex mating and/or biofilm formation by C. albicans strains, while E. faecalis exhibits increased colonization of host tissues.