Abstract
Trigger factor (TF) is the first molecular chaperone that interacts with nascent chains emerging from bacterial ribosomes. TF is a modular protein, consisting of an N-terminal ribosome binding domain, a PPIase domain, and a C-terminal domain, all of which participate in polypeptide binding. To directly monitor the interactions of TF with nascent polypeptide chains, TF variants were site-specifically labeled with an environmentally sensitive NBD fluorophore. We found a marked increase in TF-NBD fluorescence during translation of firefly luciferase (Luc) chains, which expose substantial regions of hydrophobicity, but not with nascent chains lacking extensive hydrophobic segments. TF remained associated with Luc nascent chains for 111 ± 7 s, much longer than it remained bound to the ribosomes (t½ ∼ 10–14 s). Thus, multiple TF molecules can bind per nascent chain during translation. The Escherichia coli cytosolic proteome was classified into predicted weak and strong interactors for TF, based on the occurrence of continuous hydrophobic segments in the primary sequence. The residence time of TF on the nascent chain generally correlated with the presence of hydrophobic regions and the capacity of nascent chains to bury hydrophobicity. Interestingly, TF bound the signal sequence of a secretory protein, pOmpA, but not the hydrophobic signal anchor sequence of the inner membrane protein FtsQ. On the other hand, proteins lacking linear hydrophobic segments also recruited TF, suggesting that TF can recognize hydrophobic surface features discontinuous in sequence. Moreover, TF retained significant affinity for the folded domain of the positively charged, ribosomal protein S7, indicative of an alternative mode of TF action. Thus, unlike other chaperones, TF appears to employ multiple mechanisms to interact with a wide range of substrate proteins.
Keywords: Chaperone Chaperonin, Fluorescence, Protein Folding, Ribosomes, Translation, Trigger Factor
Introduction
Proteins are synthesized as unfolded polypeptide chains on ribosomes but must fold into precise three-dimensional structures to become functionally active. While the folding of small protein modules has been studied in great detail by in vitro experiments, how de novo folding is coordinated with translation is not yet well understood (1, 2). It is generally thought that nascent polypeptide chains emerging from the ribosome must be maintained by molecular chaperones in a non-aggregated, folding-competent state, at least until a complete domain capable of independent folding has been made available (3–5). A major function of chaperones that interact with nascent chains is to shield exposed hydrophobic amino acid residues that can give rise to aggregation in the highly crowded environment of the cytosol. How nascent chain-binding chaperones affect the folding process is currently of considerable interest, with the bacterial chaperone Trigger factor serving as an important paradigm.
Trigger factor (TF)3 binds to the large ribosomal subunit close to the polypeptide exit site (6) and has been shown by chemical cross-linking to interact with a wide variety of nascent polypeptide chains (7–12). TF functionally cooperates with the Hsp70 homolog, DnaK, in chaperoning nascent chains (13–15). Deletion of either the tig or dnaK genes is not lethal, but a combined deletion of both genes results in lethality at growth temperatures above 30 °C (13, 14). TF has been shown to delay the folding of certain multidomain proteins relative to translation when expressed in Escherichia coli and to increase their folding yield (15). The concentration of TF in the cytosol exceeds that of ribosomes (∼50 μm relative to ∼30 μm). TF binds to non-translating ribosomes with a KD of ∼1 μm and is probably present in stoichiometric complexes with ribosomes in vivo. Non-ribosome-bound TF dimerizes with a KD of ∼1–2 μm, partially burying its substrate binding sites. However, the dimeric form is in rapid equilibrium with the monomer, facilitating TF recruitment to the ribosome (16, 17).
The crystal structure of E. coli TF revealed an elongated three-domain topology of the 48-kDa protein (18) (Fig. 1A). The N-domain (residues 1–148) contains the ribosome binding site and has been co-crystallized with the large ribosomal subunit (18–20). It is followed in sequence by the PPIase domain (residues 149–250) and the C-terminal domain (residues 251–432). However, the PPIase domain is connected with the N-domain via a long linker and in the folded structure is located at the opposite end of the molecule. The C-terminal domain is positioned in the middle of the molecule and provides the primary binding site for the nascent chain. The main contact of TF with the ribosome involves residues 44–46 (FRK), located in the binding loop of the N-domain, and ribosomal protein L23 (6). The second contact involves ribosomal protein L29, which is not essential for TF-ribosome interaction (6). In the Deinococcus radiodurans co-crystal structure, the TF N-domain undergoes a conformational change in the ribosome-bound form (19). As a result, the N-domain exposes hydrophobic surfaces toward the opening of the ribosomal exit tunnel, presumably providing a binding surface for the emerging nascent chain that would be absent in the non-ribosome bound form. Independent evidence for ribosome-mediated conformational rearrangements came from intramolecular FRET studies, which showed that TF attains a more elongated shape upon ribosome binding (16).
FIGURE 1.
TF interactions with nascent chains during translation in real time. A, crystal structure of E. coli TF (PDB code 1w26), with the N-terminal domain shown in red, the PPIase domain in yellow, and arm 1 and arm 2 of the C-terminal domain in green and blue, respectively. Engineered cysteines to which the NBD or BADAN probes were covalently bound are indicated by black circles and their amino acid number. B, determination of the specific activity of Luc in the absence (control) or presence of 5 μm WT TF, TF150-NBD, TF326-NBD, or TF376-NBD. The specific activity of Luc upon translation in the PURE system was normalized with respect to the control (set to 1). Standard deviations from three independent experiments are shown. C, in vitro translation of Luc in the PURE system in the presence of either 1 μm TF326-NBD (green) or TF(FRK/AAA)326-NBD (purple) and translation of α-Syn (gray) in the presence of TF326-NBD. D, in vitro translation of Luc in the presence of either 1 μm TF-B (green) or TF(FRK/AAA)-B (purple) and translation of α-Syn (gray) in the presence of TF-B. Translations were initiated by the addition of the respective DNA templates and performed at 30 °C. E, a representative autoradiograph of Luc and α-Syn translation kinetics during a real-time experiment with aliquots taken at various time points as indicated.
The C-terminal domain of TF consists of a crevice surrounded by two arm-like structures (18) similar to the N-terminal domain of the E. coli periplasmic chaperone SurA and Mycoplasma pneumoniae MPN555 (21–23). Based on photocross-linking, both arms were found to be adjacent to the nascent chain during translation (17, 24). Experiments using 1-anilino-8-naphthalene-sulfonate (ANS) as a hydrophobicity sensor showed cross-linking of the probe to residues 391–392 in arm 2 (25). Modeling of TF bound to the archaeal 50 S ribosomal subunit suggested that the C-domain provides a shielded space over the opening of the ribosomal exit tunnel for the folding of small protein domains (18). The co-crystal structure of the D. radiodurans 50 S ribosomal subunit with the N-domain revealed that an extension in ribosomal protein L24, absent in archaea, partially occupies this space (20). The proposed topology of ribosome-bound TF (20) is supported by recent cryoelectron microscopic analysis (24).
The PPIase domain is structurally similar to the FKBP family of proteins. This domain has been shown to catalyze the cis-trans isomerization of peptide bonds preceding proline residues in proteins such as RCMLA (reduced carboxymethylated lactalbumin) and RNase T1 in vitro (26, 27). The PPIase domain is dispensable in vivo, based on the finding that TF without the PPIase domain (TFNC) is sufficient to rescue the synthetic lethal phenotype of the ΔdnaKΔtig strain (28–30). However, cross-linking experiments showed that the PPIase domain interacts with nascent chains (17, 24), suggesting that it contributes to TF function.
Fluorescence spectroscopy was previously utilized as a tool to study the interactions of TF with ribosomes during translation in real time (16) and with purified ribosome-nascent chain complexes (RNCs) (31). These experiments employed TF labeled with the exogenous fluorophore, BADAN, in its ribosome-binding domain (TF-B) (32). Interestingly, it was found that the residence time of TF with the ribosomes was not significantly modulated by the presence of the nascent chain (16), however, the affinity of TF for the ribosome was greatly increased (up to ∼30-fold), apparently via an increase in the on-rate of TF binding (16, 31). This effect correlated with the presence of hydrophobic segments in the nascent chain (16). Recently it was also reported that TF can bind and recognize the hydrophilic surfaces of folded proteins, such as the small ribosomal protein S7, suggesting a role for TF in ribosome assembly (33).
To gain better insight into the properties of nascent chains that govern the interaction with TF, it is necessary to follow TF binding to nascent chains directly. To this end, we utilized TF variants that were site specifically labeled with the environmentally sensitive fluorophore NBD at the substrate binding sites. Based on experiments with model proteins, we found that the affinity of TF for nascent chains increased depending on the occurrence of hydrophobic regions in the linear sequence of nascent chains. The residence time of TF on nascent chains correlated with exposure of these hydrophobic regions. Dissociation of TF from the nascent chains showed two-phase behavior, depending on chain length and the location of the NBD fluorophore within TF. Strikingly, TF maintained its interaction with certain chains for up to ∼110 s, much longer than its residence time on the ribosome. This allows for multiple TF molecules to bind such chains, followed by post-translational dissociation. We classified the E. coli cytosolic proteome into predicted weak and strong interactors for TF binding, based on the occurrence of linear hydrophobic chain segments. Validation experiments with a series of E. coli proteins confirmed that TF binds with high affinity to nascent chains exposing linear hydrophobic regions during translation. Such hydrophobic segments include the signal sequences of secretory proteins, such as pOmpA, but not the signal anchor sequence of the inner membrane protein FtsQ. However, TF also interacts with non-linear hydrophobic epitopes, presumably formed by partial nascent chain collapse. Interestingly, our data also suggest that TF can recognize additional structural features, such as hydrophilic surfaces on certain folded domains of nascent chains. Based on these findings, TF appears to have the ability to fulfill multiple, mechanistically rather different functions, unlike most other major chaperone systems that have been studied in detail.
EXPERIMENTAL PROCEDURES
Plasmids
For in vitro translation experiments, firefly luciferase (Luc) was cloned in a pET3a plasmid with C-terminal Myc and His6 tags resulting in pET3a-Luc. TF, TF(FRK/AAA), as well as the single cysteine mutants TF14, TF150, TF326, and TF376 were cloned into pPROEX-Hta as described (16). Ribosomal protein S7 was cloned in a pET22b vector. The plasmid pET22b-S7+50 encodes the full-length S7 followed by a flexible 25-amino acid linker-TS(GGGGS)4AAA- and an ORF encoding green fluorescent protein (GFP) as described (16). FtsQ was amplified from E. coli genomic DNA and cloned into pCDF1b vector under the T7 promoter resulting in pCDF1b-FtsQ. The nucleotide sequence of Luc encoding residues 87–100 was amplified from pET3a-Luc by PCR and ligated in-frame at the region encoding residue 87 of α-synuclein in the plasmid pSTS500-α-synuclein (34). The resulting plasmid was named pSTS500-α-Syn (Luc).
Protein Purification
All TF proteins and variants contained a TEV cleavable N-terminal His6 tag, which was removed following purification on metal chelating columns (16).
Fluorescent Labeling of TF Derivatives
The TF cysteine derivatives were labeled with either BADAN (6-bromoacetyl-2-dimethylaminonaphthalene) or NBD (N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole (IANBD ester)) as previously published (16), resulting in TF-B and TF-NBD, respectively. The extent of labeling was calculated based on the molar absorbance coefficient of 23,000 m−1 cm−1 for NBD and 21,000 m−1 cm−1 for BADAN.
Fluorescence Measurements
Spectra and kinetic traces were collected on a Fluorolog 3 fluorimeter (Jobin Yvon) at the indicated temperatures. Kinetic experiments were performed as before (16) with the following modifications. Measurements with TF-B and TF-NBD were performed with a final concentration of labeled protein of 1 μm. TF-B was excited at 387 nm with emission recorded at 500 nm. TF-NBD was excited at 472 nm and emission spectra were collected at 530 nm. For monitoring changes in TF fluorescence during translation, 1 μm TF-B or TF-NBD was added to the PURE translation system (35) in the absence of the template DNA. The reactions were transferred to a cuvette prewarmed to 30 °C. Once a steady signal was reached (after ∼3 min from the start of measurement), translation was initiated upon addition of DNA at a final concentration of 10 ng/μl and fluorescence was monitored as translation proceeded.
Competition Experiments
Once the fluorescence change had reached a plateau, indicating maximum constant translation speed, excess unlabeled TF or TF (FRK/AAA) was added to a final concentration of 20 μm, and the changes in fluorescence were recorded. Data from the competition experiments were analyzed using a three-parameter, single exponential function or a five-parameter, double exponential function, depending on the best fit.
Data for a single exponential function were analyzed using Equation 1,
and data for a double exponential function were analyzed using Equation 2,
where y0 is the initial value, a and c represent the amplitudes, and b and d represent the time constants. The time constant b was used to calculate the half-time in the single exponential reactions and time constants b and d were used to calculate both the half-times in a double exponential reaction by Equations 3 and 4.
 |
 |
Preparation of DNA Templates for in Vitro Translation in PURE System
Luc DNA templates for protein production in the PURE system were amplified from the pET3a-Luc construct. DNA templates for the predicted weak and strong interactors were amplified from E. coli genomic DNA to have all the regulatory components for in vitro translation, such as the promoter for T7 RNA polymerase and the ribosomal binding site. In addition they were designed to lack the terminal stop codon.
In Vitro Translation in the PURE System
In vitro translations were performed in the PURE system consisting of all purified translation components from E. coli (35). Template DNA (10 ng/μl) was added to the reconstituted PURE system along with other additional components (1 μm TF variants) and incubated at 30 °C.
Luciferase Activity Measurements
pET3a-Luc was translated in the PURE system in the presence of either 5 μm WT TF or other TF variants and 0.8 μCi/μl of [35S]methionine for ∼50 min at 30 °C. In a control reaction, translation was performed in the absence of TF. To measure enzymatic activity, 2-μl aliquots of the translation reaction were 100-fold diluted with luciferase dilution buffer. 2 μl of the above mixture was added to 48 μl of luciferase assay buffer (Promega) and luminescence was measured in a LUMAT LB 9507 luminometer. A fraction of the translation reaction was separated by SDS-PAGE and the amount of luciferase protein synthesized was determined by autoradiography. Specific activity of luciferase was calculated by dividing the enzymatic activity with the amount of protein synthesized.
Calculation of Hydrophobicity Score
Hydrophobicity scores of the E. coli cytosolic proteome were calculated as described previously (16). The hydropathy scale of individual amino acids by Roseman was used (36). The average mean hydrophobicity, hn, for each residue n in a protein consisting of L residues over a window of w residues was calculated according to Equation 5,
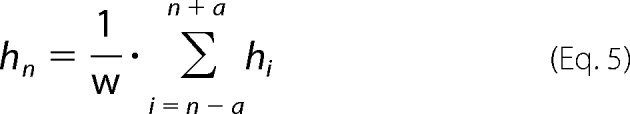 |
with a = (w)/(2) − 0.5 and a ≤ n ≤ L − a.
Limited Proteolysis of S7
Ribosome nascent chain complexes of full-length S7, with or without a carboxyl-terminal extension of 50 amino acids, were produced in the PURE system in the presence of [35S]methionine. Proteinase K was added to a final concentration of 12.5 μg/ml and the reactions were incubated for 8 min on ice. Protease action was stopped by the addition of 1 mm phenylmethylsulfonyl fluoride and incubated for 5 min on ice. Samples were TCA precipitated and separated by SDS-PAGE followed by autoradiography.
RESULTS
Monitoring the Interaction of TF with Nascent Chains during Translation
TF is the first chaperone to interact with nascent chains as they emerge from the ribosomal exit tunnel. We employed real-time fluorescence spectroscopy to monitor the interactions of TF with newly emerging nascent chains during translation. Firefly Luc was initially chosen as a model substrate for these experiments, based on previous observations indicating the high affinity of TF for Luc RNCs (16, 17, 34). TF was labeled at its substrate binding sites with the fluorophore NBD, which has previously been used to study the binding of signal recognition particle (SRP) to RNCs (37). Importantly, the fluorescence intensity of this dye increases upon transfer to a more hydrophobic environment. Positions 326 and 376 in arms 1 and 2 of the TF C-domain, respectively, and position 150 were mutated to cysteine and labeled with NBD (Fig. 1A). Residues 326 and 376 are known to be in close proximity to nascent chains based on photocross-linking experiments (17), and position 150 is located at the interface between the C-domain and PPIase domain. The labeled proteins preserved the ability of wild-type (WT) TF to bind to ribosomes (supplemental Fig. S1) and were similar to WT TF in their efficiency to assist the de novo folding of luciferase (Fig. 1B). However, when TF-NBD variants were incubated with an equimolar concentration of purified ribosomes, no change in NBD fluorescence was observed, in contrast to the fluorescence of TF labeled with BADAN at position 14 (TF-B), which was quenched upon ribosome binding (16, 32) (supplemental Fig. S2). Thus, the fluorescence of the TF-NBD variants is not altered upon ribosome binding.
Real-time fluorescence experiments were performed in the presence of TF326-NBD and TF-B during translation of full-length luciferase (FL Luc). A robust increase in the fluorescence of TF326-NBD was observed (Fig. 1C). In parallel experiments, a decrease in TF-B fluorescence, reporting on TF binding to ribosomes, occurred which preceded the increase in TF326-NBD fluorescence (Fig. 1D). This is consistent with NBD fluorescence reporting on TF properties that occur later during translation than ribosome binding. The intensity of NBD fluorescence reached a plateau when translation occurred at steady-state (Fig. 1, C and E). TF(FRK/AAA), in which residues 44–46 of the ribosome binding site of TF have been changed to alanines, has a strongly reduced ability to bind ribosomes (6). When TF326(FRK/AAA)-NBD was present during translation, no significant increase in NBD fluorescence was observed (Fig. 1C), confirming that ribosome binding is a prerequisite for the interaction of TF with nascent chains. Thus, the observed increase in NBD fluorescence results from a direct interaction between TF and Luc nascent chains and allows us to analyze the dynamics of this interaction during ongoing translation. Interestingly, no significant fluorescence change of TF326-NBD or TF-B was observed during translation of nascent chains of α-synuclein (α-Syn) (Fig. 1, C and D), consistent with the absence of significant continuous hydrophobic regions in this intrinsically unstructured protein. Previous experiments had shown that α-Syn chains can, nevertheless, be cross-linked to ribosome-bound TF (34). Thus, our measurements with fluorescence labeled TF distinguish between ribosome-associated TF being merely in close proximity to the nascent chain and actual binding.
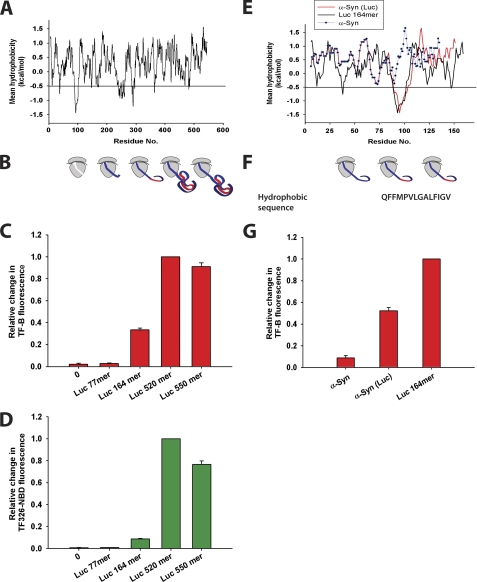
To investigate the correlation between the occurrence of hydrophobic regions in nascent chains and TF binding in more detail, Luc nascent chains of 77, 164, 520, or 550 residues were translated from mRNAs lacking the stop codon, resulting in stalling on the ribosome. Luciferase has multiple hydrophobic regions that are predicted to bind TF with high affinity (16) (Fig. 2A and supplemental Fig. S3). The different nascent chains exposed none (Luc 77-mer), one (Luc 164-mer), or all of these hydrophobic motifs (Luc 520-mer and 550-mer) outside the ribosome, taking into consideration that the ∼30–40 C-terminal amino acids are located within the ribosomal exit tunnel (Fig. 2B) (38). Upon translation of Luc 77-mer, no change in TF-B fluorescence and no increase in TF-NBD fluorescence were observed (Fig. 2, C and D), indicating that TF has no significant affinity for this short chain, exposing ∼37–47 amino acids outside the ribosome. Interestingly, while the Luc 164-mer resulted in a substantial recruitment of TF to the ribosome, as judged by the quenching of TF-B fluorescence (Fig. 2C), this nascent chain caused only a modest increase in TF-NBD fluorescence (Fig. 2D). This difference is consistent with recruitment of TF to the ribosome preceding the interaction of TF with hydrophobic regions of the nascent chain, observed in the kinetic measurements (Fig. 1, C and D). Indeed, during translation of Luc 164-mer, the fluorescence of both TF-B and TF326-NBD was saturated at around 1500 s, whereas during translation of the longer nascent chains fluorescence of TF326-NBD was saturated at 2000–2500 s (data not shown). Maximum TF-NBD fluorescence was measured with the Luc 520-mer (Fig. 2D). The increase in NBD fluorescence from the Luc 164-mer to the Luc 520-mer would be consistent with the exposure of more hydrophobic amino acid residues by the longer nascent chains. Only a moderate decrease in TF-NBD fluorescence was observed with the Luc 550-mer nascent chain, corresponding to full-length luciferase, suggesting that the burial of hydrophobic surfaces due to chain collapse is limited (Fig. 2D). The finding that TF-NBD maintains its interaction with hydrophobic regions of full-length nascent chains is consistent with earlier observations that TF delays luciferase folding relative to translation (15). Notably, the strong increase in TF-NBD fluorescence measured with the Luc 520-mer and 550-mer chains relative to Luc 164-mer may also correlate with the recruitment of multiple TF molecules by the longer chains. Such an increase in stoichiometry would not be reflected in a change in TF-B fluorescence, because only one TF can bind directly to the ribosome (16, 18).
FIGURE 2.
Recruitment of TF toward Luc RNCs of increasing length. A, hydrophobicity analysis of the firefly luciferase sequence (Luc) was performed as previously published (16). The predicted value of threshold hydrophobicity for TF binding is shown as a black line. B, schematic diagram of ribosomes displaying increasing lengths of Luc nascent chains exposing hydrophobic regions (red). The nascent chains were translated in the presence of either 1 μm TF-B or TF326-NBD. Bar diagram displaying the relative change in TF-B fluorescence (C) (red) and TF326-NBD fluorescence (D) (green) during translation of different lengths of Luc nascent chains as indicated. The fluorescence change of TF-B and TF326-NBD is shown relative to Luc 520-mer (set to 1). E, hydrophobicity analysis of α-Syn (blue), Luc 164-mer (black), and α-Syn (Luc) (red) sequences was performed as above. F, schematic representation of ribosomes displaying the hydrophobic region in α-Syn (Luc) and Luc 164-mer nascent chains (red). The sequence of the hydrophobic region present in α-Syn (Luc) and Luc 164-mer is shown. G, bar diagram displaying the relative change in TF-B fluorescence during translation of ribosome-stalled α-Syn, α-Syn (Luc), or Luc 164-mer nascent chains. TF-B fluorescence change is shown relative to Luc 164-mer (set to 1). Standard deviations from three independent experiments are shown in C, D, and G.
To confirm that linear hydrophobic regions in nascent chains can mediate the recruitment of TF to RNCs, the sequence forming the first hydrophobic region in Luc (residues 87–100) was inserted in the same position into the sequence of α-Syn, resulting in the construct α-Syn (Luc) (Fig. 2E and supplemental Fig. S3). α-Syn (Luc), along with α-Syn and Luc 164-mer, was translated in the PURE system (Fig. 2, F and G). Relative changes in TF-B fluorescence were corrected for the amounts of protein translated. Indeed, during translation of α-Syn (Luc), TF was recruited to the ribosomes with high affinity (Fig. 2G), confirming the importance of linear hydrophobic regions in mediating the interaction of TF with nascent chains. However, the hydrophobic region grafted into α-Syn-mediated TF binding with only 50% efficiency compared with TF binding to Luc 164-mer (Fig. 2G). This difference could be due to the higher average hydrophobicity of the Luc 164-mer sequence compared with α-Syn (Luc). Alternatively, the hydrophilic regions on α-Syn (Luc) flanking the grafted hydrophobic sequence may have a negative effect on TF binding (Fig. 2E).
Multiple TF Molecules Bind to Nascent Chains and Dissociate at Different Rates
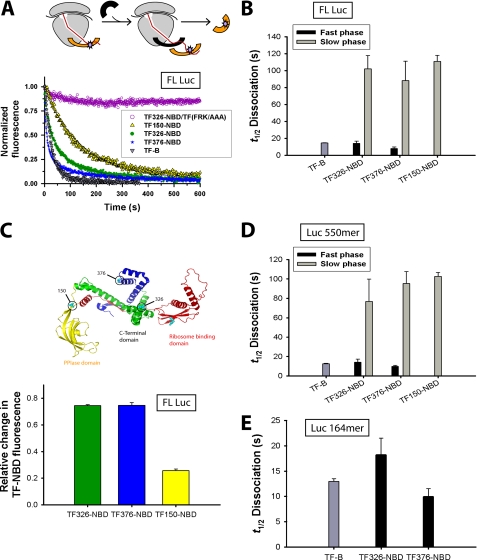
To investigate the possible interaction of multiple TF molecules with the nascent chain, we measured the kinetics of TF-nascent chain dissociation with different fluorescent labeled TF variants. To this end, a 20-fold excess of unlabeled WT TF was added to the translation reaction once translation had reached steady-state and the increase in NBD fluorescence had reached a plateau value. Interestingly, the half-time (t½) of TF326-NBD dissociation from FL Luc RNCs fit best to a model with fast and slow dissociation phases (Fig. 3A). The fast dissociation phase had a t½ value of 14 ± 2 s, identical to the dissociation of TF-B from ribosomes translating FL Luc (14 ± 0.5 s) (Fig. 3B) (16). The slow phase had a t½ value of 102 ± 16 s (Fig. 3B) (supplemental Table S1). The relative amplitudes of the two phases were 47 ± 6 and 43 ± 4%, respectively (supplemental Fig. S4). Competition experiments with TF (FRK/AAA), deficient in ribosome binding, showed no displacement of TF326-NBD, indicating that displacement required entry of a new TF molecule at the ribosome docking site (Fig. 3A).
FIGURE 3.
Dissociation of TF-NBD from Luc RNCs. A, dissociation of TF326-NBD (green), TF376-NBD (blue), TF150-NBD (yellow), or TF-B (gray) by addition of 20 μm WT TF from FL Luc RNCs once steady-state fluorescence values had been achieved during in vitro translation. Dissociation of TF326-NBD was also measured upon addition of 20 μm TF (FRK/AAA) (purple). B, bar diagram of the t½ for TF-NBD dissociation from FL Luc RNCs from reactions in A. The dissociation of TF326-NBD and TF376-NBD was best fit to a double exponential function (fast phase is shown in black and slow phase in gray) and dissociation of TF150-NBD was best fit to a single exponential function. C, top panel, crystal structure of E. coli TF displaying the sites to which the NBD probe was covalently bound. Bottom panel, bar diagram displaying the relative change in TF326-NBD (green), TF376-NBD (blue), and TF150-NBD fluorescence (yellow) during translation of FL Luc. D and E, the t½ values for TF-NBD dissociation from Luc 550-mer RNCs (D) and Luc 164-mer RNCs (E) are shown. The t½ values of TF-B dissociation from the respective RNCs are shown in B, D, and E. Standard deviations from at least three independent experiments are shown.
To investigate whether the biphasic nature of TF dissociation depended on the location of the NBD probe within TF, similar experiments were performed with TF376-NBD and TF150-NBD. The net fluorescence increase of TF376-NBD upon nascent chain binding was similar to that of TF326-NBD during translation of FL Luc, but was ∼70% lower in the case of TF150-NBD (Fig. 3C). This could suggest that only a fraction of nascent chains are in close proximity to position 150, because the PPIase domain acts only as a secondary site for nascent chain binding (16, 17, 24, 34). The dissociation of TF376-NBD from FL Luc was also biphasic, with t½ values of 8 ± 2 and 88 ± 23 s, respectively (Fig. 3B and supplemental Table S1), similar to the kinetics observed with TF326-NBD (Fig. 3, A and B). In contrast, only the slow dissociation phase (t½ value of 111 ± 7 s) was observed with TF150-NBD (Fig. 3, A and B). These results support a mechanism where TF leaves the ribosome at an intrinsic rate (t½ value ∼14 s), making the ribosome docking site available for entry of additional TF molecule, but can maintain its association with the elongating polypeptide.
The displacement of TF326-NBD and TF376-NBD from ribosome-arrested Luc 550-mer nascent chains also had two phases similar to those measured with FL Luc (Fig. 3D and supplemental Table S1). Again, only a single slow phase was measured for TF150-NBD. This indicates that the residence time of TF on the nascent chain is not affected by the translation rate per se. Interestingly, dissociation of TF326-NBD and TF376-NBD from stalled Luc 164-mer chains occurred with a single phase corresponding to a t½ values of 18 ± 3.5 and 10 ± 1.5 s, respectively (Fig. 3E and supplemental Table S1), equivalent to the fast phase observed with the Luc 550-mer and very similar to the dissociation of TF-B from the ribosome docking site (Fig. 3E). The second slow phase observed in the case of Luc 550-mer was absent during the dissociation from Luc 164-mer. No fluorescence change of TF150-NBD was detected during translation of the Luc 164-mer (data not shown) and accordingly no dissociation rates were measurable (Fig. 3E), indicating that the PPIase domain does not contact the hydrophobic region of this shorter Luc chain. Taken together, the chain length dependence of the residence time of TF-NBD variants suggests that multiple TF molecules bind to different regions of Luc and dissociate with different half-times. The fast dissociation phase, observed exclusively with shorter chains, likely reflects TF molecules that are still in contact with the ribosome docking site, whereas the slowly dissociating molecules observed with long chains represent TF molecules that maintain their interaction with hydrophobic chain segments after dissociating from the ribosome. Although TF release from the ribosomes appears to occur at an intrinsic rate, largely independent of the structural properties of the translating nascent chain, TF dissociation from the nascent chain depends on the hydrophobicity of the specific nascent chain and may occur post-translationally.
Dynamics of TF Interaction with Different Ribosome-Nascent Chain Complexes
The prolonged association of TF with Luc nascent chains led us to investigate the properties of nascent chains that govern the interaction with TF in more detail. The ability to monitor the interaction of TF with ribosomes and nascent chains independently allowed us to analyze the dynamics of TF interaction with the RNCs of different E. coli cytosolic proteins. The presence of linear hydrophobic regions in the primary sequence of nascent chains was used to classify proteins into predicted weak and strong interactors of TF. The hydrophobicity values of the individual residues were based on the Roseman hydropathy scale (36). Following our previous analysis of several model proteins (16), the threshold below which a residue is assigned a high mean hydrophobicity value was set to −0.5 kcal/mol. Hydropathy was analyzed over a window size of 15 residues (16), in accordance with the length of the proposed substrate binding crevice of TF (18), which is long enough to accommodate a peptide of 18 residues in a linear conformation. If a minimum of 5 consecutive residues in the central region of a 15-amino acid window have a mean hydrophobicity value of ≤0.5 kcal/mol, this segment was considered a potential motif for TF binding (16).
Approximately 1600 and 1400 E. coli proteins were predicted to have weak and strong interaction with TF, respectively, based on the number of linear hydrophobic segments in their sequences. Four proteins (AROE, RIMK, GATD, and DCP), having predicted interaction strengths of 0.85, 0.85, 1.0, and 1.0, respectively, were analyzed as representatives of predicted strong interactors (supplemental Table S2). Nine proteins lacking linear hydrophobic motifs above the threshold (S7, RRF, GRPE, SPOR, METF, HISG, RLMG, METK, and HEMA) were chosen as representatives of predicted weak interactors (supplemental Table S2). The proteins selected for validation cover a wide size range (Fig. 4, A and B). The respective genes were amplified from E. coli genomic DNA without the terminal stop codon. The nascent chains were translated in the PURE system (supplemental Fig. S5) and the interaction strength of the stalled nascent chains with TF-B and TF326-NBD was analyzed by monitoring the relative change in BADAN and NBD fluorescence, respectively. Relative fluorescence changes were corrected for the amount of radiolabeled protein. When the predicted strong interactors were translated, TF-B was recruited to the ribosomes, resulting in a relative change in BADAN fluorescence varying over a wide range from 0.13 to 1 (Fig. 4A). A significant increase in NBD fluorescence was also noted with these RNCs upon addition of TF326-NBD, with the relative fluorescence change varying from 0.23 to 1 (Fig. 4B). The change in NBD fluorescence, reflecting nascent chain binding, was most pronounced with DCP (78 kDa) (set to 1), the longest polypeptide sequence analyzed. It was lowest with RIMK (32 kDa), which contains only one hydrophobic segment, whereas AROE, GATD, and DCP contain two segments. The change in NBD fluorescence measured for these proteins correlated well with the change in BADAN fluorescence, reflecting TF recruitment to the ribosome. Based on these results, hydrophobic segments in nascent chains are likely to contribute substantially to TF binding, but the correlation with the binding strength predicted from the presence of such linear hydrophobic sequence elements is weak.
FIGURE 4.
Dynamics of TF interactions with predicted weak and strong interactors. Recruitment of TF-B (A) and TF326-NBD (B) toward stalled RNCs of predicted weak interactors (S7, RRF, GRPE, SPOR, METF, HISG, RLMG, METK, and HEMA) (black) or predicted strong interactors (AROE, RIMK, GATD, and DCP) (gray) of TF. The relative change in TF-B and TF326-NBD fluorescence measured after saturation was reached was corrected for the amount of radiolabeled protein. The fluorescence change of TF-B and TF326-NBD is shown relative to DCP (set to 1). Standard deviations from at least three independent experiments are shown. Molecular weight and absolute hydrophobicity of the protein sequences are shown. Hydrophobicity scale of Kyte and Doolittle (39) was used to calculate absolute hydrophobicity.
In the case of the predicted weak interactors, there was also considerable variation in TF recruitment to the ribosomes (Fig. 4A). Notably, several of these proteins, such as SPOR, METF, HISG, and RLMG bound TF-B with a relative affinity comparable with that measured with the predicted strong interactors. However, except for HISG and HEMA, all predicted weak interactors showed clearly reduced levels of TF326-NBD fluorescence, including RLMG, which recruited TF-B most efficiently to the ribosome (Fig. 4B). These results indicate that linear chain hydrophobicity is not sufficient to discriminate between weak and strong interactors, suggesting that an additional feature, such as the degree of exposure of hydrophobic segments, modulate TF binding.
As an additional criteria for binding strength we therefore analyzed the absolute hydrophobicity of the individual proteins according to Kyte and Doolittle (39). Several of the predicted weak interactors (S7, RRF, GRPE, and SPOR), lacking continuous hydrophobic segments above the threshold and having low overall hydrophobicity values, showed only weak fluorescence with TF-NBD (Fig. 4B). In contrast, proteins HISG and HEMA, which have ∼2-fold higher hydrophobicity, resulted in proportionally higher levels of TF-NBD fluorescence (Fig. 4B). On the other hand, METF, RLMG, and METK, proteins with similarly high absolute hydrophobicity, gave only low TF-NBD fluorescence (Fig. 4B). Based on these findings, both linear hydrophobic sequence elements and exposed hydrophobic surfaces are likely to contribute to TF binding. Low TF-NBD fluorescence despite relatively high overall hydrophobicity may be explained by the burial of hydrophobic residues upon nascent chain collapse.
The dissociation of TF-B from the different nascent chains generally occurred with a t½ value of ∼10–12 s (data not shown). The residence time of TF326-NBD with all the strong interactors and one of the weak interactors (SPOR) was analyzed (Fig. 5A). The dissociation of TF326-NBD for SPOR, AROE, and RIMK occurred with t½ values of 13.5 ± 1, 9.5 ± 0.5, and 10 ± 2.5 s, respectively (Fig. 5A and supplemental Table S3), equivalent to TF-B dissociation from the ribosome. In the case of GATD and DCP, the residence time of TF326-NBD was prolonged, with t½ values of 24.5 ± 1 and 28.5 ± 6 s, respectively (Fig. 5A and supplemental Table S3). No clear distinction between mono- or biphasic behavior was possible. Thus, TF remains associated with GATD and DCP nascent chains after dissociating from the ribosome.
FIGURE 5.
Dissociation of TF326-NBD from the RNCs of predicted weak and strong interactors. A, bar diagram of the t½ values for TF326-NBD dissociation from RNCs of SPOR (black) and RNCs of the predicted strong interactors AROE, RIMK, GATD, and DCP (gray). The dissociation of TF326-NBD from all the above RNCs was best fit to a single exponential function. Standard deviations from at least three independent experiments are shown. B, ribbon diagram of the crystal structure of E. coli shikimate dehydrogenase (AROE) (PDB code 1NYT). Residues 1–101, comprising domain I, and residues 102–232, comprising domain II, are shown in red and green, respectively. C-terminal 39 residues (233–272) are shown in blue. C, ribbon diagram of the crystal structure of E. coli DCP (PDB code 1Y79). Residues 1–146, 351–539, and 611–640 forming domain I are shown in red. Residues 147–350 and 540–610 forming domain II are shown in green. C-terminal residues 641–680, located in the ribosomal exit tunnel prior to the release of the full-length chain, are shown in blue. Structures were generated with PyMOL (51).
Possible Effects of Domain Topology on TF-Nascent Chain Interactions
AROE and DCP both interacted strongly with TF (Fig. 4, A and B) but the residence time of TF on their nascent chains varied substantially from 9.5 ± 0.5 to 28.5 ± 6 s, respectively (Fig. 5A), suggesting that these chains differ in their capacity to bury hydrophobic residues while attached to the ribosome. AROE (Shikimate dehydrogenase) consists of two α/β domains separated by a wide cleft (Fig. 5B). The C-terminal domain adopts a canonical Rossmann-fold and binds NAD(P). In the crystal structure, the 35 C-terminal residues of AROE are not part of the core of the protein (Fig. 5B) (40). This suggests that AROE nascent chains could undergo significant collapse and compaction, burying hydrophobic regions even before the full-length protein is synthesized and released from the ribosome. As a result, TF might dissociate faster from AROE nascent chains, with similar kinetics as its dissociation from the ribosomes. In contrast, dipetidyl carboxypeptidase (DCP) is a 78-kDa protein consisting of two half-shell-like subdomains forming a closed two-chamber cavity containing the proteolytically active site. The crystal structure of E. coli DCP shows that the protein has a discontinuous domain structure with the C terminus being part of domain I formed by regions of the N-domain (Fig. 5C) (41). DCP attains a “closed” form only when it is bound to the substrate. The formation of the “closed” form from the “open” form in the presence of substrate is accomplished by a hinge-bending mechanism. Prior to release from the ribosome, DCP may not bury its hydrophobic regions and remain unfolded, due to unavailability of the C-terminal ∼40 residues that are necessary to complete the folding of domain I. This might explain the prolonged interaction of TF with the exposed hydrophobic regions of DCP, even after dissociation of the protein from the ribosome. These considerations support the view that the rate of dissociation of TF from hydrophobic regions of a protein is determined not only by the absolute hydrophobicity of these segments but also by the structural capacity of the chain to bury hydrophobic residues while being attached to the ribosome.
Interaction of TF with Secretory and Inner Membrane Proteins
Secretory and inner membrane proteins interact via their signal sequences and signal anchor (SA) sequences with dedicated targeting factors, SecA/SecB and the SRP, respectively (42). Given the hydrophobic nature of both the signal sequence and SA, it is of interest how these factors may cooperate with TF. TF has been shown to interact with the precursor of the outer membrane protein A (pOmpA), maintaining it in a translocation competent state (43, 44). In a manner dependent on chain length, the signal sequence of pOmpA was cross-linked to TF, SecA, and SRP (45). Co-sedimentation, cross-linking, and structural studies have suggested that SRP and TF can bind to ribosomes simultaneously using L23 as a common docking site (20, 46, 47). SRP is thought to have higher affinity for the emerging SA sequences than TF (10, 11), but how SRP achieves a selective advantage over TF is not well understood.
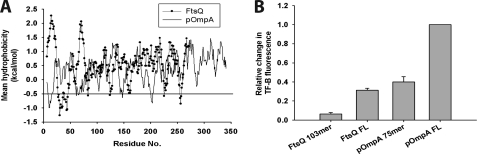
To begin to understand whether signal sequences and SA sequences are recognized by TF in the absence of targeting factors, we analyzed the recruitment of TF to RNCs of the secretory protein, pOmpA, and the inner membrane protein, FtsQ. In addition to the interaction with the FL nascent chains, the properties of the signal sequence of pOmpA and the SA sequence of FtsQ with regard to TF binding were examined. The N-terminal 103 residues of FtsQ (FtsQ 103-mer) contain the hydrophobic SA sequence (residues 25–49) (Fig. 6A). Similar lengths of FtsQ nascent chains were previously shown to interact with SRP by photocross-linking and cryoelectron microscopy (11, 48). A pOmpA 75-mer nascent chain, exposing the N-terminal hydrophobic signal sequence (residues 1–21) outside the ribosome, was chosen to study the interaction of TF with a signal sequence (Fig. 6A). The nascent chains were translated in the PURE system and the recruitment of TF-B to ribosomes was monitored.
FIGURE 6.
Interaction of TF with RNCs of inner membrane and secretory proteins. A, hydrophobicity analysis of the FtsQ (black circles) and pOmpA (black line) sequences were performed as described in the legend to Fig. 2. B, bar diagram displaying the relative change in TF-B fluorescence during translation of ribosome-stalled FtsQ 103-mer, FtsQ FL, pOmpA 75-mer, and pOmpA FL. The fluorescence change of TF-B is shown relative to pOmpA (set to 1). Standard deviations from at least three independent experiments are shown.
During translation of FL pOmpA, TF was recruited to the ribosomes with high affinity (relative change in TF-B fluorescence set to 1) (Fig. 6B). TF binding was less pronounced in the case of FL FtsQ (relative fluorescence change ∼0.3) (Fig. 6B), consistent with the lower average hydrophobicity of FtsQ (Fig. 6A). TF-B was efficiently recruited to pOmpA 75-mer chains (relative fluorescence change ∼0.4), indicating an interaction with the signal sequence. In contrast, the interaction of TF with the FtsQ 103-mer was very weak (relative fluorescence change <0.1), indicating that TF lacks high affinity for the SA sequence (Fig. 6B). A weaker interaction of TF with the hydrophobic SA sequence of FtsQ in the absence of SRP suggests that this sequence possesses additional structural features that disfavor recognition by TF. The interaction with regions of FtsQ downstream of the SA sequence may reflect a role of TF in maintaining the protein in a non-aggregated state for efficient membrane targeting.
Interaction of TF with a Folded Protein Domain
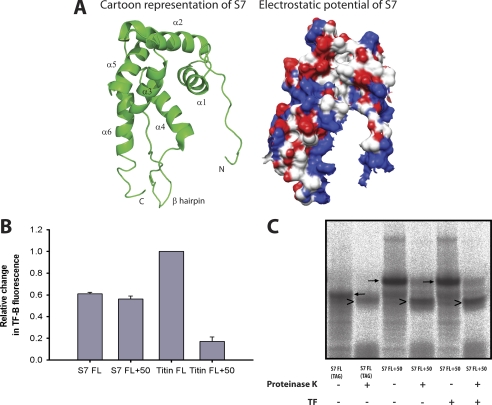
It was recently reported that TF, in addition to its association with nascent chains, can also interact with the folded domains of certain ribosomal proteins, thereby playing a role in ribosome assembly (33). Specifically, these authors obtained the x-ray crystal structure of a complex of two TF molecules encapsulating two molecules of the 20-kDa ribosomal protein S7 in a native-like conformation. The interactions between TF and S7 were predominantly hydrophilic. E. coli S7 consists of six α-helices and a β-hairpin between helices 3 and 4 (Fig. 7A). The β-hairpin along with regions of helices 1, 4, and 6 forms a large positively charged surface that binds RNA (Fig. 7A).
FIGURE 7.
Interaction of TF with RNCs of the ribosomal protein S7. A, ribbon diagram and electrostatic surface potential of the crystal structure of S7 from the intact E. coli ribosome and shown in the same orientation (PDB code 2AVY). Surfaces are shown in degrees of positive (blue) and negative (red) potential. Electrostatic potential was generated with Chimera (50). B, the maximal relative change in TF-B fluorescence, reflecting TF recruitment to the ribosome, measured with nascent chains of S7 FL, S7 FL+50, Titin FL, and Titin FL+50. Values were corrected for the amount of radiolabeled protein synthesized. The fluorescence change of TF-B with Titin FL is set to 1. C, proteinase K digestion of S7 FL (TAG) (ribosome released S7) or S7 FL+50 nascent chains. The nascent chains were translated in the PURE system for ∼50 min in the presence of [35S]methionine. S7 FL+50 nascent chains were translated in the presence or absence of 5 μm TF. The reactions were digested with Proteinase K for 8 min on ice and analyzed by SDS-PAGE. Black arrows indicate nascent chains, arrowheads indicate protease-protected fragments.
To investigate the interaction of TF with S7, we monitored the binding of TF-B to nascent chains of S7 during different stages of elongation and folding. During translation of stalled, full-length chains of S7, TF-B was recruited to ribosomes, indicating a significant affinity of TF for the S7 protein (Fig. 7B). Considering that S7 lacks continuous hydrophobic segments and has an overall low hydrophobicity (Fig. 4B), the observed binding of TF suggests that in this case TF recognizes features other than exposed hydrophobic amino acid residues. When S7 was fused to a flexible 50-amino acid linker to allow the S7 domain to fold outside the ribosomal tunnel, TF-B binding remained at a high level (Fig. 7B). For comparison, we measured the interaction of TF-B with stalled, full-length chains of the I27 domain of Titin, which is of similar size as S7 but contains two continuous hydrophobic segments that mediate high affinity TF binding (16). The relative changes in TF-B fluorescence were corrected for the amounts of the radiolabeled proteins (Fig. 7B). In contrast to the interaction with S7, in the case of Titin the affinity of TF-B for the ribosome was strongly reduced when the ribosome-bound Titin domain was allowed to fold by attaching a C-terminal 50-amino acid linker (Titin FL+50) (Fig. 7B) (16). Incubation of S7 FL+50 nascent chains with proteinase K resulted in a PK-resistant fragment of ∼17 kDa (Fig. 7C). This fragment was similar to the protease-resistant fragment of full-length S7 released from the ribosome, S7 FL (TAG) (Fig. 7C), indicating that S7 efficiently attained a protease-resistant structure when the complete domain has emerged from the ribosome. The same result was obtained in the presence of TF, demonstrating that TF binding did not interfere with the formation of this folded domain (Fig. 7C). Dissociation of TF-B from S7 FL and S7 FL+50 nascent chains occurred with t½ values of 11.7 ± 1 and 11.7 ± 2.2 s, respectively. Excess TF FRK-AAA did not displace TF-B from the S7 chains, indicating that binding to S7 was dependent on prior interaction of TF with the ribosome (data not shown). We conclude that TF initially binds to S7 when it is not yet folded, but retains a similar affinity for folded S7, consistent with the findings by Martinez-Hackert and Hendrickson (33).
DISCUSSION
The interaction of TF with ribosomes has previously been analyzed in the presence and absence of nascent chains (16, 31, 32). In the present study, we have investigated the direct interaction of TF with nascent chains during translation using TF variants that were labeled with the environmentally sensitive fluorophore NBD. Our results revealed markedly different dynamics of TF interaction with nascent chain substrates compared to its interaction with the ribosome. Although the mean residence time of TF on the ribosome (t½ values ∼10–14 s) appeared to be largely independent of the properties of the elongating polypeptide, the residence time of TF with the nascent chains varied greatly, dependent on the exposure of appropriate hydrophobic motifs in the nascent chain for TF binding. TF dissociated from nascent chains exposing extensive hydrophobic motifs, such as the heterologous firefly luciferase, substantially more slowly (t½ values ∼100 s) than from the ribosome (t½ values ∼14 s), indicating that after dissociating from the translating ribosome TF remains associated with the elongating nascent chain, thereby making the ribosomal docking site available for the entry of additional TF molecules. This mechanism would result in a post-translational release of TF for proteins that are presumably more aggregation-prone.
Our analysis of several proteins that were predicted as weak or strong interactors demonstrated the significance of linear hydrophobic regions in determining the affinity for TF. Such segments can be transplanted to other proteins, resulting in TF binding. As shown for pOmpA, TF can also interact with the signal sequences of secretory proteins, at least in the absence of dedicated targeting factors. In contrast, TF lacks high affinity for the SA sequence of the inner membrane protein FtsQ, which is recognized by SRP. On the other hand, strong recruitment of TF was also observed with translating chains lacking linear hydrophobic motifs, suggesting that additional structural features, such as non-linear hydrophobic epitopes, play a role in governing the interaction of a specific protein with TF. Notably, TF also interacted with the small, hydrophilic ribosomal protein S7 throughout its synthesis and folding, consistent with the proposed role of TF in supporting ribosome assembly (33). Based on these findings, TF appears to be mechanistically more versatile than other nascent chain-binding chaperones, such as Hsp70.
Hydrophobicity as a Driving Force for TF-Nascent Chain Interaction
The exposure of hydrophobic regions by a nascent chain was previously shown to result in the increased recruitment of TF to ribosomes (16). By employing the environmentally sensitive fluorophore NBD, we have demonstrated in the present study that multiple sites in TF are in close proximity to hydrophobic nascent chain segments, with the measured increase in NBD fluorescence correlating with the degree of hydrophobicity and the number of these segments. Interestingly, the hydrophobic character of the nascent chain only moderately affected the residence time of TF on the ribosome, as measured with TF-B, but rather increased the on-rate for TF binding to the ribosome, presumably by providing additional interaction surfaces. Thus, whereas TF binding to the L23/L29 docking site on the ribosome is a prerequisite for nascent chain interaction, resulting in a conformational change in TF that facilitates nascent chain association (16, 19), the presence of hydrophobic elements in the nascent chain emerging from the ribosome appears to increase the avidity of TF for ribosome docking.
The hydrophobic character of the nascent chain was found to strongly modulate the residence time of TF on the nascent chain after TF release from the ribosome. The dissociation kinetics differed dependent on the location of the NBD probe within TF, with TF326-NBD (t½ values of 14 ± 2 and 102 ± 16 s) and TF376-NBD (t½ values of 8 ± 2 and 88 ± 23 s) having fast and slow phases of dissociation from long chains of luciferase (Fig. 3). In contrast, only the slow dissociation phase was observed with TF150-NBD (t½ value of ∼110 s) (Fig. 3D). The dissociation of TF326-NBD and TF376-NBD from nascent chains was chain length-dependent and only the fast rate component was observed with short chains of luciferase that expose less hydrophobic residues (Fig. 3E). This suggests that for short chains of moderate or low hydrophobicity, the binding of TF to the ribosome contributes significantly to the overall affinity for the ribosome-nascent chain complex, whereas TF binding to long, hydrophobic chains is governed by the hydrophobic chain character and the capacity of the elongating chain to bury hydrophobic residues. In light of the prolonged interaction of TF150-NBD with Luc nascent chains, it seems likely that engagement of the PPIase domain contributes to the slow dissociation of TF from nascent chains. This would explain the ability of TF to delay the de novo folding of luciferase relative to its translation upon bacterial expression, shifting the folding process from the co-translational mechanism in the eukaryotic system toward a post-translational mechanism (15, 49). Moreover, the slow dissociation of TF from long nascent chains implies that multiple TF molecules can interact with such nascent chains, with a new molecule entering at the ribosome docking site when a preceding TF molecule vacates the ribosome but maintains its hydrophobic interaction with the elongating chain.
TF was also recruited toward nascent chains of pOmpA exposing the secretory signal sequence as the only hydrophobic element outside the ribosome (Fig. 6, A and B). In contrast, the similarly hydrophobic SA sequence of the inner membrane protein FtsQ was not recognized by TF. Interaction of TF with signal sequences but not SA sequences may increase the differential specificity of SRP for the latter and may help to explain how SRP can efficiently select those sequences despite its low cellular concentration relative to TF. The structural features of the SA sequence of FtsQ that disfavor TF binding remain to be investigated. The α-helical propensity of the SA sequence or the proximity of positively charged residues may play a role.
Multiple Modes of TF Action
A systematic effort was made in this study to understand the dynamics of TF interaction with different ribosome-nascent chain complexes. Based on the presence of linear hydrophobic segments in protein sequences known to recruit TF (16), we attempted to classify the E. coli cytosolic proteome into predicted weak and strong interactors of TF. During translation of the predicted strong interactors, TF was recruited to the ribosomes and interacted with the nascent chains with high affinity (Fig. 4), consistent with the ability of TF to recognize and shield exposed hydrophobic regions. However, the correlation between predicted and experimentally determined binding affinities was weak. We attribute this to the variability in the intrinsic capacity of nascent chains to bury hydrophobic regions during elongation. The propensity to bury hydrophobic segments may also determine the mean residence time of TF on a specific nascent chain. For example, both AROE and DCP bound with high affinity to TF-NBD, reflecting the hydrophobic character of the interaction, but the t½ of dissociation varied from ∼10 to 30 s, respectively (Figs. 4 and 5). This can be explained in terms of the domain topology and folding properties of the two proteins. Although the C-terminal 35 amino acids of AROE are not required for the folding of the protein into a stable structure (40), thus allowing burial of hydrophobic surfaces prior to chain release from the ribosome, DCP has a discontinuous domain structure and can only fold after ribosome release (41). The observation of fast and slow phases during TF dissociation from luciferase nascent chains may be due to the presence of multiple hydrophobic regions on luciferase (supplemental Fig. S3).
Interestingly, TF was also recruited with high affinity to the nascent chains of several proteins lacking substantial continuous hydrophobic sequences. However, some of these sequences were of high average hydrophobicity, suggesting the involvement of discontinuous hydrophobic epitopes in TF binding. It seems plausible that nascent chains undergo collapse into compact structures that may transiently expose hydrophobic surfaces, rendering them aggregation-prone. For example, the protein HEMA was found to be highly aggregation-sensitive upon expression in a bacterial translation system in the absence of chaperones.4 The ability of TF to recognize such hydrophobic surface epitopes would suggest that TF does not have a defined peptide binding cleft like the Hsp70 chaperones, but rather utilizes flexible binding surfaces in its different domains for substrate interaction.
Although continuous and discontinuous hydrophobic features play a major role in mediating high affinity interactions of nascent chains with TF, our results also provide evidence that TF can interact with the nascent chains of certain highly hydrophilic proteins that lack hydrophobic sequences and are overall of very low hydrophobicity. An example of such a protein is the small ribosomal protein S7, which was recently crystallized as a folded protein in a complex with TF (33). Based on the use of TF-B as a reporter, nascent chains of S7 recruited TF to the ribosome with moderate but clearly detectable affinity. There was only very little signal with TF-NBD, suggesting that the interaction with the S7 nascent chains may not primarily be hydrophobic. Ribosome-bound S7 chains with a 50-amino acid C-terminal linker folded into a protease-resistant domain but maintained the association with TF. These findings suggest that TF may also recognize certain hydrophilic features on nascent chains. Interestingly, in the crystal structure of Thermophilus TF with S7 the net negatively charged tmTF makes multiple electrostatic interactions with the positively charged S7 (33).
Although almost all nascent chains tested showed some measurable affinity for TF, the E. coli proteome apparently also contains proteins that do not recruit TF during translation, as shown for the nucleotide exchange protein GRPE (Fig. 4). Another example of a non-interacting protein is the heterologous model protein α-synuclein. We note that even though TF may not interact directly, it may nevertheless bind to the ribosomes translating these chains, thus potentially shielding them against degradation during synthesis and possibly folding (18, 24). In summary, our results indicate multiple modes of action for TF, dependent on the structural properties of nascent chains. In light of this versatility it is surprising that TF deletion apparently does not cause specific defects as long as the Hsp70 chaperone, DnaK, is present in cells (13, 14).
Supplementary Material
Acknowledgments
We thank A. Scaia for technical assistance with protein purification. pKSM717 pOmpA encoding pOmpA under T7 promoter was a kind gift from Prof. Matthias Mueller, University of Freiburg, Germany.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5 and Tables S1–S3.
H. Taguchi, personal communication.
- TF
- Trigger factor
- RNCs
- ribosome nascent chain complexes
- Luc
- firefly luciferase
- BADAN
- 6-bromoacetyl-2-dimethylaminonaphthalene
- NBD
- (N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole
- SRP
- signal recognition particle
- FL Luc
- full-length luciferase
- DCP
- dipetidyl carboxypeptidase
- SA
- signal anchor
- pOmpA
- outer membrane protein A
- α-Syn
- α-synuclein
- PPIase
- peptidylprolyl cis-trans isomerase.
REFERENCES
- 1.Kramer G., Boehringer D., Ban N., Bukau B. (2009) Nat. Struct. Mol. Biol. 16, 589–597 [DOI] [PubMed] [Google Scholar]
- 2.Hartl F. U., Hayer-Hartl M. (2009) Nat. Struct. Mol. Biol. 16, 574–581 [DOI] [PubMed] [Google Scholar]
- 3.Hartl F. U. (1996) Nature 381, 571–579 [DOI] [PubMed] [Google Scholar]
- 4.Frydman J., Erdjument-Bromage H., Tempst P., Hartl F. U. (1999) Nat. Struct. Biol. 6, 697–705 [DOI] [PubMed] [Google Scholar]
- 5.Lu J., Deutsch C. (2005) Nat. Struct. Mol. Biol. 12, 1123–1129 [DOI] [PubMed] [Google Scholar]
- 6.Kramer G., Rauch T., Rist W., Vorderwülbecke S., Patzelt H., Schulze-Specking A., Ban N., Deuerling E., Bukau B. (2002) Nature 419, 171–174 [DOI] [PubMed] [Google Scholar]
- 7.Jong W. S., ten Hagen-Jongman C. M., Genevaux P., Brunner J., Oudega B., Luirink J. (2004) Eur. J. Biochem. 271, 4779–4787 [DOI] [PubMed] [Google Scholar]
- 8.Eisner G., Koch H. G., Beck K., Brunner J., Muller M. (2003) J. Cell. Biol. 163, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner G., Moser M., Schäfer U., Beck K., Müller M. (2006) J. Biol. Chem. 281, 7172–7179 [DOI] [PubMed] [Google Scholar]
- 10.Ullers R. S., Houben E. N., Raine A., ten Hagen-Jongman C. M., Ehrenberg M., Brunner J., Oudega B., Harms N., Luirink J. (2003) J. Cell. Biol. 161, 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullers R. S., Houben E. N., Brunner J., Oudega B., Harms N., Luirink J. (2006) J. Biol. Chem. 281, 13999–14005 [DOI] [PubMed] [Google Scholar]
- 12.Valent Q. A., Kendall D. A., High S., Kusters R., Oudega B., Luirink J. (1995) EMBO J. 14, 5494–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deuerling E., Schulze-Specking A., Tomoyasu T., Mogk A., Bukau B. (1999) Nature 400, 693–696 [DOI] [PubMed] [Google Scholar]
- 14.Teter S. A., Houry W. A., Ang D., Tradler T., Rockabrand D., Fischer G., Blum P., Georgopoulos C., Hartl F. U. (1999) Cell 97, 755–765 [DOI] [PubMed] [Google Scholar]
- 15.Agashe V. R., Guha S., Chang H. C., Genevaux P., Hayer-Hartl M., Stemp M., Georgopoulos C., Hartl F. U., Barral J. M. (2004) Cell 117, 199–209 [DOI] [PubMed] [Google Scholar]
- 16.Kaiser C. M., Chang H. C., Agashe V. R., Lakshmipathy S. K., Etchells S. A., Hayer-Hartl M., Hartl F. U., Barral J. M. (2006) Nature 444, 455–460 [DOI] [PubMed] [Google Scholar]
- 17.Lakshmipathy S. K., Tomic S., Kaiser C. M., Chang H. C., Genevaux P., Georgopoulos C., Barral J. M., Johnson A. E., Hartl F. U., Etchells S. A. (2007) J. Biol. Chem. 282, 12186–12193 [DOI] [PubMed] [Google Scholar]
- 18.Ferbitz L., Maier T., Patzelt H., Bukau B., Deuerling E., Ban N. (2004) Nature 431, 590–596 [DOI] [PubMed] [Google Scholar]
- 19.Baram D., Pyetan E., Sittner A., Auerbach-Nevo T., Bashan A., Yonath A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlünzen F., Wilson D. N., Tian P., Harms J. M., McInnes S. J., Hansen H. A., Albrecht R., Buerger J., Wilbanks S. M., Fucini P. (2005) Structure 13, 1685–1694 [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Gahmen U., Aono S., Chen S., Yokota H., Kim R., Kim S. H. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 1343–1347 [DOI] [PubMed] [Google Scholar]
- 22.Maier T., Ferbitz L., Deuerling E., Ban N. (2005) Curr. Opin. Struct. Biol. 15, 204–212 [DOI] [PubMed] [Google Scholar]
- 23.Bitto E., McKay D. B. (2002) Structure 10, 1489–1498 [DOI] [PubMed] [Google Scholar]
- 24.Merz F., Boehringer D., Schaffitzel C., Preissler S., Hoffmann A., Maier T., Rutkowska A., Lozza J., Ban N., Bukau B., Deuerling E. (2008) EMBO J. 27, 1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Fan D. J., Li S. X., Zhang H. J., Perrett S., Zhou J. M. (2007) Protein Sci. 16, 1165–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholz C., Stoller G., Zarnt T., Fischer G., Schmid F. X. (1997) EMBO J. 16, 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakob R. P., Zoldák G., Aumüller T., Schmid F. X. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20282–20287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merz F., Hoffmann A., Rutkowska A., Zachmann-Brand B., Bukau B., Deuerling E. (2006) J. Biol. Chem. 281, 31963–31971 [DOI] [PubMed] [Google Scholar]
- 29.Genevaux P., Keppel F., Schwager F., Langendijk-Genevaux P. S., Hartl F. U., Georgopoulos C. (2004) EMBO Rep. 5, 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer G., Rutkowska A., Wegrzyn R. D., Patzelt H., Kurz T. A., Merz F., Rauch T., Vorderwülbecke S., Deuerling E., Bukau B. (2004) J. Bacteriol. 186, 3777–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutkowska A., Mayer M. P., Hoffmann A., Merz F., Zachmann-Brand B., Schaffitzel C., Ban N., Deuerling E., Bukau B. (2008) J. Biol. Chem. 283, 4124–4132 [DOI] [PubMed] [Google Scholar]
- 32.Maier R., Eckert B., Scholz C., Lilie H., Schmid F. X. (2003) J. Mol. Biol. 326, 585–592 [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Hackert E., Hendrickson W. A. (2009) Cell 138, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomic S., Johnson A. E., Hartl F. U., Etchells S. A. (2006) FEBS Lett. 580, 72–76 [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y., Inoue A., Tomari Y., Suzuki T., Yokogawa T., Nishikawa K., Ueda T. (2001) Nat. Biotechnol. 19, 751–755 [DOI] [PubMed] [Google Scholar]
- 36.Roseman M. A. (1988) J. Mol. Biol. 200, 513–522 [DOI] [PubMed] [Google Scholar]
- 37.Flanagan J. J., Chen J. C., Miao Y., Shao Y., Lin J., Bock P. E., Johnson A. E. (2003) J. Biol. Chem. 278, 18628–18637 [DOI] [PubMed] [Google Scholar]
- 38.Malkin L. I., Rich A. (1967) J. Mol. Biol. 26, 329–346 [DOI] [PubMed] [Google Scholar]
- 39.Kyte J., Doolittle R. F. (1982) J. Mol. Biol. 157, 105–132 [DOI] [PubMed] [Google Scholar]
- 40.Michel G., Roszak A. W., Sauvé V., Maclean J., Matte A., Coggins J. R., Cygler M., Lapthorn A. J. (2003) J. Biol. Chem. 278, 19463–19472 [DOI] [PubMed] [Google Scholar]
- 41.Comellas-Bigler M., Lang R., Bode W., Maskos K. (2005) J. Mol. Biol. 349, 99–112 [DOI] [PubMed] [Google Scholar]
- 42.Luirink J., Sinning I. (2004) Biochim. Biophys. Acta 1694, 17–35 [DOI] [PubMed] [Google Scholar]
- 43.Crooke E., Wickner W. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 5216–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lill R., Crooke E., Guthrie B., Wickner W. (1988) Cell 54, 1013–1018 [DOI] [PubMed] [Google Scholar]
- 45.Karamyshev A. L., Johnson A. E. (2005) J. Biol. Chem. 280, 37930–37940 [DOI] [PubMed] [Google Scholar]
- 46.Buskiewicz I., Deuerling E., Gu S. Q., Jöckel J., Rodnina M. V., Bukau B., Wintermeyer W. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7902–7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raine A., Ivanova N., Wikberg J. E., Ehrenberg M. (2004) Biochimie 86, 495–500 [DOI] [PubMed] [Google Scholar]
- 48.Halic M., Blau M., Becker T., Mielke T., Pool M. R., Wild K., Sinning I., Beckmann R. (2006) Nature 444, 507–511 [DOI] [PubMed] [Google Scholar]
- 49.Netzer W. J., Hartl F. U. (1997) Nature 388, 343–349 [DOI] [PubMed] [Google Scholar]
- 50.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 51.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.