Abstract
The innate immune system in humans consists of both cellular and humoral components that collaborate to eradicate invading bacteria from the body. Here, we discover that the Gram-positive bacterium Bacillus anthracis, the causative agent of anthrax, does not grow in human serum. Fractionation of serum by gel filtration chromatography led to the identification of human transferrin as the inhibiting factor. Purified transferrin blocks growth of both the fully virulent encapsulated B. anthracis Ames and the non-encapsulated Sterne strain. Growth inhibition was also observed in serum of wild-type mice but not of hypotransferrinemic mice that only have ∼1% circulating transferrin levels. We were able to definitely assign the bacteriostatic activity of transferrin to its iron-binding function: neither iron-saturated transferrin nor a recombinant transferrin mutant unable to bind iron could inhibit growth of B. anthracis. Additional iron could restore bacterial growth in human serum. The observation that other important Gram-positive pathogens are not inhibited by transferrin suggests they have evolved effective mechanisms to circumvent serum iron deprivation. These findings provide a better understanding of human host defense mechanisms against anthrax and provide a mechanistic basis for the antimicrobial activity of human transferrin.
Keywords: Bacteria, Innate Immunity, Iron Metabolism, Protein Chemistry, Serum
Introduction
The Gram-positive bacterium Bacillus anthracis can infect humans and is notorious for its potential as a biological weapon (1). Depending on the route of infection, B. anthracis causes respiratory, cutaneous, or gastrointestinal anthrax (2). The most lethal form, respiratory anthrax, is caused by inhalation of dormant bacterial spores, which are taken up by alveolar macrophages and dendritic cells. These spores can germinate within macrophages and hijack them for transport of vegetative bacilli to the lymph nodes. Subsequent spread into the bloodstream often results in sepsis and death (1). The ability to divide within macrophages without inducing an inflammatory reaction depends on the plasmid-encoded tripartite anthrax toxin (pXO1) that blocks intracellular signaling in immune cells (3–5). Intradermal inoculation with B. anthracis spores results in cutaneous anthrax, a milder infection that normally remains localized and resolves spontaneously (6).
Little is known about human innate immune defenses against B. anthracis (7). In cutaneous anthrax, neutrophils are essential for maintaining a localized infection (6). Recent studies have demonstrated that cathelicidin antimicrobial peptides, expressed by epithelial cells and phagocytes, are also an important component the innate immune response that targets B. anthracis (8–9). Serum proteins provide additional defense functions by activation of the complement system. Complement-depleted or C5-deficient mice are much more susceptible to infection with B. anthracis (10). However, the poly-γ-d glutamic acid capsule (pXO2) makes B. anthracis highly resistant to complement-dependent clearance (11). Here we discover that serum also provides resistance to anthrax propagation via a complement-independent mechanism. In contrast to other Gram-positive pathogens, we find that B. anthracis does not grow in the presence of human serum. We determine that serum resistance is provided by transferrin, identifying a novel antibacterial function of this human iron homeostasis protein.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
B. anthracis Sterne (pXO1+pXO2-) strains 34F2 and 7702 are both derived from the original Sterne strain isolated by Max Sterne in the 1930's. This strain was subsequently cultivated in different laboratories for many years. B. anthracis Sterne strain 7702, originally from the Pasteur Institute collection (12), was provided by S. Stibitz (Bethesda, MD). B. anthracis Sterne strain 34F2 and B. anthracis Ames were from the USAMRIID collection. B. anthracis was propagated on Todd Hewitt agar (THA) plates and grown under aerobic conditions at 30 °C; for liquid cultures, bacteria were grown in TH broth (THB) at 37 °C. Staphylococcus aureus strain Newman (NC_009641) was a kind gift from T. J. Foster (Trinity College, Dublin, Ireland) (13). Streptococcus pneumoniae serotype 2 strain D39 (NCTC 7466) was a gift from T. Mitchell (University of Glasgow) and was cultured directly from glycerol stock in Brain-Heart Infusion broth.
Sera and Transferrin
Blood was drawn from healthy human volunteers after informed consent and serum was collected. Normal human serum (nhs) was prepared by pooling sera of 6 different donors and stored at −80 °C. Heat inactivation was performed at 56 °C for 30 min and analyzed by a functional complement ELISA (14). Human apo-transferrin (apo-hTF)3 and holo-transferrin (holo-hTF) were obtained from Calbiochem (San Diego, CA). We use three different forms of recombinant transferrin prepared as previously described (15): 1) recombinant holo-transferrin (holo-rhTF), predicted to behave identically to holo-hTF from Calbiochem; 2) holo-rhTF from which iron was removed, i.e. recombinant apo-transferrin (apo-rhTF); 3) an iron-binding mutant (apo-rhTFΔFe) with two mutated tyrosines in both the N- and C-terminal lobe (Y95F/Y188F/Y426F/Y517F). All recombinant transferrins have a hexa-His tag at the N terminus and are nonglycosylated. Neither the lack of carbohydrates nor the presence of the His tag affect their function (15).
Bacterial Growth Assays
Bacterial cultures were grown to post-log phase in THB media and diluted 1:10,000 in RPMI 1640 tissue culture medium (Invitrogen, Carlsbad, CA). 50 μl of bacteria were mixed with 50 μl RPMI (untreated controls), serum or transferrin (both diluted in RPMI) in 96-well plates. Iron(III) citrate was obtained from Sigma. Plates were incubated at 37 °C under continuous shaking and surviving bacteria were enumerated by plating serial dilutions on THA. Growth index was calculated as the ratio of bacterial colony forming units (cfu) surviving after incubation versus the initial inoculum. Relative growth was used when growth of B. anthracis was compared with different bacteria (S. aureus, S. pneumonia, GAS, and GBS); in this case growth in buffer alone (RPMI) was set at 100%.
Gel Filtration Chromatography and Functional Screening
Heat-inactivated serum (250 μl) was separated on a Superdex-200 GL 10/300 (GE Healthcare, Piscataway, NJ) equilibrated with RPMI 1640 (flow 1 ml/min), and 1-ml fractions were collected. The column was calibrated with thyroglobulin (670 kDa), gamma globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.4 kDa). Fractions were incubated with 500 cfu of B. anthracis Sterne 7702 and grown overnight at 37 °C. Overnight cultures were subcultured in THB and grown for 7 h after which A600 was assessed.
Immunoblotting
Gel filtration fractions were separated by SDS-PAGE and transferred to an Immobilon PVDF membrane (Millipore, Billerica, MA). Transferrin was detected using peroxidase-labeled goat-anti-transferrin antibodies (Bethyl Laboratories, Montgomery, TX) and enhanced chemiluminescence (Thermo Fisher Scientific, Rockford, IL).
Hypotransferrinemic (Trfhpx/hpx) Mice
The Trfhpx/hpx mice, maintained on a BALBc/J background, were produced and housed as described previously (16). Trfhpx/hpx mice were treated with weekly intraperitoneal injections of 6 mg of human Trf (Roche Applied Science, Indianapolis, IN) until weaning. Serum was collected from female mice at 8 weeks of age, when all injected transferrin is degraded.
Statistical Analysis
Student's t test analysis was performed using the Microsoft Excel Software.
RESULTS
Human Serum Inhibits Growth of B. anthracis
While studying the role of humoral blood components in the defense against Gram-positive pathogens, we observed that growth of B. anthracis Sterne is strongly impaired in the presence of normal human serum (Fig. 1A). In contrast, serum did not affect growth of S. aureus nor S. pneumoniae. The two Sterne strains showed similar growth inhibition at 1% serum but B. anthracis Sterne 7702 was more sensitive at 5% serum. This difference may be due to the fact that these two strains have been cultured under different conditions for many years. Growth inhibition of B. anthracis was dose-dependent and complete at serum concentrations of 1% or higher (Fig. 1B). Serum was bacteriostatic rather than bactericidal to B. anthracis, since the initial bacterial inoculum did not decrease in its presence. Although Gram-positive bacteria have a thick peptidoglycan layer that makes them resistant to complement lysis, we wondered whether bacteriostasis could be complement-dependent. Heat-inactivated serum could still inhibit growth of B. anthracis Sterne, although the activity was somewhat lower than in normal serum (Fig. 1C). This finding suggests that complement contributes to the growth inhibitory effect, but that serum contains another component that blocks growth of anthrax bacilli.
FIGURE 1.
Human serum inhibits growth of B. anthracis. A, growth of different Gram-positive pathogens after 5 h of incubation in buffer (RPMI) or human serum. Growth in buffer alone (RPMI) was set at 100%. Impaired growth was detected for B. anthracis Sterne 34F2 and Sterne 7702. B, growth of B. anthracis Sterne 34F2 after 5 h of incubation with 0, 0.1, 1 or 10% human serum. C, growth of B. anthracis Sterne 34F2 after 5 h of growth in normal human serum (nhs) or heat-inactivated serum. Growth indices indicate ratio of bacterial cfu surviving after incubation versus the initial inoculum. Data represent mean ± S.E. of three independent experiments. *, p < 0.01; **, p < 0.001 (serum compared with buffer).
Identification of Human Transferrin as the Serum Inhibitory Factor
To identify the serum component that inhibits growth of B. anthracis, we fractionated heat-inactivated human serum by gel filtration chromatography and analyzed collected fractions for their ability to block growth of B. anthracis (Fig. 2A). We found that the bacteriostatic activity eluted from the column in a single wide peak, indicative of a single protein. The active protein was estimated to have a molecular weight between ∼15–150 kDa (Fig. 2A). (Moderate growth inhibition seen in fractions smaller than 1 kDa can most likely be attributed to high salt). Because the iron regulator human transferrin has a molecular mass of 80 kDa and is relatively abundant in serum (2–4 mg/ml) (17), we tested for the presence of transferrin in the eluted fractions. Immunoblotting with anti-transferrin antibodies shows that transferrin was indeed present in fractions with bacteriostatic activity (Fig. 2A). To confirm that human transferrin indeed causes bacteriostasis of B. anthracis, we incubated purified apo-transferrin with B. anthracis and monitored growth over time. Purified apo-transferrin completely inhibited growth of B. anthracis Sterne 34F2 at concentrations of 10 μg/ml and higher (Fig. 2B). Because B. anthracis strain Sterne is an attenuated, pXO2-minus strain, we also determined whether apo-transferrin affected growth of the fully virulent pXO2-positive Ames strain. Fig. 2C shows that apo-transferrin effectively blocked growth of the Ames strain as well. To show that transferrin is the bacteriostatic component in serum, we studied bacterial growth in serum of hypotransferrinemic (Trfhpx/hpx) mice that carry a spontaneous mutation in the murine transferrin (Trf) locus. These mice have only 1% circulating transferrin levels (16). In contrast to serum of wild-type mice, we observed that serum of hypotransferrinemic mice could not block growth of B. anthracis (Fig. 2D). We could restore the inhibitory effect by supplementing hypotransferrinemic serum with purified human apo-transferrin. Taken together, these data show that the iron homeostasis protein transferrin blocks growth of B. anthracis in serum.
FIGURE 2.
Identification of human transferrin as the bacteriostatic component. A, functional screening of serum fractions prepared by gel filtration. Fractions were incubated with B. anthracis Sterne 7702 for 12 h and subsequently subcultured in THB to assess growth after 7 h by A600. Inlay, below: immunoblot analysis of fraction 13–20 using an anti-human transferrin antibody. Representative figures of two independent experiments. B, growth of B. anthracis Sterne 34F2 after 5 h of incubation with 0, 1, 10, or 100 μg/ml human apo-transferrin. C, growth of B. anthracis Ames after 5 h of incubation with 100 μg/ml human apo-transferrin (apo-hTF). *, p < 0.01 (apo-hTF compared with buffer). D, growth of B. anthracis Sterne 34F2 after 5 h of incubation with 10% serum of wild-type mice, hypotransferrinemic mice (Trfhpx/hpx), or 10% serum of Trfhpx/hpx mice supplemented with 20 μg/ml apo-hTf. Each bar represents one mouse. B–D, growth indices indicate ratio of bacterial cfu surviving after incubation versus the initial inoculum. Data represent mean ± S.E. of three independent experiments.
Transferrin Blocks Growth of B. anthracis by Iron Deprivation
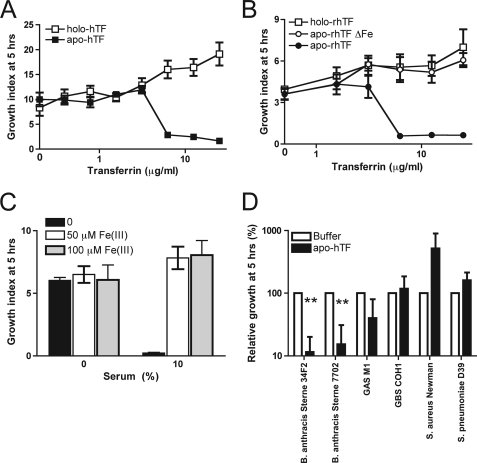
In normal persons, only ∼30% of transferrin in serum is in complex with iron (holo-transferrin), leaving a pool of transferrin with no iron, ∼40% (apo-transferrin) (17). To study whether iron binding by transferrin is important for B. anthracis growth inhibition, we compared growth in the presence of these naturally occurring forms of transferrin. We observed a dose-dependent growth inhibition by apo-transferrin, whereas growth was not affected by holo-transferrin (Fig. 3A). These data strongly suggest that transferrin blocks growth by iron sequestration. However, in the case of Gram-negative bacteria, transferrin has also been suggested to directly injure the bacterial outer membrane (18, 19). Because holo-transferrin has a different conformation than apo-transferrin (20, 21), we could not exclude that apo-transferrin has direct membrane damaging properties. However, we studied the anti-bacterial mechanism in more detail by analyzing growth in the presence of a recombinant apo-transferrin mutant that does not bind iron, but has the same conformation as wild-type apo-transferrin (15). Fig. 3B shows that, in contrast to recombinant apo-transferrin, the iron-binding mutant did not block growth of B. anthracis Sterne. Also, recombinant holo-transferrin showed no bacteriostatic activity. Furthermore we showed that growth of B. anthracis in human serum could be restored by supplementation with additional iron (Fig. 3C). Collectively these data show that transferrin effectively blocks the growth of B. anthracis by depriving the bacilli from acquiring the iron that they need to grow. Because our initial experiments in human serum showed that S. aureus and S. pneumoniae could resist the bacteriostatic effects of serum, we re-tested the growth of these and other Gram-positive bacteria in the presence of purified apo-transferrin. Growth of all tested pathogens was unaffected by purified apo-transferrin (Fig. 3D), indicating that they have evolved mechanisms to circumvent the iron scavenging effects of human transferrin.
FIGURE 3.
Transferrin blocks growth of B. anthracis by iron deprivation. A, growth of B. anthracis Sterne 34F2 after 5 h of incubation with human apo-transferrin (apo-hTF, iron-free) or holo-transferrin (holo-hTF, iron-saturated). B, growth of B. anthracis Sterne 34F2 after 5 h of incubation in the presence of recombinant apo-transferrin (apo-rhTF), recombinant holo-transferrin (holo-rhTF), or apo-rhTF mutated in its iron-binding sites (apo-rhTFΔFe). C, growth of B. anthracis Sterne 34F2 after 5 h of incubation in the presence of human serum (0 or 10%) in the presence of iron(III) citrate. D, relative growth of different Gram-positive pathogens grown for 5 h in the presence of human apo-transferrin (apo-hTF). Growth in buffer alone was set at 100%. Data represent mean ± S.E. of three independent experiments. Growth indices indicate ratio of bacterial cfu surviving after incubation versus the initial inoculum. **, p < 0.001 (apo-hTF compared with buffer).
DISCUSSION
The human innate immune system provides an essential first-line of defense against bacterial infections and is endowed with both cellular and humoral components that eradicate infectious organisms within minutes (22). In addition to directly killing the infectious organism, a common host defense strategy is to limit growth of the bacterium through deprivation of essential nutrients. The human protein lactoferrin, found at mucosal surfaces and inside neutrophil granules, has been characterized to play a role in host defense by iron sequestration and limitation of bacterial growth (23, 24). In addition, lactoferrin damages bacterial membranes directly via its highly cationic N terminus that inserts into bacterial membranes (25, 26). Transferrin, the serum-homologue of lactoferrin, is well-known for its role in iron homeostasis (27). Transferrin has a high avidity for iron (10−23 m), which ensures effective iron chelation and prevents iron-dependent formation of hydroxyl radicals. Transferrin is comprised of two homologous lobes, each of which binds ferric iron deep in a cleft. Structural studies revealed that iron-bound and iron-free transferrin are conformationally different since iron binding induces large conformational rearrangements (21, 28). Some studies (performed in the 1970–1980s) theorized that transferrin could also be an important anti-bacterial molecule, but its exact role in controlling infections remained ill-defined (29–31). For instance, it was not established whether the antibacterial activity was caused by iron deprivation or direct lysis (31–32). It has been reported that transferrin directly damages and alters the permeability of the outer membrane of Gram-negative bacteria (18, 19) even though the molecule lacks a cationic domain (33). Using recombinant forms of the molecule, we show for the first time that transferrin inhibits the growth of a bacterial pathogen, in this case the potentially lethal anthrax bacillus, through iron deprivation. Because the iron-binding mutant form of transferrin has the same conformation as apo-transferrin, we can exclude that conformational differences (as occur in the holo versus apo forms) play a role in the lack of activity. Our observation that expression of the poly-γ-d-glutamic acid capsule could not protect bacilli from transferrin-mediated growth inhibition is consistent with the idea that membrane-targeting effects are less likely to play a role.
Pathogens have found ways to counteract innate immune defenses to antimicrobial peptides, complement and phagocyte responses (34, 35). Many bacteria have also evolved iron uptake systems that allow them to specifically use host iron sources such as heme or transferrin (36). For instance, bacteria produce iron chelators that compete with transferrin (37, 38). Among Gram-positive species, only S. aureus is known to express such a molecule: the staphylococcal transferrin-binding protein A (39). Indeed, we observe that S. aureus grows even better in the presence of purified transferrin than in its absence. Our studies indicate that S. pneumoniae is also resistant to the antibacterial effects of transferrin in serum. Whereas the normal ecology of S. aureus and S. pneumoniae involves asymptomatic colonization of human mucosal surfaces, B. anthracis exists in the soil causing a zoonotic infection and only accidentally infects humans, perhaps explaining why it has not evolved transferrin resistance mechanism. In addition, many of the described bacterial iron-uptake systems are highly host-specific. Even though B. anthracis is known to express siderophores, small molecules that chelate iron (40), we observe that under the relevant physiological conditions (human serum at 37 °C) that we used these molecules are not effective in competing with transferrin in human serum.
The sensitivity of B. anthracis to human transferrin could provide an explanation for the observed differences in disease severity between B. anthracis introduced via the skin or the lungs. Anthrax spores that enter the body via the skin have been shown to germinate extracellularly, which allows serum proteins in interstitial tissue fluids to interact with the bacteria and control the infection (41, 42). In contrast, inhaled spores are quickly phagocytosed by macrophages in which they germinate intracellularly. In this case, bacilli are protected from serum resistance factors allowing them to initially grow unimpeded, multiply rapidly and potentially cause an overwhelming systemic infection. In conclusion, although the antibacterial properties of transferrin are not generally appreciated, our data show that transferrin is a critical host defense molecule that controls growth of B. anthracis in human serum.
Acknowledgments
We thank Pauline Macheboef and Partho Ghosh for practical assistance and Robert Mak for providing anti-transferrin antibodies.
This work was supported, in whole or in part, by the National Institutes of Health Grants AI077780 (to V. N.), DK084122–01 (to T. B. B.), and DK21739 (to A. B. M.). This work was also supported by the European Organization of Molecular Biology (EMBO) (to S. H. M. R.).
- apo-hTF
- human apo-transferrin
- holo-hTF
- human holo-transferrin
- holo-rhTF
- recombinant human holo-transferrin
- apo-rhTF
- recombinant human apo-transferrin
- apo-rhTFΔFe
- apo-rhTF with a mutated iron-binding site.
REFERENCES
- 1.Dixon T. C., Meselson M., Guillemin J., Hanna P. C. (1999) Anthrax N. Engl. J. Med. 341, 815–826 [DOI] [PubMed] [Google Scholar]
- 2.Friedlander A. M. (2000) Curr. Clin. Topics Inf. Dis. 20, 335–349 [PubMed] [Google Scholar]
- 3.Baldari C. T., Tonello F., Paccani S. R., Montecucco C. (2006) Trends Immunol. 27, 434–440 [DOI] [PubMed] [Google Scholar]
- 4.Pannifer A. D., Wong T. Y., Schwarzenbacher R., Renatus M., Petosa C., Bienkowska J., Lacy D. B., Collier R. J., Park S., Leppla S. H., Hanna P., Liddington R. C. (2001) Nature 414, 229–233 [DOI] [PubMed] [Google Scholar]
- 5.Ribot W. J., Panchal R. G., Brittingham K. C., Ruthel G., Kenny T. A., Lane D., Curry B., Hoover T. A., Friedlander A. M., Bavari S. (2006) Infect. Immun. 74, 5029–5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer-Scholl A., Hurwitz R., Brinkmann V., Schmid M., Jungblut P., Weinrauch Y., Zychlinsky A. (2005) PLoS Pathog. 1, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukao T. (2004) Lancet Infect. Dis. 4, 166–170 [DOI] [PubMed] [Google Scholar]
- 8.McGillivray S. M., Ebrahimi C. M., Fisher N., Sabet M., Zhang D. X., Chen Y., Haste N. M., Aroian R. V., Gallo R. L., Guiney D. G., Friedlander A. M., Koehler T. M., Nizet V. (2009) J. Innate Immun. 1, 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisanby M. W., Swiecki M. K., Dizon B. L., Pflughoeft K. J., Koehler T. M., Kearney J. F. (2008) J. Immunol. 181, 4989–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvill E. T., Lee G., Grippe V. K., Merkel T. J. (2005) Infect. Immun. 73, 4420–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welkos S. L., Keener T. J., Gibbs P. H. (1986) Infect. Immun. 51, 795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cataldi A., Labruyère E., Mock M. (1990) Mol. Microbiol. 4, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 13.Duthie E. S., Lorenz L. L. (1952) J. Gen Microbiol. 6, 95–107 [DOI] [PubMed] [Google Scholar]
- 14.Roos A., Bouwman L. H., Munoz J., Zuiverloon T., Faber-Krol M. C., Fallaux-van den Houten F. C., Klar-Mohamad N., Hack C. E., Tilanus M. G., Daha M. R. (2003) Mol. Immunol. 39, 655–668 [DOI] [PubMed] [Google Scholar]
- 15.Mason A. B., Halbrooks P. J., Larouche J. R., Briggs S. K., Moffett M. L., Ramsey J. E., Connolly S. A., Smith V. C., MacGillivray R. T. (2004) Protein Expr Purif. 36, 318–326 [DOI] [PubMed] [Google Scholar]
- 16.Trenor C. C., 3rd, Campagna D. R., Sellers V. M., Andrews N. C., Fleming M. D. (2000) Blood 96, 1113–1118 [PubMed] [Google Scholar]
- 17.Williams J., Moreton K. (1980) Biochem. J. 185, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilera O., Quiros L. M., Fierro J. F. (2003) FEBS Lett. 548, 5–10 [DOI] [PubMed] [Google Scholar]
- 19.Ellison R. T., 3rd, Giehl T. J., LaForce F. M. (1988) Infect. Immun. 56, 2774–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffrey P. D., Bewley M. C., MacGillivray R., Mason A. B., Woodworth R. C., Baker E. N. (1998) Biochemistry 37, 13978–13986 [DOI] [PubMed] [Google Scholar]
- 21.Wally J., Halbrooks P. J., Vonrhein C., Rould M. A., Everse S. J., Mason A. B., Buchanan S. K. (2006) J. Biol. Chem. 281, 24934–24944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutler B. (2004) Mol. Immunol. 40, 845–859 [DOI] [PubMed] [Google Scholar]
- 23.Legrand D., Pierce A., Elass E., Carpentier M., Mariller C., Mazurier J. (2008) Adv. Exp. Med. Biol. 606, 163–194 [DOI] [PubMed] [Google Scholar]
- 24.Valenti P., Antonini G. (2005) Cell. Mol. Life. Sci. 62, 2576–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Chávez S. A., Arévalo-Gallegos S., Rascón-Cruz Q. (2009) Int. J. Antimicrob. Agents 33, 301–308 [DOI] [PubMed] [Google Scholar]
- 26.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. (1992) Biochim. Biophys. Acta 1121, 130–136 [DOI] [PubMed] [Google Scholar]
- 27.De Domenico I., McVey Ward D., Kaplan J. (2008) Nat. Rev. Mol. Cell. Biol. 9, 72–81 [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y., Zak O., Aisen P., Harrison S. C., Walz T. (2004) Cell 116, 565–576 [DOI] [PubMed] [Google Scholar]
- 29.Oram J. D., Reiter B. (1968) Biochim. Biophys. Acta 170, 351–365 [DOI] [PubMed] [Google Scholar]
- 30.Bullen J. J., Ward C. G., Wallis S. N. (1974) Infect. Immun. 10, 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenti P., Antonini G., Fanelli M. R., Orsi N., Antonini E. (1982) Antimicrob. Agents. Chemother. 21, 840–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezkorovainy A. (1981) Adv. Exp. Med Biol. 135, 139–154 [DOI] [PubMed] [Google Scholar]
- 33.Wally J., Buchanan S. K. (2007) Biometals 20, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster T. J. (2005) Nat. Rev. Microbiol. 3, 948–958 [DOI] [PubMed] [Google Scholar]
- 35.Nizet V. (2007) J. Allergy Clin Immunol. 120, 13–22 [DOI] [PubMed] [Google Scholar]
- 36.Krewulak K. D., Vogel H. J. (2008) Biochim. Biophys. Acta 1778, 1781–1804 [DOI] [PubMed] [Google Scholar]
- 37.Cornelissen C. N. (2003) Front. Biosci. 8, d836–847 [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen C. N., Sparling P. F. (1994) Mol. Microbiol. 14, 843–850 [DOI] [PubMed] [Google Scholar]
- 39.Taylor J. M., Heinrichs D. E. (2002) Mol. Microbiol. 43, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 40.Abergel R. J., Wilson M. K., Arceneaux J. E., Hoette T. M., Strong R. K., Byers B. R., Raymond K. N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18499–18503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischof T. S., Hahn B. L., Sohnle P. G. (2007) J. Infect Dis. 195, 888–894 [DOI] [PubMed] [Google Scholar]
- 42.Rossing N., Worm A. M. (1981) Clin. Physiol. 1, 275–284 [DOI] [PubMed] [Google Scholar]