Abstract
Glutamate, the major excitatory neurotransmitter in the brain, activates receptors coupled to membrane depolarization and Ca2+ influx that mediates functional responses of neurons including processes such as learning and memory. Here we show that reversible nuclear oxidative DNA damage occurs in cerebral cortical neurons in response to transient glutamate receptor activation using non-toxic physiological levels of glutamate. This DNA damage was prevented by intracellular Ca2+ chelation, the mitochondrial superoxide dismutase mimetic MnTMPyP (Mn-5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine chloride tetrakis(methochloride)), and blockade of the permeability transition pore. The repair of glutamate-induced DNA damage was associated with increased DNA repair activity and increased mRNA and protein levels of apurinic endonuclease 1 (APE1). APE1 knockdown induced accumulation of oxidative DNA damage after glutamate treatment, suggesting that APE1 is a key repair protein for glutamate-induced DNA damage. A cAMP-response element-binding protein (CREB) binding sequence is present in the Ape1 gene (encodes APE1 protein) promoter and treatment of neurons with a Ca2+/calmodulin-dependent kinase inhibitor (KN-93) blocked the ability of glutamate to induce CREB phosphorylation and APE1 expression. Selective depletion of CREB using RNA interference prevented glutamate-induced up-regulation of APE1. Thus, glutamate receptor stimulation triggers Ca2+- and mitochondrial reactive oxygen species-mediated DNA damage that is then rapidly repaired by a mechanism involving Ca2+-induced, CREB-mediated APE1 expression. Our findings reveal a previously unknown ability of neurons to efficiently repair oxidative DNA lesions after transient activation of glutamate receptors.
Keywords: Diseases/Aging, Diseases/Neurodegeneration, DNA/Damage, DNA/Repair, Neurobiology/Neuroscience, Neurotransmitters, Nucleic Acid
Introduction
Glutamate, the major excitatory neurotransmitter in the mammalian central nervous system (CNS), plays essential roles in learning, memory, and motor function (1). Activation of glutamate receptors result in Ca2+ influx through ionotropic N-methyl-d-aspartate (NMDA) receptors and voltage-gated Ca2+ channels. Ca2+ then activates kinases such as Ca2+/calmodulin-dependent kinases (CaMK) and mitogen-activated protein kinases, resulting in activation of transcription factors such as cAMP-response element-binding protein (CREB),4 which mediate long lasting changes in neuronal structure and function (2–5). Glutamate receptor activation also stimulates an increase in mitochondrial respiration (electron transport) to generate the ATP necessary to drive the activity of ion-motive ATPases that restore ion gradients across cellular membranes (6). Mitochondrial Ca2+ uptake and increased mitochondrial respiration can result in production of the damaging free radical superoxide (7, 8), as well as mitochondrial membrane permeability changes that trigger cell death, a process called excitotoxicity (9, 10).
Damage to DNA in neurons occurs early during excitotoxicity (11, 12) and may be a pivotal event in cell death because selective inhibition or knockdown of the DNA damage response proteins p53 (13, 14) and PARP-1 (15, 16) can prevent glutamate-induced neuronal death. Ca2+ and mitochondria-derived superoxide are believed to play key roles in glutamate-induced DNA damage and cell death because PARP-1 activation is mediated by Ca2+ and mitochondrial reactive oxygen species (17), and because mitochondrial Mn-SOD and exogenous antioxidants protect neurons against excitotoxicity (18–20). However, it is not known if oxidative lesions to nuclear DNA occur in response to non-pathological subtoxic levels of glutamate receptor activation, nor is it known if and how neurons might respond to such glutamate-induced DNA damage.
Base excision repair (BER) is the primary DNA repair pathway for removal of small base modifications such as alkylation, deamination, and oxidation (21, 22). This process occurs both in the nucleus and in mitochondria. By far most information on the molecular mechanisms of DNA damage and repair comes from studies of non-neuronal cells, and the extent of DNA damage and repair in neurons under physiological and pathological conditions is largely unknown (23). Several BER enzymes, including DNA polymerase β, DNA glycosylases, and apurinic/apyrimidinic endonuclease 1 (APE1) are expressed in brain cells (24–28), but their roles in repair of oxidative DNA lesions in neurons are relatively poorly characterized.
APE1 is a multifunctional protein that plays an essential role in the DNA BER pathway in response to oxidative DNA damage. APE1 is also a redox effector factor-1, a reductive activator that responds to several transcription factors including AP-1, p53, NF-κB, HIF-1α, and PAX5 (29–31). APE1 also interacts with the calcium-responsive element (nCaRE) and is a repressor of the parathyroid hormone gene promoter and its own promoter (32, 33). Earlier studies reported that APE1 mRNA and protein are induced under oxidative stress (32, 34, 35).
In the present study we show that transient activation of glutamate receptors in cerebral cortical neurons results in oxidative DNA damage that is efficiently repaired. Glutamate-induced DNA damage results from Ca2+-mediated mitochondrial superoxide production, and is repaired in a process involving CREB-mediated up-regulation of APE1 expression. Protein levels and activities of common oxidative lesion removing glycosylases and DNA polymerase β were not affected by glutamate. Our results suggest that APE1 is the key enzyme involved in this glutamate-induced DNA repair response.
EXPERIMENTAL PROCEDURES
Cerebral Cortical Cell Cultures and Experimental Treatments
Cultures of primary cortical neurons were prepared from 18-day-old embryos of Sprague-Dawley rats using methods described previously (36). Cells were plated in 60-mm diameter plastic dishes on a polyethyleneimine substrate in minimum essential medium with Earle's salts supplemented 10% with heat-activated fetal bovine serum and containing 1 mm l-glutamine, 1 mm pyruvate, 20 mm KCl, and 26 mm sodium bicarbonate (pH 7.2). Following cell attachment (3–4 h, post-plating), the plating medium was replaced with culture maintenance medium (Neurobasal medium containing B-27 supplements (Invitrogen), 1 mm HEPES, 2 mm l-glutamine, and 0.001% gentamycin sulfate). All experiments were performed in 8- to 10-day-old cultures in which >95% of the cells were neurons. Cells that had been in culture for 7–9 days were treated with 20 μm glutamate for 10 min at 37 °C, washed twice with fresh Neurobasal medium, incubated for various periods in culture maintenance medium, and then harvested for biochemical assays.
BAPTA-AM (1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester) (AnaSpec), cyclosporin A (CsA) (Sigma), MnTMPyP (manganese(III)-5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine chloride tetrakis (methochloride) (Sigma), and KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine) (Calbiochem) were prepared as concentrated stocks in Neurobasal medium. The cortical neurons were pretreated with BAPTA-AM for 30 min, or with CsA, MnTMPyP, and KN-93 for 1 h, prior to exposure to glutamate (BAPTA-AM, CsA, and MnTMPyP were also present in the post-glutamate incubation medium). KN-93 was eliminated with glutamate after treatment.
Alkali Single-cell Gel Electrophoresis (Comet Assay)
These methods were similar to those described previously (37). Cultures were washed with cold PBS to remove dead cells and debris. Cells (∼1 million per culture) were harvested in 1 ml of PBS and gently triturated. A 10-μl aliquot of the cells (∼10,000 cells) was mixed with 75 μl of pre-warmed 0.5% low melting point agarose and the cells were spread onto an agarose-coated glass slide. A coverslip was added to the slide and the slide was placed in an ice-chilled aluminum tray for 5 min. The coverslip was removed, another 75 μl of low melting point agarose was added, a coverslip was added, and the slide was placed in the chilled aluminum tray for 5 min. The coverslip was removed and the slide incubated in lysis buffer (2.5 m NaCl, 100 mm EDTA, 10 mm Tris, and 1% Triton X-100) for at least 4 h (or overnight). The slides were then washed (three 10 min washes) with neutralization buffer (0.4 m Tris, pH 7.4), followed by a 10-min incubation with REC buffer (10 mm HEPES-KOH, 100 mm KCl, 10 mm EDTA, 0.1 mg/ml BSA, pH 7.4). Each slide was then incubated with 8 units (in 100 μl volume) of formamidopyrimidine DNA glycosylase (FPG) (New England Biolabs) at 37 °C for 1 h. The FPG-treated slides were rinsed with Alkali buffer (300 mm NaOH and 1 mm EDTA; pH 12.1) for 20 min to denature DNA. Electrophoresis was then performed at 25 volts for 15 min, and the slides were dehydrated in 100% ethanol for 5 min, and then stained with ethidium bromide (10 ng/ml). Images of nuclei were acquired under epifluorescence illumination using a Zeiss Axiovert microscope and images were analyzed using Komet 5.5 software (Kinetic Imagine).
Measurement of Cellular ATP Levels
Cortical neurons were plated in 48-well plates with 200 μl of culture maintenance medium per well. The cellular ATP level was determined using a CellTiter-Glo luminescent kit (Promega) according to the manufacturer's protocol.
DNA Repair Enzyme Incision Activity Assays
The cultured neurons were rinsed with PBS, scraped into 1 ml of PBS, and pelleted by centrifugation. Cells were then either extracted immediately or stored at −80 °C for future use. Cells were extracted by re-suspending in buffer I (10 mm Tris-HCl, 200 mm KCl, pH 7.8) and adding an equal volume of buffer II (10 mm Tris-HCl, 600 mm KCl, 2 mm EDTA, 40% (v/v) glycerol, 0.2% (v/v) Nonidet P-40, 2 mm dithiothreitol (DTT) 0.5 mm phenylmethylsulfonyl fluoride (PMSF), and 1× protease inhibitor mixture (Roche Applied Science; pH 7.8)). The lysate was briefly sonicated to completely disrupt cell and nuclear membranes. A 16,000 × g centrifugation at 4 °C for 10 min was performed to remove cellular debris and DNA. The cell extract was dialyzed overnight with buffer III (25 mm HEPES-KOH, 100 mm KCl, 12 mm MgCl2, 1 mm EDTA 17% glycerol, 1 mm DTT, pH 8.0) at 4 °C. A brief centrifugation was employed to remove precipitation after dialysis. The amounts of total protein used for incision activity assays for each enzyme were as follows: 2.5 ng for APE1, 2 μg for UDG, 6 μg for OGG1 and NEIL1. For polymerase β gap filling we used 0.5 μg of total protein. The procedures for incision assays were described previously (27, 28).
Quantitative Real Time PCR
Approximately 1 million cells were lysed in 1 ml of TRIzolTM (Invitrogen), 300 μl of chloroform (Sigma) was added and the solution was vortexed for 30 s. The tube was centrifuged at 8,000 × g for 30 min at room temperature. The upper aqueous layer was transferred and mixed with an equal volume of 70% ethyl alcohol. A RNeasy purification (Qiagen) kit was used for further total RNA isolation according to the manufacturer's protocol. One microgram of total RNA as template, 1 μl of random primer (Invitrogen), and 1 μl of 10 mm dNTPs (Invitogen) were added to a PCR tube and nuclease-free water was added to a final volume of 10 μl. The tube was heated to 70 °C for 5 min, then spun briefly, and 10 μl of master mix (4 μl of 5× first strand buffer; 2 μl of 0.1 m DTT; 1 μl of SuperScript III (Invitrogen); 1 μl of RNaseOut (Invitrogen); and 2 μl of nuclease-free water) was added to the tube. The reverse transcription reaction was allowed to proceed for 90 min at 50 °C and was stopped by incubation for 15 min at 70 °C. The tube was briefly centrifuged after the reaction and the sample was stored at −20 °C for future use.
The primers and TaqMan® probes (for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and Ape1) and TaqMan Gene Expression Master Mix were purchased from Applied Biosystems. Two microliters of 20-fold diluted reverse transcription mixture (cDNA) was used as initial template for real-time PCR. One microliter of specific TaqMan probe and primer solution, 10 μl of Universal PCR Master Mix, and 7 μl of nuclease-free water were added up to a total volume of 20 μl. The real time PCR for each RNA sample were performed in triplicate. The default setting of the ABI 7900HT Fast Real-time PCR System was used for the PCR. The Gapdh result was used as the internal control for other genes.
Lentiviral shRNA Knockdown of CREB and APE1
The packaging plasmid (psPAX2) and envelope plasmid (pMD2.G) were obtained from Addgene. The shRNA plasmids of CREB1 (5′-CCGGCGTCTAATGAAGAACAGGGAACTCGAGTTCCCTGTTCTTCATTAGACGTTTTT-3′) and Apex1 (5′-CCGGCGGGTGATTGTGGCTGAATTTCTCGAGAAATTCAGCCACAATCACCCGTTTTTG-3′) were purchased from ThermoScientific Open Biosystems. The scrambled control shRNA (5′-CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG-3′) was obtained from Addgene. All shRNAs were incorporated into the pLKO.1 vector. HEK 293T cells were transfected with shRNA, packaging, and envelope plasmids simultaneously using FuGENE 6 (Roche Applied Science) to produce lentiviral particles. The 3-day in vitro rat cortical neurons were infected with lentivirus using procedures described in the Addgene plasmid 10878 protocol.
Immunoblots
Cultured neurons were extracted in RIPA buffer (150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1× protease inhibitor mixture (Roche) and 50 mm Tris; pH 8.0) and the total protein concentration of cell extracts was determined using a BCATM protein assay kit (Pierce). Thirty micrograms of total protein from each sample was applied for immunoblotting. Precast 12% SDS-polyacrylamine gels and PVDF membrane filter paper were purchased from Invitrogen. The washing buffer was 0.1% Tween 20 in Tris-buffered saline (20 mm Tris and 150 mm NaCl; pH 7.4) and the blocking buffer was 5% skim milk (Bio-Rad) in washing buffer. The dilution factors for the primary antibodies were: APE1 (Santa Cruz), 1:500; UDG (Santa Cruz), 1:200; and pCREB (Santa Cruz), 1:500. The secondary antibodies (Vector Laboratories) were diluted 1:10,000.
Statistics
Statistical comparisons were made using a two-tail Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). All values shown in graphs are the mean ± S.D. of determinations in at least 3 separate experiments.
RESULTS
Glutamate-induced Oxidative DNA Damage Is Repaired Efficiently in Cortical Neurons
Previous studies have shown that glutamate exerts dose-dependent effects on neurons in cell culture and in vivo, with low/transient doses promoting cell survival and modifying dendritic architecture and synaptic plasticity, whereas high/sustained doses cause cell death (3, 9, 38, 39). There is a large body of literature describing the consequences of toxic doses of glutamate, whereas much less is known about the effects of lower, physiological doses. In preliminary experiments we found that when rat cortical neurons were exposed to a relatively low concentration of glutamate (20 μm) for a 10-min period in culture, there was no significant cell death during the subsequent 24-h culture period (data not shown).
FPG is a DNA glycosylase with AP-lyase activity; it specifically recognizes and removes oxidized bases from DNA including 8-oxoguanine, 8-oxoadenine, formamidopyrimidine (FAPY)-guanine, FAPY-adenine, 5-hydroxycytosine, and 5-hydroxyuracil. Comet assays demonstrated significant nuclear DNA fragmentation in cortical neurons at 6 h, but not at 24 h after exposure to glutamate (Fig. 1, A and B). As a control, we assayed for another lesion, pyrimidine dimers, that are normally introduced by UV light and are detected by the T4 endo V glycosylase. As expected, neurons that had been exposed to glutamate did not exhibit pyrimidine dimers at 6 or 24 h as indicated by lack of a comet tail in samples treated with this glycosylase (Fig. 1, A and B). These results indicate that glutamate specifically produces oxidative lesions in the nuclear DNA. Because DNA repair enzyme activities require ATP, and excessive glutamate receptor activation can result in ATP depletion (40), we measured cellular ATP levels in neurons that had or had not been exposed to 20 μm glutamate. During the first 20–30 min of glutamate exposure the ATP level was reduced by ∼40%; ATP levels subsequently returned to baseline levels 6 h after exposure to glutamate suggesting that the neurons maintain ATP in amounts sufficient to support DNA repair enzyme activities (Fig. 1C).
FIGURE 1.
Glutamate induces reversible oxidative DNA damage in cerebral cortical neurons. A and B, cortical cultures were exposed to 20 μm glutamate for 10 min, then, after 6 or 24 h the cells were harvested and comet assays were performed in the presence of no enzyme, FPG, or T4 endonuclease. Panel A shows representative images of nuclear DNA. First row of panel A is the control group, without glutamate treatment, pre-treated with FPG or T4 endo. The second and third rows of panel A are 6 and 24 h after glutamate treatment, respectively. Panel B shows the results of quantitative measurements of DNA damage (Olive Tail Moment). C, cellular ATP levels at the indicated time points after a 10-min exposure to 20 μm glutamate; values for glutamate-treated neurons were normalized to the value for untreated control cultures. Values for treated cultures are expressed as a percentage of the value for untreated control cultures (100%) (mean ± S.D.; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Glutamate-induced DNA Damage Is Mediated by Ca2+ and Mitochondrial Superoxide Radicals
Ca2+ influx through NMDA receptors and voltage-gated Ca2+ channels mediates physiological responses to glutamate including cell survival and synaptic plasticity, but Ca2+ also mediates excitotoxic cellular damage (9). To determine whether Ca2+ is involved in glutamate-induced oxidative DNA damage, we treated neurons with the intracellular Ca2+ chelator BAPTA-AM, exposed them to glutamate, and then quantified the oxidative DNA damage. BAPTA-AM treatment completely prevented glutamate-induced oxidative DNA damage (Fig. 2, A and B). No DNA fragmentation was observed in glutamate-treated neurons when FPG was not applied in the comet assay (Fig. 2B), indicating that glutamate did not induce nonspecific endonuclease activity.
FIGURE 2.
Glutamate-induced DNA damage is mediated by Ca2+ and mitochondrial superoxide, and requires opening of the mitochondrial permeability transition pore. Cortical cultures were pretreated with vehicle, 10 μm BAPTA-AM, 10 μm MnTMPyP, or 5 μm CsA, and were then exposed to 20 μm glutamate for 10 min. At designated times after glutamate exposure the cells were harvested and comet assays were performed in the presence of no enzyme or FPG. A, images of nuclear DNA from cortical cells that had been exposed to the indicated treatments for 1, 6, or 24 h. B–D, results of quantitative measurements of DNA damage in neurons pretreated with BAPTA-AM (B), MnTMPyP (C), or CsA (D), exposed to glutamate for 10 min, and then incubated for the indicated time periods (in the continued presence of BAPTA-AM, MnTMPyP, or CsA) (mean ± S.D.; ***, p < 0.001).
Glutamate-induced Ca2+ influx can elicit several changes in mitochondria including membrane depolarization, superoxide production, and increased permeability of mitochondrial membranes (41–43). To determine whether mitochondrial superoxide anion radicals were involved in glutamate-induced DNA damage, we pretreated neurons with the mitochondrial superoxide dismutase mimetic agent MnTMPyP and then exposed them to 20 μm glutamate for 10 min. Oxidative DNA damage was reduced to about 50% at 1 and 6 h after exposure to glutamate in MnTMPyP-treated neurons compared with neurons not treated with MnTMPyP (Fig. 2C), suggesting a key role for mitochondrial superoxide in formation of the DNA damage. It was recently reported that bursts of mitochondrial superoxide generation are triggered by transient openings of mitochondrial permeability transition pores (PTP) in cardiac myocytes and primary cultured hippocampal neurons (44). We found that inhibition of the PTP with cyclosporin A completely prevented glutamate-induced DNA damage (Fig. 2D), consistent with a role for PTP-mediated superoxide production in DNA damage formation.
APE1 Expression and AP Site Incision Activity Are Selectively Increased in Response to Glutamate Receptor Activation
Although considerable progress has been made in identifying DNA repair enzymes and elucidating their roles in the repair process, mechanisms that regulate the expression and activity of repair enzymes are poorly understood. We therefore measured the DNA incision and gap filling (polymerase β) activities of BER enzymes in neurons stimulated with glutamate compared with unstimulated neurons. Because 8-oxoguanine DNA-glycosylase 1 (OGG1) and nei endonuclease VIII-like 1 (NEIL1) are two major glycosylases that incise oxidative damaged bases, these activities from whole cell lysate were determined in response to glutamate treatment. The activities of OGG1 and NEIL1 were not changed after glutamate treatment (Fig. 3). The enzymatic activities and protein levels of uracil DNA glycosylase (UDG) (supplemental Fig. S1) and polymerase β (supplemental Fig. S2) were also unaffected by glutamate, although there was a trend toward increased polymerase β activity and protein level. The protein levels of OGG1 (supplemental Fig. S3) and ligase III (supplemental Fig. S4) were examined by immunoblotting and were also not affected by glutamate. In contrast, the incision activity of APE1 was significantly increased within 1 h after stimulation with glutamate, and remained elevated through 6 h, and then returned to baseline by 24 h (Fig. 4A). These results suggested that APE1 is the only protein in the BER pathway up-regulated in response to glutamate-induced oxidative DNA lesions.
FIGURE 3.
Incision activity of OGG1 and NEIL1 are not significantly changed after glutamate treatment. The 8-oxoguanine and 5-hydroxycytosine lesions are mainly produced by oxidation and predominately removed by OGG1 and NEIL1, respectively. The results of biochemical and immunoblotting assays for incision activities of OGG1 (A) and NEIL1 (B) (upper panels; *, 32P-labeled 5′-end; the filled circle marks the 8-OxoG and 5-OHC lesion sites) were not affected by a 10-min pulse glutamate treatment. Values for treated cultures are expressed as a percentage of the value for untreated control cultures (100%).
FIGURE 4.
APE1 DNA incisional activity, and mRNA and protein levels, are elevated in cortical neurons in response to glutamate. Cortical cultures were exposed to glutamate for the indicated time periods and then harvested for analyses of APE1 DNA incision activity (A), APE1 protein levels (B), and APE1 mRNA levels (C). A, an example of an APE1 DNA incision assay (upper; *, 32P-labeled 5′-end; the filled circle marks the abasic site lesion) and measurements obtained using this assay (graph). B, an example of an APE1 protein immunoblot (upper) and the results of densitometric analysis of blots (graph). C, relative levels of APE1 mRNA as determined by quantitative RT-PCR analysis. Values for treated cultures are expressed as a percentage of the value for untreated control cultures (100%) (mean ± S.D.; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Evidence that Glutamate Induces APE1 Expression via a Pathway Involving Ca2+/Calmodulin-dependent Kinase and CREB
We next measured relative levels of APE1 protein and mRNA in control and glutamate-stimulated neurons and found that glutamate induced a statistically significant increase in the amount of APE1 protein (by 6 h) (Fig. 4B) and mRNA (within 10 min) after treatment (Fig. 4C), suggesting that glutamate may induce Ape1 gene expression at the transcriptional level. Glutamate-induced Ca2+ influx is known to activate CREB, a transcription factor that mediates cell survival (38, 41, 45) and synaptic plasticity (46, 47) responses to glutamate. Analysis of the nucleotide sequence of the promoter region upstream of the Ape1 gene revealed a CREB-binding consensus sequence (35, 48), consistent with the possibility that glutamate receptor activation induces CREB-mediated APE1 expression. Indeed, we found that levels of activated CREB (phospho-CREB) increased rapidly (within 15 min with a peak increase at 30 min) in neurons in response to glutamate receptor stimulation (Fig. 5A). Thus, CREB was activated many hours in advance of the glutamate-induced increase in APE1 levels (Fig. 4B). Because Ca2+/calmodulin-dependent protein kinases (CaMK) mediate CREB activation in response to glutamate receptor stimulation, we asked whether inhibition of CaMK would prevent glutamate-induced APE1 expression. To this end, we pretreated neurons with the CaMK inhibitor KN-93 and then exposed them to glutamate. Phosphorylation of CREB in response to glutamate was significantly inhibited starting from 15 min until 24 h in neurons pretreated with KN-93 (Fig. 5B). Moreover, glutamate failed to increase APE1 levels in neurons treated with KN-93; APE1 levels remained unchanged during the first 3 h of exposure to glutamate, then decreased by 6 h (Fig. 5C).
FIGURE 5.
Glutamate induces CREB phosphorylation in a CaMK-dependent manner. The phosphorylation of CREB was increased at 15 and 30 min after transient exposure to glutamate (A). Glutamate-induced CREB phosphorylation is inhibited in neurons pretreated with the CaMK inhibitor KN-93 (B). Glutamate-induced expression of APE1 is also inhibited by KN-93 (C). Values for treated cultures are expressed as a percentage of the value for untreated control cultures (100%) (mean ± S.D.; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
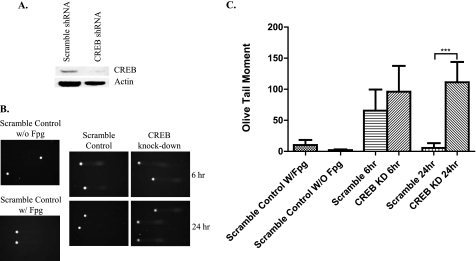
We also employed lentiviral shRNA to knockdown CREB (CREB KD) (Fig. 6A) and found that the phosphor-CREB and APE1 protein levels were very low in CREB KD neurons (data not shown). The results from the comet assay showed that the repair of oxidative DNA lesions was deficient in CREB KD neurons compared with control neurons expressing scrambled shRNA. 24 h after exposure to glutamate (Fig. 6, B and C) CREB KD clearly inhibited BER activity causing unrepaired damage and accumulation of lesions. These results suggested that CREB KD reduces BER activity via up-regulation of APE expression. We then knocked down APE1 (APE1 KD) by lentiviral shRNA (Fig. 7A) and examined the BER repair efficiency in glutamate pulse-treated neurons by the comet assay (Fig. 7B). Quantification of the comet tails showed that APE1 KD neurons accumulated oxidative DNA damage (Fig. 7C) after 24 h. The BER activity was, as expected, very low due to lack of APE1. The results suggest that glutamate regulates APE1 expression by activation of CaMK, which stimulates CREB-mediated expression of APE1.
FIGURE 6.
Depletion of CREB using RNA interference technology abolishes the ability of neurons to repair DNA damaged as a result of glutamate receptor activation. A, Western blot showing that CREB levels are greatly reduced in neurons expressing shRNA directed against the CREB mRNA compared with neurons expressing a scrambled control shRNA. B, images of nuclear DNA from neurons expressing CREB shRNA or scrambled control shRNA at 6 or 24 h after a 10-min exposure to 20 μm glutamate. C, results of quantitative measurements of DNA damage demonstrated that neurons expressing scrambled control shRNA recovered from damage, whereas oxidative lesions in neurons expressing CREB shRNA were not repaired within 24 h (mean ± S.D.; ***, p < 0.001).
FIGURE 7.
Depletion of APE1 using RNA interference technology results in accumulation of glutamate-induced oxidative DNA damage. A, Western blot showing that APE1 levels are greatly reduced in neurons expressing shRNA directed against the APE1 mRNA compared with neurons expressing a scrambled control shRNA. B, images of nuclear DNA from neurons expressing APE1 shRNA or scrambled control shRNA at 6 or 24 h after a 10-min exposure to 20 μm glutamate. C, results of quantitative analysis of DNA damage demonstrated that neurons expressing scrambled control shRNA were recovered from damage, whereas oxidative lesions in neurons expressing APE1 shRNA were not repaired within 24 h (mean ± S.D.; ***, p < 0.001).
DISCUSSION
Our findings reveal that nuclear DNA in neurons is oxidatively modified in response to transient moderate activation of glutamate receptors. This is a novel observation that links synaptic transmission to DNA damage and its processing. We detected an up-regulation of APE1 in response to glutamate receptor activation, which is likely involved in the repair of the oxidative DNA lesions. Extensive unrepaired nuclear DNA damage occurs in neurons during the process of excitotoxic cell death (11–16). In contrast, we found that a subtoxic transient glutamate exposure induces DNA damage evident for up to 6 h and is completely repaired by 24 h. We found that MnTMPyP prevents DNA damage caused by glutamate, suggesting that mitochondrial superoxide plays a pivotal role in the reversible DNA damage caused by physiological levels of glutamate receptor activation. The abilities of cyclosporin A and BAPTA-AM to prevent glutamate-induced DNA damage suggest a scenario in which glutamate induces Ca2+ influx resulting in opening of the mitochondrial membrane PTP and superoxide generation (Fig. 8). Previous studies have shown that elevated intracellular Ca2+ levels can induce mitochondrial superoxide production (41, 49) and that oxidative stress can trigger opening of the PTP (50). However, PTP opening can stimulate superoxide production in isolated mitochondria (51), and recent findings suggest that spontaneous bursts of mitochondrial superoxide occur in response to transient openings of mitochondrial PTP in excitable cells (44). Our findings are consistent with PTP-mediated superoxide production causing reversible DNA damage induced by transient activation of glutamate receptors.
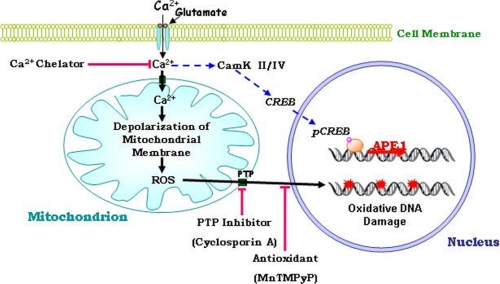
FIGURE 8.
Model for the mechanisms of glutamate-induced DNA damage and repair in neurons. Activation of glutamate receptors results in Ca2+ influx which, in turn, induces mitochondrial superoxide production and opening of PTP in the mitochondrial membranes. The superoxide or related reactive oxygen species then cause nuclear DNA damage. The DNA damage can be prevented by chelating intracellular Ca2+, by removing mitochondrial superoxide and blocking the PTP. Glutamate-induced Ca2+ influx also triggers the activation of CaMK and CREB resulting in increased expression of APE1 and repair of the damaged DNA.
The increased APE1 expression and DNA incision activity in response to glutamate receptor activation is, to our knowledge, the first evidence that a neurotransmitter can stimulate DNA repair. Although it increased the APE1 activity, glutamate did not affect the repair activity levels of UDG, OGG1, and NEIL1, and also did not significantly affect the gap-filling activity level of polymerase β. APE1 is the major eukaryotic apurinic/apyrimidinic endonuclease involved in the repair of oxidative DNA lesions (52). APE1 is also an essential BER enzyme that plays a key role in the DNA repair process in both nuclei and mitochondria. Studies of cancer cells have shown that APE1 expression is increased in response to oxidative stress (53, 54), but is decreased by the anti-apoptotic protein Bcl2 (55). It was recently shown that selective knockdown of APE1 expression increases the vulnerability of cultured neurons to oxidative stress (56). Our APE1 KD neurons accumulated significant oxidative DNA damage without efficient repair, which demonstrates that APE1 is essential for repair of oxidative DNA damage in neurons. It has been reported that when APE1 is oxidized by hydrogen peroxide and diamide, its activity is reduced (57). Thus, under conditions of oxidative stress, APE1 could become a rate-limiting protein for the BER pathway, which protects neurons against oxidative stress. Other repair enzymes, including XRCC1, polymerase β, and ligase III have also been suggested to play neuroprotective roles (58, 59).
Glutamate receptor-mediated Ca2+ influx regulates major structural and functional changes in neurons including dendrite outgrowth/retraction and synaptic plasticity (1, 3). We found that a transient moderate stimulation of the glutamate receptors induced Ca2+-mediated oxidative DNA damage. This damage was repaired in cortical neurons, suggesting the possibility that DNA damage occurs in response to physiological activation of glutamate receptors. Superoxide is generated during normal spontaneous glutamate-mediated synaptic activity and, indeed, data suggest that superoxide plays an important role in synaptic plasticity and in learning and memory (41, 60). Moreover, some evidence suggests a key role for mitochondrial PTP opening in synaptic plasticity (61). It is therefore possible that oxidative DNA damage occurs in response to the increased excitatory synaptic transmission involved in learning and memory processes. Our findings suggest that physiological levels of glutamate receptor activation can induce APE1 expression via a CaMK- and CREB-mediated pathway to elevate BER incision activity. It has been demonstrated that transcriptional activation of the Ape1 gene occurs in response to oxidative stress (34, 35). Another group reported that Ca2+ influx through NMDA receptors or calcium channels leads to multiple downstream signaling pathways, one of them involving the Ca2+-dependent phosphorylation of the transcription factor CREB (2). CaMKs mediate CREB phosphorylation to regulate downstream gene expression in hippocampal neurons (48, 49). Knockdown of CREB clearly affected BER efficiency and caused increased accumulation of nuclear oxidative DNA damage. This effect of CREB deficiency resulted from impaired up-regulation of APE1 expression. Taken together with our findings, the available information suggests the APE1 expression is regulated via a Ca2+-mediated CaMK-CREB signaling pathway.
Our findings suggest that normal amounts of glutamate receptor stimulation may elevate oxidative DNA repair capability in neurons thereby preventing the accumulation of potentially harmful or lethal amounts of DNA damage. Compromised DNA repair mechanisms could therefore render neurons vulnerable to glutamate receptor-mediated dysfunction and death. Indeed, patients with genetic defects in DNA repair often exhibit major neurodegenerative phenotypes (63) and DNA damage occurs in neurodegenerative disorders such as Alzheimer disease in which overactivation of glutamate receptors is implicated (15, 27, 62, 64). A better understanding of the signaling pathways that enhance DNA repair may therefore lead to novel approaches for protecting neurons against injury and neurodegenerative disorders.
Supplementary Material
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program, NIA.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- CREB
- cAMP-response element-binding protein
- BER
- base excision repair
- BAPTA-AM
- 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester
- CsA
- cyclosporin A
- MnTMPyP
- manganese(III)-5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine chloride tetrakis(methochloride)
- FPG
- formamidopyrimidine DNA glycosylase
- PTP
- permeability transition pore
- OGG1
- 8-oxoguanine DNA-glycosylase 1
- NEIL1
- nei endonuclease VIII-like 1
- UDG
- uracil DNA glycosylase
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- APE1
- apurinic endonuclease 1
- PARP-1
- poly(ADP-ribose) polymerase-1
- KD
- knockdown.
REFERENCES
- 1.Bliss T. V., Collingridge G. L. (1993) Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A., Ginty D. D., Bading H., Greenberg M. E. (1994) J. Neurobiol. 25, 294–303 [DOI] [PubMed] [Google Scholar]
- 3.Mattson M. P., Dou P., Kater S. B. (1988) J. Neurosci. 8, 2087–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soderling T. R., Tan S. E., McGlade-McCulloh E., Yamamoto H., Fukunaga K. (1994) J. Neurobiol. 25, 304–311 [DOI] [PubMed] [Google Scholar]
- 5.Wei F., Vadakkan K. I., Toyoda H., Wu L. J., Zhao M. G., Xu H., Shum F. W., Jia Y. H., Zhuo M. (2006) J. Neurosci. 26, 851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadava N., Nicholls D. G. (2007) J. Neurosci. 27, 7310–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinopoulos C., Tretter L., Rozsa A., Adam-Vizi V. (2000) J. Neurosci. 20, 2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengpiel B., Preis E., Krieglstein J., Prehn J. H. (1998) Eur. J. Neurosci. 10, 1903–1910 [DOI] [PubMed] [Google Scholar]
- 9.Mattson M. P. (2003) Neuromolecular. Med. 3, 65–94 [DOI] [PubMed] [Google Scholar]
- 10.Reynolds I. J. (1999) Ann. N.Y. Acad. Sci. 893, 33–41 [DOI] [PubMed] [Google Scholar]
- 11.Didier M., Bursztajn S., Adamec E., Passani L., Nixon R. A., Coyle J. T., Wei J. Y., Berman S. A. (1996) J. Neurosci. 16, 2238–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwag B. J., Koh J. Y., DeMaro J. A., Ying H. S., Jacquin M., Choi D. W. (1997) Neuroscience 77, 393–401 [DOI] [PubMed] [Google Scholar]
- 13.Culmsee C., Zhu X., Yu Q. S., Chan S. L., Camandola S., Guo Z., Greig N. H., Mattson M. P. (2001) J. Neurochem. 77, 220–228 [DOI] [PubMed] [Google Scholar]
- 14.Miller F. D., Pozniak C. D., Walsh G. S. (2000) Cell Death Differ. 7, 880–888 [DOI] [PubMed] [Google Scholar]
- 15.Kruman I. I., Culmsee C., Chan S. L., Kruman Y., Guo Z., Penix L., Mattson M. P. (2000) J. Neurosci. 20, 6920–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandir A. S., Poitras M. F., Berliner A. R., Herring W. J., Guastella D. B., Feldman A., Poirier G. G., Wang Z. Q., Dawson T. M., Dawson V. L. (2000) J. Neurosci. 20, 8005–8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Y., Gross R. A., Sheu S. S. (2007) J. Physiol 585, 741–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller J. N., Kindy M. S., Holtsberg F. W., St. Clair D. K., Yen H. C., Germeyer A., Steiner S. M., Bruce-Keller A. J., Hutchins J. B., Mattson M. P. (1998) J. Neurosci. 18, 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Copin J. C., Reola L. F., Calagui B., Gobbel G. T., Chen S. F., Sato S., Epstein C. J., Chan P. H. (1998) Brain Res. 814, 164–170 [DOI] [PubMed] [Google Scholar]
- 20.Uz T., Giusti P., Franceschini D., Kharlamov A., Manev H. (1996) Neuroscience 73, 631–636 [DOI] [PubMed] [Google Scholar]
- 21.Baute J., Depicker A. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 239–276 [DOI] [PubMed] [Google Scholar]
- 22.Wilson D. M., 3rd, Bohr V. A. (2007) DNA Repair 6, 544–559 [DOI] [PubMed] [Google Scholar]
- 23.Englander E. W. (2008) Mech. Ageing Dev. 129, 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imam S. Z., Karahalil B., Hogue B. A., Souza-Pinto N. C., Bohr V. A. (2006) Neurobiol. Aging 27, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 25.Raffoul J. J., Cabelof D. C., Nakamura J., Meira L. B., Friedberg E. C., Heydari A. R. (2004) J. Biol. Chem. 279, 18425–18433 [DOI] [PubMed] [Google Scholar]
- 26.Wei W., Englander E. W. (2008) J. Neurochem. 107, 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissman L., Jo D. G., Sørensen M. M., de Souza-Pinto N. C., Markesbery W. R., Mattson M. P., Bohr V. A. (2007) Nucleic Acids Res. 35, 5545–5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissman L., de Souza-Pinto N. C., Mattson M. P., Bohr V. A. (2008) Neurobiol. Aging 30, 2080–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans A. R., Limp-Foster M., Kelley M. R. (2000) Mutat. Res. 461, 83–108 [DOI] [PubMed] [Google Scholar]
- 30.Tell G., Damante G., Caldwell D., Kelley M. R. (2005) Antioxid. Redox Signal. 7, 367–384 [DOI] [PubMed] [Google Scholar]
- 31.Xanthoudakis S., Curran T. (1992) EMBO J. 11, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumi T., Henner W. D., Mitra S. (1996) Biochemistry 35, 14679–14683 [DOI] [PubMed] [Google Scholar]
- 33.Okazaki T., Chung U., Nishishita T., Ebisu S., Usuda S., Mishiro S., Xanthoudakis S., Igarashi T., Ogata E. (1994) J. Biol. Chem. 269, 27855–27862 [PubMed] [Google Scholar]
- 34.Grösch S., Fritz G., Kaina B. (1998) Cancer Res. 58, 4410–4416 [PubMed] [Google Scholar]
- 35.Grösch S., Kaina B. (1999) Biochem. Biophys. Res. Commun. 261, 859–863 [DOI] [PubMed] [Google Scholar]
- 36.Mattson M. P., Barger S. W., Begley J. G., Mark R. J. (1995) Methods Cell Biol. 46, 187–216 [DOI] [PubMed] [Google Scholar]
- 37.Morris E. J., Dreixler J. C., Cheng K. Y., Wilson P. M., Gin R. M., Geller H. M. (1999) BioTechniques 26, 282–289 [DOI] [PubMed] [Google Scholar]
- 38.Balazs R. (2006) Curr. Top. Med. Chem. 6, 961–968 [DOI] [PubMed] [Google Scholar]
- 39.Choi D. W. (1992) J. Neurobiol. 23, 1261–1276 [DOI] [PubMed] [Google Scholar]
- 40.Mattson M. P., Zhang Y., Bose S. (1993) Exp. Neurol. 121, 1–13 [DOI] [PubMed] [Google Scholar]
- 41.Hongpaisan J., Winters C. A., Andrews S. B. (2004) J. Neurosci. 24, 10878–10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lafon-Cazal M., Pietri S., Culcasi M., Bockaert J. (1993) Nature 364, 535–537 [DOI] [PubMed] [Google Scholar]
- 43.Mattson M. P., Gleichmann M., Cheng A. (2008) Neuron 60, 748–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Wang W., Mattson M. P., Kao J. P., Lakatta E. G., Sheu S. S., Ouyang K., Chen J., Dirksen R. T., Cheng H. (2008) Cell 134, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabuchi T., Kitagawa K., Kuwabara K., Takasawa K., Ohtsuki T., Xia Z., Storm D., Yanagihara T., Hori M., Matsumoto M. (2001) J. Neurosci. 21, 9204–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deisseroth K., Bito H., Tsien R. W. (1996) Neuron 16, 89–101 [DOI] [PubMed] [Google Scholar]
- 47.Leutgeb J. K., Frey J. U., Behnisch T. (2005) Neuroscience 131, 601–610 [DOI] [PubMed] [Google Scholar]
- 48.Bito H., Deisseroth K., Tsien R. W. (1996) Cell 87, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 49.Hongpaisan J., Winters C. A., Andrews S. B. (2003) Mol. Cell. Neurosci. 24, 1103–1115 [DOI] [PubMed] [Google Scholar]
- 50.Kim J. S., He L., Lemasters J. J. (2003) Biochem. Biophys. Res. Commun. 304, 463–470 [DOI] [PubMed] [Google Scholar]
- 51.Maciel E. N., Vercesi A. E., Castilho R. F. (2001) J. Neurochem. 79, 1237–1245 [DOI] [PubMed] [Google Scholar]
- 52.Tell G., Quadrifoglio F., Tiribelli C., Kelley M. R. (2008) Antioxid. Redox Signal. 11, 602–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianchi M., Bellini A., Cervelli M., Degan P., Marcocci L., Martini F., Scatteia M., Mariottini P., Amendola R. (2007) Biochim. Biophys. Acta 1773, 774–783 [DOI] [PubMed] [Google Scholar]
- 54.Yoo D. G., Song Y. J., Cho E. J., Lee S. K., Park J. B., Yu J. H., Lim S. P., Kim J. M., Jeon B. H. (2008) Lung Cancer 60, 277–284 [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Gao F., Zhang Y., Wei K., Liu Y., Deng X. (2008) J. Biol. Chem. 283, 9925–9932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasko M. R., Guo C., Kelley M. R. (2005) DNA Repair 4, 367–379 [DOI] [PubMed] [Google Scholar]
- 57.Bhakat K. K., Mantha A. K., Mitra S. (2008) Antioxid. Redox. Signal. 11, 621–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkarni A., McNeill D. R., Gleichmann M., Mattson M. P., Wilson D. M., 3rd (2008) Nucleic Acids Res. 36, 5111–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N., Wu H., Yang S., Chen D. (2007) DNA Repair 6, 1297–1306 [DOI] [PubMed] [Google Scholar]
- 60.Thiels E., Urban N. N., Gonzalez-Burgos G. R., Kanterewicz B. I., Barrionuevo G., Chu C. T., Oury T. D., Klann E. (2000) J. Neurosci. 20, 7631–7639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levy M., Faas G. C., Saggau P., Craigen W. J., Sweatt J. D. (2003) J. Biol. Chem. 278, 17727–17734 [DOI] [PubMed] [Google Scholar]
- 62.Culmsee C., Bondada S., Mattson M. P. (2001) Brain Res. Mol. Brain Res. 87, 257–262 [DOI] [PubMed] [Google Scholar]
- 63.Rass U., Ahel I., West S. C. (2007) Cell 130, 991–1004 [DOI] [PubMed] [Google Scholar]
- 64.Adamec E., Vonsattel J. P., Nixon R. A. (1999) Brain Res. 849, 67–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.