Abstract
Unlike neurons in the central nervous system (CNS), injured neurons in the peripheral nervous system (PNS) can regenerate their axons and reinnervate their targets. However, functional recovery in the PNS often remains suboptimal, especially in cases of severe damage. The lack of regenerative ability of CNS neurons has been linked to down-regulation of the mTOR (mammalian target of rapamycin) pathway. We report here that PNS dorsal root ganglial neurons (DRGs) activate mTOR following damage and that this activity enhances axonal growth capacity. Furthermore, genetic up-regulation of mTOR activity by deletion of tuberous sclerosis complex 2 (TSC2) in DRGs is sufficient to enhance axonal growth capacity in vitro and in vivo. We further show that mTOR activity is linked to the expression of GAP-43, a crucial component of axonal outgrowth. However, although TSC2 deletion in DRGs facilitates axonal regrowth, it leads to defects in target innervation. Thus, whereas manipulation of mTOR activity could provide new strategies to stimulate nerve regeneration in the PNS, fine control of mTOR activity is required for proper target innervation.
Keywords: Axon, Neurobiology, Neurodevelopment, TOR Complex (TORC), Translation Regulation, DRG Neuron, TSC2, Axon Regeneration, Gap43, mTOR
Introduction
Whereas neurons in the central nervous system (CNS) have limited capacity for regrowth after damage, neurons in the peripheral nervous system (PNS)3 have a robust ability to regenerate their axons following injury. However, functional recovery of injured peripheral nerves often remains suboptimal, especially in cases of damage to a significant length of a peripheral nerve. Successful regeneration depends upon both extrinsic cues in the environment and the activation of intrinsic mechanisms to promote regrowth. The glial environment of the adult CNS includes inhibitory factors that prevent axon regrowth (1–3). Components of the glial scar, which forms after CNS injury, act as additional barriers to axon regeneration (4). Furthermore, CNS neurons display a decreased intrinsic capacity to regenerate, as removal of extracellular inhibitory cues is not sufficient to promote successful regeneration (5–7).
Injured peripheral neurons benefit from the absence of inhibitory signals in their environment and, in addition, activate intracellular signaling pathways that enable axonal regrowth. The “conditioning injury” paradigm observed in dorsal root ganglial neurons (DRGs) provides evidence for the existence of such intracellular signaling pathways induced by injury, which enhance axonal growth capacity (8–10). Injury to the sciatic nerve several days prior to dissection allows affected DRGs to extend more elongated, rapidly growing axons in culture compared with DRGs not subjected to a conditioning injury (9). This suggests that the robust response of peripheral axons to injury is not merely a “default” state, but results from activation of injury signals to increase axonal growth capacity.
Injury signals elicited both locally at the injury site and in DRG cell bodies increase the intrinsic growth capacity (11). Members of the MAPK family including JNK (12, 13), protein kinase G (14), and Erk1/Erk2 (15) (16) are activated in injured axons and retrogradely transported to the cell body, where they activate downstream effectors required for regeneration. In DRG cell bodies, transcription factors including c-Jun (17, 18), cAMP-response element-binding protein (19), STAT3 (20), and ATF3 (21) are activated and initiate transcriptional changes that contribute to regeneration. Despite the growing number of molecules identified that play a role in regeneration, no single signaling pathway or transcription factor alone has been shown to be sufficient for complete regeneration in the PNS, suggesting that multiple pathways work in concert to maximize axonal growth capacity.
Recent evidence implicates the control of protein synthesis by the mTOR pathway in the ability of neurons to regenerate. In non-neuronal cells, the mTOR pathway plays a critical role in the regulation of cellular growth, proliferation, and survival during development via the regulation of protein synthesis (22, 23). Upstream regulators of mTOR include Akt and the TSC1-TSC2 complex, which sense the level of growth factors, nutrients, ATP, and reactive oxygen species to either inhibit or activate mTOR (24). Activation of mTOR leads to the downstream phosphorylation of S6 ribosomal protein and 4EBP-1 to initiate protein translation (24). The developmental decline in mTOR activity observed in retinal ganglion cells correlates with their decreased growth capacity (25). Moreover, axotomy to retinal ganglion cells leads to a further down-regulation of mTOR activity (25). Activation of mTOR by deletion of the upstream negative regulators PTEN (phosphatase and tensin homolog) or TSC1 promotes regeneration of retinal ganglion cells, suggesting that mTOR activity is sufficient to increase growth capacity in normally nonregenerating central neurons (25). Although inactivation of mTOR activity is associated with decreased growth ability in the CNS, it is not yet known whether this pathway is activated in the PNS after injury and whether it contributes to increased axonal growth capacity.
In this study, we report that the mTOR pathway is activated in DRG neurons after injury. Inhibition of mTOR activity by rapamycin partially blocks the conditioning injury effect in DRGs, suggesting that mTOR activity contributes to the enhancement of axonal growth capacity upon injury. Furthermore, deletion of TSC2, a negative regulator of mTOR activity, leads to increased basal level of mTOR activity, which is sufficient to mimic the conditioning injury effect and enhance regeneration in vitro and in vivo. We further report that mTOR activation increases the expression of the growth-associated protein GAP-43, suggesting that mTOR-dependent increased protein synthesis contributes to enhance regeneration of injured peripheral neurons.
Although peripheral nerves display remarkable regenerative abilities, functional recovery often remains suboptimal. The time required for a complete recovery generally depends on the distance the axons must regenerate to their targets. In cases of damage to a significant length of a peripheral nerve, recovery is slow and limited, even when nerve grafts are inserted surgically to provide a bridge to promote axon regeneration. Therefore, manipulation of mTOR activity could provide new strategies to stimulate nerve regeneration in the PNS. However, our results also suggest that constitutive mTOR activation by TSC2 deletion lead to major defects in target innervation and axon morphology. Thus, mTOR activity must be controlled carefully to allow for proper axon regeneration, targeting, and functional recovery.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The following antibodies were used: anti-tuberin/TSC2 (Santa Cruz Biotechnology, C-terminal), anti-phosphorylated S6 ribosomal protein (Cell Signaling, serine 240/244), anti-S6 ribosomal (Cell Signaling Technology), anti-α-tubulin (Sigma), SMI-31 and SMI-32 (Sternberger Monoclonals, Inc.), anti-β-actin (Sigma), anti-peripherin (Millipore), anti-caspase-3 (Millipore), anti-GAP-43 (Abcam), anti-Tau (Synaptic Systems), and anti-GAP-43 (Chemicon) when used with anti-Tau. For TUNEL staining, an in situ cell death detection kit (Roche Applied Science) was used.
Animals
For experiments involving wild-type animals, C57B6 6-to-9-month-old females from Harlan were used. Tsc2flox/flox animals were described previously (26) and AdvillinCre/Cre mice were used to drive expression of Cre in sensory neurons, based on the previous characterization of the advillin-human placenta alkaline phosphatase reporter mouse (27, 28). To generate Tsc2flox/flox;AdvillinCre/+ conditional knock-out mice, we crossed Tsc2flox/flox females to AdvillinCre/Cre males to generate Tsc2flox/+;AdvillinCre/+ animals. Then, Tsc2flox/flox;Advillin+/+ females were crossed to Tsc2flox/+;AdvillinCre/+ males to generate Tsc2flox/flox;AdvillinCre/+ conditional knock-out animals and Tsc2flox/flox;Advillin+/+ littermate control animals. Genotype was confirmed by tail PCR at weaning age. 4–8-Week-old animals and sex-matched littermate controls were used for all experiments.
Surgical Procedures and Drug Treatment
All surgical procedures were approved by the Washington University in St. Louis, School of Medicine Animal Studies Committee. Sciatic nerve injury experiments were performed as described previously (12). Briefly, the sciatic nerves of mice were ligated, axotomized, or crushed unilaterally at the midpoint, and mice were sacrificed at the indicated time after surgery. For biochemistry on DRG cell bodies, L4, L5, and L6 DRGs were dissected from both the injured side and the contralateral uninjured side for control. For biochemistry on the sciatic nerve, equal lengths (5 mm) of the proximal and distal parts were homogenized. DRGs and sciatic nerves were homogenized in lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin) with phosphatase inhibitor mixtures 1 and 2 (Invitrogen). Equal protein amounts were loaded and analyzed by SDS-PAGE and Western blot.
Rapamycin was delivered by intraperitoneal injection at 5 mg/kg body weight. Rapamycin was dissolved in 200 μl DMEM from a 10 mg/ml stock solution in dimethyl sulfoxide. An equivalent volume of dimethyl sulfoxide was dissolved into 200 μl DMEM for vehicle control. Intraperitoneal injection was performed 1 h before sciatic nerve ligation and was repeated 2 days following ligation. Animals were sacrificed, and DRGs and nerves were dissected 4 days following ligation.
Primary DRG Culture and Immunofluorescence
L4, L5, and L6 DRGs were dissected and dissociated in 0.7 mg/ml Liberase Blendzyme 3 (Roche Applied Science), 600 μg/ml DNase, 10 mg/ml BSA, in DMEM air (DMEM/F12 supplemented with 10 mm glucose and 1% penicillin/streptomycin) at 37 °C for 15 min, followed by trypsinization with 0.25% trypsin in DMEM air at 37 °C for 15 min. DRGs were then triturated and placed in culture medium (DMEM, 10% FBS, 1% penicillin/streptomycin) with 50 ng/ml NGF (Invitrogen) on poly-d-lysine-coated plates.
After 20–24 h, DRGs were fixed in 4% paraformaldehyde and 4% sucrose in PBS for 10 min. For immunofluorescence staining, cells were permeabilized and blocked in 10% goat serum, 0.1% Triton X-100 in PBS for 15 min. Staining was performed with the indicated primary antibodies for 30 min at room temperature and with Alexa-conjugated secondary antibodies for 20 min. Images were acquired with a Nikon Eclipse TE2000-E inverted microscope and analyzed using Nikon NIS Elements Advanced Research 2.30 Imaging software. To quantify levels of axonal outgrowth, we measured the distance between the cell body and the tip of the longest axon and annotated this measurement as the radial projection length. For each condition, radial projection lengths of all cells imaged were measured, and both means and histograms were used for comparison across conditions.
Immunohistochemistry
Crushed sciatic nerves were dissected and fixed for 1–2 h in 4% PFA in PBS and then cryoprotected overnight in 20% sucrose. Posterior hind paw skin sections were fixed in 15% picric acid, 2% paraformaldehyde in PBS for 3–4 h and cryoprotected in 30% sucrose for 48 h. Nerves and skin were embedded in Tissue-Tek O.C.T. and frozen in dry ice-cooled methanol. Serial 10-μm (nerve) or 30-μm (skin) cryostat sections were cut and mounted onto coated slides (Thermo Fisher Scientific). Sections were permeabilized and blocked with 10% goat serum, 0.1% Triton X-100 in PBS for 30 min. Primary staining with the indicated antibodies was performed in the blocking solution overnight at 4 °C. Staining with Alexa-conjugated secondary antibody was performed for 1 to 3 h. Sciatic nerve images were acquired with Nikon Eclipse TE2000-E inverted epifluorescence microscope and analyzed using Metamorph 6.2. Fluorescent images from hind paw skin sections were acquired using an Olympus 500 confocal microscope. Confocal images were acquired at 1-μm intervals, and all images from one 30-μm stack were compressed into one image using Metamorph 6.2.
RESULTS
The mTOR Pathway Is Activated in DRG Cell Bodies following Injury and Contributes to Enhance Axonal Growth Capacity after Injury
To test whether peripheral neurons up-regulate the mTOR pathway following injury, we induced sciatic nerve injury by ligation and then dissected and analyzed L4, L5, and L6 DRG cell bodies 1 to 4 days later. Phosphorylation of ribosomal S6 protein (S6), a downstream effector of mTOR, was used as a marker for mTOR activity. One day following sciatic nerve ligation, we observed an ∼2-fold increase in the level of phosphorylated S6 protein in DRG cell bodies from the ligated nerve compared with those from the contralateral unligated nerve (Fig. 1, A and B). By 4 days post-ligation, S6 phosphorylation levels returned to basal (Fig. 1B), revealing the transient nature of the mTOR pathway activation. We also observed increases in the total level of S6 protein (Fig. 1A), suggesting that the mTOR pathway might regulate the expression of S6 protein itself.
FIGURE 1.
The mTOR pathway contributes to enhance axonal growth capacity after peripheral nerve injury. Injury to the sciatic nerve of wild-type mice was induced by ligation, and S6 phosphorylation levels were assessed in L4, L5, and L6 DRG cell bodies at the indicated time points. A, time course of S6 phosphorylation in DRG cell bodies showed increased S6 phosphorylation 1 to 2 days following ligation. A representative Western blot is shown for each time point. Cytochrome c (cyt c)was used as loading control. B, quantification of fold change in S6 phosphorylation levels between DRG cell bodies from injured and uninjured nerves shows a 2-fold increase in S6 phosphorylation 1 day after ligation, which reached basal level 4 days following ligation. S6 phosphorylation levels were normalized to loading control. At least four mice were tested for each time point. Data are mean ± S.E. *, p < 0.05 (Student's t test). C, quantification of mean radial projection length of cultured DRGs from wild-type mice treated with rapamycin or dimethyl sulfoxide by intraperitoneal injection and then subjected to sciatic nerve ligation to provoke injury (see “Experimental Procedures” for details). L4, L5, and L6 DRGs were dissected and cultured 4 days later in the presence of NGF. DRGs cultured from injured sciatic nerves showed enhanced axonal outgrowth at 24 h in culture. This effect was blocked partially by rapamycin (n = 4 mice per condition). 100–350 neurons were analyzed per condition. Data are mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Student's t test). D, as in C, but a histogram of the distribution in radial projection lengths of injury-conditioned DRGs for one representative experiment. E, selected images of cultured DRGs from C. Images of injury-conditioned DRGs were selected from the far right tail of histograms shown in D. For a comprehensive set of images, see supplemental Fig. 1. Axons were stained with a SMI-31 antibody. Bar, 100 μm.
Activation of the mTOR pathway was shown to mediate in part the increased growth capacity of retinal ganglion cells (25). To determine whether increased mTOR activity after injury also contributes to the enhanced axonal growth capacity of DRGs, we used the conditioning injury paradigm, in which prior injury causes a transition in neuronal morphology (or “growth pattern”) from compact, branched arbors to elongated, more sparsely branched axons (9). We tested whether the presence of rapamycin, a potent inhibitor of mTOR activity during the conditioning injury, blocks the increase in axonal outgrowth. To measure the extent of axon outgrowth, we calculated the distance between the cell body and the tip of the longest axon (“radial projection length”). DRGs cultured from mice injected intraperitoneally with vehicle as a control showed the expected ∼2-fold increase in mean radial projection length upon a conditioning injury (Fig. 1, C–E, and supplemental Fig. 1). In contrast, intraperitoneal injection of rapamycin partially blocked the enhanced radial projection of axons upon a conditioning injury (Fig. 1, C–E, and supplemental Fig. 1). These results suggest that the mTOR pathway contributes at least in part to increase axonal growth capacity following injury.
Deletion of TSC2 in DRG Leads to Elevated mTOR Activity
To test whether mTOR activation is sufficient to increase axonal growth capacity, we genetically increased basal level of mTOR activity by deleting TSC2, a negative regulator of mTOR activity, in DRG. Tsc2flox/flox mice (26) were crossed to mice expressing Cre under the control of the advillin promoter, which is expressed almost exclusively in peripheral sensory neurons (27, 28). Tsc2flox/flox;AdvillinCre/+ mice are hereafter referred to as TSC2KO. Tsc2flox/flox;Advillin+/+ littermates are referred to as control. Deletion of TSC2 was confirmed by analyzing protein expression in DRG cell bodies and brain extracts by Western blot (Fig. 2A). As TSC2 is a negative regulator of mTOR activity, we predicted that TSC2 deletion would up-regulate mTOR activity. As expected, deletion of TSC2 in DRG led to enhanced basal level of S6 phosphorylation, specifically in DRG cell bodies (Fig. 2, A and B). We measured an ∼2-fold increase in basal S6 phosphorylation levels in DRG cell bodies isolated from TSC2KO mice compared with control littermates, a level similar to the increase in S6 phosphorylation levels we observed in DRG cell bodies 1 day after injury in wild-type mice (Fig. 2B).
FIGURE 2.
TSC2KO DRGs display enhanced axonal outgrowth in vitro. A, Western blot of DRG cell bodies and brain lysates from TSC2KO and control animals show that TSC2 protein levels are dramatically reduced in DRGs but not in brain. As expected, S6 phosphorylation level is increased in TSC2KO compared with control animals. A representative Western blot is shown. B, quantification of A (n = 5 mice per genotype). Data are mean ± S.E. **, p < 0.01 (Student's t test). C, quantification of mean radial projection length of naïve and injury-conditioned DRGs cultured from TSC2KO mice and controls in the presence of NGF (see “Experimental Procedures” for details). TSC2KO DRGs show enhanced axonal outgrowth in the absence of a conditioning injury. Injury to the sciatic nerve 4 days prior to dissociation does not further increase axonal outgrowth in TSC2KO DRGs (n = 3 mice per genotype). 60–370 neurons were analyzed per condition. Data are mean ± S.E. n.d., no statistically significant difference. *, p < 0.05; **, p < 0.01 (Student's t test). D, as in C, but a histogram of the distribution in radial projection lengths of cultured naive TSC2KO and control DRGs is shown for one representative experiment. E, selected images of cultured DRGs from C. Images of naive and injury-conditioned TSC2KO DRGs and injury-conditioned control DRGs were selected from the far right tail of their respective radial projection length histogram. A comprehensive set of images is shown in supplemental Fig. 2. Axons were stained with a SMI-31 antibody. F, there was no significant difference in radial projection length between DRGs cultured from wild-type;AdvillinCre/+ mice and those cultured from wild-type;Advillin+/+ mice (n = 3 mice per genotype). 100–200 neurons were measured per genotype. Data are mean ± S.E. n.d., no statistically significant difference (Student's t test). Bar, 100 μm.
DRGs Lacking TSC2 Display Enhanced Axonal Outgrowth in Vitro and in Vivo
To test whether increased mTOR activity is sufficient to increase axonal growth capacity in DRGs in the absence of a conditioning injury, we cultured DRGs from TSC2KO and control mice and assessed axonal outgrowth. TSC2KO DRGs grew more elongated, less arborized axons compared with those of control DRGs, mimicking the morphology of DRGs subjected to a conditioning injury (Fig. 2, C–E, and supplemental Fig. 2). The radial projection length of TSC2KO DRG axons was greater than that of control littermate and similar to that of control littermate DRGs subjected to a conditioning injury (Fig. 2, C–E, and supplemental Fig. 2). Conditioning injury to TSC2KO DRGs did not significantly increase radial projection length compared with the uninjured condition, suggesting that even without the presence of a conditioning injury, TSC2KO DRGs have reached their maximal growth capacity in this assay (Fig. 2, C–E, and supplemental Fig. 2). The presence of the Cre recombinase is not sufficient to enhance axonal growth capacity, as wild-type;AdvillinCre/+ mice did not show significant difference with wild-type;Advillin+/+ littermate controls (Fig. 2F).
To test whether TSC2 deletion facilitates axonal regeneration in vivo, we crushed the sciatic nerve in TSC2KO mice and control littermates and assessed the level of regeneration past the crush site 12 or 24 h later. To visualize regrowth of damaged axons, longitudinal sciatic nerve sections were stained for the growth-associated protein GAP-43. The reported concentration of GAP-43 in axonal growth cone (29) together with its strong expression in regenerating axons (30) makes it an ideal marker to track nerve regeneration in vivo (31). The length of GAP-43-expressing axons past the crush site was markedly increased in TSC2KO sciatic nerve compared with that of control littermates (Fig. 3, A and B). We quantified this increase by normalizing GAP-43 fluorescence intensity to that at the crush site to control for the increased GAP-43 expression level observed from TSC2KO DRGs (Fig. 4, B and C). We calculated a regeneration index by measuring the distance away from the crush site in which the average GAP-43 intensity is half that observed at the crush site. The regeneration index was significantly higher in TSC2KO sciatic nerves compared with control nerves for both the 12 and 24 h time points (Fig. 3C). Thus, this result suggests that enhanced mTOR activity is sufficient to facilitate axon regeneration in vivo.
FIGURE 3.
TSC2KO DRGs display enhanced regeneration in vivo. TSC2KO and control mice were subject to a sciatic nerve crush, and regeneration of crushed axons was assessed 12 or 24 h later. A, longitudinal section of sciatic nerve dissected 12 or 24 h after crush reveals increased length of GAP-43 positive axons past the crush site in TSC2KO mice compared with control mice for both time points. A dashed line indicates a crush site. B, average GAP-43 intensity at various distances distal to crush sites 12 and 24 h after crush reveal regenerating axons grew longer distances in TSC2KO mice compared with controls. GAP-43 intensity values were normalized to that at the crush site to control for the increased GAP-43 expression level observed from TSC2KO DRGs. Data are mean ± S.E (n = 3 mice per genotype). 3–5 longitudinal sections were analyzed per mouse for both time points. C, regeneration index was measured as the distance away from the crush site in which the average GAP-43 intensity is half that observed at the crush site. TSC2KO mice show a higher regeneration index compared with controls at both 12 and 24 h time points. *, p < 0.05; **, p < 0.01 (Student's t test). Bar, 200 μm.
FIGURE 4.
The mTOR pathway regulates GAP-43 expression. A, naïve TSC2KO DRGs show enhanced cell body and axonal GAP-43 expression in culture. Arrows indicate cell body, and arrowheads indicate axonal tips. B, injury to the sciatic nerve of TSC2KO and control mice was induced by axotomy and GAP-43 levels in nerve portions proximal and distal to the axotomy site and in contralateral noninjured sciatic nerves were analyzed by Western blot 24 h later. Control mice show an increase in GAP-43 expression in sciatic nerve proximal to the injury site. Both the basal level and injury-induced level of GAP-43 expression is enhanced in TSC2KO mice. GAP-43 levels were normalized to loading control (tubulin). Data are mean ± S.E. (n = 3 mice per genotype). *, p < 0.05 (Student's t test). C, representative Western blot from one experiment (one mouse per genotype) from B. D, as in B, but DRG cell bodies were analyzed. GAP-43 levels were increased in DRG cell bodies of TSC2KO mice compared with control. Axotomy did not increase GAP-43 levels in DRG cell bodies in either genotype (n = 3 mice per genotype). **, p < 0.01. (Student's t test). E, representative Western blot from one experiment (one mouse per genotype) from C. F, rapamycin blocks increase in GAP-43 expression after injury. Wild-type mice were injected intraperitoneally with rapamycin or dimethyl sulfoxide vehicle control, and then sciatic nerve injury was induced by ligation. Four days following ligation, unligated and ligated nerve distal and proximal to the ligation site were analyzed for GAP-43 expression. Data are mean ± S.E. (n = 3 mice per condition). *, p < 0.05; *, p < 0.01 (Student's t test). G, representative Western blot from one experiment from F. Bar, 100 μm. a.u., arbitrary units; high exp., high exposure; low exp., low exposure.
mTOR Activity Regulates GAP-43 Expression following Injury
GAP-43 is a crucial component of axonal outgrowth in developing and regenerating neurons (30, 32, 33). The enhanced growth capacity of neurons lacking TSC2 may thus result from an enhanced expression of GAP-43. To directly test whether mTOR activity regulates GAP-43 expression following injury, we analyzed GAP-43 levels in nerve portions proximal and distal to an axotomy site and in contralateral noninjured sciatic nerve by Western blot. 24 h after injury, the GAP-43 protein level increased in the sciatic nerve proximal to the injury site in both TSC2KO and control mice (Fig. 4, B and C). Accumulation of GAP-43 in the proximal nerve stump is consistent with its role in promoting axonal outgrowth. However, both the basal and injury-induced levels of GAP-43 in the sciatic nerve were significantly higher in TSC2KO mice compared with controls (Fig. 4, B and C). The basal level of GAP-43 expression also was enhanced in TSC2KO DRG cell bodies (Fig. 4, D and E), but injury did not increase GAP-43 levels in the DRG cell bodies of TSC2KO mice or controls. Cultured TSC2KO DRGs also showed a higher GAP-43 expression in both the cell bodies and the tip of growing axons (Fig. 4A).
To confirm that mTOR activity is required for the increase in GAP-43 expression after injury, we tested whether inhibition of mTOR by rapamycin reduces GAP-43 protein levels in injured and uninjured sciatic nerve. Intraperitoneal injection of rapamycin 1 h prior to injury partially blocked the increase in GAP-43 expression in the proximal injured nerve stump (Fig. 4, F and G). These results suggest that mTOR activity regulates GAP-43 expression in peripheral nerves following injury.
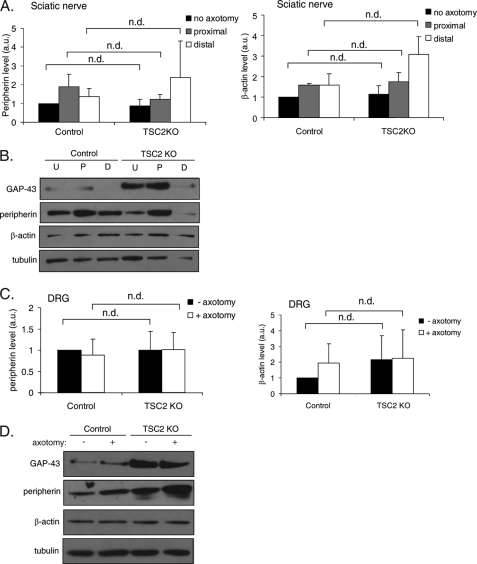
To determine whether the effect of TSC2 deletion on GAP-43 expression is specific to GAP-43 or is due to a global up-regulation of protein translation, we determined whether the levels of other proteins also are increased in TSC2KO DRGs. We examined the levels of peripherin and β-actin, whose translation after injury has been implicated in axon regeneration (16, 34). The basal level of peripherin and β-actin in naive sciatic nerve were not significantly different between control and TSC2KO mice (Fig. 5, A and B). Furthermore, the elevated level of these proteins in injured sciatic nerve also were unaffected by TSC2 deletion (Fig. 5, A and B). The expression levels of these proteins also were not significantly altered in DRG cell bodies of TSC2 KO mice compared with control (Fig. 5, C and D). Thus, mTOR may be regulating the expression level of a specific subset of proteins that include GAP-43.
FIGURE 5.
TSC2 deletion in DRGs does not affect peripherin or β-actin expression. A, injury to the sciatic nerve of TSC2KO and control mice was induced by axotomy. Peripherin and β-actin levels in nerve portions proximal and distal to the axotomy site and in contralateral noninjured sciatic nerve were analyzed by Western blot 24 h later. No significant difference in basal or injury-induced levels of peripherin or β-actin between TSC2KO and control mice was observed. Peripherin and β-actin levels were normalized to loading control (tubulin). Data are mean ± S.E. (n = 3 mice per genotype). n.d., no statistically significant difference. B, representative Western blot from one experiment (one mouse per genotype) from A. C, as in A, but DRG cell bodies were analyzed. Peripherin and β-actin levels in DRG were not significantly different between TSC2KO and control. Data are mean ± S.E. (n = 3 mice per genotype). D, representative Western blot from one experiment (one mouse per genotype) from C. U, no axotomy; P, proximal; D, distal; a.u., arbitrary units.
Deletion of TSC2 Leads to Abnormal Target Innervation and Axon Morphology in Vivo
Although genetic enhancement of mTOR activity can facilitate axon regeneration, persistent elevated levels of mTOR activity may cause deleterious effects on axon target innervation and morphology. To assess whether constitutive mTOR activity alters target innervation, we examined sensory nerve innervation of hindlimb glabrous foot pad skin in adult TSC2KO and control littermates. TSC2 deletion led to a significant loss of epidermal innervation (Fig. 6, A and B). We found no gross effects of TSC2 deletion on neuronal survival (supplemental Fig. 3) or the number of axons in the peripheral branch of L5 DRG (Fig. 6, E and F), suggesting that the loss of innervation is due to axon targeting defects rather than to degeneration or neuronal loss. Furthermore, of the sensory nerves that innervated the epidermis, a large proportion displayed excessive branching at the axon terminal (Fig. 6, A and C). Not only was the proportion of endings with excessive branching higher in TSC2KO mice, the absolute number of these endings was also greater (Fig. 6D). The presence of the Cre recombinase does not alter skin innvervation, as wild-type;AdvillinCre/+ mice did not show significant difference from wild-type;Advillin+/+ littermate controls. These results suggest that TSC2 deletion leads to abnormal target innervation and axonal branching.
FIGURE 6.
TSC2 deletion leads to abnormal target innervation and axon morphology. A, posterior hind paw glaborous skin sections were stained with α-GAP-43 to visualize axonal endings innervating skin. Red dashed lines indicate epidermal layer. Low magnification (15×) confocal images reveal fewer axons penetrating the epidermal layer in TSC2KO mice compared with control. High magnification (60×) confocal images reveal enrichment of endings with excessive branching (arrow) and sharp turning (arrowhead) compared with controls. The presence of Cre recombinase does not contribute to the innvervation defects, as wild-type;AdvillinCre/+ mice did not show significant difference from wild-type;Advillin+/+ littermates or control. Bar, 200 μm for 15× images, 50 μm for 60× images. B, quantification of innervation density reveals loss of skin innervation in TSC2KO mice. Data are mean ± S.E. (n = 3 mice per genotype). 17–56 sections were analyzed per mouse. Quantification of percentage (C) and number (D) of nerve endings with excessive branching (axons with more than two branches at tip or those that turned 90 degrees were counted). *, p < 0.05; n.d., no statistically significant difference (Student's t test). E, cross-section of the peripheral branch of L5 DRG stained with the axonal marker SMI-31 revealed no significant difference in axon number between TSC2 KO and controls. F, quantification of E. (n = 3 mice per genotype). Two sections analyzed per mouse. Bar, 100 μm (E).
DISCUSSION
The extent of axonal regeneration depends not only on the presence or absence of inhibitory cues in the environment but also on the intrinsic growth capacity of the damaged neurons. Research on nerve regeneration has focused largely on identifying the inhibitory molecules in the CNS environment that act as barriers to regeneration. Recent evidence suggests that in addition to differences in their environments, CNS and PNS neurons differ in their intrinsic ability to regrow their axons after injury.
Work in retinal ganglion cells has implicated the mTOR pathway in regulating the growth capacity of neurons. Axotomy to retinal ganglion cells markedly reduces mTOR activity (25), whereas genetic activation of mTOR activity by PTEN or TSC1 deletion is sufficient to boost the regenerative ability in these normally nonregenerating neurons (25). We found that in contrast to retinal ganglion cells, DRG neurons in the PNS activate the mTOR pathway in response to injury. Activation of the mTOR pathway in DRGs implies that the ability of PNS neurons to regenerate is not solely due to the lack of inhibitory molecules in the environment but also to an active intracellular mechanism that enhance growth capacity. Indeed, we find that rapamycin blocks the enhancement in axonal outgrowth following a conditioning injury, suggesting that mTOR activity contributes to boost the regenerative potential after damage. The observation that mTOR activation is transient and returns to basal level 3 to 4 days following injury suggests that prolonged mTOR activity may further enhance PNS regeneration.
Similar to what was observed in CNS neurons, we found that up-regulation of the mTOR pathway is sufficient to enhance axonal growth capacity in PNS neurons. Genetic ablation of TSC2, a negative regulator of mTOR activity, led to enhanced axonal outgrowth and regenerative capacity in DRGs. TSC2KO DRGs grew faster, more elongated, and sparsely branched axons in culture, similarly to wild-type DRGs subjected to a conditioning injury (9). Thus, increased mTOR activity is sufficient to enhance axonal growth potential even in the absence of a conditioning injury. We also observed that injured TSC2KO DRGs grew farther past the injury site in vivo compared with control DRGs. Based on our observation that TSC2 deletion primes DRGs to grow long axons in vitro in the absence of a conditioning injury, TSC2KO DRGs also may be primed to respond to injury in vivo and initiate axon regrowth earlier than control DRGs. Alternatively, TSC2 deletion may affect injury- induced retraction that occurs prior to axon elongation. Nevertheless, the observation that TSC2 deletion can facilitate the regrowth of crushed peripheral axons in vivo supports the notion that regeneration of injured axons in the PNS can be further enhanced. A more detailed analysis will determine the precise mechanisms by which TSC2 deletion regulates regenerative growth.
The major downstream targets of mTOR are components of translation machinery, including those that regulate the recruitment of ribosomes to mRNA. Protein synthesis plays a critical role in both injury signaling (16, 35–37) and the formation of new growth cones during regeneration (38). Several proteins translated in peripheral neurons after injury, including vimentin and importin β, have been shown to be critical for regeneration (16). However, whether their expression is regulated by mTOR activity has not been explored. Our present study identified GAP-43 as a downstream target of mTOR activity. TSC2KO neurons showed enhanced GAP-43 levels both in naïve and injury-induced conditions. In addition, rapamycin treatment partially blocked the increase in GAP-43 protein levels after injury, showing that the mTOR pathway regulates GAP-43 expression. As GAP-43 plays a key role in axon sprouting and outgrowth in regenerating axons (32, 33, 39, 40), it is likely that the enhancement of axon growth capacity by the mTOR pathway is at least in part due to the regulation of GAP-43 expression. However, overexpression of GAP-43 alone is not sufficient to fully stimulate regrowth of axons in the central branch of DRGs into the spinal cord (10). Thus, the mTOR pathway likely regulates the translation of a number of proteins in addition to GAP-43 to maximize the axonal growth capacity.
Our work indicates that PNS neurons turn on the mTOR pathway following injury, whereas CNS neurons do not (25). It will therefore be important in future studies to identify the upstream regulators of mTOR that are activated specifically by PNS injury to better understand the poor regenerative ability of CNS neurons. In non-neuronal cells, a number of upstream regulators of mTOR have been identified and include the serine/threonine protein kinase Akt and the TSC1-TSC2 complex. Growth factors, nutrients, and insulin, among other factors, activate PI3K, which leads to the phosphorylation and activation of Akt. Active Akt in turn phosphorylates and inhibits TSC2 activity leading to mTOR activation via the small GTPase Rheb (41–45). Surprisingly, we did not detect Akt phosphorylation in DRG cell bodies 24 h after injury (supplemental Fig. 4), a time point at which S6 already is phosphorylated (Fig. 1A). Furthermore, we found that TSC2KO DRGs, which have an increased basal level of S6 phosphorylation, are able to further increase S6 phosphorylation levels upon sciatic nerve injury even in the absence of TSC2 (supplemental Fig. 5). Thus, inactivation of TSC2 by Akt may not account fully for mTOR activation in DRG cell bodies after injury, and other pathways independent of TSC2 may converge to activate mTOR.
It is thus possible that injury-induced mTOR activation occurs via TSC2-independent pathways. One TSC2-independent mode of mTOR regulation occurs through phosphatidic acid (46). Phosphatidic acid production is mediated by phospholipase D1, which is activated by the small GTPase Cdc42 (47). Interestingly, Cdc42 mRNA is up-regulated in DRGs following axotomy, and Cdc42 overexpression induces enhanced axonal outgrowth (48). Another possible TSC2-independent regulator of mTOR activity is the JAK-STAT-SOCS3 signaling pathway. SOCS3 is a negative regulator of JAK-STAT signaling, which suppresses regeneration in retinal ganglion cells (49). Genetic deletion of SOCS3 in retinal ganglion cells is sufficient to activate the mTOR pathway and promotes regeneration (49). In DRGs, injury induces activation of JAK-STAT signaling through the cytokine ciliary neurotrophic factor and its receptor gp130 (50), suggesting that CNTF-mediated JAK-STAT signaling may interact with the mTOR pathway after peripheral nerve injury.
Enhancement of axonal regeneration in both CNS and PNS neurons by activation of the mTOR pathway presents an exciting therapeutic target for facilitating recovery from nerve injury. However, it remains unclear whether up-regulation of mTOR activity can promote successful target reinnervation in addition to axonal regrowth. Recent evidence implicates the importance of TSC2-mTOR signaling in various developmental processes in the CNS, such as axonal targeting of retinal ganglion cells (51), growth cone dynamics during axon navigation (52, 53), and axon specification and neuronal polarity (54, 55). Our study suggests that TSC2-mTOR signaling also regulates axon targeting and branching in peripheral neurons, as we found that TSC2KO mice have marked defects in skin innervation and abnormal morphology of axonal endings. Although we cannot exclude the possibility that these abnormalities are due to mTOR-independent effectors of TSC2, it is likely that constitutive activation of mTOR contributes to axon innervation defects given the importance of protein synthesis and degradation in growth cone dynamics (56, 57, 58). Consequently, proper targeting of regenerating axons also may be affected by constitutive mTOR activation. Thus, while targeting the mTOR pathway to increase the speed and extent of recovery of both PNS and CNS neurons may represent an attractive clinical strategy, it will be important to control the duration and level of mTOR activity to allow for proper reinnervation of targets and functional recovery.
Supplementary Material
Acknowledgments
We thank Drs. Tim Holy and Vitaly Klyachko for comments on the manuscript and Drs. David Gutmann and Narendrakumar Ramanan for reagents. We are grateful to Tammy Kershner for technical assistance.
This work was supported in part by NINDS, National Institutes of Health Grant RO1NS060709 (to V. C.). This work was also supported by the Wings for Life Foundation (to F. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- PNS
- peripheral nervous system
- DRG
- dorsal root ganglial neurons
- TSC2
- tuberous sclerosis complex 2.
REFERENCES
- 1.Case L. C., Tessier-Lavigne M. (2005) Curr. Biol. 15, R749–753 [DOI] [PubMed] [Google Scholar]
- 2.Filbin M. T. (2003) Nat. Rev. Neurosci. 4, 703–713 [DOI] [PubMed] [Google Scholar]
- 3.Yiu G., He Z. (2006) Nat. Rev. Neurosci. 7, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Y., Tenney A. P., Busch S. A., Horn K. P., Cuascut F. X., Liu K., He Z., Silver J., Flanagan J. G. (2009) Science 326, 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng B., Ho C., Li S., Keirstead H., Steward O., Tessier-Lavigne M. (2003) Neuron 38, 213–224 [DOI] [PubMed] [Google Scholar]
- 6.Zheng B., Atwal J., Ho C., Case L., He X. L., Garcia K. C., Steward O., Tessier-Lavigne M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf C. J. (2003) Neuron 38, 153–156 [DOI] [PubMed] [Google Scholar]
- 8.Richardson P. M., Issa V. M. (1984) Nature 309, 791–793 [DOI] [PubMed] [Google Scholar]
- 9.Smith D. S., Skene J. H. (1997) J. Neurosci. 17, 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann S., Woolf C. J. (1999) Neuron 23, 83–91 [DOI] [PubMed] [Google Scholar]
- 11.Abe N., Cavalli V. (2008) Curr. Opin. Neurobiol. 18, 276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli V., Kujala P., Klumperman J., Goldstein L. S. (2005) J. Cell Biol. 168, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindwall C., Kanje M. (2005) Mol. Cell Neurosci. 29, 269–282 [DOI] [PubMed] [Google Scholar]
- 14.Sung Y. J., Chiu D. T., Ambron R. T. (2006) Neuroscience 141, 697–709 [DOI] [PubMed] [Google Scholar]
- 15.Hanz S., Perlson E., Willis D., Zheng J. Q., Massarwa R., Huerta J. J., Koltzenburg M., Kohler M., van-Minnen J., Twiss J. L., Fainzilber M. (2003) Neuron 40, 1095–1104 [DOI] [PubMed] [Google Scholar]
- 16.Perlson E., Hanz S., Ben-Yaakov K., Segal-Ruder Y., Seger R., Fainzilber M. (2005) Neuron 45, 715–726 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins R., Hunt S. P. (1991) Neurosci. Lett. 129, 107–110 [DOI] [PubMed] [Google Scholar]
- 18.Broude E., McAtee M., Kelley M. S., Bregman B. S. (1997) Exp. Neurol. 148, 367–377 [DOI] [PubMed] [Google Scholar]
- 19.White D. M., Walker S., Brenneman D. E., Gozes I. (2000) Brain Res. 868, 31–38 [DOI] [PubMed] [Google Scholar]
- 20.O'Brien J. J., Nathanson N. M. (2007) J. Neurochem. 103, 288–302 [DOI] [PubMed] [Google Scholar]
- 21.Tsujino H., Kondo E., Fukuoka T., Dai Y., Tokunaga A., Miki K., Yonenobu K., Ochi T., Noguchi K. (2000) Mol. Cell Neurosci. 15, 170–182 [DOI] [PubMed] [Google Scholar]
- 22.Martin D. E., Hall M. N. (2005) Curr. Opin. Cell Biol. 17, 158–166 [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Curr. Opin. Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 24.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 25.Park K. K., Liu K., Hu Y., Smith P. D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., He Z. (2008) Science 322, 963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez O., Way S., McKenna J., 3rd, Gambello M. J. (2007) Genesis 45, 101–106 [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa H., Abbott S., Han B. X., Qi Y., Wang F. (2007) J. Neurosci. 27, 14404–14414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X., Wang L., Hasegawa H., Amin P., Han B. X., Kaneko S., He Y., Wang F. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9424–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goslin K., Schreyer D. J., Skene J. H., Banker G. (1988) Nature 336, 672–674 [DOI] [PubMed] [Google Scholar]
- 30.Fu S. Y., Gordon T. (1997) Mol. Neurobiol. 14, 67–116 [DOI] [PubMed] [Google Scholar]
- 31.Ackermann P. W., Ahmed M., Kreicbergs A. (2002) J. Orthop. Res. 20, 849–856 [DOI] [PubMed] [Google Scholar]
- 32.Aigner L., Arber S., Kapfhammer J. P., Laux T., Schneider C., Botteri F., Brenner H. R., Caroni P. (1995) Cell 83, 269–278 [DOI] [PubMed] [Google Scholar]
- 33.Caroni P. (1997) Bioessays. 19, 767–775 [DOI] [PubMed] [Google Scholar]
- 34.Vogelaar C. F., Gervasi N. M., Gumy L. F., Story D. J., Raha-Chowdhury R., Leung K. M., Holt C. E., Fawcett J. W. (2009) Mol. Cell Neurosci. 42, 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis D. E., Twiss J. L. (2006) Current opinion in neurobiology 16, 111–118 [DOI] [PubMed] [Google Scholar]
- 36.Wang W., van Niekerk E., Willis D. E., Twiss J. L. (2007) Dev. Neurobiol. 67, 1166–1182 [DOI] [PubMed] [Google Scholar]
- 37.Michaelevski I., Medzihradszky K. F., Lynn A., Burlingame A. L., Fainzilber M. (2010) Mol. Cell Proteomics 9, 976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma P., Chierzi S., Codd A. M., Campbell D. S., Meyer R. L., Holt C. E., Fawcett J. W. (2005) J. Neurosci. 25, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aigner L., Caroni P. (1995) J. Cell Biol. 128, 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusik B. W., Hammond D. R., Udvadia A. J. (2010) Dev. Dyn. 239, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoki K., Li Y., Xu T., Guan K. L. (2003) Genes Dev. 17, 1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Gao X., Saucedo L. J., Ru B., Edgar B. A., Pan D. (2003) Nat. Cell Biol. 5, 578–581 [DOI] [PubMed] [Google Scholar]
- 43.Manning B. D., Tee A. R., Logsdon M. N., Blenis J., Cantley L. C. (2002) Mol. Cell 10, 151–162 [DOI] [PubMed] [Google Scholar]
- 44.Tee A. R., Fingar D. C., Manning B. D., Kwiatkowski D. J., Cantley L. C., Blenis J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13571–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 46.Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. (2001) Science 294, 1942–1945 [DOI] [PubMed] [Google Scholar]
- 47.Fang Y., Park I. H., Wu A. L., Du G., Huang P., Frohman M. A., Walker S. J., Brown H. A., Chen J. (2003) Curr. Biol. 13, 2037–2044 [DOI] [PubMed] [Google Scholar]
- 48.Tanabe K., Tachibana T., Yamashita T., Che Y. H., Yoneda Y., Ochi T., Tohyama M., Yoshikawa H., Kiyama H. (2000) J. Neurosci. 20, 4138–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith P. D., Sun F., Park K. K., Cai B., Wang C., Kuwako K., Martinez-Carrasco I., Connolly L., He Z. (2009) Neuron 64, 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwaiger F. W., Hager G., Schmitt A. B., Horvat A., Hager G., Streif R., Spitzer C., Gamal S., Breuer S., Brook G. A., Nacimiento W., Kreutzberg G. W. (2000) Eur. J. Neurosci. 12, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 51.Nie D., Di Nardo A., Han J. M., Baharanyi H., Kramvis I., Huynh T., Dabora S., Codeluppi S., Pandolfi P. P., Pasquale E. B., Sahin M. (2010) Nat. Neurosci. 13, 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu K. Y., Hengst U., Cox L. J., Macosko E. Z., Jeromin A., Urquhart E. R., Jaffrey S. R. (2005) Nature 436, 1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell D. S., Holt C. E. (2001) Neuron 32, 1013–1026 [DOI] [PubMed] [Google Scholar]
- 54.Choi Y. J., Di Nardo A., Kramvis I., Meikle L., Kwiatkowski D. J., Sahin M., He X. (2008) Genes Dev. 22, 2485–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wildonger J., Jan L. Y., Jan Y. N. (2008) Genes Dev. 22, 2447–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brittis P. A., Lu Q., Flanagan J. G. (2002) Cell 110, 223–235 [DOI] [PubMed] [Google Scholar]
- 57.Leung K. M., van Horck F. P., Lin A. C., Allison R., Standart N., Holt C. E. (2006) Nat. Neurosci. 9, 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao J., Sasaki Y., Wen Z., Bassell G. J., Zheng J. Q. (2006) Nat. Neurosci. 9, 1265–1273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.