Abstract
Akt is an important oncoprotein, and data suggest a critical role for nuclear Akt in cancer development. We have previously described a rapid (3–5 min) and P2X7-dependent depletion of nuclear phosphorylated Akt (pAkt) and effects on downstream targets, and here we studied mechanisms behind the pAkt depletion. We show that cholesterol-lowering drugs, statins, or extracellular ATP, induced a complex and coordinated response in insulin-stimulated A549 cells leading to depletion of nuclear pAkt. It involved protein/lipid phosphatases PTEN, pleckstrin homology domain leucine-rich repeat phosphatase (PHLPP1 and -2), protein phosphatase 2A (PP2A), and calcineurin. We employed immunocytology, immunoprecipitation, and proximity ligation assay techniques and show that PHLPP and calcineurin translocated to the nucleus and formed complexes with Akt within 3 min. Also PTEN translocated to the nucleus and then co-localized with pAkt close to the nuclear membrane. An inhibitor of the scaffolding immunophilin FK506-binding protein 51 (FKBP51) and calcineurin, FK506, prevented depletion of nuclear pAkt. Furthermore, okadaic acid, an inhibitor of PP2A, prevented the nuclear pAkt depletion. Chemical inhibition and siRNA indicated that PHLPP, PP2A, and PTEN were required for a robust depletion of nuclear pAkt, and in prostate cancer cells lacking PTEN, transfection of PTEN restored the statin-induced pAkt depletion. The activation of protein and lipid phosphatases was paralleled by a rapid proliferating cell nuclear antigen (PCNA) translocation to the nucleus, a PCNA-p21cip1 complex formation, and cyclin D1 degradation. We conclude that these effects reflect a signaling pathway for rapid depletion of pAkt that may stop the cell cycle.
Keywords: Calcineurin, PP2A, Protein Phosphatase, Purinergic Receptor, Signal Transduction, Akt, PHLPP, PTEN
Introduction
The serine/threonine kinase Akt is a central regulator of apoptosis and cell growth and is activated by insulin, growth factors, and cellular stress. Many studies indicate a key role for Akt in carcinogenesis and as a target for therapeutic agents (1–4). 3-Hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, statins, have anticancer properties (5), and we have shown that statins decrease levels of phosphorylated Akt (pAkt)3 in lung and pancreatic cancer cells (6–9). A conspicuous finding was that dephosphorylation of nuclear Akt was induced within minutes and that this effect was associated with inhibited cell proliferation. Extracellular ATP also induced nuclear pAkt depletion, and several lines of evidence indicated that the rapid pAkt depletion was mediated by the purinergic receptor P2X7 (7, 8).
A family of protein phosphatases, pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP), was identified as a phosphatase for Akt (10). Two isoforms, PHLPP1 and PHLPP2, have been shown to dephosphorylate distinct Akt isoforms, at one (Ser473) of two phosphorylation sites required for activation (11). This dephosphorylation is regulated by the immunophilin FKBP51 (FK506-binding protein 51), which acts as scaffolding protein for Akt and PHLPP (12). Other phosphatases shown to dephosphorylate Akt are calcineurin (13) and PP2A (14). Earlier studies show that calcineurin also interacts with FKBP51 (15).
Yet another regulator of nuclear Akt is the lipid phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome ten). PTEN targets phosphatidylinositol (3,4,5)-trisphosphate, a product of the phosphatidylinositol 3-kinase. The PTEN regulation is complex, involving phosphorylations, ubiquitinations, and protein complex formation (16). Although questioned (17), recent data indicate that monoubiquitination of PTEN by the E3 ubiquitin ligase NEDD4-1 mediates its nuclear localization. This effect was correlated with tumor suppression and inhibition of nuclear pAkt (18). p21cip1 is one of several nuclear targets for Akt (19, 20), and Akt may counteract p21cip1 by inducing phosphorylations at Thr145 and Ser146 (21). These phosphorylations can translocate p21cip1 to the cytoplasm (19) or prevent its binding to PCNA (20) and stop the cell cycle (22, 23).
In the present study, we have investigated the mechanism underlying the rapid depletion of nuclear pAkt. We report that ATP or pharmacologically relevant concentrations of statins activate PHLPP1 and 2, calcineurin, PP2A, and PTEN within minutes. We also show that activation of these phosphatases is associated with nuclear translocation of PCNA and its binding to p21cip1 and suggest that these signaling events regulate the cell cycle.
EXPERIMENTAL PROCEDURES
Cell Culture
Non-small cell lung cancer cells, A549, were purchased from ATCC. Cells were grown in Dulbecco's modified Eagle's medium (DMEM), with 10% inactivated calf serum, penicillin/streptomycin, 1 mm sodium pyruvate. Serum-starved cells were cultured with medium supplemented with 0.1% serum for 24 h. The androgen-sensitive human prostate adenocarcinoma LNCaP cells were purchased from ATCC. Cells were grown in RPMI 1640 supplemented with 10% inactivated calf serum, 1 mm sodium pyruvate, 10 mm HEPES, 2 mm l-glutamine, and penicillin/streptomycin.
Reagents
Atorvastatin was provided by Pfizer (New York, NY). BzATP, insulin, cycloheximide, FK-506 monohydrate, adenosine 5′-triphosphatase (porcine cerebral cortex), adenosine 5′-triphosphate disodium salt, benzyloxycarbonyl-Leu-Leu-Leu-al (MG132), and KN-62 were purchased from Sigma-Aldrich. BAPTA-AM was provided by Molecular Probes (Invitrogen). Okadaic acid sodium salt and leptomycin B were provided by Calbiochem. The final concentration of DMSO added to the cells was <0.4%. No effect of DMSO was observed.
Western Blotting
Western blotting was performed as described previously (6). In brief, the samples were subjected to SDS-PAGE and thereafter blotted onto a PVDF membrane (Bio-Rad). Some samples were subfractionated. The protein bands were probed using antibodies against Akt-1, Akt phosphorylated at residue Ser473 or Thr308, α-tubulin, PTEN, NEDD4-1, and Cdk2 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); α-calcineurin and β-calcineurin from Sigma-Aldrich; PTEN from Cell Signaling (Beverly, MA); and PHLPP1 and PHLPP2 from Bethyl Laboratories Inc. (Montgomery, TX). Cyclin D1 was purchased from Calbiochem. Proteins were visualized with the ECL procedure (Amersham Biosciences). The Western blotting results were analyzed with NIH Image 1.62 software.
siRNA Transfection
Cells were transfected with PHLPP, PTEN, or NEDD4-1 small interfering RNA (siRNA) (Cell Signaling Technology, Beverly, MA) for 72 h according to the TranIT-TKO protocol from the manufacturer (Mirus, Madison, WI) (Lipofectin). Control siRNA was purchased from Santa Cruz Biotechnology, Inc.
Transfection with PTEN-HA-tagged Vector
Cells were transfected with pSG5L or pSG5L-HA-PTEN (Addgene) for 24 h according to the Lipofectamine 2000 protocol (Mirus).
Immunocytochemical Staining
Cells were fixed in 3.7% formaldehyde. After fixation, the cells were stained with antibodies anti-PTEN (Cell Signaling, Beverly, MA), pAkt Thr308, PCNA, ubiquitin (Santa Cruz Biotechnology, Inc.), α-calcineurin, β-calcineurin (Sigma-Aldrich), and PHLPP1 (Bethyl Laboratories, Inc.). After incubation with primary antibodies at 4 °C overnight, secondary antibody conjugated with FITC or Texas Red were applied (Dako, Glostrup, Denmark). No staining was detected when the primary antibodies were omitted. The staining intensity was analyzed with NIH Image 1.62 software.
Proximity Ligation Assay
The proximity ligation assay (PLA) was performed according to the manufacturer's protocol using the Duolink detection kit with PLA PLUS and MINUS probes for mouse and rabbit (Olink Bioscience, Uppsala, Sweden).
Immunoprecipitation
Immunoprecipitation was performed by using FKBP51 (BD Biosciences), PTEN, (total Akt), Akt-1, p21cip1, PCNA, and pAkt Ser473 antibody (Santa Cruz Biotechnology, Inc.), α-calcineurin (Sigma-Aldrich), PHLPP1 and PHLPP2 from Bethyl Laboratories, Inc., and protein A/G PLUS-agarose (Santa Cruz Biotechnology, Inc.). Cells were washed with PBS and lysed in IPB-7. The cell lysates were incubated for 1 h with antibodies and then with protein A/G PLUS-agarose for 24 h at 4 °C.
Statistical Analysis
Statistical analysis was conducted using Student's t test. The data were presented as mean ± S.D. Experiments were performed at least three times with different batches of cells. Results were considered to be statistically significant at p ≤ 0.05.
RESULTS
ATP and Atorvastatin Rapidly Recruit PHLPP via P2X7
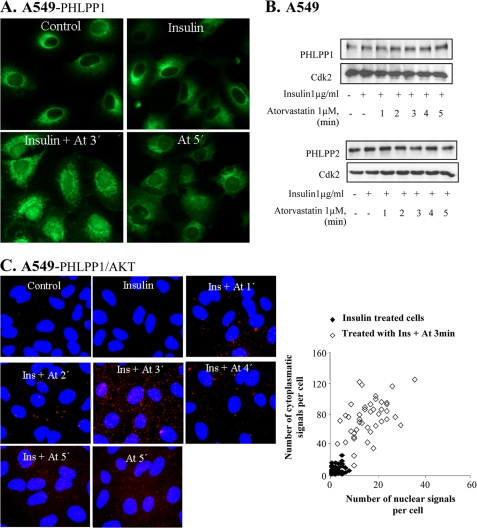
We have previously shown that insulin-induced nuclear pAkt levels were decreased within 1 min after the addition of ATP or atorvastatin, whereas the total Akt levels were not affected (8). This effect was associated with altered phosphorylations of downstream targets of pAkt, including GSK-3β and cyclin D1 (7). Here we investigated the mechanism behind this effect. PHLPP has been shown to dephosphorylate pAkt (10), and studies of colon and pancreatic cancers indicate a tumor suppressor function of this protein (24). Both PHLPP isoforms (PHLPP1 and -2) are present in the cytosolic, nuclear, and membrane fractions of the cell (11). As shown in Fig. 1A, atorvastatin induced a nuclear localization of PHLPP1 within 3 min of statin treatment in insulin-stimulated cells. A similar effect was found for PHLPP2 (data not shown). The total protein levels of PHLPP1 or PHLPP2 were not affected (Fig. 1B).
FIGURE 1.
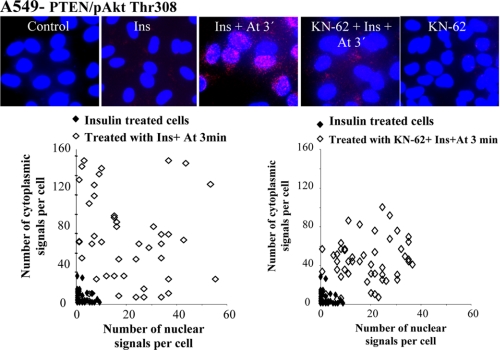
Atorvastatin and BzATP induce nuclear localization of PHLPP and binding to Akt. In A–D, A549 cells were treated with insulin (1 μm for 15 min) and thereafter with atorvastatin (1 μm for 1–5 min) as indicated. In E, cells were transfected with siRNA against PHLPP (50 nm) for 72 h and thereafter treated with insulin (1 μg/ml for 15 min) and atorvastatin (1 μm for 5 min). In F, cells were treated with KN-62 (100 nm for 10 min) and thereafter with BzATP or ATP (100 μm for 1–5 min) as indicated. In A and F, cells were stained for PHLPP1, and in E, they were stained for pAkt Thr308. In B, samples were then analyzed by Western blotting, employing antibodies for PHLPP1 and PHLPP2. Cdk2 was used as a loading control. In C, protein-protein interactions between Akt and PHLPP1 were studied employing PLA. Purple dots indicate proximity between cellular bound antibodies. The purple dots were counted using MATLAB, and the distribution of PHLPP1/AKT PLA signals between the nucleus and the cytoplasm is shown in insulin-treated (n = 50) or insulin plus atorvastatin-treated cells (n = 50) (each square represents a single cell). In D, lysates were immunoprecipitated (IP) using an Akt1 antibody and analyzed employing antibodies for PHLPP1 or PHLPP2. Total lysates were analyzed for Cdk2 (loading control).
To study the binding of PHLPP to Akt, described previously (11), PLA was performed to image protein-protein interaction between Akt and PHLPP. PLA analysis showed a binding between Akt and PHLPP1, as seen by the appearance of purple stained dots in Fig. 1C. This event was coordinated with the dephosphorylation. At 5 min, the binding between PHLPP1 and Akt returned to background levels. The visualization of individual protein complexes as distinct fluorescent spots allows computer-assisted image analysis. As shown in the PLA diagram (Fig. 1C, each square in the diagram represents a single cell), the dots representing PHLPP1/Akt-binding proteins tended to be localized in the cytoplasm (Fig. 1C). Atorvastatin treatment alone induced PHLPP1-Akt interaction although not to the same extent as in insulin-stimulated cells (Fig. 1C). To further investigate interactions between the proteins, cell lysates were immunoprecipitated for PHLPP1 or PHLPP2 with anti-Akt1 antibody. Western blot analyses of the samples show increased binding between PHLPP2 and Akt1 at 3 and 4 min after statin treatment (Fig. 1D) and no binding between PHLPP1 and Akt1. This was expected because PHLPP2 dephosphorylates Akt1 and Akt3, and PHLPP1 dephosphorylates Akt2 and Akt3 (11).
The role of PHLPP for depletion of pAkt was also investigated by using siRNA for PHLPP. Silencing PHLPP1 and -2 inhibited the statin-induced depletion of nuclear pAkt (Fig. 1E), suggesting that PHLPP was needed for dephosphorylation of insulin-induced nuclear pAkt. Also shown in Fig. 1E is that silencing PHLPP resulted in phosphorylation of Akt at Ser473, detected by Western blotting. Furthermore, pAkt Thr308 accumulated in the nucleus.
We have previously shown that statins regulate nuclear pAkt via the P2X7 purinergic receptor in epithelial cells (8). As shown in Fig. 1F, a 1-min incubation with the P2X7 agonist BzATP induced nuclear localization of PHLPP1. A similar effect was induced by the natural agonist for P2X receptors ATP (Fig. 1F). The results in Fig. 1F also show that ATP-induced nuclear localization of PHLPP1 was inhibited by the P2X7 receptor antagonist, KN-62. These data indicate that this statin- and ATP-induced nuclear localization of PHLPP1 was mediated by P2X7.
Inhibition of FKBP51, Calcineurin, and PP2A and Effects of BAPTA-AM
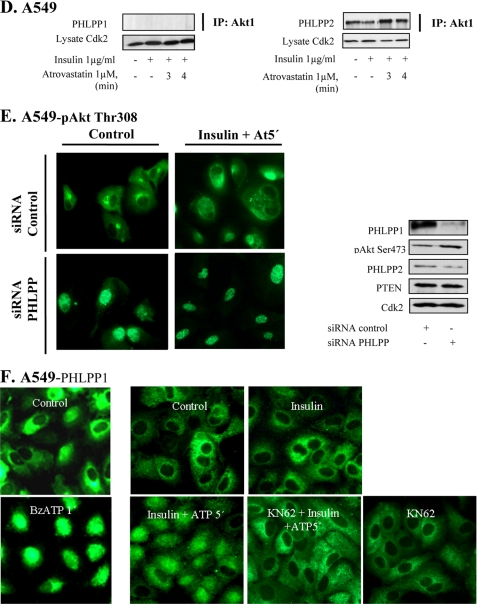
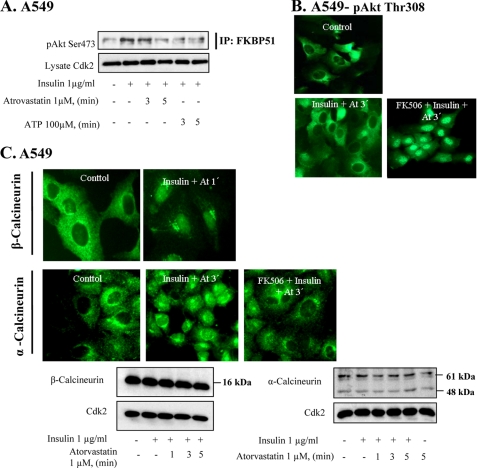
FKBP51 acts as a scaffolding protein for PHLPP and Akt (12). The binding between these proteins was studied here in immunoprecipitation experiments. Fig. 2A shows an increased binding between FKBP51 and pAkt when cells were stimulated with insulin. This binding was decreased at 5 min after the addition of statin. Next, we tested an inhibitor of FKBP51, FK506. We found that preincubation with FK506 completely prevented the effect of atorvastatin on nuclear pAkt Thr308 (Fig. 2B). These data indicate that FKBP51 is critical for the regulation of nuclear Akt in A549 cells.
FIGURE 2.
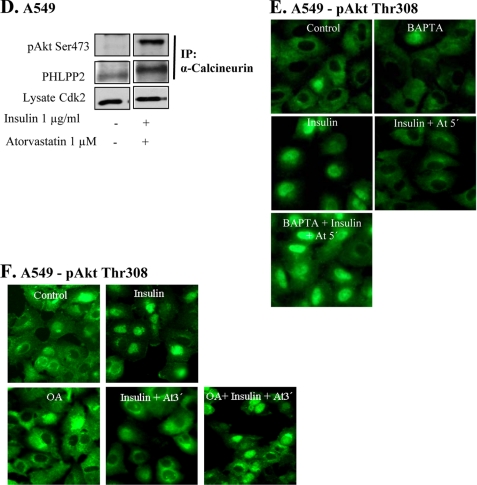
FK506 inhibits statin-induced depletion of pAkt and nuclear localization of calcineurin. In A–C, cells were treated with FK 506 (20 μm) prior to the addition of insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 3 min) or ATP. In B, cells were stained for pAkt Thr308, and in C, they were stained for α or β-calcineurin. In A, cells were immunoprecipitated (IP) using FKBP51 antibody and analyzed employing pAkt Ser473. In C, Western blotting, employing antibodies for α-calcineurin and β-calcineurin, was also used, and Cdk2 was the loading control. In D, cells were immunoprecipitated by using a α-calcineurin antibody. PHLPP2 and pAkt Ser473 antibodies were used for detection. Total lysates were analyzed for Cdk2. In E, cells were pretreated with BAPTA (2 μm for 15 min) followed by insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 5 min). Cells were stained for pAkt Tyr308. In F, cells were pretreated with okadaic acid (100 nm) for 4 h followed by insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 3 min). Cells were stained for pAkt Tyr308.
FKBP51 has also been shown to be regulated by calcineurin (15). Calcineurin is activated by Ca2+, and we have shown previously that atorvastatin induces increased Ca2+ levels in epithelial cells (8). Studies on myocardial myocytes also indicate that calcineurin can dephosphorylate Akt (25, 26). Thus, we investigated effects of statins and purins on calcineurin. We found that incubation of A549 cells with atorvastatin (1 μm) for 1–3 min increased nuclear levels of α- and β-calcineurin (Fig. 2C). This was induced without affecting total amounts of the proteins as indicated by Western blotting (Fig. 2C). Also shown in Fig. 2C is that atorvastatin-induced nuclear localization of calcineurin was inhibited by FK506. NFAT is a downstream target of calcineurin, and we found that NFAT was translocated to the nucleus by statin treatment, confirming that calcineurin was activated (data not shown). These experiments were also repeated with ATP or BzATP. Both purins mimicked the effects of statins (not shown). Immunoprecipitation experiments (Fig. 2D) showed that after a 3-min incubation with atorvastatin, an increased binding of Akt and PHLPP was detected in lysates immunoprecipitated with a α-calcineurin antibody. There was also an increased binding between α-calcineurin and pAkt Ser473 (Fig. 2D). The use of the pAkt Ser473 or pAkt Thr308 antibodies in this experiment confirms the observations from our previous reports that both phosphospecific Akt antibodies gave the same results. The immunoprecipitation experiments shown in Fig. 2D support the results shown in Fig. 2, B and C.
BAPTA-AM was used to block a previously shown (8) increase of intracellular Ca2+ levels. As shown in Fig. 2E, the atorvastatin-induced depletion of nuclear pAkt was completely abolished by BAPTA-AM. This supports a role of Ca2+ in this process. We also found that BAPTA-AM inhibited the statin-induced nuclear localization of PHLPP (data not shown).
PP2A is a phosphatase that has been shown to dephosphorylate Akt at both Ser473 and Thr308 and that is regulated by Ca2+ (27). It is inhibited by okadaic acid, and we found that okadaic acid prevented the statin-induced depletion of nuclear pAkt (Fig. 2F), indicating a role for PP2A in the depletion of nuclear pAkt.
P2X7 Receptor Activation Induces Relocations of PTEN and pAkt Binding
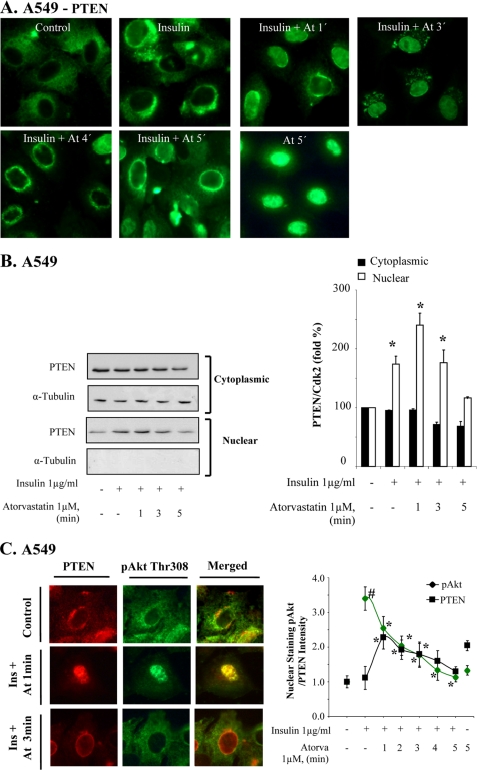
The lipid phosphatase PTEN is a major regulator of Akt phosphorylation. We found that in insulin-stimulated A549 cells, atorvastatin affected PTEN localization within a few minutes. As shown in Fig. 3A, atorvastatin decreased the cytoplasmic PTEN staining, but vesicle-like cytoplasmic compartments remained at 3 min. The nuclear PTEN staining increased (Fig. 3A). The earliest effects were seen at 1 min and peaked at 3 min. 4 or 5 min after the addition of atorvastatin, the PTEN staining was located close to the nuclear membrane, leaving the center of the nucleus unstained. It thus seemed that atorvastatin triggered a rapid translocation of PTEN to the nucleus, followed by an accumulation in a perinuclear compartment. The pattern of nucleocytoplasmic shuttling of PTEN is consistent with previous reports (4, 28). BzATP and ATP induced similar effects (data not shown). In other experiments, we also replaced ATP by UTP. ATP activates both P2Y and P2X receptors, whereas UTP activates P2Y receptors only (29). We found that UTP did not have any effect on PTEN (data not shown).
FIGURE 3.
Statin induces rapid nuclear PTEN localization. In A–C, cells were treated with insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 1–5 min) as indicated. In A, cells were stained with a PTEN antibody. In B, samples were analyzed by Western blotting, employing antibodies for PTEN and α-tubulin. The diagram shows means (columns) ± S.D. (bars) from three independent experiments. *, significantly different from control, p ≤ 0.05. In C, cells were stained for PTEN (red) and pAkt Thr308 (green), and the cells from one experiment are shown separately or merged. Note the yellow nuclear corona in merged pictures at 3 min. The diagram shows results from 50 cells expressed as the relative nuclear staining intensity (mean ± S.D. from 20 nuclei). *, significantly different from control; #, significantly different from insulin, p < 0.05.
In fractionation experiments and employing Western blots, we found that statin increased nuclear PTEN levels at 1 min (Fig. 3B). PTEN was thereafter decreased without a concomitant increase of PTEN in the cytoplasmic fraction. Fig. 3B also shows the densitometric analysis of three different experiments, confirming results shown in Fig. 3A. The purity of the cytoplasmic extract was confirmed by analyzing α-tubulin.
Double immunofluorescence staining of pAkt and PTEN was performed to compare the localizations of these two antigens (Fig. 3C). In line with our earlier data (8), we observed that insulin-stimulated nuclear pAkt levels (green staining) significantly decreased after the addition of atorvastatin. Nuclear PTEN levels (red staining) were first increased by atorvastatin (as already shown in Fig. 3A) and then decreased. Fig. 3C also shows that the increased nuclear PTEN staining co-located with the pAkt staining at 1 min. At 3 min, the center of the nucleus was unstained, but a rim close to the nuclear membrane indicated co-localization (yellow) as well as separate PTEN staining. These and other data from additional time points are summarized in the diagram in Fig. 3C. Taken together, these data indicate a rapid translocation of PTEN with nuclear levels peaking even earlier than, for example, PHLPP and calcineurin. A causative association between PTEN and the previously described down-regulation of pAkt was indicated.
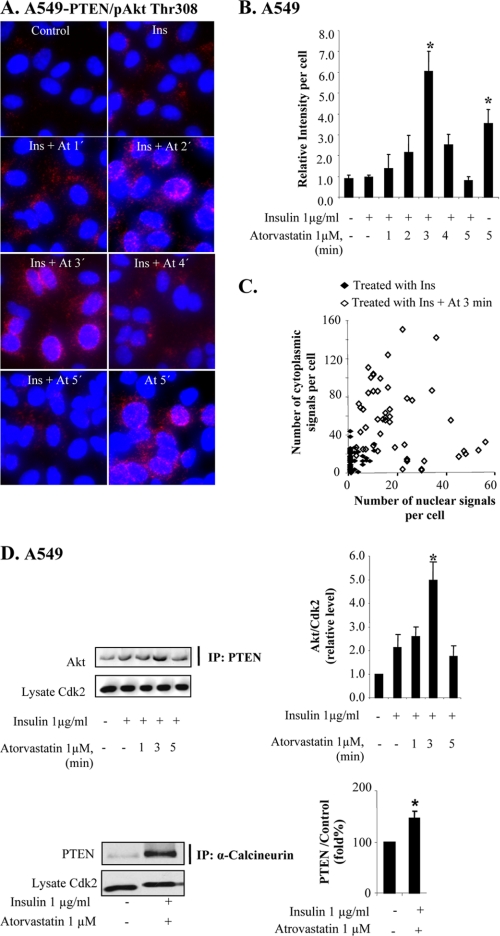
To study an involvement of PTEN in the depletion of nuclear pAkt more in detail, PLA was performed. As seen by the appearance of purple-stained dots in Fig. 4A, atorvastatin induced a rapid interaction between pAkt Thr308 and PTEN. Most dots were centered on the nuclear membrane. As compared with the PLA picture produced by PHLPP/Akt antibodies (Fig. 1C), more dots were seen in the nucleus at 2 min. Replacing the pAkt Thr308 with an antibody against pAkt Ser473 did not markedly change the staining pattern (data not shown). This confirms the results shown in Fig. 3 and indicates that PTEN forms a complex with pAkt a few min after the addition of atorvastatin and that the complex is localized close to the nuclear membrane within 3 min. This nuclear membrane localization is decreased at 4 min, and the binding is reversed to background levels at 5 min. Atorvastatin alone also gave rise to pAkt-PTEN interactions, seen after 5 min (Fig. 4A), suggesting that insulin stimulation speeded up the PTEN and pAkt encounter. Fig. 4B shows the highest level of binding at 3 min after atorvastatin treatment. Fig. 4C shows the distribution (at 3 min) of spots in the cytoplasmic or the nuclear area. It can be seen that the distribution varied between single cells but that the nuclear localization was more predominant than in the experiment with PHLPP and Akt (Fig. 1C).
FIGURE 4.
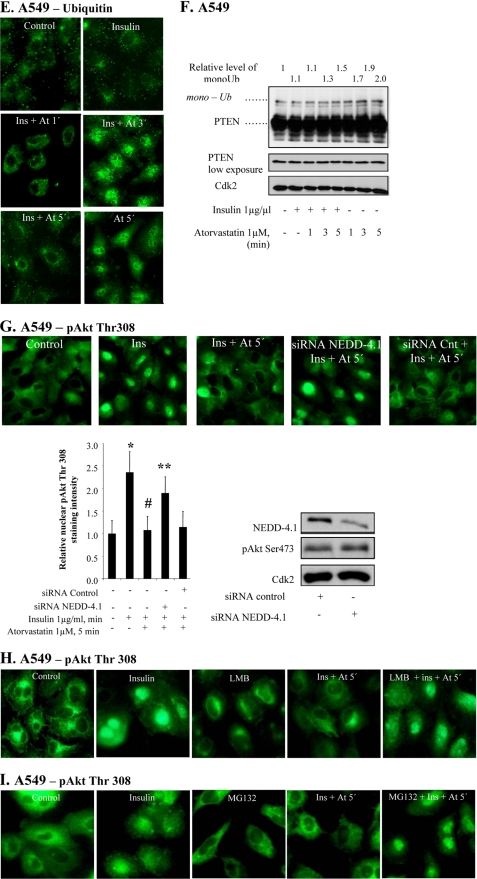
Statin-induced increased PTEN-pAkt binding correlates to PTEN ubiquitination. In A–F, cells were treated with insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 1–5 min) as indicated. A shows cells subjected to PLA analysis. Purple dots indicate proximity between cellular bound antibodies for PTEN and pAkt Thr308. In several photographs, many dots are seen in or on the nuclear membrane. In B, columns summarize the total number of PTEN/pAkt Thr308 PLA signals in 50 cells 3 min after the addition of atorvastatin (using MATLAB), and in C, the distribution of pAkt/PTEN PLA signals between the nucleus and the cytoplasm in 50 cells is shown (each square represents a single cell). In D, lysates were immunoprecipitated (IP) with PTEN or α-calcineurin antibodies and analyzed for Akt or PTEN. Total lysates were analyzed for Cdk2. Error bars, S.D. from three independent experiments; *, significantly different from control, p ≤ 0.05. In E, cells were stained for ubiquitin. In F, a Western blot analysis employing PTEN antibody is shown. In G, cells were transfected with siRNA against NEDD4-1 (50 nm) for 72 h and thereafter treated with insulin (1 μg/ml for 15 min) and atorvastatin (1 μm for 5 min). Cells were stained for pAkt Thr308. The diagram shows the results expressed as the relative nuclear staining intensity (mean ± S.D. from 50 nuclei). *, significantly different from control; #, significantly different from insulin; **, significantly different from insulin and atorvastatin, p < 0.05. The Western blot in G shows a decreased level of NEDD4-1 in NEDD4-1 siRNA-transfected cells. In H, cells were pretreated with leptomycin B (10 ng/ml for 5 min) followed by insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 5 min). Cells were stained for pAkt Tyr308. In I, cells were pretreated with MG132 (10 μmol/liter for 4 h) followed by insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 5 min). Cells were stained for pAkt Tyr308.
The binding between proteins was further studied in immunoprecipitation experiments (Fig. 4D). After a 3-min incubation with atorvastatin, an increased binding of Akt was detected in cell lysates immunoprecipitated with a PTEN antibody. After 5 min, the binding was reduced to basal levels (Fig. 4D). These results corroborate the findings described in the legend to Fig. 4, A–C. Fig. 4D also shows an increased binding between calcineurin and PTEN induced by insulin and statin.
PTEN Ubiquitination
Recent data show that monoubiquitination can mediate nuclear localization of PTEN (4, 18, 30), and a binding between PTEN and NEDD4 has been confirmed (30). As shown in Fig. 4E, accumulation of ubiquitin in the nucleus was induced at 3 min by atorvastatin in insulin-pretreated cells. After 5 min, nuclear levels of ubiquitin were reversed (Fig. 4E). Atorvastatin alone had a more long lasting effect. This pattern was confirmed by Western blotting (Fig. 4F), showing increased levels of monoubiquitinated PTEN. These findings indicate that P2X7 affects monoubiquitination of PTEN. Western blotting for pAkt also showed increased levels of ubiquitinated protein (data not shown). The role of NEDD4-1 in statin-induced pAkt depletion was also studied by employing siRNA for NEDD4-1. The results in Fig. 4G show that statin-induced depletion of nuclear pAkt was inhibited by silencing NEDD4-1, whereas control siRNA did not affect pAkt localization. Fig. 4G also shows that the siRNA treatment down-regulated NEDD4-1 protein levels. Our data are in line with a monoubiquination by NEDD4-1 regulating the nuclear localization of PTEN, although indirect effects (17) cannot be excluded.
The involvement of nuclear export and proteasomal degradation of Akt was also studied. When nuclear export was inhibited by leptomycin B, statin-induced nuclear depletion of pAkt was inhibited (Fig. 4H). This was also the case when proteasomal degradation was inhibited by MG132 (Fig. 4I). These data indicate that nuclear export and degradation was involved in the rapid nuclear depletion of pAkt. A coupling between dephosphorylation and protein degradation of Akt has been reported previously in response to oxidative stress (31).
PTEN Is Required for Depletion of Nuclear pAkt
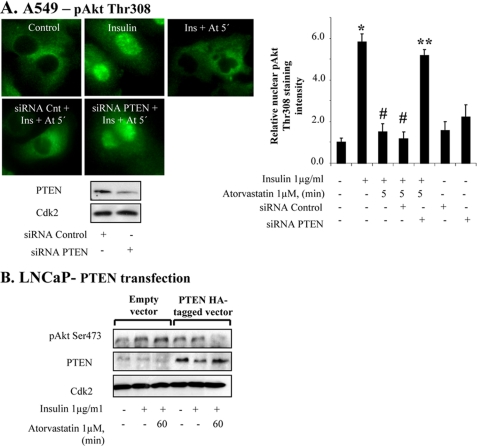
The role of PTEN for inhibition of pAkt was further investigated by using siRNA for PTEN. As shown in Fig. 5A, siRNA lowered the levels of PTEN and inhibited the statin-induced depletion of pAkt (Fig. 5A). The densitometric analysis of the nuclear intensity of pAkt staining shows that siRNA treatment prevented the effect of atorvastatin on pAkt (Fig. 5A). We also tested statins on LNCaP cells, a PTEN-negative prostatic cancer cell line. In LNCaP cells, statin did not induce any effect on insulin-induced pAkt (Fig. 5B). However, when LNCaP cells were transfected for PTEN, atorvastatin decreased pAkt levels (Fig. 5B). These data indicate that PTEN is necessary for the statin-induced down-regulation of nuclear pAkt levels in the cell.
FIGURE 5.
PTEN is required for inhibition of statin-induced depletion of pAkt. In A, A549 cells were transfected with siRNA against PTEN (50 nm) for 72 h and thereafter treated with insulin (15 min) and atorvastatin as indicated. Cells were stained for pAkt Thr308. The diagram shows the results expressed as the relative nuclear staining intensity (mean ± S.D. (error bars) from 50 nuclei). *, significantly different from control; #, significantly different from insulin; **, significantly different from insulin and atorvastatin. p < 0.05. In B, PTEN-deficient LNCaP cells were transfected with PTEN-HA-tagged vector (24 h) and thereafter treated with insulin (1 μg/ml for 15 min) and atorvastatin (1 μm for 60 min). Samples were analyzed by Western blotting, employing antibodies for PTEN and pAkt Ser473. Total lysates were analyzed for Cdk2.
We tested the effect of a P2X7 antagonist, KN-62, on the pAkt-PTEN protein interaction. The results in Fig. 6 show that the protein-protein interaction, as displayed by PLA, was partly inhibited by KN-62. This indicates that this statin-induced interaction is mediated by P2X7 signaling.
FIGURE 6.
KN-62 treatment decreases PTEN-pAkt binding induced by atorvastatin. Cells were treated with KN-62 (100 nm for 10 min) and thereafter with insulin (1 μg/ml for 15 min) and atorvastatin (1 μm for 3 min). Photographs show cells subjected to PLA analysis. Purple dots indicate proximity between cellular bound antibodies for PTEN and pAkt Thr308. Diagrams show the distribution of PLA signals between the nucleus and the cytoplasm in individual cells (each square represents a single cell). 50 cells from each treatment category were included in the analysis.
P2X7-mediated Effects on pAkt Are Associated with Rapid Nuclear Translocation of PCNA
The effect of phosphatidylinositol 3-kinase inhibitor LY294002 was examined. We used a high concentration of LY294002 (25 μm) in insulin-pretreated A549 cells. We did not observe an effect on pAkt levels as rapid as that seen with atorvastatin. The pAkt levels were unaffected 5 min after the addition of LY294002 but were decreased at later time points (data not shown). This supports an involvement of active phosphatases in the P2X7-mediated response and suggests that rapidity is important.
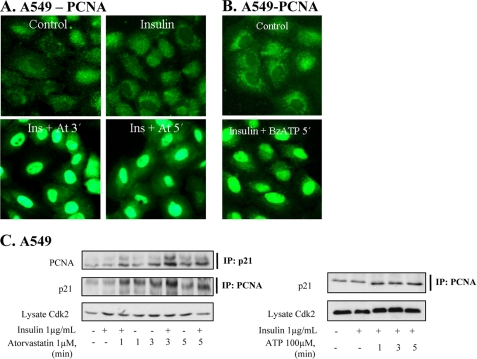
We have earlier shown that statins induce cell cycle block in cancer cell lines (6), and next we studied PCNA and p21cip1 because they are nuclear targets of Akt and may regulate the cell cycle (20). A cytoplasmic location of PCNA has been observed in starved cells (32), and a translocation of PCNA to the nucleus involves binding of PCNA to PP2A (33). As shown in Fig. 7, A and B, BzATP and statin induced rapid nuclear translocation of PCNA in insulin-stimulated cells. Within 3–5 min, a decrease in cytoplasmic staining and a marked increase in nuclear staining for PCNA were seen. The PCNA translocation was blocked by okadaic acid (not shown). In immunoprecipitation experiments (Fig. 7C), it was found that atorvastatin increased the level of p21cip1 detected in cell lysates immunoprecipitated with a PCNA antibody. An increased binding was also detected when cell lysates were immunoprecipitated with p21cip1. In staining experiments, we also found that p21cip1 localized to the nucleus after ATP stimulation (data not shown). PCNA nuclear translocation and formation of PCNA-p21cip1 complexes has been shown to result in cycle arrest (22, 23). In line with previous reports (7), we also found that statin (30 min) decreased the level of cyclin D1 by 29% (data not shown). These data are in line with a previously shown rapid degradation of cyclin D1 (34, 35).
FIGURE 7.
Atorvastatin and BzATP induce nuclear localization of PCNA and binding to p21cip1. In A and B, cells were pretreated with insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 3 or 5 min) or with BzATP (100 μm for 5 min). In C, cells were pretreated with insulin (1 μg/ml for 15 min) and thereafter with atorvastatin (1 μm for 1–5 min) or ATP (100 μm for 1–5 min), and then the lysates were immunoprecipitated (IP) using PCNA or p21cip1 antibodies. The samples were analyzed by Western blotting, employing antibodies for p21cip1 and PCNA. Total lysates were analyzed for Cdk2.
DISCUSSION
In this study, we provide evidence that five phosphatases (PTEN, PHLPP1, PHLPP2, calcineurin, and PP2A) were activated in a concerted action that depleted nuclear pAkt within 5 min. We show that this effect was associated with nuclear localization of PCNA, increased binding between PCNA and p21cip1, and decreasing cyclin D1 levels. This suggests a coupling of the nuclear pAkt depletion with cell cycle stop. We also provide evidence that this rapid response to external stimuli was mediated by the purinergic receptor P2X7.
Our data strongly implicate PHLPP in P2X7-mediated effects. PHLPP has been shown to inactivate Akt by dephosphorylating Ser473 (11) but not in the narrow time frame we showed here. By employing immunoprecipitation and PLA, we show that statins or ATP increased binding between PHLPP2 and Akt1 in a proper time frame. In insulin-stimulated cells, the PLA signal peaked at 3 min and was terminated within 5 min. We also show that knockdown of PHLPP inhibited the depletion of nuclear pAkt induced by statin. Furthermore, the knockdown of PHLPP alone resulted in pAkt accumulation in the nucleus, suggesting that PHLPP is involved in keeping the steady state level of pAkt low in the nucleus. The involvement of PP2A was indicated by okadaic acid, which prevented pAkt depletion.
Perhaps more surprising was the finding that PTEN seemed necessary for the pAkt depletion. PTEN is a lipid phosphatase, catalyzing the dephosphorylation of phosphatidylinositol (3,4,5)-trisphosphate (36), so it can be anticipated that the PTEN translocation protected dephosphorylated Akt from de novo phosphorylation. However, PTEN may also have phosphatase-independent activity (e.g. as a scaffolding protein (37)). PTEN has been shown to bind at least 93 proteins (30).
Relatively little has been published on the dephosphorylation or degradation of Akt, and in a recent comprehensive review (38) we find no data specifying the dephosphorylation or degradation of nuclear Akt. Our data indicate that four protein phosphatases and one lipid phosphatase collaborated with proteasomal degradation in the P2X7-mediated response. How was this complex response coordinated? As indicated by our BAPTA-AM experiments, increased intracellular Ca2+ concentrations can explain several events. We have shown previously that statin and ATP increase intracellular Ca2+ levels (8), and literature data show that Ca2+ can activate PP2A and calcineurin (27). Ca2+ can also activate NEDD4 ubiquitin ligases (39), so this may explain the nuclear accumulation of PTEN. Whether Ca2+ can activate FKBP51 is not known, but FKBP51 may act as scaffolding protein for PHLPP and Akt and promote dephosphorylation of pAkt Ser473 (12). FKBP51 can also bind calcineurin (15), so it seems likely that FKBP51 coordinated the activation of these two phosphatases. Interestingly, a FK506-like dipeptide (Leu-Ile) protects from a rapid (10 min) Akt ubiquitination and dephosphorylation. This effect was ascribed to an increased binding of Akt to Hsp90 (40), but it is conceivable that the peptide also affected the FKBP51 scaffolding activity. Taken together, our data suggest that P2X7 mediates a complex and rapid response that involves several phosphatases activated by Ca2+ and FKBP51. It can be assumed that specificity for pAkt was obtained via scaffolding properties of FKBP51 (12) and perhaps also of PTEN (37).
Further studies are needed to fully understand the effects triggered by statins or ATP. It seems clear, however, that the action of several phosphatases is essential for a robust depletion of nuclear pAkt. Thus, knocking down either PTEN or PHLPP or inhibiting either FKBP51 or PP2A blocked the response. Although PHLPP dephosphorylates only one of the two activating phosphorylation sites, PP2A may dephosphorylate both (Ser473 and Thr308) (14), so the involvement of additional phosphatases may seem superfluous. However, it has been demonstrated that for robust activation of Akt in cardiomyocytes, inhibition of two phosphatases, PP2A and calcineurin, was needed (13). The simultaneous activation of several phosphatases suggests that rapidity is essential, as most clearly shown here in insulin-stimulated cells.
Several targets for nuclear pAkt have been discussed in the literature. Previously, we have shown that statins induce a cell cycle block in G1 (9) and that statins decrease GSK-3β phosphorylation and rapidly degrade cyclin D1 (7). Here we show that the depletion of pAkt was associated with a nuclear translocation of PCNA and binding to p21cip1. Both of these proteins bind cyclin D1 (35, 41), and drug-induced cyclin D1 degradation mechanisms are not fully understood (34), so additional studies seem warranted. Both GSK-3β-dependent and -independent mechanisms for cyclin D1 degradation have been described (35), and our model might be particular useful for clarifying their mutual relationship. It is clear, however, that a rapid down-regulation of cyclin D1 leads to G1 arrest (34, 35). Further studies may, for example, show if this effect can explain an ATP-dependent prevention of metastatic spread seen in mammary cancer (42).
Atorvastatin was used here in a concentration (1 μm) relevant for lowering cholesterol levels and for preventing cardiovascular disease. However, statins may also have anticancer effects, and several epidemiological studies indicate that statins prevent aggressive prostate cancer (5). The mechanisms are unknown, but we showed that atorvastatin depleted nuclear pAkt also in prostate cancer cells, and previous studies indicate a critical role for nuclear Akt in the development of aggressive prostate cancer. Thus, the catalytic subunit of phosphoinositide 3-kinase β p110β regulates nuclear Akt (43) and is needed for prostate cancer development in a PTEN-deficient background in transgenic mouse models (44). Furthermore, clinical data indicate that the expression of p110β increases with aggressiveness and castration resistance in human prostate cancer (45). Taken together, these data suggest a role for nuclear Akt in aggressive prostate cancer, and our data raise the hypothesis that statins prevent prostate cancer via nuclear pAkt depletion.
This work was supported by the Swedish Research Council for the Environment, Agriculture Science, and Spatial Planning (Formas) and funds from Karolinska Insititutet.
- pAkt
- phosphorylated Akt
- PHLPP
- pleckstrin homology domain leucine-rich repeat protein phosphatase
- PLA
- proximity ligation assay
- PCNA
- proliferating cell nuclear antigen
- PP2A
- protein phosphatase 2A
- BzATP
- 2′-(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt
- P2X7
- purinergic 2X7 receptor
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester.
REFERENCES
- 1.Crowell J. A., Steele V. E., Fay J. R. (2007) Mol. Cancer Ther. 6, 2139–2148 [DOI] [PubMed] [Google Scholar]
- 2.Lian Z., Di Cristofano A. (2005) Oncogene 24, 7394–7400 [DOI] [PubMed] [Google Scholar]
- 3.Déléris P., Gayral S., Breton-Douillon M. (2006) J. Cell. Biochem. 98, 469–485 [DOI] [PubMed] [Google Scholar]
- 4.Trotman L. C., Wang X., Alimonti A., Chen Z., Teruya-Feldstein J., Yang H., Pavletich N. P., Carver B. S., Cordon-Cardo C., Erdjument-Bromage H., Tempst P., Chi S. G., Kim H. J., Misteli T., Jiang X., Pandolfi P. P. (2007) Cell 128, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton R. J., Freedland S. J. (2008) Curr. Urol. Rep. 9, 189–196 [DOI] [PubMed] [Google Scholar]
- 6.Pääjärvi G., Roudier E., Crisby M., Högberg J., Stenius U. (2005) FASEB J. 19, 476–478 [DOI] [PubMed] [Google Scholar]
- 7.Mistafa O., Stenius U. (2009) Biochem. Pharmacol. 78, 1115–1126 [DOI] [PubMed] [Google Scholar]
- 8.Mistafa O., Högberg J., Stenius U. (2008) Biochem. Biophys. Res. Commun. 365, 131–136 [DOI] [PubMed] [Google Scholar]
- 9.Roudier E., Mistafa O., Stenius U. (2006) Mol. Cancer Ther. 5, 2706–2715 [DOI] [PubMed] [Google Scholar]
- 10.Gao T., Furnari F., Newton A. C. (2005) Mol. Cell 18, 13–24 [DOI] [PubMed] [Google Scholar]
- 11.Brognard J., Sierecki E., Gao T., Newton A. C. (2007) Mol. Cell 25, 917–931 [DOI] [PubMed] [Google Scholar]
- 12.Pei H., Li L., Fridley B. L., Jenkins G. D., Kalari K. R., Lingle W., Petersen G., Lou Z., Wang L. (2009) Cancer Cell 16, 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni Y. G., Wang N., Cao D. J., Sachan N., Morris D. J., Gerard R. D., Kuro-O M., Rothermel B. A., Hill J. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20517–20522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andjelković M., Jakubowicz T., Cron P., Ming X. F., Han J. W., Hemmings B. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5699–5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T. K., Baksh S., Cristillo A. D., Bierer B. E. (2002) J. Cell. Biochem. 84, 460–471 [DOI] [PubMed] [Google Scholar]
- 16.Rabinovsky R., Pochanard P., McNear C., Brachmann S. M., Duke-Cohan J. S., Garraway L. A., Sellers W. R. (2009) Mol. Cell. Biol. 29, 5377–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fouladkou F., Landry T., Kawabe H., Neeb A., Lu C., Brose N., Stambolic V., Rotin D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8585–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Trotman L. C., Koppie T., Alimonti A., Chen Z., Gao Z., Wang J., Erdjument-Bromage H., Tempst P., Cordon-Cardo C., Pandolfi P. P., Jiang X. (2007) Cell 128, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B. P., Liao Y., Xia W., Spohn B., Lee M. H., Hung M. C. (2001) Nat. Cell Biol. 3, 245–252 [DOI] [PubMed] [Google Scholar]
- 20.Rössig L., Jadidi A. S., Urbich C., Badorff C., Zeiher A. M., Dimmeler S. (2001) Mol. Cell. Biol. 21, 5644–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Dowbenko D., Lasky L. A. (2002) J. Biol. Chem. 277, 11352–11361 [DOI] [PubMed] [Google Scholar]
- 22.Ando T., Kawabe T., Ohara H., Ducommun B., Itoh M., Okamoto T. (2001) J. Biol. Chem. 276, 42971–42977 [DOI] [PubMed] [Google Scholar]
- 23.Cayrol C., Knibiehler M., Ducommun B. (1998) Oncogene 16, 311–320 [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Weiss H. L., Rychahou P., Jackson L. N., Evers B. M., Gao T. (2009) Oncogene 28, 994–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C. H., Kim Y. S., Kim Y. H., Choi M. Y., Yoo J. M., Kang S. S., Choi W. S., Cho G. J. (2008) Brain Res. 1234, 148–157 [DOI] [PubMed] [Google Scholar]
- 26.Teruel T., Hernandez R., Lorenzo M. (2001) Diabetes 50, 2563–2571 [DOI] [PubMed] [Google Scholar]
- 27.Ahn J. H., Sung J. Y., McAvoy T., Nishi A., Janssens V., Goris J., Greengard P., Nairn A. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9876–9881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung J. H., Eng C. (2005) Cancer Res. 65, 8096–8100 [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G. (2007) Cell Mol. Life Sci. 64, 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn Y., Hwang C. Y., Lee S. R., Kwon K. S., Lee C. (2008) Biochem. J. 412, 331–338 [DOI] [PubMed] [Google Scholar]
- 31.Martin D., Salinas M., Fujita N., Tsuruo T., Cuadrado A. (2002) J. Biol. Chem. 277, 42943–42952 [DOI] [PubMed] [Google Scholar]
- 32.Vriz S., Lemaitre J. M., Leibovici M., Thierry N., Méchali M. (1992) Mol. Cell. Biol. 12, 3548–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow P. W., Tung H. Y., Hemmings H. C., Jr. (2004) Biochem. Biophys. Res. Commun. 323, 645–651 [DOI] [PubMed] [Google Scholar]
- 34.Pawar S. A., Sarkar T. R., Balamurugan K., Sharan S., Wang J., Zhang Y., Dowdy S. F., Huang A. M., Sterneck E. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9210–9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alao J. P. (2007) Mol. Cancer 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maehama T., Dixon J. E. (1998) J. Biol. Chem. 273, 13375–13378 [DOI] [PubMed] [Google Scholar]
- 37.Freeman D. J., Li A. G., Wei G., Li H. H., Kertesz N., Lesche R., Whale A. D., Martinez-Diaz H., Rozengurt N., Cardiff R. D., Liu X., Wu H. (2003) Cancer Cell 3, 117–130 [DOI] [PubMed] [Google Scholar]
- 38.Liao Y., Hung M. C. (2010) Am. J. Transl. Res. 2, 19–42 [PMC free article] [PubMed] [Google Scholar]
- 39.Plant P. J., Yeger H., Staub O., Howard P., Rotin D. (1997) J. Biol. Chem. 272, 32329–32336 [DOI] [PubMed] [Google Scholar]
- 40.Cen X., Nitta A., Ohya S., Zhao Y., Ozawa N., Mouri A., Ibi D., Wang L., Suzuki M., Saito K., Ito Y., Kawagoe T., Noda Y., Ito Y., Furukawa S., Nabeshima T. (2006) J. Neurosci. 26, 3335–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukami-Kobayashi J., Mitsui Y. (1999) Exp. Cell Res. 246, 338–347 [DOI] [PubMed] [Google Scholar]
- 42.Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., Perfettini J. L., Schlemmer F., Tasdemir E., Uhl M., Génin P., Civas A., Ryffel B., Kanellopoulos J., Tschopp J., André F., Lidereau R., McLaughlin N. M., Haynes N. M., Smyth M. J., Kroemer G., Zitvogel L. (2009) Nat. Med. 15, 1170–1178 [DOI] [PubMed] [Google Scholar]
- 43.Marqués M., Kumar A., Poveda A. M., Zuluaga S., Hernández C., Jackson S., Pasero P., Carrera A. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7525–7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S. H., Zhang J., Signoretti S., Loda M., Roberts T. M., Zhao J. J. (2008) Nature 454, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Q., Youn H., Tang J., Tawfik O., Dennis K., Terranova P. F., Du J., Raynal P., Thrasher J. B., Li B. (2008) Oncogene 27, 4569–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]