Abstract
Daptomycin (Cubicin®) is a branched cyclic lipopeptide antibiotic of nonribosomal origin and the prototype of the acidic lipopeptide family. It was approved in 2003 for the nontopical treatment of skin structure infections caused by Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), and in 2006 for the treatment of bacteremia. Understanding the ribosome-independent biosynthesis of daptomycin assembly will provide opportunities for the generation of daptomycin derivatives with an altered pharmaceutical spectrum to address upcoming daptomycin-resistant pathogens. Herein, the structural properties of daptomycin, its biosynthesis, recent efforts for the generation of structural diversity, and its proposed mode of action are discussed.
Keywords: Drug Action, Drug Resistance, Enzyme Catalysis, Multifunctional Enzymes, Peptide Biosynthesis, MRSA, NRPS, Antibiotic, Combinatorial Biosynthesis, Daptomycin
Introduction
The rapid bacterial acquisition of resistance to conventional antibiotics represents an increasing challenge in treating infections with the contemporary drug arsenal (1). Especially the rise of drug-resistant Gram-positive pathogens, exemplified by methicillin-resistant Staphylococcus aureus (MRSA)2 or vancomycin-resistant enterococci, underlines the urgent demand for antibiotics with alternative modes of action (2). The discovery of natural products as antibacterial drugs had a drastic impact on fatality rates and gave rise to a variety of antibacterial drug classes (3–5). Although the effort for the identification of new bioactive compounds has drastically increased in the past decades, only three new antibacterial classes have been approved by the Food and Drug Administration since the 1970s, one of them being daptomycin (Cubicin®, Cubist Pharmaceuticals) (6). Daptomycin, a decanoic acid-inheriting acidic lipopeptide, was isolated as a member of an antibiotic complex, termed A21978C factors, from cultures of Streptomyces roseosporus (7). Daptomycin is a nontopically used natural lipopeptide antibiotic approved by the Food and Drug Administration in 2003 for the treatment of skin and skin structure infections caused by Gram-positive pathogens and for the treatment of bacteremia and right-sided endocarditis caused by S. aureus strains and MRSA in 2006 (8). Daptomycin does not meet non-inferiority criteria for the treatment of community-acquired pneumonia (9). Its low efficacy against community-acquired pneumonia is considered to be due to inhibition by pulmonary surfactants (10). Recently, Eisenstein et al. (11) provided an interesting historical overview on how daptomycin became available to the market. As the development of daptomycin resistance in Enterococcus faecium and S. aureus has been reported, concerns about decreasing clinical effectiveness of daptomycin will have to be addressed (12–14). Comprehensive knowledge about the biosynthesis of the lead compound offers the opportunity to enhance the structural diversity and the corresponding bioactivity of daptomycin (15–18). In this minireview, the structural and functional properties of daptomycin as a member of the acidic lipopeptide family are presented, with focus on daptomycin biosynthesis. In addition, recent efforts and advances in the generation of novel daptomycin derivatives by means of genetic engineering and chemoenzymatic approaches are highlighted. In the last part of this minireview, the mode of action (MOA) of daptomycin is discussed in detail.
Daptomycin, a Prototype of the Acidic Lipopeptide Family
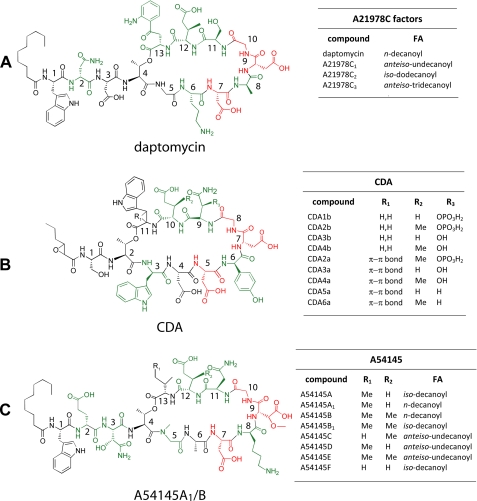
Daptomycin is a member of the A21978C factor family, isolated from cultures of S. roseosporus, and was initially isolated as a minor component of the A21978C factors (7). Precursor-directed fermentation, by supplementing cultures of S. roseosporus with decanoic acid, optimized daptomycin production and facilitated purification (19). The A21978C factors consist of 13 amino acids and share a 10-member macrolactone ring and three exocyclic residues. The factors can be distinguished by the fatty acyl moiety attached to the N-terminal Trp1, which ranges from 10 to 13 carbon atoms. These fatty acyl moieties comprise n-decanoyl, anteiso-undecanoyl, iso-dodecanoyl, and anteiso-tridecanoyl, respectively (Fig. 1A). The peptide core is composed of a set of non-proteinogenic amino acids, including d-Asn2 (20), Orn6, d-Ala8, d-Ser11, (2S,3R)-methylglutamate (MeGlu), and kynurenine (Kyn13), that forms an ester bond with Thr4 and builds up the macrolactone ring. Daptomycin inherits a specific EF-hand motif (DXDG) initially found in ribosomally assembled calmodulin, which is proposed to be involved in Ca2+ binding (21). This is also a common feature of a set of other acidic lipopeptides, namely the macrolactones CDA (Streptomyces coelicolor A3 (2)) and A54145 (Streptomyces fradiae (22, 23)) (Fig. 1, B and C). In addition, the relative position of d-configured amino acids is conserved within this family, as is the long chain fatty acid attached to the cyclic core. Although the A21978C factors were first described in 1987, it took 18 years until the biosynthetic machinery was unveiled on a genetic level, paving the way for biosynthetic engineering of the pathway (7, 20).
FIGURE 1.
Selected members of the acidic lipopeptides. Non-proteinogenic amino acids are shown in green, and the conserved DXDG motif responsible for Ca2+ binding is highlighted in red. The peptide cores with the relative position of each residue are given for daptomycin (A), CDA (B), and A54145 (C). The tables summarize the variants of each acidic lipopeptide and cover alterations within the FA-moieties or amino acid residues.
Biosynthesis of Daptomycin
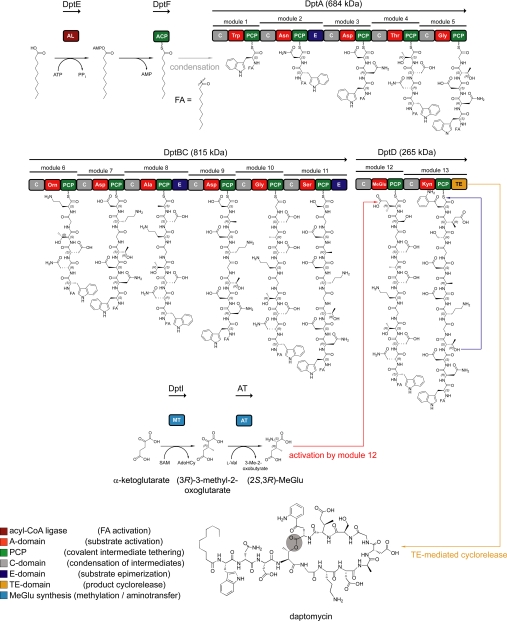
The biosynthesis of daptomycin by S. roseosporus is governed by three nonribosomal peptide synthetases (NRPSs), DptA, DptBC, and DptD, and in trans acting enzymes (Fig. 2) (20, 24, 25). This modularly organized enzymatic machinery assembles the natural product in a thiotemplate-directed manner, in which the enzyme at once represents the template and the biosynthetic machinery. The multidomain organization of the NRPS can be subdivided into modules and domains (1, 26, 27). Each module is responsible for the specific recognition, activation, covalent binding, and incorporation of a building block into the oligopeptide chain and can be furthermore dissected into catalytic domains (28). The structural and functional diversity of NRPS-derived natural products arises from the numerous building blocks recognized and incorporated into the oligopeptide as well as post-synthetic modifications to the peptide core introduced by tailoring enzymes (29). A comprehensive and detailed overview of the mechanistic and structural aspects of nonribosomal product assembly has been provided (1, 26, 27).
FIGURE 2.
Schematic overview of daptomycin biosynthesis by S. roseosporus. The assembly of the peptide core is governed by the three NRPSs DptA, DptBC, and DptD, comprising 43 catalytic domains. Initiation of daptomycin biosynthesis is mediated by DptE and DptF, both responsible for the activation and incorporation of the FA moiety. In this model, the N-terminal CIII-domain of DptA catalyzes the transfer of the DptF-bound FA onto the α-amino group of Trp1. The synthesis of MeGlu12 is carried out by the S-adenosylmethionine (SAM)-dependent methyltransferase DptI and a currently unknown aminotransferase. Cyclorelease is mediated by the C-terminal TE domain of DptD. The cyclization position is shown in gray. A, adenylation; AL, acyl-CoA ligase; AT, aminotransferase; C, condensation; E, epimerization; MT, methyltransferase; PCP, peptidyl carrier protein.
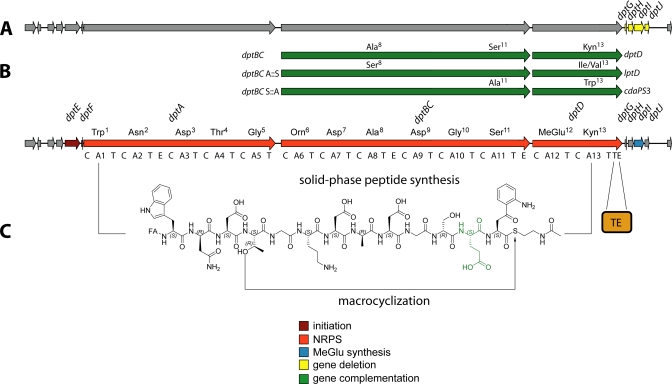
The analysis of the biosynthetic gene clusters responsible for acidic lipopeptide assembly revealed a set of genes putatively involved in the acylation of the N-terminal amino acid. In a model proposed for the initiation mechanism, the machinery thus consists of an acyl-CoA ligase that activates the fatty acid (FA) and an acyl carrier protein (ACP) to which the FA is covalently tethered (20). The N-terminal C-domain (type CIII) of the initiation module subsequently catalyzes the condensation between the FA and Trp1, and chain elongation commences (30). Initiation of daptomycin biosynthesis is mediated by the action of the two distinct enzymes DptE and DptF, encoded upstream of dptD (25). DptE, which shares a high degree of homology with the acyl-CoA ligase superfamily, was shown to activate the FA moiety attached to the N terminus of daptomycin in an ATP-dependent manner. The activated FA is subsequently transferred onto the 4′-phosphopantetheine group of DptF, the cognate ACP. The N-terminal C-domain of the DptA initiation module is predicted to catalyze the condensation of the ACP-bound FA and tryptophan. The broad substrate tolerance of DptE toward the length and type of FAs is believed to be reflected in the composition of the A21978C factors (7). After initiation, chain elongation is mediated by the linearly operating NRPSs DptA, DptBC, and DptD. The three epimerization domains present in the synthetases correlate with the d-configured amino acids d-Asn2, d-Ala8, and d-Ser11, respectively (20). Apart from the non-proteinogenic amino acids Orn6 and Kyn13, a β-methylated glutamate residue (MeGlu12) is located within the 10-member macrolactone ring (31). This residue, which contributes to the bioactivity of the corresponding compound, is a common feature of daptomycin, A54145, and CDA and is located at the same relative position within the peptide ring (18, 31, 32). Initially, a gene located within the CDA biosynthetic gene cluster was predicted to encode S-adenosylmethionine-dependent GlmT (glutamate 3-methyltransferase). A gene sharing a high degree of homology with glmT, namely dptI, was also identified in the daptomycin gene cluster. The construction of S. roseosporus ΔdptGHIJ deletion mutants and the subsequent analysis of fermentation products revealed the presence of A21978C analogs lacking MeGlu12, which was substituted with Glu (Fig. 3A) (31). Complementation of the ΔdptGHIJ mutant with dptI restored daptomycin production and proved that DptI is indeed the methyltransferase involved in MeGlu biosynthesis. Recently, a mechanistic route for the generation of MeGlu has been established by the characterization of recombinant DptI (Fig. 2) (24). Intriguingly, DptI did not catalyze the methylation of Glu directly. Instead, the substrate for DptI-mediated methyl transfer was shown to be α-ketoglutarate, leading to 3-methyl-2-oxoglutarate. Subsequently, an additional transamination step is required for the conversion of 3-methyl-2-oxoglutarate to MeGlu. A branched chain aminotransferase from the primary metabolism of S. coelicolor A3 (2) was demonstrated to catalyze this reaction, with Val being the amino group donor. Taking the results from DptI-mediated methyl transfer and subsequent transamination together, the mechanism for MeGlu synthesis starting from α-ketoglutarate was established. The activation of MeGlu and its incorporation into the peptide intermediate are subsequently carried out by module 12 of DptD. Another round of chain elongation leads to the peptidyl carrier protein-bound tridecapeptide intermediate. This oligopeptide is then transferred onto the active-site serine of the C-terminal thioesterase (TE) domain of DptD. The intramolecular nucleophilic attack of the side chain hydroxyl functionality of Thr4 on the acyl-O-TE oxoester intermediate leads to the release of the cyclic lipopeptide (32). The excised recombinant TE domain was shown to be responsible for this macrolactonization and represents a robust and versatile catalyst for the chemoenzymatic generation of daptomycin analogs (18).
FIGURE 3.
Overview of the methods utilized for the generation of daptomycin derivatives employing the native gene cluster. Structural diversity can be generated in vivo, as depicted by gene deletion (A) and trans-complementation (B) approaches. For trans-complementation, dptBC and dptD were substituted with homologous genes, leading to amino acid substitutions. Combinations of this set of genes with deletions and module exchanges afforded numerous daptomycin analogs. C, in vitro chemoenzymatic synthesis of daptomycin derivatives employing linear activated peptidyl thioesters that substitute the assembly line and the recombinant TE domain. The modified MeGlu12 residue is shown in green.
Efforts for the Generation of Structural Diversity
This part of the minireview focuses on the methods applied to obtain daptomycin derivatives with an altered or improved pharmaceutical spectrum. In the first part, semisynthetic modifications as the initial approach to introduce structural diversity are presented, followed by the more recent chemoenzymatic synthesis. In the last part, the sophisticated combinatorial biosynthesis is discussed in detail.
Semisynthetic Modifications
Initially, daptomycin was isolated as a member of a series of lipopeptides, the A21978C factors. The direct utilization of this complex was impeded by rising toxicity caused by different acyl groups attached to the exocyclic amino acids but paved the way to alter the bioactivity through acyl group exchange (33). Deacylation was achieved by a highly efficient deacylase derived from Actinoplanes utahensis (34). Reacylation was performed chemically with activated acyl esters after protection of side chain nucleophiles. The generated derivatives varied not only in the acyl functionality but also in the number of exocyclic amino acids (e.g. α-N-acylated Phe). The derivatives were evaluated for in vitro and in vivo activities against the Gram-positive pathogens S. aureus and Streptococcus pyogenes, but none of the semisynthetic compounds, except daptomycin, could meet pharmaceutical criteria. As already mentioned, the precursor-directed fermentation enabled high quantity production of daptomycin and substituted for the tedious process of deacylation and reacylation (19). Further modifications of the deacylated A21978C peptide core included removal of the two N-terminal exocyclic amino acids by Edman-type degradation and coupling of N-decanoylated dipeptides to the Orn-protected undecapeptide. Using this approach, Miao et al. (20) investigated the impact of Asn2 stereochemistry on bioactivity. In this experiment, the l-Asn isomer displayed a 10-fold reduced antibacterial activity. The modification of side chains represents an alternative approach and has been applied for the acylation of the Orn6 δ-amino group (35). In a series of acylations and subsequent evaluation of antibacterial activities, it was shown that incorporation of long chain alkyls was not tolerated, whereas coupling of Trp retained bioactivity. As it was observed that the conversion of the free Orn6 amine to an amide increased the minimum inhibitory concentration (MIC), an alternative side chain modification was investigated (36, 37). This approach took advantage of a reductive alkylation of the Orn6 amine to afford numerous benzylic substitutions including amide or sulfonamide groups as polar functionalities. Structure-activity relationship (SAR) studies conducted with these derivatives suggest that an increased electron deficiency of the aryl moiety as well as heterocyclic spacers and polar groups contributes to maintaining antibacterial properties (37).
Chemoenzymatic Synthesis
Insights into daptomycin biosynthesis enabled a chemoenzymatic approach for the generation of daptomycin analogs by combining organic synthesis and enzymatic mechanisms. The catalytic unit used is the TE domain usually located in the C-terminal module of the ultimate NRPS. As it has been shown that the excised TE domains catalyze the macrocyclization of activated thioester substrates, mimicking the native peptidyl carrier protein-bound oligopeptide, the entire enzymatic machinery can be substituted via solid-phase peptide synthesis (18, 32, 38–40). Initial studies toward the production of daptomycin analogs were carried out utilizing the recombinant TE from the CDA biosynthetic machinery (32). It was shown that the excised domain catalyzes the macrolactonization of linear daptomycin analogs (Fig. 3C). In the course of this project, seven positions within daptomycin were varied, including the acidic residues of the Ca2+-binding motif DXDG, and SAR studies were carried out against Bacillus subtilis. The substitution of MeGlu12 with Glu led to a 7-fold increase in the MIC compared with the native compound and proved to be in full agreement with the results of Nguyen et al. (41). Furthermore, the substitution of Kyn13 with Trp increased the MIC. Disruption of the Ca2+-binding motif by a single substitution of Asp7 or Asp9 with Asn completely abolished bactericidal activity, confirming the importance of the acidic residues. The substitution of exocyclic Asp3 with Asn did not decrease bioactivity dramatically, suggesting that only Asp7 and Asp9, located within the conserved EF-hand motif DXDG, are essential for cation binding (21). A more recent study focused on the generation of daptomycin and A54145 hybrid molecules through enzymatic cyclization of the linear thioesters by the TE domains from A54145 and daptomycin NRPSs (18). SAR studies were conducted with a set of acidic lipopeptide variants (daptomycin, CDA, A54145), including an alternative macrolactam. Again, the importance of an intact DXDG motif and MeGlu12 was confirmed because of a complete loss of antibacterial activity in the case of Asp7 and Asp9 substitutions and a 6-fold increase in the MIC when omitting MeGlu12. Hybrid molecules consisting of an exocyclic daptomycin peptide and endocyclic A54145 peptide cores displayed similar MICs, thus illustrating the opportunity to construct structural hybrids. Summarizing the chemoenzymatic approaches, it can be stated that this method offers the possibility to rapidly construct daptomycin analogs that can be investigated for SAR. As the main drawback of this method in vitro is the low quantity of derivative generated, scientists in collaboration with the pharmaceutical industry set out to exploit the known biosynthetic machinery in vivo.
Combinatorial Biosynthesis
Combinatorial biosynthesis describes the targeted reprogramming of genes encoding the enzymatic machinery involved in natural product assembly (42). Apart from the genetic accessibility of the target strain, combinatorial biosynthesis relies on the knowledge of the biosynthetic mechanisms for the structural redesign of the target compound. Both requirements are met by S. roseosporus, as it is accessible to genetic manipulations, and the gene cluster has been sequenced, cloned, and heterologously expressed (20, 43). Combinatorial biosynthesis of daptomycin and hybrid molecules in S. roseosporus has already been extensively carried out (41, 44, 45). The modular NRPS assembly line logic offers a set of possible manipulation targets, ranging from single-module to multimodule substitutions and manipulation of tailoring steps. Initially, dptA and dptD were deleted from the original locus (46). These genes were subsequently introduced into S. roseosporus to trans-complement deletions of dptA and dptD by construction of plasmid-cloning vectors, allowing conjugal transfer of genetic information from Escherichia coli to the target strain (46, 47). After conjugation, the plasmids inserted site-specifically into the S. roseosporus chromosome under the control of inducible promoters and restored A21978C factor production. This system was exploited for complete subunit exchange by substitution of dptD with lptD or cdaPS3, generating hybrid lipopeptides inheriting Ile13 or Trp13 instead of Kyn13 (Fig. 3) (45, 46). LptD and CDAPS3 are NRPSs involved in the assembly of A54145 and CDA, respectively (48, 49). Complementation of DptD with LptD restored 25% of the original production, whereas CDAPS3 complementation afforded 50%. The reduced production of lipopeptide hybrids is most likely due to disturbed communication between DptBC and the complementing synthetase. Cognate protein-protein interaction between NRPSs is mediated via specific C- and N-terminal COM helices (50, 51). The production of the latter compounds was shown to be increased when complementing DptD with hybrid recombinants consisting of CA12T derived from DptD and CA13TTE from LptD or CDAPS3, respectively (52). This approach restored native NRPS interactions and ensured functional communication between DptBC E11 and DptD C12. An intact hybrid synthetase consisting of CA12T::TTe of DptD and CA11 of LptC (activating Asn) gave rise to A21978C factors containing Asn13 instead of Kyn13. The generated derivatives were assayed for antibacterial activity against S. aureus, demonstrating that substitution of Kyn13 with Trp does not increase MICs, whereas Kyn13 exchange with Asn reduces bioactivity (16, 52). Kyn13 substitution with Ile led to a 4-fold decrease in antimicrobial activity, illustrating that the introduction of an aliphatic residue instead of an aromatic residue might interfere with intermolecular π-stacking during daptomycin micelle formation. Micelle formation strongly contributes to daptomycin bioactivity in vivo (see “Mode of Action” below) (41).
Entire module exchanges were achieved by combining the trans-complementation system with engineered dptBC genes to generate lipopeptide hybrids with substitutions of d-Ala8 and d-Ser11 (Fig. 3) (41, 53, 54). These residues were altered to d-Ser8 and d-Ala11, respectively. In addition, hybrid synthetases derived from DptBC and LptC afforded substitutions of positions 8 and 11 with d-Asn or d-Lys (41, 54). The combination of all methods mentioned, including the deletion of dptI, allowed the generation of a multitude of A21978C factor analogs. The most recent study in this field took advantage of λ Red-mediated module substitutions within the A54145 producer strain S. fradiae (17, 23). It was also shown that the methyltransferases DptI and LptI are interchangeable with GlmT, showing a 50% reduced methylation rate (17, 24). This approach was chosen to generate lipopeptide hybrids to combine the high bactericidal activity of daptomycin with the low inhibition rate of A54145 for pulmonary surfactants (9, 10). The generated analogs were isolated, and the antibacterial activity was evaluated in the presence and absence of bovine pulmonary surfactant. Derivatives carrying Ile13 or Val13 had 8-fold decreased MICs in the presence of surfactant, whereas the MICs in the absence of surfactant increased by 4- and 8-fold, respectively. These compounds represent the most promising daptomycin derivatives to evade pulmonary surfactant inhibition. Substitution of MeGlu12 with Glu, achieved by deletion of the corresponding methyltransferase, resulted in a 16-fold less active compound in the absence of surfactant, showing a 4-fold increase in the MIC in the presence of surfactant. Substitution of d-Ala8 or d-Ser11 with d-Ser/d-Lys or d-Ala/d-Asn, respectively, did not improve bioactivity in a surfactant-containing environment. In summary, modifications of positions 12 and 13 strongly influence the degree of surfactant inhibition, whereas the substitution of MeGlu12 with Glu greatly reduces antibacterial activity.
Mode of Action
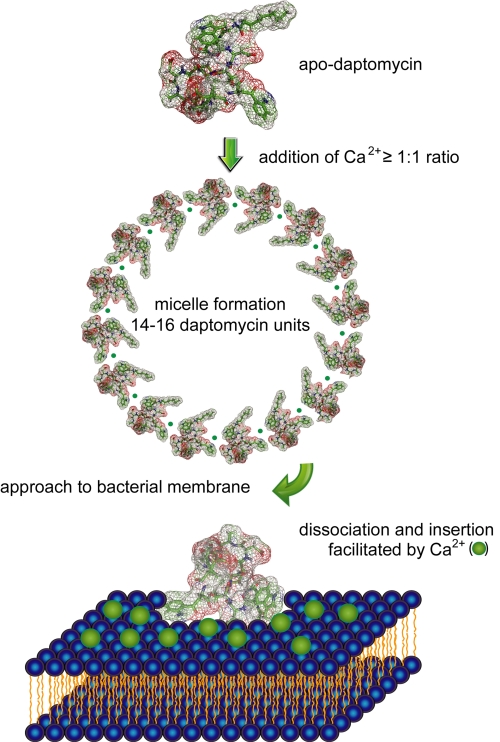
Daptomycin is a still reliable ally in the combat against clinical pathogens, as it displays a strong bactericidal activity against high inoculum MRSA, leading to a more rapid cell death than the reference antibiotics nafcillin, vancomycin, and linezolid (55, 56). Nevertheless, the discrete MOA of daptomycin is still not thoroughly elucidated, although it is known that calcium ions are essential for the antimicrobial activity (57). Daptomycin was also investigated for antibacterial activity in the presence of other divalent ions. It was shown that the bioactivity was not completely abolished but MICs increased at least 32-fold (58). Currently, MOA studies largely focus on NMR structures of daptomycin in the presence and absence of Ca2+ in combination with membrane insertion models. Three different structures of apo-daptomycin have been published up to now (59–62). The series of proposed apo-structures seems to reflect the high degree of mobility within the peptide backbone and the lipid tail. In addition, NMR studies of apo-daptomycin showed that the four acidic residues Asp3, Asp7, Asp9, and MeGlu12 are not in close spatial proximity to form a distinct pre-organized Ca2+-binding motif (60). In one of the first studies, Jung et al. (59) proposed a two-step mechanism of action derived from structural changes observed in NMR experiments, CD measurements, and fluorescence spectroscopy. In the first step, Ca2+ binds to daptomycin in solution and induces a conformational change, increasing amphipathicity and decreasing its charge. This process facilitates oligomerization and leads to micelle formation, which allows daptomycin to interact with neutral or acidic membranes. In a second step, Ca2+ bridges the gap between daptomycin and the acidic phospholipids. As indicated by CD measurements, daptomycin undergoes a second structural transition, allowing a deeper insertion into the membrane bilayer. In contrast to the results of Silverman et al. (63), it was proposed that cytoplasmic membrane depolarization is not the main cause of cell death, as it occurred subsequently. More recent studies contradict this MOA on the basis of an NMR structure of daptomycin in the presence of Mg2+ (58). It was found that Mg2+ also promotes micelle formation as proven by equilibrium sedimentation experiments but does not induce a conformational change. Daptomycin was found to form oligomers consisting of 14–16 monomers upon addition of Ca2+ at a 1:1 ratio. It was speculated that the nuclear Overhauser effect contacts observed by Jung et al. (59) arise from inter- rather than intramolecular contacts. In accordance with Jung et al., it was proposed that divalent cations mask the negatively charged residues and enable micelle formation either by π-stacking interactions between aromatic residues or by arrangement of the lipid tails toward the interior of the micelle (58). Results obtained by Scott et al. (64) confirmed that daptomycin does not undergo a major conformational transition prior to membrane insertion. The NMR-based investigation of daptomycin in 1,2-dihexanoyl-sn-glycero-3-phosphocholine micelles showed that the structure of the inserted daptomycin resembles the apo-structure. Therefore, it is assumed that daptomycin experiences only a minor conformational rearrangement upon binding to 1,2-dihexanoyl-sn-glycero-3-phosphocholine in the presence of Ca2+.
Based on the data obtained from these experiments, a revised model for the mechanism of action has been proposed (Fig. 4). In this model, the micelles are vehicles to deliver daptomycin to the bacterial cell membranes in high local concentrations and a functional conformation (62). The close proximity of the daptomycin micelle and the bacterial membrane induces the dissociation of the multimer and allows daptomycin insertion into the bilayer, which is promoted by the lipid tail. It is speculated that daptomycin might oligomerize inside the membrane to generate larger pores. This process would also lead to potassium efflux, membrane depolarization, and finally to cell death (63). Although daptomycin has been approved for nearly 7 years, fundamental aspects of its MOA remain ambiguous. It is still unclear if daptomycin oligomerizes within the membrane and if this process is essential for its antibacterial activity. In addition, little information is available as to whether membrane depolarization is the main cause of cell death or if other membrane-associated processes, e.g. cell wall biosynthesis, cell division, and energetics, are disturbed.
FIGURE 4.
Proposed mechanism of action of daptomycin. The moderately amphiphilic daptomycin builds up 14–16-mers upon addition of Ca2+ at a 1:1 ratio (58–61). The complex approaches the membrane and dissociates in close proximity of the lipid bilayer. Daptomycin subsequently inserts into the membrane and might oligomerize. The formation of pores within the bilayer depolarizes the membrane through potassium efflux and disturbs membrane-associated processes, ultimately leading to cell death.
Supplementary Material
Acknowledgment
We gratefully acknowledge Dr. Matthias Strieker (Department of Chemistry, Philipps-University Marburg) for the preparation of Fig. 4.
This work was supported by the Deutsche Forschungsgemeinschaft and the LOEWE Center for Synthetic Microbiology. This is the first article in the Thematic Minireview Series on Antibacterial Natural Products. This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- MRSA
- methicillin-resistant S. aureus
- MOA
- mode of action
- CDA
- calcium-dependent antibiotic
- MeGlu
- (2S,3R)-methylglutamate
- Kyn
- kynurenine
- NRPS
- nonribosomal peptide synthetase
- FA
- fatty acid
- ACP
- acyl carrier protein
- TE
- thioesterase
- MIC
- minimum inhibitory concentration
- SAR
- structure-activity relationship.
REFERENCES
- 1.Walsh C. T., Fischbach M. A. (2010) J. Am. Chem. Soc. 132, 2469–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enoch D. A., Bygott J. M., Daly M. L., Karas J. A. (2007) J. Infect. 55, 205–213 [DOI] [PubMed] [Google Scholar]
- 3.Newman D. J., Cragg G. M., Snader K. M. (2000) Nat. Prod. Rep. 17, 215–234 [DOI] [PubMed] [Google Scholar]
- 4.Newman D. J., Cragg G. M. (2007) J. Nat. Prod. 70, 461–477 [DOI] [PubMed] [Google Scholar]
- 5.Newman D., Cragg G. (2009) Bioorg. Med. Chem. 17, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick P., Raja A., LaBonte J., Lebbos J. (2003) Nat. Rev. Drug Discov. 2, 943–944 [DOI] [PubMed] [Google Scholar]
- 7.Debono M., Barnhart M., Carrell C. B., Hoffmann J. A., Occolowitz J. L., Abbott B. J., Fukuda D. S., Hamill R. L., Biemann K., Herlihy W. C. (1987) J. Antibiot. 40, 761–777 [DOI] [PubMed] [Google Scholar]
- 8.Sauermann R., Rothenburger M., Graninger W., Joukhadar C. (2008) Pharmacology 81, 79–91 [DOI] [PubMed] [Google Scholar]
- 9.Pertel P. E., Bernardo P., Fogarty C., Matthews P., Northland R., Benvenuto M., Thorne G. M., Luperchio S. A., Arbeit R. D., Alder J. (2008) Clin. Infect. Dis. 46, 1142–1151 [DOI] [PubMed] [Google Scholar]
- 10.Silverman J. A., Mortin L. I., Vanpraagh A. D., Li T., Alder J. (2005) J. Infect. Dis. 191, 2149–2152 [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein B. I., Oleson F. B., Jr., Baltz R. H. (2010) Clin. Infect. Dis. 50, Suppl. 1, S10–S15 [DOI] [PubMed] [Google Scholar]
- 12.Montero C. I., Stock F., Murray P. R. (2008) Antimicrob. Agents Chemother. 52, 1167–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman L., Alder J. D., Silverman J. A. (2006) Antimicrob. Agents Chemother. 50, 2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy M. H., Olson M. E., Wickert R. W., Fey P. D., Jalali Z. (2008) J. Med. Microbiol. 57, 1036–1038 [DOI] [PubMed] [Google Scholar]
- 15.Baltz R. H. (2009) Methods Enzymol. 458, 511–531 [DOI] [PubMed] [Google Scholar]
- 16.Baltz R. H. (2009) Curr. Opin. Chem. Biol. 13, 144–151 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen K. T., He X., Alexander D. C., Li C., Gu J. Q., Mascio C., Van Praagh A., Mortin L., Chu M., Silverman J. A., Brian P., Baltz R. H. (2010) Antimicrob. Agents Chemother. 54, 1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp F., Grünewald J., Mahlert C., Marahiel M. A. (2006) Biochemistry 45, 10474–10481 [DOI] [PubMed] [Google Scholar]
- 19.Huber F. M., Pieper R. L., Tietz A. J. (1988) J. Biotechnol. 7, 283–292 [Google Scholar]
- 20.Miao V., Coëffet-LeGal M. F., Brian P., Brost R., Penn J., Whiting A., Martin S., Ford R., Parr I., Bouchard M., Silva C. J., Wrigley S. K., Baltz R. H. (2005) Microbiology 151, 1507–1523 [DOI] [PubMed] [Google Scholar]
- 21.Yazawa M., Yagi K. (1980) Biochem. Biophys. Res. Commun. 96, 377–381 [DOI] [PubMed] [Google Scholar]
- 22.Lakey J. H., Lea E. J., Rudd B. A., Wright H. M., Hopwood D. A. (1983) J. Gen. Microbiol. 129, 3565–3573 [DOI] [PubMed] [Google Scholar]
- 23.Counter F. T., Allen N. E., Fukuda D. S., Hobbs J. N., Ott J., Ensminger P. W., Mynderse J. S., Preston D. A., Wu C. Y. (1990) J. Antibiot. 43, 616–622 [DOI] [PubMed] [Google Scholar]
- 24.Mahlert C., Kopp F., Thirlway J., Micklefield J., Marahiel M. A. (2007) J. Am. Chem. Soc. 129, 12011–12018 [DOI] [PubMed] [Google Scholar]
- 25.Wittmann M., Linne U., Pohlmann V., Marahiel M. A. (2008) FEBS J. 275, 5343–5354 [DOI] [PubMed] [Google Scholar]
- 26.Marahiel M. A., Essen L. O. (2009) Methods Enzymol. 458, 337–351 [DOI] [PubMed] [Google Scholar]
- 27.Marahiel M. A. (2009) J. Pept. Sci. 15, 799–807 [DOI] [PubMed] [Google Scholar]
- 28.Fischbach M. A., Walsh C. T. (2006) Chem. Rev. 106, 3468–3496 [DOI] [PubMed] [Google Scholar]
- 29.Sieber S. A., Marahiel M. A. (2005) Chem. Rev. 105, 715–738 [DOI] [PubMed] [Google Scholar]
- 30.Rausch C., Hoof I., Weber T., Wohlleben W., Huson D. H. (2007) BMC Evol. Biol. 7, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen K. T., Kau D., Gu J. Q., Brian P., Wrigley S. K., Baltz R. H., Miao V. (2006) Mol. Microbiol. 61, 1294–1307 [DOI] [PubMed] [Google Scholar]
- 32.Grünewald J., Sieber S. A., Mahlert C., Linne U., Marahiel M. A. (2004) J. Am. Chem. Soc. 126, 17025–17031 [DOI] [PubMed] [Google Scholar]
- 33.Debono M., Abbott B. J., Molloy R. M., Fukuda D. S., Hunt A. H., Daupert V. M., Counter F. T., Ott J. L., Carrell C. B., Howard L. C., Boeck L. V., Hamill R. L. (1988) J. Antibiot. 41, 1093–1105 [DOI] [PubMed] [Google Scholar]
- 34.Boeck L. D., Fukuda D. S., Abbott B. J., Debono M. (1988) J. Antibiot. 41, 1085–1092 [DOI] [PubMed] [Google Scholar]
- 35.Hill J., Siedlecki J., Parr I., Morytko M., Yu X., Zhang Y., Silverman J., Controneo N., Laganas V., Li T., Lai J. J., Keith D., Shimer G., Finn J. (2003) Bioorg. Med. Chem. Lett. 13, 4187–4191 [DOI] [PubMed] [Google Scholar]
- 36.Silverman J. A., Oliver N., Andrew T., Li T. (2001) Antimicrob. Agents Chemother. 45, 1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siedlecki J., Hill J., Parr I., Yu X., Morytko M., Zhang Y., Silverman J., Controneo N., Laganas V., Li T., Li J., Keith D., Shimer G., Finn J. (2003) Bioorg. Med. Chem. Lett. 13, 4245–4249 [DOI] [PubMed] [Google Scholar]
- 38.Grünewald J., Marahiel M. A. (2006) Microbiol. Mol. Biol. Rev. 70, 121–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieber S. A., Walsh C. T., Marahiel M. A. (2003) J. Am. Chem. Soc. 125, 10862–10866 [DOI] [PubMed] [Google Scholar]
- 40.Sieber S. A., Tao J., Walsh C. T., Marahiel M. A. (2004) Angew. Chem. Int. Ed. Engl. 43, 493–498 [DOI] [PubMed] [Google Scholar]
- 41.Nguyen K. T., Ritz D., Gu J. Q., Alexander D., Chu M., Miao V., Brian P., Baltz R. H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17462–17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh C. T. (2002) ChemBioChem 3, 125–134 [DOI] [PubMed] [Google Scholar]
- 43.Penn J., Li X., Whiting A., Latif M., Gibson T., Silva C. J., Brian P., Davies J., Miao V., Wrigley S. K., Baltz R. H. (2006) J. Ind. Microbiol. Biotechnol. 33, 121–128 [DOI] [PubMed] [Google Scholar]
- 44.Baltz R. H., Brian P., Miao V., Wrigley S. K. (2006) J. Ind. Microbiol. Biotechnol. 33, 66–74 [DOI] [PubMed] [Google Scholar]
- 45.Miao V., Coëffet-Le Gal M. F., Nguyen K., Brian P., Penn J., Whiting A., Steele J., Kau D., Martin S., Ford R., Gibson T., Bouchard M., Wrigley S. K., Baltz R. H. (2006) Chem. Biol. 13, 269–276 [DOI] [PubMed] [Google Scholar]
- 46.Coëffet-Le Gal M. F., Thurston L., Rich P., Miao V., Baltz R. H. (2006) Microbiology 152, 2993–3001 [DOI] [PubMed] [Google Scholar]
- 47.McHenney M. A., Baltz R. H. (1996) Microbiology 142, 2363–2373 [DOI] [PubMed] [Google Scholar]
- 48.Miao V., Brost R., Chapple J., She K., Gal M. F., Baltz R. H. (2006) J. Ind. Microbiol. Biotechnol. 33, 129–140 [DOI] [PubMed] [Google Scholar]
- 49.Hojati Z., Milne C., Harvey B., Gordon L., Borg M., Flett F., Wilkinson B., Sidebottom P. J., Rudd B. A., Hayes M. A., Smith C. P., Micklefield J. (2002) Chem. Biol. 9, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 50.Tanovic A., Samel S. A., Essen L. O., Marahiel M. A. (2008) Science 321, 659–663 [DOI] [PubMed] [Google Scholar]
- 51.Hahn M., Stachelhaus T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15585–15590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doekel S., Coëffet-Le Gal M. F., Gu J. Q., Chu M., Baltz R. H., Brian P. (2008) Microbiology 154, 2872–2880 [DOI] [PubMed] [Google Scholar]
- 53.Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu J. Q., Nguyen K. T., Gandhi C., Rajgarhia V., Baltz R. H., Brian P., Chu M. (2007) J. Nat. Prod. 70, 233–240 [DOI] [PubMed] [Google Scholar]
- 55.LaPlante K. L., Rybak M. J. (2004) Antimicrob. Agents Chemother. 48, 4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sader H. S., Moet G., Jones R. N. (2009) J. Chemother. 21, 500–506 [DOI] [PubMed] [Google Scholar]
- 57.Baltz R. H., Miao V., Wrigley S. K. (2005) Nat. Prod. Rep. 22, 717–741 [DOI] [PubMed] [Google Scholar]
- 58.Ho S. W., Jung D., Calhoun J. R., Lear J. D., Okon M., Scott W. R., Hancock R. E., Straus S. K. (2008) Eur. Biophys. J. 37, 421–433 [DOI] [PubMed] [Google Scholar]
- 59.Jung D., Rozek A., Okon M., Hancock R. E. (2004) Chem. Biol. 11, 949–957 [DOI] [PubMed] [Google Scholar]
- 60.Ball L. J., Goult C. M., Donarski J. A., Micklefield J., Ramesh V. (2004) Org. Biomol. Chem. 2, 1872–1878 [DOI] [PubMed] [Google Scholar]
- 61.Rotondi K. S., Gierasch L. M. (2005) Biopolymers 80, 374–385 [DOI] [PubMed] [Google Scholar]
- 62.Straus S. K., Hancock R. E. (2006) Biochim. Biophys. Acta 1758, 1215–1223 [DOI] [PubMed] [Google Scholar]
- 63.Silverman J. A., Perlmutter N. G., Shapiro H. M. (2003) Antimicrob. Agents Chemother. 47, 2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott W. R., Baek S. B., Jung D., Hancock R. E., Straus S. K. (2007) Biochim. Biophys. Acta 1768, 3116–3126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.