Abstract
Damaged mitochondria can be eliminated by autophagy, i.e. mitophagy, which is important for cellular homeostasis and cell survival. Despite the fact that a number of factors have been found to be important for mitophagy in mammalian cells, their individual roles in the process had not been clearly defined. Parkin is a ubiquitin-protein isopeptide ligase able to translocate to the mitochondria that are to be removed. We showed here in a chemical hypoxia model of mitophagy induced by an uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP) that Parkin translocation resulted in mitochondrial ubiquitination and p62 recruitment to the mitochondria. Small inhibitory RNA-mediated knockdown of p62 significantly diminished mitochondrial recognition by the autophagy machinery and the subsequent elimination. Thus Parkin, ubiquitin, and p62 function in preparing mitochondria for mitophagy, here referred to as mitochondrial priming. However, these molecules were not required for the induction of autophagy machinery. Neither Parkin nor p62 seemed to affect autophagy induction by CCCP. Instead, we found that Nix was required for the autophagy induction. Nix promoted CCCP-induced mitochondrial depolarization and reactive oxygen species generation, which inhibited mTOR signaling and activated autophagy. Nix also contributed to mitochondrial priming by controlling the mitochondrial translocation of Parkin, although reactive oxygen species generation was not involved in this step. Deletion of the C-terminal membrane targeting sequence but not mutations in the BH3 domain disabled Nix for these functions. Our work thus distinguished the molecular events responsible for the different phases of mitophagy and placed Nix upstream of the events.
Keywords: Autophagy, Cellular Regulation, Mitochondria, Reactive Oxygen Species (ROS), Signal Transduction, Mitophagy, Nix, p62
Introduction
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved “self-degradation process” characterized by the formation of double-membraned autophagosomes, which sequester macromolecules or subcellular organelles and deliver them to the lysosome for degradation (1, 2). Autophagy is important for a number of cellular functions to maintain cellular homeostasis.
Although autophagy is generally considered to be a nonselective process, accumulating evidence now supports that it can also selectively degrade specific targets (3). These selective targets include proteins, mitochondria, peroxisomes, endoplasmic reticulum, ribosomes, and even invading bacteria. Notably, most of the subcellular materials can be also nonspecifically sequestered in autophagosomes during the bulk degradation process, such as in starvation. Selective autophagy would thus play a different role in addressing specific biological or pathological concerns.
The selective degradation of mitochondria, or mitophagy (4–6), can occur in a specific developmental process, such as in the maturation of erythroid cells (7, 8), or can occur following pathological mitochondrial damage (9, 10). Mitophagy in the latter case is considered to be important for the quality control of the mitochondrial population and therefore cellular homeostasis (4, 9, 11, 12). Genetic analysis indicated that yeast cells with deletion of key autophagy genes exhibit a variety of mitochondrial defects, including an increased rate of mitochondrial mutation and ROS generation, with deleterious impact on cell growth (13). Failure of mitophagy may lead to abnormal cell death, which can contribute to the pathogenesis of Parkinson disease (9, 12), or may lead to genomic instability and tumorigenesis (11, 14).
The molecular mechanism of mitophagy has not been clearly elucidated, although a number of contributing events have been identified. In yeast, early studies suggested that Uth1, a protein localized to the mitochondrial outer membrane; Aup1, a putative protein phosphatase localized in the mitochondrial intermembrane space, and Mdm38p, an integral mitochondrial inner membrane protein participating in the mitochondrial K+/H+ exchange, could participate in mitophagy (6). More recently, Atg32 was identified to be required for selective mitochondrial degradation in the yeast (15, 16). Atg32 has a mitochondrial transmembrane domain and is anchored to the mitochondrial membranes. Atg32 can directly interact with Atg8 and Atg11 that are located on the autophagosome membranes and thus can serve as a specific “eat me” signal for the mitochondria to be degraded.
No Atg32 homologues have been identified in mammalian cells. In contrast, Parkin, a E32 ubiquitin ligase whose loss-of-function mutations represent the most common recessive cause for Parkinson disease (17), can be recruited selectively to depolarized mitochondria to promote their degradation via autophagy (9, 12). In addition, Nix, a BH3-only Bcl-2 family protein (7, 8), and ULK1 (18), a component of autophagy machinery, have been found to be important for mitochondrial elimination during erythroid cell maturation. Overexpression of another BH3-only Bcl-2 family protein, Bnip3, could also increase mitochondrial inclusion in autophagosomes (19). Finally, the mitochondrial fission/fusion machinery is able to affect mitochondrial elimination (20, 21).
The exact relationships of these events in regulating mitophagy have not been clearly defined. It is not clear how autophagy is activated to initiate the removal process and how the mitochondria being removed are specifically recognized by the activated autophagy machinery in a coordinated way.
Here we used a chemical hypoxia model to address these questions and defined the basic molecular events for these two aspects of mitophagy. We showed that Parkin-ubiquitin-p62 constituted the signaling events to prime the damaged mitochondria for recognition, whereas Nix and ROS were required for the induction of autophagy. Nix, but not ROS, also participated in mitochondrial priming by promoting Parkin translocation to the mitochondria, thus coordinating the two aspects of a successful process of mitophagy.
EXPERIMENTAL PROCEDURES
Reagents
The following primary antibodies were used: monoclonal anti-p62 (BD Biosciences); anti-Tom20, monoclonal anti-ubiquitin and anti-Parkin (Santa Cruz Biotechnology); and anti-phosphorylated p70 S6 kinase, anti-p70 S6 kinase, and anti-GAPDH (Cell Signaling). Secondary antibodies were conjugated with Alexa Fluo 340 (blue), Alexa Fluo 488 (green) (Invitrogen), or Cy3 (red) (Jackson ImmunoResearch). siRNA (Qiagen) for human p62/SQSTM1 gene was 5′-CCGAATCTACATTAAAGAGAA-3′ and for the scrambled control was 5′-UUCUCCGAACGUGUCACGU-3′.
Cell Culture and Transfection
Mouse embryonic fibroblasts (MEF), HCT116 (Bax−/−) cells, and HeLa cells stably expressing GFP-LC3 were established as described previously (22, 23). Nix−/− MEF were generated from day 13 Nix-deficient embryos (24). MEF and HeLa cells were maintained in DMEM, whereas HCT116 cells were maintained in McCoy's 5A medium with 10% FBS and the routine supplements. For transient expression, the cells were cultured on coverslips and were transfected with DNA constructs using TurboFect (Fermentos) and analyzed 24–48 h later. For gene expression knockdown, siRNA (120 nm) was transfected into 1 × 106 cells using Oligofectamine (Invitrogen) for 48 h before analysis.
Immunofluorescence Staining
After the indicated treatments, the cells were fixed with 4% paraformaldehyde in PBS. They were then subjected to immunofluoresence staining using standard protocols. All of the images were obtained using an inverted confocal microscope (Olympus Fluoview 1000). The cells with “less or no mitochondria” are defined as those cells, when stained with anti-Tom20, presenting no or very weak Tom-20 staining because of mitochondrial loss or significant reduction in size. Quantification of GFP-LC3 punctation and its co-localization with other markers was based on enumeration of the number of GFP-LC3 dots in each cell, and ∼30–100 cells from repeated experiments were counted for each condition.
Immunoblot Assay
The cells were washed in PBS and lysed in radioimmune precipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl (lauryl) sulfate). Forty micrograms of protein was separated by SDS-PAGE and transferred to PVDF membranes. The membranes were stained with the indicated antibodies and developed with SuperSignal West Pico chemiluminescent substrate (Pierce). The images were acquired with a Kodak Image Station 4000 MM and analyzed by the companion Software (Carestream Health, Inc.).
Statistical Analysis
The data were subjected to Z test, Student's t test, or one-way ANOVA with Scheffe's post hoc test where appropriate.
RESULTS
CCCP-induced Autophagy
CCCP is a lipid-soluble weak acid and a potent mitochondrial uncoupling agent that increases the proton permeability across the mitochondrial inner membranes, thus dissipating the transmembrane potential and depolarizing the mitochondria. CCCP could induce the lysosomal removal of depolarized mitochondria (9, 12), which was considered to be mediated by autophagy.
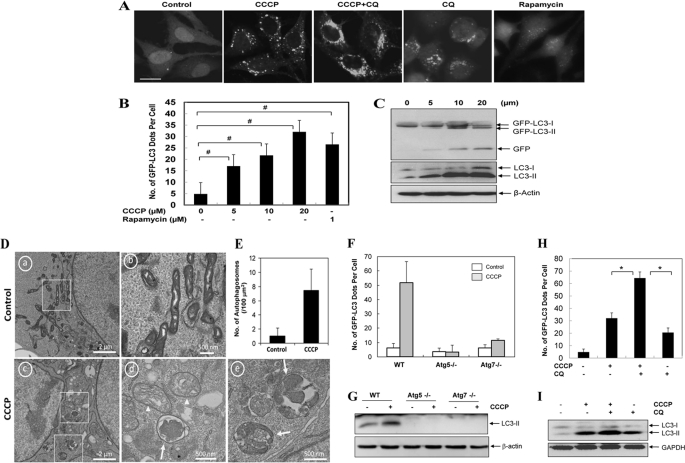
To further investigate the molecular events in CCCP-induced mitochondrial clearance, we first sought for the definitive evidence that CCCP could indeed induce autophagy. Using LC3 as the marker for autophagy activation, we found that CCCP induced GFP-LC3 punctation and the formation of lipidated LC3 in a dose-dependent (Fig. 1, A–C and supplemental Figs. S1, A and B, and S2, A–C) and time-dependent (supplemental Fig. S2, D and E) manner in HeLa cells (Fig. 1), HCT116 cells (supplemental Fig. S1), and MEF (supplemental Fig. S2). In HeLa cells the magnitude of activation was comparable with that induced by rapamycin (Fig. 1, A and B). Electron microscopy confirmed a significant increase of autophagomes in CCCP-treated cells (Fig. 1, D and E). The activation of autophagy by CCCP required specific autophagy genes, Atg5 and Atg7, and was inhibited in cells deficient in either of the genes (Fig. 1, F and G, and supplemental Fig. S2A).
FIGURE 1.
CCCP induces autophagy and mitophagy. A–C, HeLa cells stably expressing GFP-LC3 (GFP-LC3-HeLa) were treated with CCCP at 20 μm (A) or at the indicated doses (B and C) in the presence or absence of CQ (10 μm) for 16 h or with rapamycin (1 μm) for 6 h, followed by confocal microscopy (A). Bar, 20 μm. The number of GFP-LC3/cell was quantified (B), and total lysates were subjected to Western blot analysis with anti-GFP and anti-LC3 (C). D, HCT116 cells were treated with control (panels a and b) or CCCP (20 μm, panels c–e) for 16 h and examined by electron microcopy. Panel b was enlarged from the boxed area in panel a; panels d and e were enlarged from the boxed areas in panel c. E, the number of autophagosomes was counted from more than 12 different cell sections. F and G, wild type (WT), Atg5-deficient, and Atg7-deficient MEF were infected with Ad-GFP-LC3 overnight and then treated with vehicle control or CCCP (30 μm) for 6 h before being analyzed for the number of GFP-LC3 puncta (F) and LC3-II formation (G). H and I, GFP-LC3 HeLa cells were treated with CCCP (30 μm) in the absence or presence of CQ (10 μm) for 16 h. The number of GFP-LC3 dots/cell was quantified (H), and the total lysates were subjected to Western blot analysis with anti-LC3 (I). For B, F, and H, the data shown are the means ± S.D. #, p < 0.05; *, p < 0.01, one-way ANOVA.
To examine whether CCCP-induced autophagy was functional or completed through the lysosome stage, we first determined that GFP-LC3 puncta could co-localize with the lysosome (supplemental Fig. S2F). Flux analysis with CCCP treatment in the presence of lysosomal inhibiting agents, chloroquine (CQ), or E64D together with pepstatin, resulted in a significantly higher level of accumulation of GFP-LC3 puncta and the lipidated LC3-II than that caused by CCCP alone (Fig. 1, A, H, and I, and supplemental Figs. S1D and S2, G and H), indicating that at least a portion of autophagosomes induced by CCCP were degraded in the lysosome. Indeed, GFP-LC3 was found to undergo dose-dependent (Fig. 1C and supplemental Figs. S1B and S2C) and time-dependent (supplemental Fig. S2E) degradation, resulting in the appearance of the free GFP moiety, a lysosomal degradative product of GFP-LC3, which can be suppressed by E64D and pepstatin (supplemental Fig. S2H). Finally, the application of the GFP-RFP-LC3 construct in CCCP-treated HeLa cells showed a significant accumulation of LC3 dots that were only positive for RFP signals but not GFP signals (supplemental Fig. S3), suggesting that they were localized in the autolysosomes because the GFP signals were preferentially quenched by the acidic degradative environment (25). Taken together, these observations demonstrated that CCCP was able to induce a functional autophagy in several cell lines based on a variety of parameters.
Parkin Promotes Mitochondrial Degradation but Not Autophagy Induction
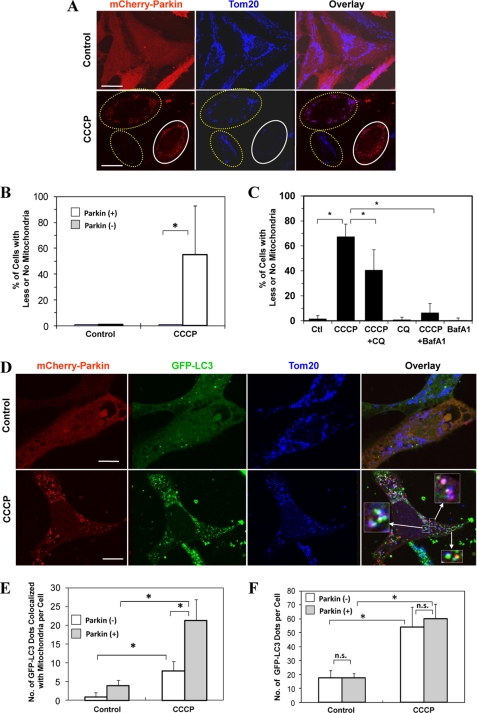
EM images also indicated the presence of damaged mitochondria and its engulfment by the autophagosome (Fig. 1D), but the event was infrequent. Efficient autophagic degradation of mitochondria requires the participation of Parkin (9, 12). Notably, Parkin was below the detectable level in HeLa cells, HCT116 cells, and MEF, and its expression was not up-regulated by CCCP treatment (supplemental Fig. S4). When exogenous Parkin was introduced into HeLa cells, mitochondrial removal was significantly increased following CCCP treatment, compared with cells without the exogenous Parkin (Fig. 2, A and B). Immunostaining with an antibody recognizing a mitochondrial outer membrane molecule, Tom20, indicated a significant reduction in mitochondrial number and/or size in Parkin-positive cells, which, however, was reversed in the presence of CQ or bafilomycin A1 (Fig. 2C), suggesting that the reduction was largely mediated by lysosomal degradation. Bafilomycin A1 is a more potent inhibitor than CQ and suppressed most, if not all, of the mitochondrial degradation. We sought for additional evidence of autophagy involvement in the mitochondrial removal by transfecting Parkin into HeLa cells stably expressing GFP-LC3. We found that the presence of Parkin promoted the co-localization of GFP-LC3 puncta with the mitochondria (Fig. 2, D and E), suggesting that Parkin increased the recognition of the mitochondria by the autophagy machinery for degradation. We noted that there was also a mild but significant increase in the association of GFP-LC3 dots with the mitochondria in cells negative for the exogenous Parkin following CCCP treatment (Fig. 2E). This might indicate that the very low level of endogenous Parkin could still have an effect or that there could be a Parkin-independent mechanism for mitophagy.
FIGURE 2.
Parkin promotes mitophagy but not autophagy induction. A, HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and stained with anti-Tom20, followed by confocal microscopy. The cells marked with the yellow dotted ovals expressed no or low levels of Parkin and still had mitochondria, whereas cells marked with white solid ovals expressed a high level of Parkin and had no mitochondria. Bars, 20 μm. B, the percentage of cells with less or no mitochondria were quantified in Parkin-positive and -negative cells. *, p < 0.01, Z test. C, HEK 293-mCherry-Parkin cells were treated as indicated with CQ (10 μm) or bafilomycin A1 (BafA1, 100 nm). The percentages of cells with less or no mitochondria were quantified in Parkin-positive cells. *, p < 0.05, Z-test. Ctl, control. D, GFP-LC3-HeLa cells were transfected with mCherry-Parkin (red) for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 (blue). Bars, 10 μm. Enlarged images from the boxed areas demonstrate the co-localization of Tom20 with GFP-LC3 and/or with Parkin. E, the number of GFP-LC3 dots that were co-localized with mitochondria was quantified in Parkin-positive and Parkin-negative cells. *, p < 0.01, one-way ANOVA. F, the total number of GFP-LC3 dots was quantified in Parkin-positive and -negative cells. *, p < 0.01. n.s., not significant (p > 0.05), one-way ANOVA. All of the data shown are the means ± S.D.
On contrary, Parkin did not seem to affect the total number of GFP-LC3 puncta induced by CCCP, which was no different between cells overexpressing Parkin and cells without the overexpressed Parkin (Fig. 2F). In both groups, GFP-LC3 puncta was equally well induced by CCCP treatment. This observation indicated that Parkin per se did not affect the general autophagy induction. Instead Parkin might promote autophagic removal of mitochondria by “preparing” the mitochondria for autophagic recognition, a step here referred to as mitochondrial priming.
Parkin Promotes Mitochondrial Ubiquitination and p62 Recruitment to the Mitochondria
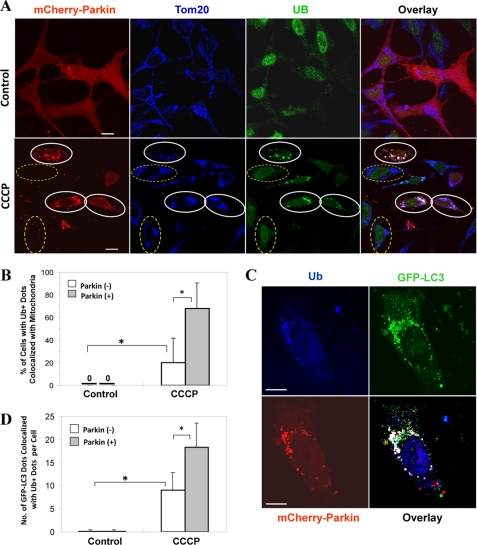
Because Parkin is an ubiquitin E3 ligase, we examined whether Parkin translocation could promote the ubiquitination of mitochondria as a mechanism for priming the mitochondria. Ubiquitin signals were mainly in the nucleus of untreated HeLa cells, but they were found in discrete cytoplasmic locations following CCCP treatment, particularly in Parkin-positive cells (Fig. 3A). The ubiquitin signals were located at the mitochondria because they were co-localized with Tom20, suggesting that mitochondria were ubiquitinated. This process was dependent on both the presence of Parkin and the CCCP treatment (Fig. 3B). Furthermore, CCCP treatment and Parkin also caused an increased GFP-LC3 co-localization with the ubiquitin signals (Fig. 3, C and D). We noted that such association could also be observed in cells negative for the exogenous Parkin following CCCP treatment, but at a much lower level. This event would be consistent with the association of GFP-LC3 dots with the mitochondria in cells negative for exogenous Parkin (Fig. 2E).
FIGURE 3.
Parkin promotes mitochondrial ubiquitination following CCCP treatment. A, HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 and ubiquitin (UB). Bars, 10 μm. The cells marked with yellow dotted ovals had no Parkin expression, in which ubiquitin signals were within the nucleus, whereas cells marked with white solid ovals had Parkin expression/translocation and ubiquitin labeling of Tom20-positive mitochondria. B, the percentage of cells with mitochondrial ubiquitin-positive dots was determined. *, p < 0.01, Z test. C and D, GFP-LC3-HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for ubiquitin (Ub). Bars, 10 μm. The number of GFP-LC3 dots that were co-localized with ubiquitin-positive dots was quantified. *, p < 0.01, one-way ANOVA. All of the data shown are the means ± S.D.
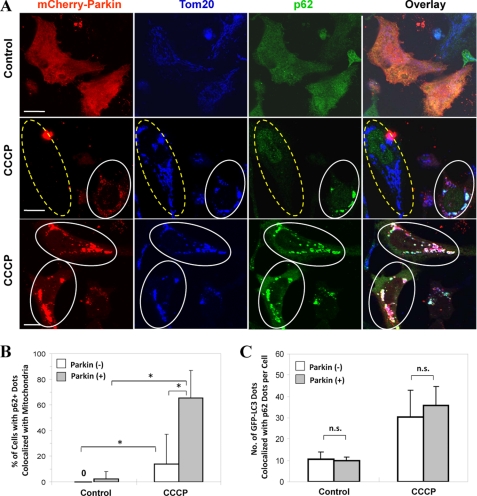
Parkin-promoted mitochondrial ubiquitination would be an important step of the mitochondrial priming for autophagy recognition. The multi-signaling adaptor molecule p62/SQSTM1 can directly interact with ubiquitin and LC3, promoting autophagic degradation of ubiquitinated aggregated proteins, peroxisome, and bacteria (3). It was thus possible that p62 could be recruited to the mitochondria as a result of their ubiquitination. Indeed, when we examined the distribution of the endogenous p62 in CCCP-treated cells, we found that there was a dramatic increase in the number of cells in which p62 was recruited to the mitochondria (Fig. 4, A and B). In untreated cells, Parkin and p62 were diffusively distributed. In cells treated with CCCP, p62 was recruited to the mitochondria together with Parkin. The occurrence of translocation was significantly lower in cells negative for the exogenous Parkin, although not completely absent (Fig. 4B).
FIGURE 4.
Parkin promotes p62/SQSTM1 mitochondrial targeting following CCCP treatment. A, HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 and p62/SQSTM1. Bars, 10 μm. The cells marked with yellow dotted ovals had no Parkin expression, whereas cells marked with white solid ovals had Parkin expression and translocation, in which p62/SQSTM1 was also recruited to the mitochondria. B, the numbers of p62/SQSTM1 dots that co-localized with mitochondria per cell were quantified in Parkin-positive and -negative cells. *, p < 0.01, Z test. C, GFP-LC3-HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and stained for p62/SQSTM1. The number of GFP-LC3 dots that were co-localized with p62 were quantified. n.s., no significant difference (p > 0.05): one-way ANOVA. All of the data shown are the means ± S.D.
There were good correlations of p62 and GFP-LC3 with Parkin-positive puncta (i.e. mitochondria) in cellular locations (Fig. 4C), consistent with the finding that co-localization of GFP-LC3 with the ubiquitinated mitochondria (Fig. 3D) was promoted by Parkin and suggesting that it was mediated by p62. However, a careful quantitative analysis indicated that Parkin did not affect the total number of GFP-LC3 dots that were co-localized with p62, although this number was increased in response to CCCP (Fig. 4, A and C, and supplemental Fig. S5). This observation once again indicated that the total number of GFP-LC3 puncta, whether they were associated with p62 or not, was not affected by Parkin but was elevated by CCCP as a general autophagy response. Furthermore, whereas p62 recruitment to the mitochondria was dependent on Parkin, its general association with LC3 was not dependent on Parkin or CCCP. CCCP affected the level of punctated GFP-LC3, thus increasing the level of p62 that was associated with LC3, whereas Parkin induced p62 recruitment to the mitochondria via ubiquitination, thus co-recruiting the p62-associated LC3 and possibly the autophagosome membranes.
Knockdown of p62 Inhibites CCCP-induced Mitophagy but Not General Autophagy Induction
We then examined the importance of p62 in CCCP-induced mitophagy. HeLa cells that stably expressed GFP-LC3 were transfected with a specific siRNA against p62, which led to an effective knockdown of p62 and significantly reduced the level of mitochondria that had recruited GFP-LC3 (Fig. 5, A–C). Notably, this inhibition of p62 expression did not affect the total number of GFP-LC3 puncta in CCCP-treated cells (Fig. 5D), confirming that autophagy induction was not affected by the p62 level and/or its modification of mitochondria.
FIGURE 5.
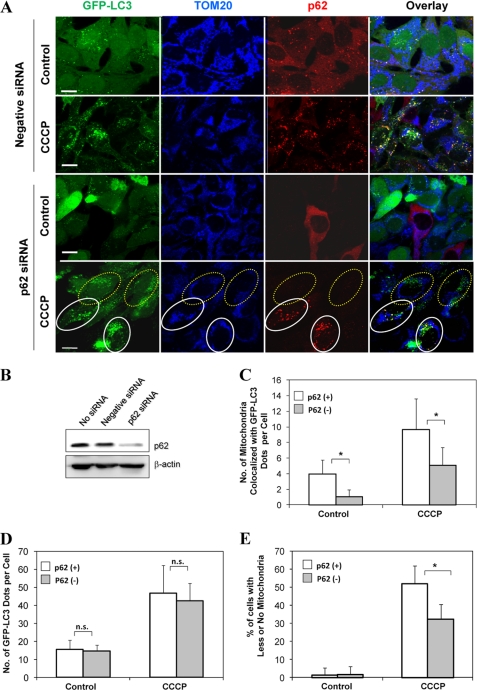
Knockdown of p62 attenuates CCCP-induced mitophagy. A, GFP-LC3-HeLa cells were transfected with either negative siRNA or p62/SQSTM1-specific siRNA (120 nm) for 36 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 and p62/SQSTM1. Bars, 10 μm. The cells marked with yellow dotted ovals had no p62/SQSTM1 expression in which LC3 was not co-localized with Tom20. The cells marked with white solid ovals expressed p62/SQSTM1 in which LC3 was co-localized with Tom20. B, the expression level of p62/SQSTM1 in control, negative, and specific siRNA-transfected cells. C and D, the number of mitochondria co-localized with GFP-LC3 dots/cell (C) and the total number of GFP-LC3 dots/cell (D) were quantified in p62/SQSTM1-positive and knockdown cells. *, p < 0.01; n.s., no significance (p > 0.05), one-way ANOVA. E, HEK 293 cells stably expressing mCherry-Parkin cells were transfected with either negative siRNA or p62/SQSTM1-specific siRNA for 36 h. The cells were then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 and p62/SQSTM1. The number of Parkin-positive cells with less or no mitochondria was quantified in p62-positive and knockdown status. *, p < 0.01, Z test. All of the data shown are the means ± S.D.
The level of GFP-LC3 co-localized with mitochondria and that of mitophagy in HeLa cells following CCCP treatment were relatively low because of the negligible level of endogenous Parkin (9) (supplemental Fig. S4). To facilitate our analysis of the importance of p62 in mitophagy, we established a HEK 293 cell line in which a significant portion of cells stably expressed mCherry-Parkin. Cells with no mCherry-Parkin expression served as controls under the same culture condition. HEK 293 cells behaved in the same way as HeLa cells, in which Parkin was translocated to the mitochondria in response to CCCP and mitochondria were reduced in amount in Parkin-positive cells (supplemental Fig. S6). We then transfected these cells with p62 siRNA followed by CCCP treatment and immunostained for p62 and Tom20. In Parkin-positive cells, we found that CCCP treatment increased the number of cells with less or no mitochondria following negative siRNA transfection, whereas the number of cells with less or no mitochondria was significantly decreased in Parkin-positive, p62 knockdown cells (Fig. 5E). Thus p62 critically participated in Parkin-mediated mitochondrial degradation.
CCCP-induced Autophagy Requires Nix and ROS
The above data indicated that although Parkin and p62 were both important for autophagic removal of mitochondria induced by CCCP, they apparently did not directly affect autophagy induction. Thus they were only involved in the priming of the mitochondria for the recognition by the autophagy machinery. To explore the upstream signaling that affected autophagy induction, we investigated the role of Nix, a BH3-only Bcl-2 family protein, in CCCP-induced mitophagy. Nix had been known to be important for the autophagic removal of mitochondria during erythroid cell maturation (7, 8).
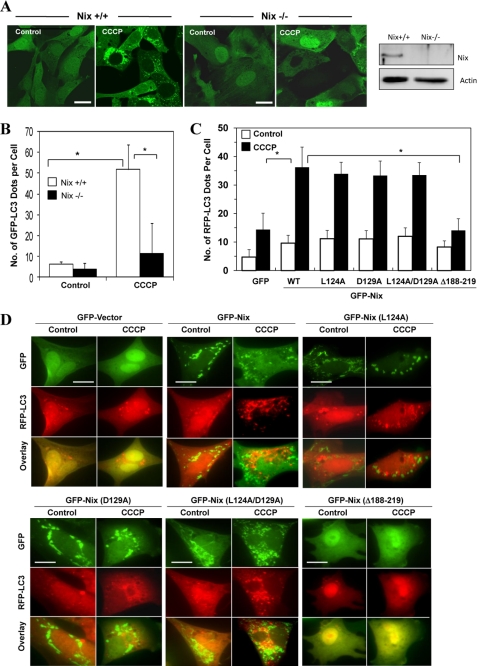
We found that CCCP-induced GFP-LC3 puncta formation was almost completely eliminated in the absence of Nix (Fig. 6, A and B). To confirm the importance of Nix in the general induction of autophagy, we reconstituted Nix-deficient MEFs with GFP or GFP-Nix, which were then treated with CCCP. We found that CCCP induced RFP-LC3 puncta only in GFP-Nix-positive cells but not in GFP-Nix-negative cells or in Nix-deficient cells that were reconstituted with the GFP vector only (Fig. 6, C and D). To determine which structural elements were important for Nix in CCCP-induced autophagy, we reconstituted the Nix-deficient cells with the BH3 mutants (L124A and/or D129A) or the C-terminal deletion mutant (Δ188–219) of Nix and found that the C-terminal sequence, which includes the transmembrane domain but not the BH3 domain, was required for Nix to participate in CCCP-induced RFP-LC3 punctation (Fig. 6, C and D). Notably, the mitochondrial location pattern of Nix was lost for the Δ188–219 mutant but remained unchanged for the BH3 mutants. This would be consistent with the early finding that the C-terminal sequence of Nix is required for its mitochondrial targeting (26).
FIGURE 6.
CCCP-induced autophagy requires Nix. A, wild type (Nix+/+) and Nix-deficient (Nix−/−) MEFs expressing GFP-LC3 were treated with CCCP (30 μm) for 6 h. The cells were fixed followed by confocal microscopy. Bars, 20 μm. The right panel shows an immunoblot analysis of Nix expression in these cells. B, the number of GFP-LC3 dots/cell was quantified. *, p < 0.01, one-way ANOVA. C and D, Nix−/− MEFs were co-transfected with the GFP vector, GFP-Nix, GFP-NixL124A, GFP-NixD129A, GFP-NixL124A/D129, or GFP-NixΔ188–219, together with RFP-LC3 for 24 h. The cells were then treated with CCCP (30 μm) for 6 h followed by fluorescence microscopy. The numbers of RFP-LC3 dots in each cell were quantified. *, p < 0.01, one-way ANOVA. All of the data shown are the means ± S.D. Bars, 20 μm. Note that both Leu124 and Asp129 are highly conserved among the BH3-only Bcl-2 family proteins in the BH3 domain. NixΔ188–219 is a C-terminal deletion mutant that also lacks the conserved transmembrane domain. WT, wild type.
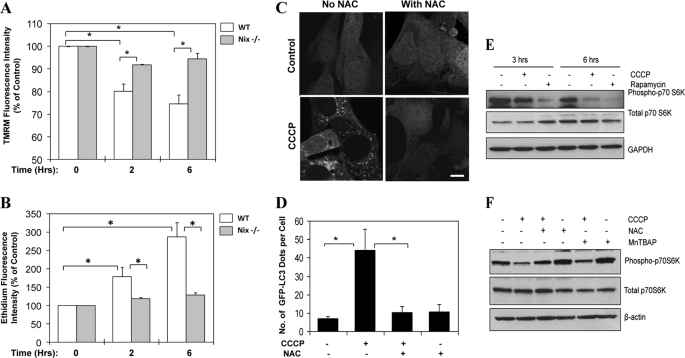
Nix had been suggested to cause mitochondrial depolarization during erythroid cell maturation (8). As an uncoupler, CCCP is able to rapidly induce mitochondrial depolarization (Fig. 7A). We found that CCCP-induced depolarization was also dependent on Nix at the early stage (in the first 6 h after treatment) (Fig. 7A), although a prolonged treatment of CCCP led to collapse of the potentials regardless of the absence of Nix (data not shown). However, the first 6 h of CCCP treatment were crucial to the induction of autophagy and mitophagy in this model (Fig. 1 and supplemental Figs. S1 and S2). During this period, as a result of depolarization and mitochondrial respiration dysfunction, CCCP induced a superoxide burst as determined by flow cytometry using dihydroethidium (Fig. 7B). This burst could be suppressed by the deletion of Nix. Importantly, CCCP-induced GFP-LC3 punctation could be almost completely suppressed by an anti-oxidant, N-acetylcysteine (NAC) in MEF (Fig. 7, C and D) and in HeLa cells (supplemental Fig. S7), as effectively as the deletion of Nix (Fig. 6, A and B).
FIGURE 7.
CCCP-induced autophagy requires ROS and is accompanied with mTOR inhibition. A and B, wild type (WT) and Nix−/− MEF were treated with CCCP for different times as indicated. The cells were harvested and stained for 15 min with tetramethylrhodamine methyl ester (TMRM, 2.5 μm, A), which incorporates into mitochondria in a potential-dependent way, or dihydroethidium (2.5 μm, B), which is converted to the red fluorescent product, ethidium, in the presence of superoxide, followed by flow cytometry. The mean fluorescence intensity was measured. *, p < 0.01, one-way ANOVA. C, wild type MEF stably expressing GFP-LC3 were treated with CCCP (30 μm) in the presence or absence of NAC (20 mm) for 6 h. The cells were examined by confocal microscopy. Bar, 10 μm. D, the number of GFP-LC3 dots/cell was quantified. *, p < 0.01, one-way ANOVA. E, HeLa cells were treated as indicated with CCCP (30 μm) or rapamycin (1 μm) for 3 or 6 h. Total cell lysates were subjected to immunoblot analysis. F, HeLa cells were treated as indicated with CCCP (30 μm) and/or NAC (10 mm) or MnTBAP (0.5 mm) for 6 h. Total cell lysates were subjected to immunoblot analysis. All of the data shown are the means ± S.D.
We also found that CCCP treatment resulted in suppression of mTOR signaling, much like rapamycin, leading to the inhibition of p70 S6 kinase phosphorylation in HeLa cells (Fig. 7E). This suppression seemed to be at least in part mediated by ROS, because it could be reversed by NAC or another antioxidant, MnTBAP (Fig. 7F). This result suggested that Nix-ROS-mTOR inhibition was involved in the activation of autophagy by CCCP.
Nix but Not ROS Affects Parkin Translocation
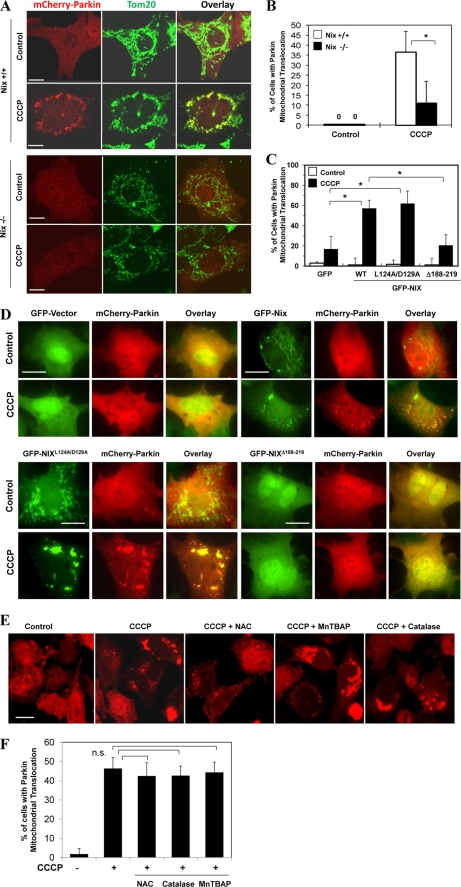
To examine whether Nix and ROS had any effect on mitochondrial priming, we first examined whether there were any differences in Parkin translocation in wild type and Nix-deficient cells. We found that CCCP-induced Parkin translocation was significantly inhibited in cells deficient in Nix (Fig. 8, A and B), which could be rescued by wild type Nix and its BH3 mutant (NixL124A/D129A), but not the C-terminal deletion mutant (NixΔ188–219) (Fig. 8, C and D). This clearly demonstrated the importance of Nix and its ability to recruit Parkin to the mitochondria. However, CCCP-induced Parkin translocation was not suppressed by NAC at concentrations ranging from 5 to 20 mm (Fig. 8, E and F, and data not shown). We further tested two other anti-oxidants, MnTBAP and catalase, and found that neither had any apparent effects (Fig. 8, E and F). Thus these observations indicated that Nix was required for both autophagy induction and mitochondrial priming. Although ROS mediated the function of Nix in the mobilization of autophagy machinery, it did not mediate the function of Nix in inducing Parkin translocation and therefore mitochondrial priming.
FIGURE 8.
CCCP-induced Parkin translocation is controlled by Nix but not by ROS. A and B, wild type (Nix +/+) and Nix-deficient (Nix −/−) MEFs were transfected with mCherry-Parkin for 24 h and then treated with CCCP (30 μm) for 6 h. The cells were fixed and immunostained for Tom20 (A). Bars, 10 μm. The percentage of cells with Parkin mitochondrial translocation was quantified in Parkin-positive cells. *, p < 0.01, Z test. C and D, Nix−/− MEF cells were co-transfected with GFP vector or GFP-NIX or the mutants, together with mCherry-Parkin for 24 h, and then treated with CCCP (30 μm) for 6 h followed by fluorescence microscopy. Bars, 20 μm. The percentages of cells that had mitochondrial mCherry-Parkin translocation were quantified (means ± S.D.). *, p < 0.01, Z test. E and F, HeLa cells were transfected with mCherry-Parkin for 24 h and then treated with CCCP (20 μm) for 6 h in the presence or absence of NAC (10 mm), MnTBAP (0.5 mm), or catalase (1000 units/ml). The cells were examined by confocal microscopy (C). Bars, 20 μm. The percentage of cells with Parkin translocation was quantified in Parkin-positive cells (D). n.s., no significant difference (p > 0.05), one-way ANOVA. All of the data shown are the means ± S.D.
DISCUSSION
A number of factors have been found to affect mitochondrial clearance during a developmental or pathological process. In the case of macroautophagic removal of mitochondria, or mitophagy, the contributing factors had not been clearly determined for their specific roles in the process. The present study distinguished and investigated two separate but related steps of mitophagy, the mobilization of autophagy machinery and the priming of the mitochondria for the recognition by the autophagy machinery. By directly disturbing mitochondrial oxidative phosphorylation as a way to damage mitochondria, we demonstrated that autophagy mobilization involved Nix and ROS, whereas mitochondrial priming was promoted by Parkin-dependent mitochondrial ubiquitination and p62 recruitment. These two processes are coupled in that Nix is also important for Parkin translocation to the depolarized mitochondria.
The Mobilization of Autophagy Machinery by Nix and ROS
Developmental clearance of mitochondria has been reported during the differentiation of lens cells (27, 28) and erythrocytes (7, 8, 18, 29) and in sperms after fertilization (30), which can be mediated by autophagic (7, 8, 18, 29) and nonautophagic mechanisms (28, 29). In the more defined case of erythrocyte maturation, the deletion of a key autophagy gene, ULK1/Atg1 (18) or Atg7 (29), resulted in significant defects in mitochondrial clearance, suggesting that mitochondrial clearance in erythrocyte development involves autophagy machinery.
Such an involvement of autophagy in mitochondrial clearance in the nondevelopment context has also been demonstrated in yeast (13, 15, 16) and in some mammalian experiment systems (4, 19). The model of CCCP-induced mitophagy in mammalian cells is particularly interesting because the critical role of Parkin and Pink1, two Parkinson disease risk factors, in mitophagy could be clearly defined in this system (9, 12, 31), but whether CCCP actually activated autophagy had not been clearly defined before. We showed here with definite evidence that CCCP indeed activated autophagy based on the lipidation and translocation of LC3 and the formation of autophagosomes, which were dependent on the classical macroautophagy machinery (Fig. 1 and supplemental Figs. S1 and S2). In addition, we showed by a number of criteria including flux analysis that CCCP-induced autophagy was functional, leading to lysosomal degradation.
Notably, we have identified that Nix is required for the mobilization of autophagy machinery. This function depends on the C-terminal sequence that includes the transmembrane domain, which was also critical for Nix to target to the mitochondria (Fig. 6D) and its other cellular function (26). In contrast, mutation of the conserved Leu124 and/or Asp129 in the BH3 domain did not affect the ability of Nix to induce autophagy and to target to the mitochondria. Mutagenesis of the BH3 domain of Nix had not been specifically conducted before for its importance in the function of Nix, although works done on the BH3 domain of Bnip3, a close homologue, had indicated that this domain was not critical to the function of Bnip3 (32). Overall, these results would thus indicate that the autophagy induction of Nix is intimately connected with the mitochondrial targeting of Nix and the mitochondrial alterations medicated by Nix.
Consistently, Nix is directly responsible for mitochondrial depolarization following CCCP treatment. Nix has been shown to promote autophagy under hypoxia conditions (10, 33), in which mitochondrial depolarization could be expected. Depolarization seems to be a consistent feature in other models of mammalian mitophagy, although this does not seem to be as important in yeast (6).
Our study further indicated that mitochondrial ROS generation, a consequence of depolarization, was an important factor in mediating the activation of autophagy machinery. CCCP-induced autophagy could be suppressed by an antioxidant, NAC. In addition, Nix-deficient MEF had less ROS production, and Nix could thus activate autophagy via ROS. ROS have been implicated to be important for autophagy and mitophagy (34), but the actual mechanisms are not clear. Our studies indicated that ROS could cause inhibition of the mTOR signaling pathway and thus the induction of autophagy (Fig. 7E). Other studies had indicated that ROS could inactivate Atg4 to reduce its deconjugation activity to increase LC3 association with the autophagosomal membrane (35).
There could be other ways by which Nix activates autophagy. Beclin1, a human homologue of yeast Atg6 essential for autophagy induction, can form a complex with Bcl-2 through its BH3 domain (36, 37). It is possible that Nix may be able to dissociate a Bcl-2-Beclin1 complex through competitive binding to Bcl-2 via its BH3 domain (33). From this point of view, it is interesting to note that deletion of Nix also affected rapamycin-induced autophagy to a noticeable degree (supplemental Fig. S8), which could suggest that the normal presence of Nix in cells increases the availability of Bcl-2-free Beclin 1 to autophagy induction by upstream signals such as rapamycin. This possibility is not necessarily incompatible with the finding that mutation in the BH3 domain of Nix did not affect its function in autophagy induction in CCCP-treated cells because these mutant molecules still possessed a functional TM domain, which would allow the BH3 mutants of Nix to target to the mitochondria (Fig. 6D) and trigger mitochondrial depolarization following CCCP treatment. Thus it is possible that the TM and BH3 domains of Nix may have independent autophagy-inducing function, with the TM domain being the dominant element.
Notably, Nix has also been shown to be involved in the removal of mitochondria in the context of development, i.e. during erythroid cell maturation (7, 8), but its mechanistic role seems to be less clear. The deficiency of mitochondrial removal in Nix-deficient erythroid cells could be overcome by the treatment with CCCP, which caused mitochondrial depolarization (8). This suggests that on one hand Nix may normally be required for mitochondrial depolarization triggered by developmental signals, but on the other hand, CCCP-induced depolarization could bypass Nix in these erythroid cells. Nix-independent depolarization by CCCP could be also observed in the present study in the nonerythroid MEF, which, however, required substantially longer treatment time (data not shown). Interestingly, it was found that Nix could be dispensed for the induction of autophagy during terminal erythroid differentiation (8), although this does not rule out that Nix would normally be contributory to autophagy activation during the differentiation. Thus it seems that in this developmental scenario, there are redundant mechanisms for autophagy activation.
Mitochondrial Priming and Parkin-mediated Ubiquitination and p62/SQSTM1
Although Parkin has been suggested to be important for mitophagy, the molecular mechanisms for regulating Parkin mitochondrial translocation had not been elucidated until recently using the CCCP model. In this model Parkin translocation was independent of mitochondrial fragmentation and ROS (9) (Fig. 8 and data not shown) but dependent on mitochondrial depolarization (9) and Pink1 (12, 31). The present study now further showed that CCCP-induced Parkin translocation was also dependent on Nix (Fig. 8). Notably, this function of Nix also requires the C-terminal mitochondrial targeting sequence, but not the BH3 domain, as in its autophagy induction function, once again suggesting the importance of mitochondrial changes caused by Nix in Parkin translocation. Consistently, although mitochondrial fragmentation could be induced by CCCP based on Tom20 staining (Figs. 2–5 and 8A and supplemental Figs. S5 and S6), Parkin distribution (Figs. 2–4 and 8D supplemental Fig. S6), and Nix distribution (Figs. 6D and 8D), this did not require Nix because it could be observed in Nix-deficient cells (Fig. 8A). On the other hand Nix is instrumental in the rapid mitochondrial depolarization following CCCP treatment (Fig. 7), and depolarization is important for the accumulation of Pink1 on the mitochondria (31). Thus Pink1 recruitment would likely depend on Nix. As a mitochondria-located kinase, Pink1 is both necessary and sufficient to recruit Parkin to the mitochondria (12, 31). In this capacity, Nix is not only important for the mobilization of the autophagy machinery but also important for mitochondrial priming.
The expression level of Parkin is quite low in several cell lines tested, including the neuronal cell line SH-SY5Y (this study and Refs. 9 and 12). Exogenous introduction of Parkin may not necessarily result in a level of expression consistent with endogenous needs and function. Nevertheless, as an E3 ligase, Parkin had been suspected to mediate ubiquitination of specific targets on the mitochondria once it is translocated there. The results reported here and in other studies (12) demonstrated that mitochondrial ubiquitination was indeed significantly increased following enforced Parkin expression and translocation. Approximately 70% of these Parkin-positive cells had ubiquitinated mitochondria, whereas only 20% Parkin-negative cells did (Fig. 4B). The latter, however, suggests either that the very low level of endogenous Parkin is still effective or that there is Parkin-independent mitochondrial ubiquitination, perhaps mediated by other mitochondrial E3 ligases. Among the possible mitochondrial proteins, voltage-dependent anion channel (VDAC) has been identified as a key target for Parkin-mediated ubiquitination required for mitochondrial clearance (12).
The association of p62/SQSTM1 with the mitochondria is clearly promoted by Parkin translocation and ubiquitination following CCCP treatment (Fig. 4B), indicating that the recruitment of p62 to the mitochondria was mediated by its ability to bind to ubiquitin. p62 can directly interact with LC3. Therefore it is not surprising that the co-localization of GFP-LC3 with p62 was in general independent of Parkin (Fig. 4C). However, the number of GFP-LC3 puncta that were co-localized with the mitochondria were significantly increased in Parkin-positive cells (Fig. 2E), which was well correlated with the association of p62 with mitochondria (Fig. 4B). Knockdown of p62 in CCCP-treated cells significantly decreased the number of GFP-LC3 puncta that were co-localized with mitochondria and the number of cells that had removed mitochondria following CCCP treatment (Fig. 5). Our results thus indicated that p62 served as an adaptor for the ubiquitinated mitochondria to be recognized by the autophagy machinery. Mitochondrial ubiquitination followed by lysosomal destruction had also been observed in the fertilized sperm (38), suggesting that this pathway could be well conserved in the context of development as well.
p62 is known to mediate autophagic degradation of other ubiquitinated targets, including misfolded proteins, peroxisomes, and invading bacteria in mammalian cells (3). Overall, our findings together with those of others support that ubiquitination and recruitment of adaptor molecules, such as p62, represent a general selective degradation signal in mammalian cells not only for protein aggregates but also for membrane-bound organelles (3).
It has also been reported that Nix could directly interact with LC3 and GABARAP (39, 40). Thus Nix could participate in mitochondrial priming in a more direct way without the involvement of Parkin and p62. The in vivo evidence for such a mechanism was shown in the context of reticulocyte maturation (39). This mechanism may in part explain the presence of the Parkin- and p62-independent mitochondrial clearance (Figs. 2E and 4B). In either scenario (via Parkin-ubiquitin-p62-LC3 or via direct interaction with LC3), Nix is important to mitochondrial priming in addition to its role in autophagy induction.
The molecular events of mitophagy described here are not limited to the CCCP model. We have also observed these phenomena using various respiratory chain toxins and in cells under hypoxia (data not shown). Taken together, mitochondrial respiration disturbance is an important trigger of mitophagy, and this process involves distinguished steps in the mobilization of autophagy machinery and in priming dysfunctional mitochondria for recognition by the autophagy machinery.
Supplementary Material
Acknowledgments
We thank Dr. R. Youle (National Institutes of Health, Bethesda, MD) for providing the Parkin plasmid, Dr. N. Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) for Atg5−/− MEFs, and Dr. M. Komatsu (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan) for Atg7−/− MEFs. We thank Sun Min for expert technical assistance in electron microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA83817 and R01 CA111456 (to X.-M. Y.) and R21-AA017421, P20 RR021940, and P20 RR016475 (to W.-X. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- E3
- ubiquitin-protein isopeptide ligase
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- CQ
- chloroquine
- MEF
- mouse embryonic fibroblast(s)
- MnTBAP
- manganese (III) tetrakis (4-benzoic acid)porphyrin chloride
- NAC
- N-acetylcysteine
- ROS
- reactive oxygen species
- ANOVA
- analysis of variance
- RFP
- red fluorescent protein.
REFERENCES
- 1.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y. (2009) Nat. Rev. Mol. Cell Biol. 10, 458–467 [DOI] [PubMed] [Google Scholar]
- 3.Kirkin V., McEwan D. G., Novak I., Dikic I. (2009) Mol. Cell 34, 259–269 [DOI] [PubMed] [Google Scholar]
- 4.Kim I., Rodriguez-Enriquez S., Lemasters J. J. (2007) Arch. Biochem. Biophys. 462, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanki T., Klionsky D. J. (2008) J. Biol. Chem. 283, 32386–32393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolkovsky A. M. (2009) Biochim. Biophys. Acta 1793, 1508–1515 [DOI] [PubMed] [Google Scholar]
- 7.Schweers R. L., Zhang J., Randall M. S., Loyd M. R., Li W., Dorsey F. C., Kundu M., Opferman J. T., Cleveland J. L., Miller J. L., Ney P. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19500–19505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval H., Thiagarajan P., Dasgupta S. K., Schumacher A., Prchal J. T., Chen M., Wang J. (2008) Nature 454, 232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Ney P. A. (2009) Cell Death Differ. 16, 939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin S. (2006) Autophagy 2, 80–84 [DOI] [PubMed] [Google Scholar]
- 12.Geisler S., Holmström K. M., Skujat D., Fiesel F. C., Rothfuss O. C., Kahle P. J., Springer W. (2010) Nat. Cell Biol. 12, 119–131 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Qi H., Taylor R., Xu W., Liu L. F., Jin S. (2007) Autophagy 3, 337–346 [DOI] [PubMed] [Google Scholar]
- 14.Mathew R., Karantza-Wadsworth V., White E. (2007) Nat. Rev. Cancer 7, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanki T., Wang K., Cao Y., Baba M., Klionsky D. J. (2009) Dev. Cell 17, 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto K., Kondo-Okamoto N., Ohsumi Y. (2009) Dev. Cell 17, 87–97 [DOI] [PubMed] [Google Scholar]
- 17.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998) Nature 392, 605–608 [DOI] [PubMed] [Google Scholar]
- 18.Kundu M., Lindsten T., Yang C. Y., Wu J., Zhao F., Zhang J., Selak M. A., Ney P. A., Thompson C. B. (2008) Blood 112, 1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamacher-Brady A., Brady N. R., Logue S. E., Sayen M. R., Jinno M., Kirshenbaum L. A., Gottlieb R. A., Gustafsson A. B. (2007) Cell Death Differ. 14, 146–157 [DOI] [PubMed] [Google Scholar]
- 20.Twig G., Elorza A., Molina A. J., Mohamed H., Wikstrom J. D., Walzer G., Stiles L., Haigh S. E., Katz S., Las G., Alroy J., Wu M., Py B. F., Yuan J., Deeney J. T., Corkey B. E., Shirihai O. S. (2008) EMBO J. 27, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagda R. K., Cherra S. J., 3rd, Kulich S. M., Tandon A., Park D., Chu C. T. (2009) J. Biol. Chem. 284, 13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding W. X., Ni H. M., Gao W., Hou Y. F., Melan M. A., Chen X., Stolz D. B., Shao Z. M., Yin X. M. (2007) J. Biol. Chem. 282, 4702–4710 [DOI] [PubMed] [Google Scholar]
- 23.Ding W. X., Ni H. M., Gao W., Yoshimori T., Stolz D. B., Ron D., Yin X. M. (2007) Am J. Pathol. 171, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diwan A., Koesters A. G., Odley A. M., Pushkaran S., Baines C. P., Spike B. T., Daria D., Jegga A. G., Geiger H., Aronow B. J., Molkentin J. D., Macleod K. F., Kalfa T. A., Dorn G. W., 2nd (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6794–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura S., Noda T., Yoshimori T. (2007) Autophagy 3, 452–460 [DOI] [PubMed] [Google Scholar]
- 26.Chen G., Cizeau J., Vande Velde C., Park J. H., Bozek G., Bolton J., Shi L., Dubik D., Greenberg A. (1999) J. Biol. Chem. 274, 7–10 [DOI] [PubMed] [Google Scholar]
- 27.Bassnett S. (2002) Exp. Eye Res. 74, 1–6 [DOI] [PubMed] [Google Scholar]
- 28.Matsui M., Yamamoto A., Kuma A., Ohsumi Y., Mizushima N. (2006) Biochem. Biophys. Res. Commun. 339, 485–489 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Randall M. S., Loyd M. R., Dorsey F. C., Kundu M., Cleveland J. L., Ney P. A. (2009) Blood 114, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutovsky P., Moreno R. D., Ramalho-Santos J., Dominko T., Simerly C., Schatten G. (1999) Nature 402, 371–372 [DOI] [PubMed] [Google Scholar]
- 31.Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., Youle R. J. (2010) PLoS Biol. 8, e1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray R., Chen G., Vande Velde C., Cizeau J., Park J. H., Reed J. C., Gietz R. D., Greenberg A. H. (2000) J. Biol. Chem. 275, 1439–1448 [DOI] [PubMed] [Google Scholar]
- 33.Bellot G., Garcia-Medina R., Gounon P., Chiche J., Roux D., Pouysségur J., Mazure N. M. (2009) Mol. Cell. Biol. 29, 2570–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherz-Shouval R., Elazar Z. (2007) Trends Cell Biol. 17, 422–427 [DOI] [PubMed] [Google Scholar]
- 35.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. (2007) EMBO J. 26, 1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiuri M. C., Le Toumelin G., Criollo A., Rain J. C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N., Hickman J. A., Geneste O., Kroemer G. (2007) EMBO J. 26, 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberstein A., Jeffrey P. D., Shi Y. (2007) J. Biol. Chem. 282, 13123–13132 [DOI] [PubMed] [Google Scholar]
- 38.Sutovsky P., Moreno R. D., Ramalho-Santos J., Dominko T., Simerly C., Schatten G. (2000) Biol. Reprod. 63, 582–590 [DOI] [PubMed] [Google Scholar]
- 39.Novak I., Kirkin V., McEwan D. G., Zhang J., Wild P., Rozenknop A., Rogov V., Löhr F., Popovic D., Occhipinti A., Reichert A. S., Terzic J., Dötsch V., Ney P. A., Dikic I. (2010) EMBO Rep. 11, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarten M., Mohrluder J., Ma P., Stoldt M., Thielmann Y., Stangler T., Hersch N., Hoffmann B., Merkel R., Willbold D. (2009) Autophagy 5, 690–698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.