Abstract
Angiotensin II (ANG II) stimulates renal tubular reabsorption of NaCl by targeting Na+/H+ exchanger NHE3. We have shown previously that inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) plays a critical role in stimulation of NHE3 in response to elevated intracellular Ca2+ concentration ([Ca2+]i). In this study, we investigated the role of IRBIT in mediating NHE3 activation by ANG II. IRBIT is abundantly expressed in the proximal tubules where NHE3 is located. ANG II at physiological concentrations stimulates NHE3 transport activity in a model proximal tubule cell line. ANG II-induced activation of NHE3 was abrogated by knockdown of IRBIT, whereas overexpression of IRBIT enhanced the effect of ANG II on NHE3. ANG II transiently increased binding of IRBIT to NHE3 at 5 min but became dissociated by 45 min. In comparison, it took at least 15 min of ANG II treatment for an increase in NHE3 activity and NHE3 surface expression. The stimulation of NHE3 by ANG II was dependent on changes in [Ca2+]i and Ca2+/calmodulin-dependent protein kinases II. Inhibition of CaMKII completely blocked the ANG II-induced binding of IRBIT to NHE3 and the increase in NHE3 surface abundance. Several serine residues of IRBIT are thought to be important for IRBIT binding. Mutations of Ser-68, Ser-71, and Ser-74 of IRBIT decreased binding of IRBIT to NHE3 and its effect on NHE3 activity. In conclusion, our current findings demonstrate that IRBIT is critically involved in mediating activation of NHE3 by ANG II via a Ca2+/calmodulin-dependent protein kinases II-dependent pathway.
Keywords: Calcium, Calcium Calmodulin-dependent Protein Kinase (CaMK), Kidney, Protein-Protein Interactions, Sodium Proton Exchange
Introduction
The mammalian kidney plays an essential role in the control of systemic water, ion, and acid-base balance. Na+/H+ exchanger type 3 (NHE3) plays a pivotal role in salt and fluid reabsorption in the proximal tubule (1), accounting for ∼50% of NaCl and 70% of NaHCO3 reabsorbed from the glomerular filtrate (2). In fact, mice deficient in NHE3 expression are relatively hypotensive, even when NHE3 in the intestine is rescued by transgenic expression (3).
The renin-angiotensin system is critically involved in regulation of body blood pressure and fluid balance. The kidney secretes renin when blood pressure is low that stimulates the production of ANG3 I, which is subsequently converted into ANG II by angiotensin-converting enzyme. The presence of ANG II receptor on the membrane of renal proximal tubules was first reported in the 1980s (4). Through the binding with its cognate receptor(s), ANG II is importantly involved in fluid reabsorption in the proximal tubules. Substantial earlier evidence from Cogan and co-workers (5–8) has demonstrated that ANG II is a potent agonist of H+ secretion and HCO3− absorption in rat proximal tubules through mechanisms dependent on decrease of cAMP, increase of [Ca2+]i, and activation of protein kinase C (PKC). It was later shown that ANG II, at low concentrations, stimulates NHE3 activity in renal proximal tubule cells (9–11). Studies have implicated the roles of PKC and c-Src in ANG II-mediated activation of NHE3 (9, 12). In addition, ANG II stimulates NHE3 activity through increased exocytotic insertion of NHE3 in a phosphatidylinositol 3-kinase (PI3K)-dependent manner (13). Nevertheless, the molecular mechanisms underlying NHE3 activation by ANG II remain incompletely delineated.
IRBIT was initially identified as an IP3 receptor-binding protein and was shown to be a competitive inhibitor of Ca2+ release by IP3 receptor (14, 15). We have recently identified IRBIT as a novel NHE3-interacting protein by yeast two-hybrid screening of a kidney library (16). Our study demonstrated that IRBIT binds the C-terminal domain of NHE3 and activates NHE3 activity in response to thapsigargin or ionomycin-induced rise of [Ca2+]i in PS120 fibroblast cells (16). IRBIT mRNA is ubiquitously present in all tissues, but the highest expression was reported in the brain, reproductive tissues, and kidney (14). These findings prompted us to hypothesize that IRBIT might play an important role in NHE3 regulation in the kidney. In this work, we investigated the role of IRBIT in the regulation of NHE3 by ANG II. Our findings show that IRBIT is critically involved in the activation of NHE3 by ANG II, and this regulation is Ca2+-CaMKII-dependent.
EXPERIMENTAL PROCEDURES
Cell Culture
Opossum kidney proximal tubule (OKP) cells (17) were kindly provided by Dr. Orson Moe, Texas Southwestern Medical Center. OKP cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1 mm sodium pyruvate, 50 units/ml penicillin, 50 μg/ml streptomycin, 20 mm HEPES, and 10% fetal bovine serum in a 5% CO2 humidified incubator at 37 °C. In all experiments, cells were grown 4–6 days post-confluence.
Plasmids
pcDNA3.1 harboring HA-tagged human IRBIT, pcDNA3.1/HA-IRBIT, was described previously (16). The lentiviral vector PLKO.1 harboring short hairpin RNA (shRNA) targeting IRBIT, sh-IRBIT, was purchased from Sigma. The pcDNA3.1/FLAG-CaMKIIα was subcloned from CaMKII-CS2+, which was obtained from Addgene (Cambridge, MA). pEGFP/CaMKIIN2 carrying the β isoform inhibitory protein of CaMKII (18) was kindly provided by Dr. Thomas Soderling, Oregon Health and Sciences University.
Immunoprecipitation
OKP cells were washed twice in cold phosphate-buffered saline (PBS), scraped, and lysed in lysis buffer (Cell Signaling, Danvers, MA), containing 20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm β-glycerophosphate, 2.5 mm sodium pyrophosphate, 1 mm Na2EDTA, 1 mm EGTA, 1 mm Na3VO4, 1 μg/ml leupeptin, 1% Triton X-100, and protease inhibitors mixture tablets (Roche Applied Science). The crude lysate was sonicated two times for 15 s and spun at 14,000 × g for 30 min. Protein concentration was determined by the bicinchoninic acid (BCA) assay (Sigma). Lysate (800 μg) was then incubated overnight with either an anti-IRBIT serum (16), an anti-HA antibody (Covance, Denver, PA), or an anti-CaMKII antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The generation of the anti-IRBIT antibody against the N-terminal 104 amino acids of IRBIT was described previously (16). The following morning, the lysate was incubated with 50 μl of protein A-Sepharose beads for 1 h, followed by three washes in lysis buffer and two washes in PBS. All the above steps were performed at 4 °C or on ice. Bound immunocomplex was eluted by incubating the protein-A beads in Laemmli sample buffer for 10 min at 95 °C and was separated by SDS-PAGE. Western blotting was performed as described previously (16).
Surface Biotinylation
Surface biotinylation of NHE3 was performed as described previously (16, 19). Briefly, OKP cells were serum-starved, and the cells were treated with 1 nm ANG II for up to 45 min. Cells were rinsed twice in PBS and a 10-min incubation in borate buffer composed of 154 mm NaCl, 7.2 mm KCl, 1.8 mm CaCl2, and 10 mm H3BO3, pH 9.0. Cells were then incubated for 40 min with 0.5 mg/ml NHS-SS-biotin (Pierce) in borate buffer. Unbound NHS-SS-biotin was quenched with Tris buffer (20 mm Tris, 120 mm NaCl, pH 7.4). Cells were then rinsed with PBS, scraped, lysed in the lysis buffer described above, and sonicated two times for 15 s. The lysate was agitated for 30 min and spun at 14,000 × g for 30 min to remove the insoluble cell debris. An aliquot was retained as the total fraction representing the total cellular NHE3. Protein concentration was determined, and 1 mg of lysate was then incubated with streptavidin-agarose beads (Pierce) for 2 h. The streptavidin-agarose beads were washed three times in lysis buffer and twice in PBS. All the above procedures were performed at 4 °C or on ice. Biotinylated surface proteins were then eluted by boiling the beads at 95 °C for 10 min. Dilutions of the total and surface NHE3 were resolved by SDS-PAGE and immunoblotted with an anti-NHE3 antibody, 3H3 (20). Densitometric analysis was performed using Scion Image software (National Institutes of Health, Bethesda).
Na+-dependent Intracellular pH Recovery
The Na+-dependent changes in intracellular pH (pHi) by NHE3 was determined with the use of the ratio-fluorometric (excitation, 495 nm and 440 nm, and emission, 530 nm) pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester as described previously (19, 20). Briefly, OKP cells were seeded on coverslips, grown to 4–6 days post-confluence, and then serum-starved overnight. Cells were washed in Na+ buffer (30 mm NaCl, 20 mm HEPES, 5 mm KCl, 1 mm tetramethylammonium-PO4 (TMA-PO4), 2 mm CaCl2, 1 mm MgSO4, and 18 mm glucose) and then were dye-loaded by incubating with 6.5 μm 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester in the same solution. The coverslips were mounted on a perfusion chamber mounted on an inverted microscope and were superfused with NH4+ buffer (40 mm NH4Cl, 90 mm NaCl, 20 mm HEPES, 5 mm KCl, 1 mm TMA-PO4, 2 mm CaCl2, 1 mm MgSO4, and 18 mm glucose) and subsequently with TMA+ buffer (130 mm TMA-Cl, 20 mm HEPES, 5 mm KCl, 1 mm TMA-PO4, 2 mm CaCl2, 1 mm MgSO4, and 18 mm glucose). One nm ANG II was added to Na+ buffer during dye loading and/or NH4+ and TMA+ buffer for the indicated time before the reintroduction of Na+ buffer to drive Na+-dependent pH recovery. The rate of Na+-dependent pHi recovery was calculated by determining slopes along the early stage of pHi recovery by linear least squares analysis over a minimum of 9 s (19). Calibration of the fluorescence signal was performed using the K+/H+ ionophore nigericin as described previously (21). Comparisons of Na+/H+ exchange were made between measurements made on the same day. The microfluorimetry was performed on a Nikon TE200 inverted microscope with a Nikon CFI Super Fluor ×40 objective, coupled to a Lambda 10-2 filter wheel controller equipped with a multiwavelength filter set designed for 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester. Photometric data were acquired using the Metafluor software (Molecular Devices, Sunnyvale, CA).
Calcium Imaging
OKP cells grown on coverslips were incubated with a perfusion buffer (in mm: 145 NaCl, 6 KCl, 1 MgSO4, 1 CaCl2, 10 glucose, 10 HEPES, pH 7.3) supplemented with 3 μm Fura-2 acetoxymethyl ester (Fura-2 AM, Invitrogen) for 20 min in the dark. Coverslips were thoroughly rinsed with the perfusion buffer without Fura-2, mounted on a perfusion chamber placed on a Nikon TE200 microscope, and perfused with the perfusion buffer containing ANG II or a carrier. Emission images at 510 nm were taken at 340- and 380-nm excitation wavelengths for ratiometric measurement of intracellular Ca2+ concentration ([Ca2+]i) according to previous studies (14, 22).
Confocal Immunofluorescence
Animals were maintained and experiments were performed under institutional guidelines of Emory University. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 200–250 g, received free access to water and standard rat chow (Purina). Preparation of rat kidney sections was as reported previously (23). Briefly, a rat was anesthetized with pentobarbital sodium and was perfused via the ascending aorta with PBS, followed by paraformaldehyde lysine periodate fixative (2% paraformaldehyde, 75 mm lysine, and 10 mm sodium periodate, pH 7.4). Kidneys were removed and post-fixed in the same fixative for 4 h at 4 °C and then incubated overnight in 30% sucrose in PBS for cryoprotection. Six-micron cryostat sections were prepared and stored at −80 °C until needed. The kidney tissues were washed twice with cold PBS, permeabilized in 0.5% Triton X-100 in PBS for 5 min, and blocked in PBS containing 5% normal goat serum for 30 min at room temperature. For OKP, cells were grown on Transwells (Corning Glass, Lowell, MA) until 4–6 days post-confluence. Cells were fixed and permeabilized as described previously (16). Tissues and cells were then stained with an affinity-purified polyclonal anti-IRBIT antibody, a monoclonal anti-NHE3 antibody (3H3 (24) for OKP cells or 4F5 (Chemicon, Temecula, CA) for tissues), or a monoclonal anti-FLAG antibody (Sigma) for 1 h at room temperature. Following three washes of 10 min each with PBS, the cells were incubated with Alexa 488-conjugated goat anti-rabbit IgG and Alexa 555-conjugated goat anti-mouse IgG (Invitrogen) for 1 h at room temperature. After three 10-min washes with PBS, the specimens were mounted with ProLong Gold Antifade Reagent (Invitrogen) and observed under a Zeiss LSM510 laser confocal microscope (Zeiss Microimaging, Thornwood, NY) coupled to a Zeiss Axioplan2e with ×100 Pan-Apochromat oil lenses.
Statistical Analysis
Results were presented as the mean ± S.E. Statistical significance was assessed by Student's t test using the SPSS statistic program (SPSS, Inc). p < 0.05 was considered significant.
RESULTS
Co-expression of IRBIT with NHE3 in the Rat Kidney
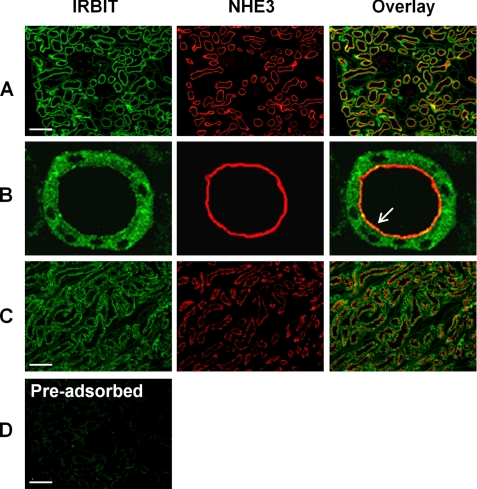
The presence of IRBIT protein in total cellular extract of mouse kidney was shown previously (14). Herein, we investigated the expression of IRBIT protein in rat kidney by indirect confocal immunofluorescence microscopy. The staining of IRBIT (green) in the outer portion of rat renal cortex is illustrated in Fig. 1. Positive staining for IRBIT was evident in the proximal convoluted tubules. On the contrary, the staining was absent in the glomeruli or distal convoluted tubules (Fig. 1A). The localization of IRBIT at the proximal tubules was confirmed by co-labeling of NHE3 (Fig. 1A, red), which is present in all sections of proximal tubules (1, 25). The specificity of IRBIT staining was confirmed by preadsorption of the anti-IRBIT antibody with a recombinant IRBIT peptide (Fig. 1D). High magnification images show that most of IRBIT was present in the cytosol, but the localization of IRBIT in the luminal membrane of proximal tubules can be seen (Fig. 1B). Co-localization of IRBIT and NHE3 was also observed in the outer medulla (Fig. 1C). The expression of NHE3 in the thick ascending limb and thin descending limb of the loop of Henle has been shown previously (25). Similarly to the expression pattern of NHE3 and IRBIT in the cortex, all the renal tubules expressing NHE3 showed positive staining for IRBIT.
FIGURE 1.
Expression of IRBIT and NHE3 in the rat kidney. Cellular distribution of IRBIT and NHE3 in the rat kidney was determined by indirect fluorescence microscopic analysis using an affinity-purified polyclonal anti-IRBIT antibody and a monoclonal anti-NHE3 antibody, respectively. A, IRBIT (green) and NHE3 (red) expression in the cortex is shown. B, enlarged view of the proximal tubule. Arrow indicates co-localization of IRBIT with NHE3 at the luminal membrane. C, IRBIT (green) and NHE3 (red) expression in outer medulla is shown. D, immunofluorescence labeling of a cortical section was performed using an anti-IRBIT antibody that was preadsorbed with the recombinant IRBIT peptide antigen (16). Scale bar, 20 μm.
Role of IRBIT in the Activation of NHE3 by Low Dose ANG II
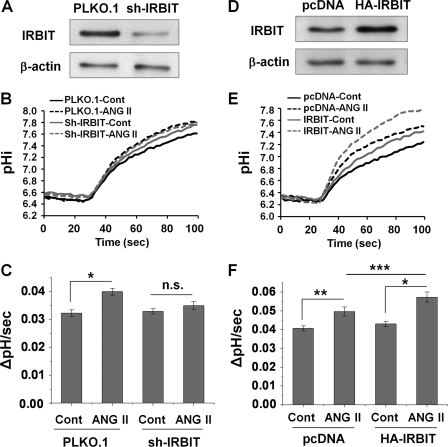
As shown previously (10), low concentrations of ANG II (0.1–1 nm) stimulated NHE3 activity in OKP cells (data not shown). One nm ANG II resulted in larger and more consistent stimulation of NHE3 than with 0.1 nm ANG II; therefore, we used 1 nm for the rest of the study. To determine the importance of IRBIT in ANG II-induced regulation of NHE3, the endogenous IRBIT was silenced by lentivirus-driven shRNA targeting IRBIT, sh-IRBIT. Fig. 2A shows that IRBIT expression in OKP/sh-IRBIT cells was decreased by ∼80% compared with the control cells treated with empty lentivirus. Knockdown of IRBIT abrogated the ANG II-mediated stimulation of NHE3 in OKP/sh-IRBIT cells as compared with the stimulation (22 ± 4%) in control transfected OKP/PLKO.1 cells (Fig. 2, B and C). Conversely, ectopic expression of HA-IRBIT in OKP cells, resulting in about 2-fold increase of total IRBIT (Fig. 2D), significantly (p < 0.05) enhanced the effect of ANG II on NHE3 (33 ± 5% versus 23 ± 4% for the control, Fig. 2, E and F). Together these data indicate that IRBIT enhances NHE3 regulation by ANG II.
FIGURE 2.
IRBIT is necessary for ANG II-mediated activation of NHE3 in OKP cells. A, OKP cells were infected with lentivirus carrying shRNA targeting IRBIT (sh-IRBIT) or the lentivirus PLKO.1 as a control. Western blot shows the expression level of IRBIT. β-Actin was used as a loading control. B, representative traces of Na+-dependent pHi recovery in OKP/PLKO.1 and OKP/sh-IRBIT cells treated with or without 1 nm ANG II for 45 min. Cont, control. C, NHE3 activity in response to 1 nm ANG II for 45 min was determined in OKP/PLKO.1 and OKP/sh-IRBIT cells. The rates of pHi recovery, ΔpH/s, at pHi 6.8 are shown. n = 10 or more. *, p < 0.001 compared with the control. n.s., not significant. D, expression level of IRBIT in OKP cells transfected with pcDNA/HA-IRBIT (HA-IRBIT) or a control vector (pcDNA) is shown. E, representative traces of Na+-dependent pHi recovery in OKP/pcDNA and OKP/HA-IRBIT treated with or without 1 nm ANG II for 45 min. F, NHE3 activities in response to 1 nm ANG II for 45 min were determined in OKP/pcDNA and OKP/HA-IRBIT cells. The rates of pHi recovery, ΔpH/s, at pHi 6.6 are shown. n = 10 or more. *, p < 0.001; **, p < 0.01; ***, p < 0.05.
Interaction between IRBIT and NHE3 Is Enhanced by ANG II
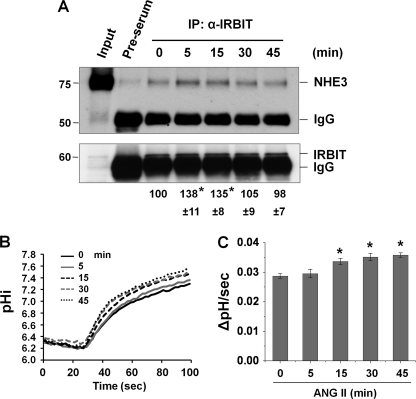
To explore the underlying mechanisms whereby IRBIT modulates NHE3 regulation by ANG II, we examined the effect of ANG II on the interaction between NHE3 and IRBIT. OKP cells were treated with ANG II for 0–45 min, followed by co-immunoprecipitation of NHE3 by an anti-IRBIT antibody. Fig. 3A shows that the interaction between NHE3 and IRBIT was significantly stimulated at 5–15 min of ANG II treatment. Surprisingly, the NHE3-IRBIT association gradually diminished to the basal level at 45 min. These results suggest that the interaction between NHE3 and IRBIT is transient.
FIGURE 3.
NHE3-IRBIT interaction is stimulated by ANG II. A, OKP cells were treated with 1 nm ANG II for 0–45 min, and the cell lystes were incubated with an anti-IRBIT antibody or a pre-serum. Upper panel, the amount of co-immunoprecipitated NHE3 was examined by Western blot with an anti-NHE3 antibody. Lower panel, total amount of immunoprecipitated (IP) IRBIT determined using an anti-IRBIT antibody is shown. The amount of co-immunoprecipitated NHE3 was normalized to the total amount of IRBIT, and the relative changes are shown below the immunoblots. Data are represented as means ± S.E. of three independent experiments. *, p < 0.01 compared with 0 min. B, representative traces of Na+-dependent pHi recovery in OKP cells treated with ANG II for 0–45 min. C, NHE3 activity in response to ANG II was determined in OKP cells for up to 45 min. The rates of pHi recovery, ΔpH/s, at pH 6.6 are shown. n = 8 or more. *, p < 0.001 compared with 0 min.
To determine the temporal relationship between the NHE3-IRBIT interaction and NHE3 transport activation, we determined the time course of NHE3 activation by ANG II in OKP cells. Fig. 3, B and C, shows that ANG II did not alter NHE3 activity at 5 min, the time at which a significant increase in binding of IRBIT to NHE3 was observed. The earliest time at which ANG II stimulated NHE3 was 15 min, and NHE3 activity remained elevated at 45 min, although IRBIT and NHE3 were dissociated based on the co-immunoprecipitation study. Therefore, the changes in NHE3 transport activity trail the interaction of NHE3 protein with IRBIT.
ANG II Increases NHE3 Surface Expression
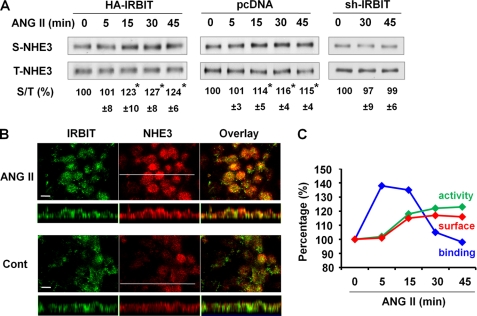
Because a major mechanism regulating NHE3 activity involves a change in NHE3 protein expression in the surface membrane, we investigated whether ANG II stimulates NHE3 by altering NHE3 surface expression. OKP/pcDNA, OKP/HA-IRBIT, and OKP/sh-IRBIT cells were treated with ANG II for up to 45 min, and NHE3 expression in the apical membrane was determined by surface biotinylation. ANG II treatment resulted in an increase in NHE3 surface expression by 23% (n = 3) as early as 15 min in OKP/HA-IRBIT cells (Fig. 4A, left panel). The corresponding changes in surface NHE3 expression in OKP/pcDNA cells were not as evident as in OKP/HA-IRBIT cells, but an ∼14% increase (n = 6, p < 0.01) following 15 min of ANG II treatment was observed (Fig. 4A, middle panel). In comparison, in cells infected with lentiviral sh-IRBIT, ANG II-induced NHE3 apical expression was completely abrogated (Fig. 4A, right panel).
FIGURE 4.
ANG II increases NHE3 protein abundance in the apical membrane. A, OKP/pcDNA, OKP/HA-IRBIT, or OKP/sh-IRBIT cells were treated with ANG II for 0–45 min, and the amount of NHE3 on the apical membrane was determined by surface biotinylation as detailed under “Experimental Procedures.” Aliquots of surface and total protein were resolved by SDS-PAGE, and the amounts of surface (S) and total (T) NHE3 proteins were determined by Western blot using an anti-NHE3 antibody. The amount of surface NHE3 was normalized to total NHE3, and the relative changes are shown as the mean ± S.E. of at least three independent experiments (n = 3 for OKP/HA-IRBIT and OKP/sh-IRBT, n = 6 for OKP/pcDNA). *, p < 0.01 compared with 0 min. B, OKP/HA-IRBIT cells were treated with or without 1 nm ANG II for 45 min, and the expression of NHE3 (red; anti-NHE3) and IRBIT (green; anti-IRBIT) was determined by indirect immunofluorescence confocal microscopy. Confocal focal planes (x-y) and cross-sections (x-z) are shown. Bar, 10 μm. Representative images from three independent experiments are shown. C, summary of the changes in NHE3-IRBIT binding, NHE3 activity, and NHE3 surface expression in OKP cells is shown.
Increased NHE3 apical expression in OKP/HA-IRBIT cells by ANG II was confirmed by an indirect confocal immunofluorescence analysis. Treatment with ANG II for 45 min intensified NHE3 staining in the apical membrane compared with the nontreated control cells (Fig. 4B). On the contrary, IRBIT labeling was not significantly altered. The time course of the changes in OKP cells during the ANG II treatment is summarized in Fig. 4C. The changes in NHE3 activity and the surface expression correlate well, but it is evident that the NHE3-IRBIT interaction precedes the changes in NHE3 activity and surface expression, suggesting that there is a temporal separation between these two series of events.
ANG II Activation of NHE3 Is Ca2+- and CaMKII-dependent
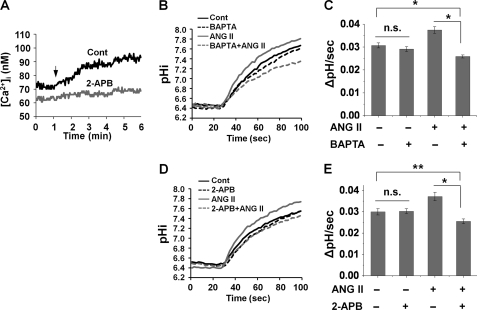
ANG II acting on angiotensin receptor subtype 1 (AT1R) increases phosphatidylinositol breakdown (26) and [Ca2+]i in kidney epithelial cells (22, 27). Application of 1 nm ANG II to OKP cells induced a gradual increase in [Ca2+]i, which reached a plateau and persisted at the elevated level for the next several minutes (Fig. 5A). The presence of 2-aminoethoxydiphenyl borate (2-APB), an IP3 receptor antagonist, abolished Ca2+ mobilization (Fig. 5A), indicating that the Ca2+ mobilization is AT1 receptor-dependent. Preincubation of the cells with the Ca2+ chelator BAPTA-AM prevented the stimulation of NHE3 by ANG II (Fig. 5, B and C), suggesting the obligatory role of Ca2+. Similarly, 2-APB completely blocked the effect of ANG II (Fig. 5, D and E). Interestingly, in the experiment using BAPTA-AM or 2-APB, NHE3 activity was lowered below the basal level when the cells were treated with an inhibitor together with ANG II, although the inhibitors by themselves had no intrinsic effect on NHE3 activity.
FIGURE 5.
Activation of NHE3 by ANG II is Ca2+-dependent. A, OKP cells were pretreated with or without 50 μm 2-APB during Fura-2 loading. Changes in [Ca2+]i in response to 1 nm ANG II were determined by ratiometric measurements. Arrow indicates where ANG II was applied. Representative images are shown. n = 16 or more. OKP cells were pretreated with 20 μm BAPTA (B and C) or 50 μm 2-APB (D and E) for 20 min, and NHE3 activities were determined after 45 min of treatment with or without 1 nm ANG II. B and D, representative traces of Na+-dependent pHi recovery. C and E, NHE3 activity is presented as ΔpH/s, and the pHi recovery rates at pHi 6.8 are shown. n = 8 or more. *, p < 0.001; **, p < 0.01; n.s., not significant; Cont, control.
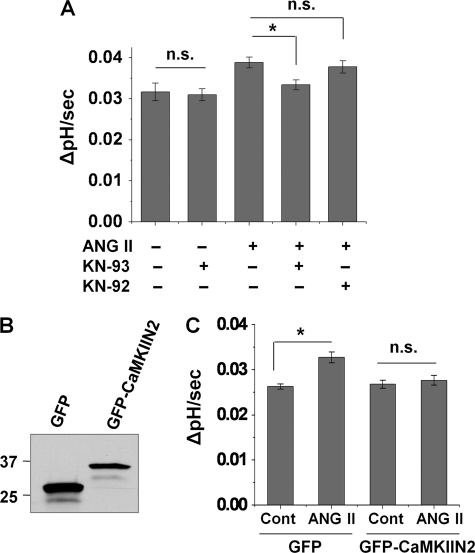
Previously, the necessity of IRBIT phosphorylation for its interaction with the IP3 receptor was shown and a potential involvement of CaMK was suggested (15, 28). We sought to determine a potential role of CaMK in ANG II-induced activation of NHE3. We initially employed the classical chemical inhibitor of CaMKII, KN-93, to probe the involvement of CaMKII. KN-93 alone did not alter the basal NHE3 activity, but it prevented activation of NHE3 by ANG II (Fig. 6A). In comparison, the inactive analog of KN-93, KN-92, showed no effect on the ANG II-induced activation of NHE3 (Fig. 6A).
FIGURE 6.
Activation of NHE3 by ANG II is CaMKII-dependent. A, OKP cells were pretreated with KN-93 or KN-92 for 20 min, and NHE3 activity was measured after 45 min of treatment with or without 1 nm ANG II. The rates of pHi recovery at pH 6.6 are shown. *, p < 0.01; n.s., not significant. B, OKP cells were transfected with pEGFP/CaMKIIN2 or pEGFP as a control (Cont). Western blot shows expression of GFP or GFP-CaMKIIN2. C, NHE3 activity in response to ANG II was determined in OKP/pEGFP and OKP/CaMKIIN2. The rates of pHi recovery, ΔpH/s, at pH 6.8 are shown. n = 8 or more. *, p < 0.001; n.s., not significant.
Despite the broad use of KN-93 as a CaMKII inhibitor, it is a direct blocker of voltage-gated K+ channels (29, 30). To further substantiate the role of CaMKII, we used the natural CaMKII inhibitor protein CaMKIIN2, which specifically inhibits CaMKII but not other CaMKs, PKC or protein kinase A (18, 31). OKP cells were stably transfected with pEGFP-CaMKIIN2 or pEGFP as a control (Fig. 6B). As shown in Fig. 6C, ANG II did not stimulate NHE3 activity in GFP-CaMKIIN2-expressing cells, which is in contrast to the GFP-expressing control cells. These findings demonstrate that CaMKII is required for ANG II-induced activation of NHE3.
IRBIT Associates with CaMKII in OKP Cells
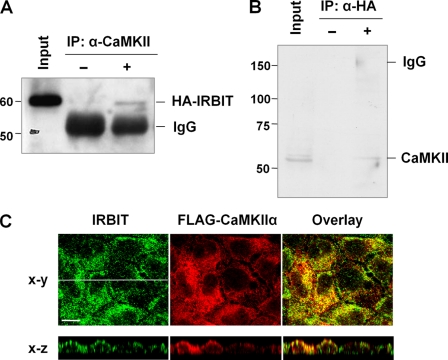
We postulated that CaMKII regulates NHE3 activity at least in part through its effect on IRBIT. To test this, we determined whether IRBIT and CaMKII associate in OKP cells. OKP/HA-IRBIT cell lysate was immunoprecipitated with an anti-CaMKII polyclonal antibody or rabbit sera as a control. Western blotting with an anti-HA monoclonal antibody showed the presence of co-immunoprecipitated IRBIT with the anti-CaMKII antibody but not with rabbit sera (Fig. 7A). The interaction between CaMKII and IRBIT was corroborated by a reciprocal experiment (Fig. 7B) where CaMKII was co-immunoprecipitated with HA-IRBIT. Of a technical note for the latter experiment, because the molecular weight of CaMKII is similar to that of the heavy chain of IgG, the bound protein complex was resuspended in Laemmli buffer in the absence of a reducing reagent to prevent the dissociation of heavy and light chains of IgG. Fig. 7C shows the subcellular localization of IRBIT and FLAG-CaMKIIα in OKP cells. IRBIT co-localized to some extent with CaMKIIα in both the cytoplasmic and apical membrane compartments as evidenced in the x-z images.
FIGURE 7.
IRBIT interacts with CaMKII in OKP cells. A, cell lysates from OKP/HA-IRBIT cells were incubated with an anti-CaMKII antibody (+) or a rabbit serum (−), and co-immunoprecipitated (IP) IRBIT was detected by immunoblotting using an anti-HA antibody. B, cell lysates from OKP/HA-IRBIT cells were incubated with or without an anti-HA antibody, and the presence of co-immunoprecipitated CaMKII was determined. C, OKP cells were ectopically expressed with FLAG-CaMKIIα, and subcellular localization of IRBIT and CaMKIIα were determined by indirect immunofluorescence with anti-IRBIT and anti-FLAG antibodies, respectively. Representative images of focal (x-y) and cross-sectional (x-z) views from three independent experiments are shown. Bar, 10 μm.
ANG II-mediated IRBIT-NHE3 Interaction and Trafficking of NHE3 Are CaMKII-dependent
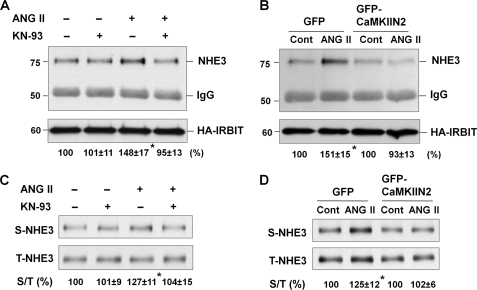
Our data in Fig. 3 show that the interaction of NHE3 with IRBIT is induced by ANG II. We tested whether the interaction between NHE3 and IRBIT is CaMKII-dependent. Fig. 8A shows that ANG II-induced interaction between NHE3 and IRBIT was abrogated by inhibition of CaMKII by KN-93. Similarly, expression of GFP-CaMKIIN2 blocked the NHE3-IRBIT interaction (Fig. 8B).
FIGURE 8.
ANG II-mediated IRBIT-NHE3 interaction and trafficking of NHE3 are CaMKII-dependent. Inhibition of CaMKII was achieved by KN-93 (A and C) or CaMKIIN2 (B and D). A and B, interaction between NHE3 and IRBIT was determined by treating cells with ANG II for 5 min. Co-immunoprecipitation of NHE3 with HA-IRBIT was determined by Western blotting. The amount of co-immunoprecipitated NHE3 was normalized to the total amount of IRBIT, and data are represented as means ± S.E. of three independent experiments. *, p < 0.01. C and D, NHE3 protein expression in the apical membrane was determined by surface biotinylation in cells treated with ANG II for 45 min. Aliquots of surface and total protein were resolved by SDS-PAGE, and the amount of surface (S) NHE3 and total (T) NHE3 proteins were determined by Western blot using an anti-NHE3 antibody. The amounts of surface NHE3 were normalized to total NHE3, and the relative changes are shown below the Western blots as means ± S.E. of three independent experiments. *, p < 0.01; Cont, control.
We next examined whether CaMKII is required for ANG II-induced increase in NHE3 surface expression. Similarly to the effect on NHE3-IRBIT interaction, the expression of CaMKIIN2 or pretreatment with KN-93 abrogated the increase in NHE3 abundance in the apical membrane of OKP/HA-IRBIT cells (Fig. 8, C and D). Taken together, these findings provide convincing evidence that CaMKII plays a pivotal role in activation of NHE3 by ANG II.
Phosphorylation of IRBIT Is Necessary for NHE3 Activation
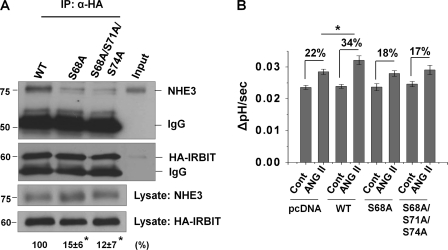
The N-terminal domain of IRBIT contains a PEST domain enriched with serine residues that can be potentially phosphorylated by multiple protein kinases (15, 28). Of these, Ser-68, Ser-71, and Ser-74 of IRBIT have been implicated as the major sites of phosphorylation (15, 28). Although we tried phospho-Ser/Thr antibodies from two commercial sources, neither antibody recognized phospho-IRBIT. Alternatively, we mutated Ser-68, Ser-71, or Ser-74 into alanine and assayed the capacity of the mutant IRBITs to bind NHE3. As shown in Fig. 9A, the amount of NHE3 co-immunoprecipitated with the S68A mutant was reduced by 85% compared with wild-type (WT) HA-IRBIT. The interaction of S68A/S71A/S74A mutant with NHE3 was similarly decreased, although it is interesting that the additional mutations at Ser-71 and Ser-74 did not impair the binding more than the S68A mutant. Importantly, in comparison with the potentiation of ANG II-mediated NHE3 activity by overexpression of WT HA-IRBIT (34% for WT IRBIT versus 22% for pcDNA control), overexpression of either mutant IRBIT did not enhance NHE3 activity (Fig. 9B). These data demonstrate that the interaction of IRBIT with NHE3 is sensitive to the phosphorylation state of IRBIT, and the IRBIT-NHE3 interaction is important for the effect of ANG II on NHE3 activity.
FIGURE 9.
NHE3-IRBIT interaction and activation of NHE3 by ANG II are dependent on phosphorylation of IRBIT. OKP cells were ectopically expressed with HA-tagged wild-type IRBIT (WT), S68A, or S68A/S71A/S74A IRBIT mutants. A, cell lysates were incubated with an anti-HA antibody, and the co-immunoprecipitated NHE3 is shown in the upper panel. Immunoprecipitated (IP) HA-IRBIT and NHE3 and HA-IRBIT in the lysate are shown in the lower panels. The relative amounts of co-immunoprecipitated NHE3 are indicated below the immunoblots. Data are represented as mean ± S.E. of three independent experiments. *, p < 0.001. B, NHE3 activity was determined in OKP cells expressing WT or mutant HA-IRBIT after exposure to ANG II or carrier for 45 min. n = 8 or more. Percent increases in the rate of pHi recovery at pHi 6.8 are shown above the vertical bars. *, p < 0.01 comparing OKP/pcDNA + ANG II and OKP/WT + ANG II.
DISCUSSION
The proximal tubule represents an important location where ANG II regulates salt and water transport. Although new and exciting developments have emerged in the past decades, the mechanisms by which ANG II stimulates the proximal tubule apical Na+/H+ exchanger NHE3 remain incompletely delineated. We initially cloned IRBIT as an NHE3-interacting protein from a yeast two-hybrid screen of a human kidney library (16). We demonstrated here that ANG II stimulates Na+/H+ exchange by NHE3 in a mechanism dependent on the presence of IRBIT. Our data show that silencing of IRBIT resulted in significant inhibition of NHE3 transport activity, whereas ectopic expression of IRBIT potentiated the effect of ANG II. Moreover, the involvement of IRBIT in modulating NHE3 activity was dependent on Ca2+-CaMKII signaling, which facilitated the interaction of IRBIT with NHE3 and the translocation of NHE3 to the apical membrane of OKP cells. These results indicate the physiological relevance of IRBIT as an NHE3-interacting protein and its critical importance in regulation of NHE3 activity at least in vitro by ANG II.
A large body of studies has shown that NHE3 is regulated through its interaction with a family of scaffold proteins containing PDZ (postsynaptic density 95, discs large, and zonula occludens-1) domains, such as Na+/H+ regulatory factor 1 and 2 (NHERF1 and -2), and PDZ domain-containing protein in kidney 1 (PDZK1) (21, 32–34). In all cases, the PDZ proteins constitutively bind to NHE3, and their interactions with NHE3 were not regulated by hormones or second messengers. One novel finding of this study is that ANG II acutely induces the binding of IRBIT to NHE3 in OKP cells. The interaction between NHE3 and IRBIT was acutely stimulated (5–15 min) but declined to the basal level by 45 min of ANG II treatment. The interaction between NHE3 and IRBIT in OKP cells differs from our earlier work in PS120 fibroblasts where the NHE3-IRBIT association was not regulated by thapsigargin or ionomycin (16). These contradicting data indicate that a similar effect, a rise in [Ca2+]i by the physiological agonist ANG II and the nonphysiological agonists, thapsigargin or ionomycin, does not result in an identical physiological outcome. The basis for the difference between OKP cells and PS120 fibroblasts is unclear, but the difference might be due to the pattern of Ca2+ mobilization induced by ANG II versus thapsigargin or ionomycin. ANG II in OKP cells induced a slow rise in [Ca2+]i (Fig. 5A), whereas thapsigargin or ionomycin has been shown to evoke a rapid and robust change in intracellular [Ca2+]i (35, 36).
Although the interaction between NHE3 and IRBIT was acutely regulated, NHE3 activity or NHE3 expression in the apical membrane was not elevated until 15 min and instead remained elevated long after the decline of IRBIT-NHE3 interaction. Moreover, ANG II treatment for 45 min potentiated NHE3 immunofluorescence labeling in the apical membrane of OKP cells (Fig. 4B), but not IRBIT, suggesting that IRBIT might be targeted to the apical membrane in a transient manner. Clearly, these data show that there is a temporal separation between the transient protein-protein interaction of NHE3 and IRBIT and the change in ion transport activity.
The effects of Ca2+ in regulation of NHE3 have been previously reported. For example, endothelin 1 stimulates NHE3 activity through a Ca2+-CaMK-dependent pathway (37). On the other hand, increased Ca2+ mobilization by low dose adenosine resulted in NHE3 activation via a negative control on adenylate cyclase activity (38). In our previous study using PS120 fibroblasts, we showed that increased [Ca2+]i by thapsigargin or ionomycin stimulated NHE3 via IRBIT (16). In the current study, we report that low dose ANG II induced an apparent rise in [Ca2+]i, and the obligatory role of the cellular Ca2+ response in NHE3 regulation was demonstrated by clamping cellular Ca2+ with BAPTA or inhibition of IP3 receptor by 2-APB (Fig. 4). Our data are consistent with previous studies where ANG II stimulates IP3 generation and subsequent Ca2+ release from the endoplasmic reticulum in a variety of cell lines (39–41). However, it is intriguing that NHE3 activity was decreased to a level below the basal level when Ca2+ mobilization was blocked in the presence of ANG II. This was unexpected as BAPTA or 2-APB alone had no observable effect on NHE3 activity. The exact reason for these observations is not known, but we postulate that ANG II might exert opposing effects on NHE3, Ca2+-dependent stimulation and Ca2+-independent inhibition, such that the blockade of Ca2+ mobilization revealed the Ca2+-independent inhibitory effect of ANG II. In support of this, it was shown that inhibition of NHE3 activity by high concentrations of ANG II (>10−6 m) was not sensitive to Ca2+-CaM inhibition but was blocked by inhibition of the cytochrome p-450 (9).
IRBIT contains a cluster of 16 serine residues within its PEST domain (14). The importance of phosphorylation of the serine residues within the PEST domain of IRBIT has been shown for interaction of IRBIT with the IP3 receptor, pNBC1, or the cleavage and polyadenylation specificity factor (14, 42, 43). Protein kinases phosphorylating IRBIT in vivo remain unknown, but several candidate protein kinases, including CaMKII, casein kinase, and protein kinase A, have been suggested (44). The decreased NHE3-IRBIT interaction when CaMKII was inhibited makes CaMKII a strong candidate protein kinase that phosphorylates IRBIT in OKP cells (Fig. 8). Although we could not provide unequivocal evidence that IRBIT is phosphorylated by the ANG II-induced signaling cascade, our finding that mutations in either Ser-68 or Ser-68/Ser-71/Ser-74 drastically reduced the affinity of IRBIT for NHE3 suggests for phosphorylation-dependent regulation of the NHE3-IRBIT interaction. The triple mutations at Ser-68, Ser-71, and Ser-74 did not have a more severe effect than the mutation at Ser-68. However, this might not be unexpected in light of a previous report that phosphorylation at Ser-68 is prerequisite for subsequent phosphorylation of Ser-71 and Ser-74 (15). Of note, it is interesting that the mutant IRBITs did not function as dominant negatives blocking the effect of ANG II. This outcome is not surprising, as the mutant IRBIT proteins do not compete with WT IRBIT for NHE3, the mutants should not affect the binding property of WT IRBIT.
What initiates the dissociation of NHE3 from IRBIT? IRBIT is able to interact with protein phosphatase 1 (PP1), which can dephosphorylate Ser-68 in vitro (28). Hence, the diminished NHE3-IRBIT interaction at later times could be mediated by dephosphorylation of IRBIT by a protein phosphatase, such as PP1. It is known that CaMKII, as a decoder of Ca2+ signaling, is able to phosphorylate the IP3 receptor and inhibits its activity (45). Given that IRBIT is a competitive inhibitor of Ca2+ release by the IP3 receptor (15), it is conceivable that the phosphorylation of IRBIT by CaMKII, or other kinases downstream of CaMKII, provides a negative feedback loop for the release of Ca2+ from the endoplasmic reticulum by enhanced binding of phosphorylated IRBIT to the IP3 receptor.
Changes in surface abundance of NHE3 protein through regulated endocytosis and exocytosis are the primary mechanism controlling NHE3 function (16, 19, 46). ANG II-induced regulation of NHE3 also involves protein trafficking as demonstrated by both in vitro and in vivo studies; du Cheyron et al. (13) showed that ANG II stimulates the exocytotic insertion of NHE3 into the apical membrane of cultured mouse kidney cortical tubule cells; infusion of rats with ANG II increases NHE3 expression in the proximal tubular brush border (23, 47). Similarly, we showed that ANG II stimulated apical membrane expression of NHE3 in OKP cells. The enhanced NHE3 surface expression by ANG II in OKP/HA-IRBIT cells and the abrogation by silencing of IRBIT suggest that IRBIT plays an important role in facilitating NHE3 trafficking in OKP cells by ANG II. The exocytic trafficking of NHE3 partially delineates the mechanism underlying the IRBIT-dependent NHE3 regulation. However, other mechanisms, such as phosphorylation of NHE3, cannot be ruled out. There are multiple putative CaMKII phosphorylation sites in NHE3 as well as IRBIT. Together with the interaction of IRBIT with NHE3 and CaMKII, it is possible that CaMKII could phosphorylate both NHE3 and IRBIT in response to ANG II.
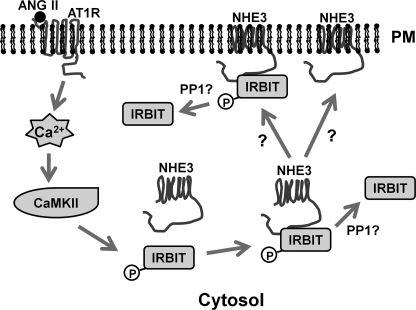
In summary, we propose the following model of IRBIT-dependent regulation of NHE3 in response to ANG II (Fig. 10). Stimulation of AT1R activates CaMKII, which induces the interaction of IRBIT with NHE3. The NHE3-IRBIT association is a necessary event for stimulation of NHE3 activity. The NHE3-IRBIT association is transient, and the dissociation of IRBIT from NHE3 is matched by the translocation of NHE3 to the apical membrane of the OKP cells and the increase in NHE3 activity. However, there remain unanswered questions regarding the precise spatiotemporal timing of the dissociation of IBRIT from NHE3 and the necessity of this dissociation for NHE3 regulation. Future analyses are required to address these issues.
FIGURE 10.
Putative model for ANG II-mediated activation of NHE3. Activation of angiotensin receptor (AT1R) induces mobilization of intracellular Ca2+ and subsequent activation of CaMKII. CaMKII phosphorylates IRBIT enhancing its interaction between NHE3 and trafficking of NHE3 to the apical membrane. PP1, protein phosphatase 1.
Acknowledgments
We acknowledge the Emory Digestive Disease Research Development Center (supported by National Institutes of Health Grant DK064399) for the use of the confocal microscope. We are grateful to Dr. Tom Soderling for the CaMKIIN2 construct.
This work was supported, in whole or in part, by National Institutes of Health Grants DK061418 and DK061418S1.
- ANG
- angiotensin
- IP3
- inositol 1,4,5-triphosphate
- IRBIT
- IP3 receptor-binding protein released with IP3
- CaMKII
- calmodulin-dependent kinase II
- [Ca2+]i
- intracellular Ca2+ concentration
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- OKP
- opossum kidney proximal tubule
- TMA-PO4
- tetramethylammonium-PO4.
REFERENCES
- 1.Biemesderfer D., Pizzonia J., Abu-Alfa A., Exner M., Reilly R., Igarashi P., Aronson P. S. (1993) Am. J. Physiol. 265, F736–F742 [DOI] [PubMed] [Google Scholar]
- 2.Aronson P. S. (1996) Kidney Int. 49, 1665–1670 [DOI] [PubMed] [Google Scholar]
- 3.Schultheis P. J., Clarke L. L., Meneton P., Miller M. L., Soleimani M., Gawenis L. R., Riddle T. M., Duffy J. J., Doetschman T., Wang T., Giebisch G., Aronson P. S., Lorenz J. N., Shull G. E. (1998) Nat. Genet. 19, 282–285 [DOI] [PubMed] [Google Scholar]
- 4.Brown G. P., Douglas J. G. (1982) Endocrinology 111, 1830–1836 [DOI] [PubMed] [Google Scholar]
- 5.Liu F. Y., Cogan M. G. (1988) J. Clin. Invest. 82, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F. Y., Cogan M. G. (1989) J. Clin. Invest. 84, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F. Y., Cogan M. G. (1990) Am. J. Physiol. 258, F927–F933 [DOI] [PubMed] [Google Scholar]
- 8.Liu F. Y., Cogan M. G. (1990) Am. J. Physiol. 259, F451–F457 [DOI] [PubMed] [Google Scholar]
- 9.Houillier P., Chambrey R., Achard J. M., Froissart M., Poggioli J., Paillard M. (1996) Kidney Int. 50, 1496–1505 [DOI] [PubMed] [Google Scholar]
- 10.Harris P. J., Young J. A. (1977) Pflugers Arch. 367, 295–297 [DOI] [PubMed] [Google Scholar]
- 11.Schuster V. L., Kokko J. P., Jacobson H. R. (1984) J. Clin. Invest. 73, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuganezawa H., Preisig P. A., Alpern R. J. (1998) Kidney Int. 54, 394–398 [DOI] [PubMed] [Google Scholar]
- 13.du Cheyron D., Chalumeau C., Defontaine N., Klein C., Kellermann O., Paillard M., Poggioli J. (2003) Kidney Int. 64, 939–949 [DOI] [PubMed] [Google Scholar]
- 14.Ando H., Mizutani A., Matsu-ura T., Mikoshiba K. (2003) J. Biol. Chem. 278, 10602–10612 [DOI] [PubMed] [Google Scholar]
- 15.Ando H., Mizutani A., Kiefer H., Tsuzurugi D., Michikawa T., Mikoshiba K. (2006) Mol. Cell 22, 795–806 [DOI] [PubMed] [Google Scholar]
- 16.He P., Zhang H., Yun C. C. (2008) J. Biol. Chem. 283, 33544–33553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole J. A., Forte L. R., Krause W. J., Thorne P. K. (1989) Am. J. Physiol. 256, F672–F679 [DOI] [PubMed] [Google Scholar]
- 18.Chang B. H., Mukherji S., Soderling T. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D., Sun H., Lang F., Yun C. C. (2005) Am. J. Physiol. Cell Physiol. 289, C802–C810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Zhang H., Lang F., Yun C. C. (2007) Am. J. Physiol. Cell Physiol. 292, C396–C404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamprecht G., Weinman E. J., Yun C. H. (1998) J. Biol. Chem. 273, 29972–29978 [DOI] [PubMed] [Google Scholar]
- 22.Welsh C., Dubyak G., Douglas J. G. (1988) J. Clin. Invest. 81, 710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riquier A. D., Lee D. H., McDonough A. A. (2009) Am. J. Physiol. Cell Physiol. 296, C900–C910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Lee H. J., Cooper D. S., Cebotaro L., Walden P. D., Choi I., Yun C. C. (2006) Am. J. Physiol. Renal Physiol. 290, F428–F437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biemesderfer D., Nagy T., DeGray B., Aronson P. S. (1999) J. Biol. Chem. 274, 17518–17524 [DOI] [PubMed] [Google Scholar]
- 26.Kobori H., Nangaku M., Navar L. G., Nishiyama A. (2007) Pharmacol. Rev. 59, 251–287 [DOI] [PubMed] [Google Scholar]
- 27.Carraro-Lacroix L. R., Malnic G. (2006) Pflugers Arch. 452, 728–736 [DOI] [PubMed] [Google Scholar]
- 28.Devogelaere B., Beullens M., Sammels E., Derua R., Waelkens E., van Lint J., Parys J. B., Missiaen L., Bollen M., De Smedt H. (2007) Biochem. J. 407, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezazadeh S., Claydon T. W., Fedida D. (2006) J. Pharmacol. Exp. Ther. 317, 292–299 [DOI] [PubMed] [Google Scholar]
- 30.Ledoux J., Chartier D., Leblanc N. (1999) J. Pharmacol. Exp. Ther. 290, 1165–1174 [PubMed] [Google Scholar]
- 31.Chang B. H., Mukherji S., Soderling T. R. (2001) Neuroscience 102, 767–777 [DOI] [PubMed] [Google Scholar]
- 32.Yun C. H., Oh S., Zizak M., Steplock D., Tsao S., Tse C. M., Weinman E. J., Donowitz M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3010–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinman E. J., Steplock D., Donowitz M., Shenolikar S. (2000) Biochemistry 39, 6123–6129 [DOI] [PubMed] [Google Scholar]
- 34.Gisler S. M., Pribanic S., Bacic D., Forrer P., Gantenbein A., Sabourin L. A., Tsuji A., Zhao Z. S., Manser E., Biber J., Murer H. (2003) Kidney Int. 64, 1733–1745 [DOI] [PubMed] [Google Scholar]
- 35.Purkiss J. R., Willars G. B. (1996) Cell Calcium 20, 21–29 [DOI] [PubMed] [Google Scholar]
- 36.Williams M. R., Riach R. A., Collison D. J., Duncan G. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1009–1017 [PubMed] [Google Scholar]
- 37.Chu T. S., Peng Y., Cano A., Yanagisawa M., Alpern R. J. (1996) J. Clin. Invest. 97, 1454–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Sole F., Cerull R., Petzke S., Casavola V., Burckhardt G., Helmle-Kolb C. (2003) J. Am. Soc. Nephrol. 14, 1720–1730 [DOI] [PubMed] [Google Scholar]
- 39.Rössig L., Zólyomi A., Catt K. J., Balla T. (1996) J. Biol. Chem. 271, 22063–22069 [DOI] [PubMed] [Google Scholar]
- 40.Sudo T., Sakuma Y., Kato M. (2001) J. Neuroendocrinol. 13, 942–950 [DOI] [PubMed] [Google Scholar]
- 41.Feng Z., Wei C., Chen X., Wang J., Cheng H., Zhang X., Hong Q., Shi S., Fu B., Wei R. (2006) Kidney Int. 70, 130–138 [DOI] [PubMed] [Google Scholar]
- 42.Shirakabe K., Priori G., Yamada H., Ando H., Horita S., Fujita T., Fujimoto I., Mizutani A., Seki G., Mikoshiba K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9542–9547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiefer H., Mizutani A., Iemura S., Natsume T., Ando H., Kuroda Y., Mikoshiba K. (2009) J. Biol. Chem. 284, 10694–10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devogelaere B., Sammels E., De Smedt H. (2008) BioEssays 30, 642–652 [DOI] [PubMed] [Google Scholar]
- 45.Bare D. J., Kettlun C. S., Liang M., Bers D. M., Mignery G. A. (2005) J. Biol. Chem. 280, 15912–15920 [DOI] [PubMed] [Google Scholar]
- 46.Bobulescu I. A., Dwarakanath V., Zou L., Zhang J., Baum M., Moe O. W. (2005) Am. J. Physiol. Renal Physiol. 289, F685–F691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon T. H., Nielsen J., Kim Y. H., Knepper M. A., Frøkiaer J., Nielsen S. (2003) Am. J. Physiol. Renal Physiol. 285, F152–F165 [DOI] [PubMed] [Google Scholar]