Abstract
Although lead has been associated with hearing loss in occupational settings and in children, little epidemiologic research has been conducted on the impact of cumulative lead exposure on age-related hearing loss in the general population. We determined whether bone lead levels, a marker of cumulative lead exposure, are associated with decreased hearing ability in 448 men from the Normative Aging Study, seen between 1962 and 1996 (2,264 total observations). Air conduction hearing thresholds were measured at 0.25 to 8 kHz and pure tone averages (PTA) (mean of 0.5, 1, 2 and 4 kHz) were computed. Tibia and patella lead levels were measured using K x-ray fluorescence between 1991 and 1996. In cross-sectional analyses, after adjusting for potential confounders including occupational noise, patella lead levels were significantly associated with poorer hearing thresholds at 2, 3, 4, 6 and 8 kHz and PTA. The odds of hearing loss significantly increased with patella lead levels. We also found significant positive associations between tibia lead and the rate change in hearing thresholds at 1, 2, and 8 kHz and PTA in longitudinal analyses. Our results suggest that chronic low-level lead exposure may be an important risk factor for age-related hearing loss and reduction of lead exposure could help prevent or delay development of age-related hearing loss.
Keywords: bone lead, general population, hearing loss, occupational noise
INTRODUCTION
Since lead was phased out of gasoline, environmental lead exposure has decreased considerably in the United States: geometric means of blood lead levels in adults declined from 13.1 µg/dL in the second National Health and Nutrition Examination Survey (NHANES-II, 1976–1980) to 1.64 µg/dL in NHANES 1999–2000 (Muntner et al., 2005; Pirkle et al., 1994). However, lead toxicity still has a significant public health concern in particular among older adults because they were already exposed to high levels of lead which accumulates in bone and stays there for decades (Hu et al., 2007). With advancing age, lead can be mobilized from bone into the circulation which may damage the central nervous and cardiovascular systems. A number of recent epidemiologic studies have demonstrated that cumulative lead exposure is associated with age-related diseases, such as cognitive decline, hypertension, age-related cataract, myocardial infarction and cardiovascular mortality (Hu et al., 1996; Jain et al., 2007; Schaumberg et al., 2004; Weisskopf et al., 2004; Weisskopf et al., 2009).
Lead exposure could also increase the risk of hearing loss, a leading chronic health condition experienced by older adults (Liu et al., 2007). Experimental studies suggest that lead exposure, even at low levels, can impair the inner ear receptor cells and the auditory neuronal function (Bertoni et al., 1988; Lasky et al., 1995; Yamamura et al., 1989). Epidemiologic studies of occupationally exposed workers have shown associations of lead exposure with hearing loss and impaired hearing ability (Chuang et al., 2007; Counter et al., 2002; Discalzi et al., 1993; Discalzi et al., 1992; Forst et al., 1997; Hwang et al., 2009; Wu et al., 2000). Studies of children have also demonstrated associations with hearing impairment (Schwartz, 1991; Schwartz et al., 1987). No study of lead and age-related hearing loss has been conducted in the general adult population, however, and it is not clear whether chronic, nonoccupational, low-level exposure to lead contributes to the development of hearing loss among older populations.
Hearing loss is one of the most profound common disabling conditions that older adults can suffer. It is estimated that more than 35 million among individuals aged 18 and older have hearing trouble in the U.S. in 2008 (age-adjusted prevalence 15.1%), and the prevalence of hearing loss increase dramatically with advancing age (27.8% in age 65–74 years; 42.7% in age 75 years and over) (Pleis et al., 2009). Hearing loss, by itself, lowers the quality of life, and for most hearing-impaired people the hearing loss has psychological, physical and social consequences (Gates et al., 2005).
In the present study, we investigated whether cumulative lead exposure, as measured in either cortical bone (tibia) or trabecular bone (patella) using K x-ray fluorescence (KXRF) between 1991 and 1996, was associated with elevations in hearing thresholds and higher prevalence of hearing loss in a community-based cohort of men, the Normative Aging Study. We also examined the relationship between tibia lead levels and the rate of change in hearing thresholds which had been measured between 1962 and 1996 with the median follow-up of 23 years. Because the bone lead measurements were conducted at the end of the follow-up time, the decades-long half-life of lead in cortical bone may be more relevant for exposure assessment than the shorter half-life of lead in trabecular bone (Hu, 1998; Hu et al., 2007). For this reason, we used lead measured in cortical bone (tibia lead) as the exposure variable in longitudinal analyses. The present study controlled for occupational noise exposure and audiometric notch, important potential confounding factors, using participants’ job titles and notch criteria (Coles et al., 2000).
METHODS
Study Population
The Normative Aging Study is a multidisciplinary longitudinal study of 2280 healthy, community-dwelling men aged 21 to 80 years in Eastern Massachusetts established by the Veterans Administration in the 1960s (Bell et al., 1966). The participants have undergone detailed evaluation every five years since their enrollment and every three years since 1984 including extensive physical examinations, laboratory tests and completion of questionnaires on smoking history and other factors that may influence health. Between 1962 and 1969, audiometric tests commenced and were repeated every 3–5 year through April 1996. Occupation was ascertained at all visits from the enrollment until 1980. The verbatim response regarding occupation was recorded and later coded into one of 45 possible categories.
Beginning in 1991, the active cohort members (n=1171) were invited to participate in a study of bone lead measurement; 801 (68%) gave informed consent and completed bone lead measurements. Of these, 539 men had audiometric test results within 5 years before the bone lead measurement and were considered the base population of the present study. 5 subjects who had an audiometric test 2–3 years after the bone lead measurements were included. We excluded 73 subjects with unilateral loss which was defined as pure-tone mean thresholds at 0.5, 1.0, 2.0, and 4.0 kHz that differed between ears by more than 10 decibel hearing level (dB HL); 3 subjects who had tibia and patella bone lead measurements with estimated uncertainties greater than 10 and 15 µg/g of bone, respectively, indicators of unreliable measurement (Hu, 1998); 15 who had missing values in the potential confounding factors. Hence, 448 participants were included in the cross-sectional data analyses. Those 448 men had repeated audiometric test measurements since 1962, resulting in a total of 2420 observations. We excluded 107 observations with unilateral loss at any visit and 49 observations with missing data in the potential confounders, and thus, 2264 observations were available for the longitudinal data analyses. Most subjects (94%) had at least 4 repeated audiometric data (median=5 and maximum=6) and the follow-up time ranged from 13 to 32 years with the median follow-up time of 23 years. All participants had given written informed consent. This study was reviewed and approved by the institutional review boards of all participating institutions.
Hearing Threshold Examination
Pure-tone audiometric examinations with the modified Hughson-Westlake procedure were obtained under standard conditions in a double-walled sound-proof chamber by audiologists. Air conduction hearing thresholds in decibels were measured for each ear at the following frequencies (kHz) 0.25, 0.5, 1, 2, 3, 4, 6 and 8, using either a Beltone 15C (Beltone Electronics Corp., Chicago, IL) or a Grason-Stadler 1701 (Grason-Stadler, Inc., Milford, NH) audiometer, both calibrated to the 1964 ISO 389 standard reference zero and followed standard clinical audiometric procedures. We used the better (lower) hearing thresholds between the left and right ears for each frequency. We also calculated a pure-tone average (PTA) of thresholds at 0.5, 1, 2 and 4 kHz for each ear, and defined hearing loss as a PTA greater than 25 dB HL in either ear (Cruickshanks et al., 1998).
Bone Lead Measurement
Bone lead levels were measured with a KXRF instrument at two sites: the mid-tibial shaft and the patella. The physical principles, technical specifications, and validation of this instrument were described in detail elsewhere (Burger et al., 1990). The KXRF instrument provides an unbiased estimate of bone lead levels (normalized for bone mineral contents as micrograms of lead per gram of bone mineral) and an estimate of the uncertainty associated with each measurement. The tibia and patella are targeted for bone lead research because they consist mainly of pure cortical and pure trabecular bone, respectively, and thus represent the two main bone compartments.
Assessment of Occupational Noise and Audiometric Notch
Occupational noise exposure might be an important potential confounder in the association between lead and hearing loss. Direct measurements of noise at workplaces had not been taken in this cohort. Because crude occupation categories were available, we used their job titles as a surrogate for occupational noise exposure. A recent study conducted by Tak and Calvert using data from the National Health Interview Survey reported differential risks of hearing difficulty by 41 occupational categories (Tak et al., 2008). This study showed 41 occupation-specific adjusted prevalence ratio of hearing difficulty compared to the reference category (finance, commodities and sales representatives). For example, mechanics/repairers, machine operators, and transportation equipment operators had highest occupation-specific adjusted prevalence ratios of hearing difficulty (1.7 to 2.6). We, therefore, used the reported occupation-specific adjusted prevalence ratios as occupational noise indices of each occupation group in our cohort, and then categorized our 45 occupation groups into three noise exposure groups (low, medium, high). Administrative, managerial, and professional occupations were classified into the low group; protective service (e.g., police and firefighter), sales (e.g., salesman and retail), motor vehicle operators and mail delivery occupations into the medium group; and most blue collar occupations (e.g., construction, mechanics, farming, etc.) into the high group.
A notch in the high frequencies of the audiogram may be evidence of noise-induced hearing loss. Audiometric notch was defined as a high-frequency notch where the hearing threshold at 3, 4, and/or 6 kHz is at least 10 dB HL greater than at 1 or 2 kHz and at least 10 dB HL greater than at 6 or 8 kHz (Coles et al., 2000). We used the Coles et al. (2000) criteria because their agreement with expert consensus was highest and because they essentially quantify what physicians and audiologists are taught in training (Rabinowitz et al., 2006).
Statistical Analysis
We determined the distributions of demographic and clinical characteristics at the times of the first audiometric test and the bone lead measurement. We computed age-adjusted means and standard errors (SEs) of bone lead levels by the characteristics of the study participants, and assessed the significance of a linear trend using linear regression models for ordinal variables and the significance of difference using t-tests for dichotomous variables.
In cross-sectional analyses, hearing threshold outcomes (continuous variables) were handled as linear models. Distributions of hearing thresholds at low frequencies (0.25, 0.5, 1 and 2 kHz) appeared to be right-skewed but those at high frequencies (3, 4, 6 and 8 kHz) seemed to be normal. Because zero and negative values in hearing thresholds exist (better-than-normal hearing) which precluded log-transformations for the low frequency hearing thresholds, we used absolute measured values (non-log-transformed) for all frequencies, but we also performed analyses using log-transformed hearing thresholds for low frequencies after adding a constant as a sensitivity analysis. For hearing loss, we determined the odds ratio (OR) and 95% confidence interval (CI) using logistic regression models. We developed three sequential models to identify the influence of potential confounders: a) age-adjusted; b) models additionally adjusted for body mass index (BMI), race (white or other), education (college graduate or not), cumulative cigarette smoking (pack-years), and status of diabetes and hypertension; c) models additionally adjusted for occupational noise (low, medium, high) and audiometric notch (yes or no). In each model, we computed β coefficients of hearing thresholds and OR of hearing loss for an interquartile range (IQR) increase in each bone lead marker. To evaluate whether occupational noise modified the association between bone lead and hearing loss outcomes, we introduced interaction terms between three categories of noise exposure and each lead marker along with the main effects in the models. Effect modification by noise notch was not evaluated because of the small number of subjects with noise notch (n=42) and thus low power.
In longitudinal associations between tibia lead and hearing thresholds, we used linear mixed effects models. To test whether lead affected the rate of change in hearing thresholds over time, an interaction term between time elapsed from the first audiometric test and tibia lead was included in the models. To make the regression coefficient for the time trend variable interpretable, tibia lead was centered so that the main effect of time is at the mean tibia lead concentrations, not at the tibia lead concentration of 0. We fit time as linear because a linear term is easier to interpret and understand and the nonlinear change in subjects’ thresholds over time can vary from individual to individual which would make it difficult to draw an overall conclusion. However, we also add a square of time into the model to examine graphical associations (and possible nonlinear associations) of the change in hearing threshold over time by quartiles of tibia lead levels. We also included random intercepts to capture correlation in audiometry tests within subject and random slopes for the time variable to capture differences in the rate of decline of hearing across subjects not explained by lead. The likelihood ratio tests and Akaike Information Criterion were used to compare different models. On the joint disbribution of the random intercept and random slope corresponding to time, we assumed an unstructured covariance whereas the measurement error correlation structure was decaying exponentially across time as specified by the power family of correlation structures. All statistical analyses were performed using SAS (version 9.1; SAS Institute, Inc., Cary, North Carolina) and R (version 2.9.2; R Foundation for Statistical Computing, http://www.w-project.org).
RESULTS
Table 1 shows characteristics of the study population at the times of bone lead measurement and the first audiometric test. Means of age at the times of the first audiometric test and bone lead measurement were 42.5 (SD=8.4) and 64.9 (SD=7.3) years, respectively. During the follow-up, PTA increased from 11.7 (SD=8.5) dB HL to 20.5 (SD=10.3) dB HL and the prevalence of hearing loss increased from 15.2% to 45.3%. Mean concentrations of tibia lead and patella lead were 22.5 (SD=14.2) and 32.5 (SD=20.4), respectively.
Table 1.
Characteristics [mean ± SD or N (%)] of study participants at the times of bone lead measurement and the first audiometric test (n=448).
| At bone lead measurement | At the first audiometric test | |
|---|---|---|
| Time varying variables | ||
| Age, yrs | 64.9 ± 7.3 | 42.5 ± 8.4 |
| Body mass index, kg/m2 | 27.5 ± 3.8 | 25.9 ± 2.8 |
| Smoking (pack-yrs) | ||
| 0 | 150 (33.5) | 151 (33.7) |
| <30 | 173 (38.6) | 220 (49.1) |
| ≥30 | 125 (27.9) | 77 (17.2) |
| Diabetes | 58 (13.0) | 9 (2.0) |
| Hypertension | 175 (39.1) | 23 (10.5)** |
| Hearing threshold, dB HL | ||
| 0.25 kHz | 12.5 ± 7.6 | 6.5 ± 7.3 |
| 0.5 kHz | 9.8 ± 7.3 | 6.5 ± 7.5 |
| 1 kHz | 10.4 ± 8.6 | 5.5 ± 7.0 |
| 2 kHz | 17.4 ± 14.9 | 7.6 ± 10.1 |
| 3 kHz | 30.2 ± 18.8 | 24.0 ± 18.6 |
| 4 kHz | 39.7 ± 20.5 | 23.4 ± 18.8 |
| 6 kHz | 44.7 ± 20.6 | 36.1 ± 23.0 |
| 8 kHz | 49.3 ± 22.6 | 21.4 ± 20.7 |
| Pure-tone average | 20.5 ± 10.3 | 11.7 ± 8.5 |
| Hearing loss* | 203 (45.3) | 68 (15.2) |
| Noise notch | 42 (9.4) | 114 (25.5) |
| Time invariant variables | ||
| Nonwhite | 14 (3.1) | – |
| Education (>12 yrs) | 234 (52.2) | – |
| Occupational noise | ||
| Low | 144 (32.1) | – |
| Medium | 175 (39.1) | – |
| High | 129 (28.8) | – |
| Tibia lead, µg/g | 22.5 ± 14.2 | – |
| Patella lead, µg/g | 32.5 ± 20.4 | – |
Hearing loss is defined as pure-tone average ≥ 25 dB HL. The prevalence of hearing loss at the time of first audiometric test was based on 447 subjects (1 missing).
Hypertension at the time of first audiometric test was based on 220 (228 missing).
Because bone lead levels and hearing thresholds were highly correlated with age, we computed age-adjusted bone lead levels and age-adjusted PTA by study characteristics (Table 2). Older age, non-white, lower educational attainment, a greater number of pack-years of cigarette smoking, higher occupational noise exposure, and having a noise notch were associated with both higher tibia and patella lead levels (p<0.05). Older age, lower educational attainment, and higher occupational noise exposure were also associated with higher PTA (p<0.05).
Table 2.
Age-adjusted mean bone lead levels by characteristics of the study participants.
| N (%) | Mean (SE) | |||
|---|---|---|---|---|
| Tibia lead, µg/g | Patella lead, µg/g | PTA, dB HL | ||
| Age (yrs)* | ||||
| <60 | 127 (28.4) | 17.1 (1.2)§ | 24.9 (1.8)§ | 14.8 (0.84)§ |
| 60–69 | 207 (46.2) | 23.6 (0.96) | 34.1 (1.4) | 21.0 (0.66) |
| 70+ | 114 (25.5) | 26.6 (1.3) | 38.1 (1.9) | 25.8 (0.89) |
| Race | ||||
| White | 434 (96.9) | 22.3 (0.65)† | 32.2 (0.95) | 20.6 (0.45) |
| Non-white | 14 (3.1) | 30.1 (3.6) | 41.2 (5.3) | 16.8 (2.5) |
| Education (yrs) | ||||
| ≤12 | 214 (47.8) | 26.3 (0.90)§ | 37.7 (1.3)§ | 21.5 (0.64)† |
| >12 | 234 (52.8) | 19.0 (0.86) | 27.8 (1.3) | 19.5 (0.61) |
| Smoking (pack-yrs) | ||||
| 0 | 150 (33.5) | 19.7 (1.1)§ | 28.8 (1.6)§ | 20.2 (0.76) |
| <30 | 173 (38.6) | 22.4 (1.0) | 31.7 (1.5) | 19.5 (0.71) |
| 30+ | 125 (27.9) | 26.1 (1.2) | 38.1 (1.8) | 22.1 (0.83) |
| Occupational noise | ||||
| Low | 144 (32.1) | 18.2 (1.1)§ | 26.3 (1.6)§ | 18.8 (0.78)† |
| Medium | 175 (39.1) | 24.4 (1.0) | 34.6 (1.5) | 21.2 (0.71) |
| High | 129 (28.8) | 24.8 (1.2) | 36.7 (1.7) | 21.4 (0.82) |
| Noise notch | ||||
| No | 406 (90.6) | 22.0 (0.68)† | 31.8 (0.98)† | 20.2 (0.46) |
| Yes | 42 (9.4) | 27.5 (2.1) | 39.7 (3.1) | 22.7 (1.4) |
| BMI | ||||
| <25 | 104 (23.2) | 19.4 (1.3) | 28.0 (1.9) | 19.2 (0.92) |
| 25–29.9 | 254 (56.7) | 23.8 (0.85) | 34.3 (1.2) | 20.8 (0.59) |
| 30+ | 90 (20.1) | 22.3 (1.4) | 32.6 (2.1) | 21.2 (0.99) |
| Hypertension | ||||
| No | 273 (60.9) | 22.1 (0.83) | 31.1 (1.2) | 19.9 (0.57) |
| Yes | 175 (39.6) | 23.2 (1.0) | 34.7 (1.5) | 21.3 (0.71) |
| Diabetes | ||||
| No | 390 (87.1) | 22.2 (0.69) | 32.1 (1.0) | 20.3 (0.47) |
| Yes | 58 (12.9) | 24.8 (1.8) | 35.3 (2.6) | 21.4 (1.2) |
For the age category, unadjusted mean bone lead levels and pure-tone average (PTA) (SE) were presented.
p<0.05,
p<0.01,
p<0.001.
Table 3 presents associations between hearing thresholds at each frequency and lead marker in different covariate-adjusted models. In age-adjusted models (Model 1), both tibia lead and patella lead were significantly associated with elevations in hearing thresholds at 2, 3 and 4 kHz and PTA. Patella lead was also associated with poorer hearing thresholds at 1, 6 and 8 kHz. The magnitudes of the associations were attenuated after additional adjustment for race, education, BMI, cigarette smoking, history of diabetes and hypertension (Model 2). All such the statistical significances except at 1 kH were retained in patella lead, but the statistical significance of the association with tibia lead remained only at 4 kH. After further controlling for occupational noise exposure and noise notch (Model 3), both tibia lead and patella lead remained significant predictors of poorer hearing thresholds at the frequencies found in Model 2. In Model 3, IQR increases in tibia lead (15 µg/g) and patella lead (21 µg/g) were associated with 2.18 (95% CI, 0.13, 4.23) dB HL and 3.43 (95% CI, 1.46, 5.41) dB HL increases in hearing thresholds at 4 kH, respectively. An IQR increase in patella lead was associated with a 1.58 (95% CI, 0.62, 2.55) dB HL increase in air conduction PTA.
Table 3.
Adjusted effect estimates and 95% confidence intervals in hearing thresholds (dB HL) with one interquartile range increment in bone lead measure in the Normative Aging Study.
| Model 1* | Model 2* | Model 3* | ||||
|---|---|---|---|---|---|---|
| Tibia lead (n=448; IQR=15 µg/g) | ||||||
| Frequency | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| 0.25 kHz | 0.45 | −0.29, 1.19 | 0.41 | −0.37, 1.20 | 0.42 | −0.38, 1.21 |
| 0.5 kHz | 0.24 | −0.47, 0.94 | 0.07 | −0.68, 0.81 | 0.07 | −0.68, 0.82 |
| 1 kHz | 0.47 | −0.35, 1.29 | 0.25 | −0.62, 1.11 | 0.22 | −0.65, 1.10 |
| 2 kHz | 1.47 | 0.05, 2.89† | 1.17 | −0.34, 2.68 | 1.10 | −0.43, 2.62 |
| 3 kHz | 1.98 | 0.19, 3.77† | 1.41 | −0.47, 3.29 | 1.17 | −0.72, 3.06 |
| 4 kHz | 3.09 | 1.14, 5.04‡ | 2.60 | 0.55, 4.64† | 2.18 | 0.13, 4.23† |
| 6 kHz | 1.80 | −0.13, 3.73 | 1.46 | −0.55, 3.46 | 1.71 | −0.28, 3.71 |
| 8 kHz | 1.24 | −0.80, 3.29 | 1.22 | −0.93, 3.37 | 1.75 | −0.35, 3.86 |
| PTA | 1.26 | 0.32, 2.20‡ | 0.96 | −0.04, 1.96 | 0.83 | −0.18, 1.83 |
| Patella lead (n=447; IQR=21 µg/g) | ||||||
| Frequency | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| 0.25 kHz | 0.60 | −0.12, 1.31 | 0.54 | −0.22, 1.30 | 0.56 | −0.21, 1.33 |
| 0.5 kHz | 0.67 | −0.01, 1.35 | 0.49 | −0.23, 1.21 | 0.50 | −0.23, 1.23 |
| 1 kHz | 0.99 | 0.20, 1.78† | 0.80 | −0.03, 1.63 | 0.80 | −0.05, 1.64 |
| 2 kHz | 2.12 | 0.75, 3.49‡ | 1.85 | 0.40, 3.30† | 1.81 | 0.34, 3.28† |
| 3 kHz | 3.06 | 1.33, 4.78§ | 2.55 | 0.74, 4.36‡ | 2.32 | 0.50, 4.15† |
| 4 kHz | 4.24 | 2.37, 6.11§ | 3.83 | 1.86, 5.79§ | 3.43 | 1.46, 5.41§ |
| 6 kHz | 2.61 | 0.75, 4.47‡ | 2.26 | 0.32, 4.19† | 2.54 | 0.61, 4.47† |
| 8 kHz | 2.16 | 0.18, 4.14† | 2.09 | 0.01, 4.16† | 2.65 | 0.61, 4.68† |
| PTA | 1.97 | 1.07, 2.88§ | 1.70 | 0.75, 2.66§ | 1.58 | 0.62, 2.55‡ |
Model 1: adjusted for age; Model 2: Model 1 + race (white or other), education (>12 yr of education or not), body mass index (kg/m2), pack-years of cigarettes (0, <30, 30+), diabetes and hypertension; Model 3: Model 2 + occupational noise (low, medium, high) and noise notch (yes or no).
p<0.05,
p<0.01,
p<0.001.
We also found similar trends in the associations between bone lead and the risk of hearing loss (Table 4). An IQR increment in patella lead was associated with a 48% (OR = 1.48, 95% CI, 1.14, 1.91) higher odds of hearing loss in air conduction PTA after controlling for all covariates including occupational noise exposure and noise notch. There was no significant association between tibia lead and hearing loss after adjusting for confounders.
Table 4.
Adjusted odds ratios and 95% confidence intervals in hearing loss.
| Tibia lead (n=448; IQR=15 µg/g) | Patella lead (n=447; IQR=21 µg/g) | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Model 1 | 1.30 | 1.03, 1.65† | 1.59 | 1.25, 2.02§ |
| Model 2 | 1.22 | 0.95, 1.57 | 1.52 | 1.18, 1.96‡ |
| Model 3 | 1.19 | 0.92, 1.53 | 1.48 | 1.14, 1.91‡ |
Model 1: adjusted for age; Model 2: Model 1 + race (white or other), education (>12 yr of education or not), body mass index (kg/m2), pack-years of cigarettes (0, <30, 30+), diabetes and hypertension; Model 3: Model 2 + occupational noise (low, medium, high) and noise notch (yes or no).
p<0.05,
p<0.01,
p<0.001
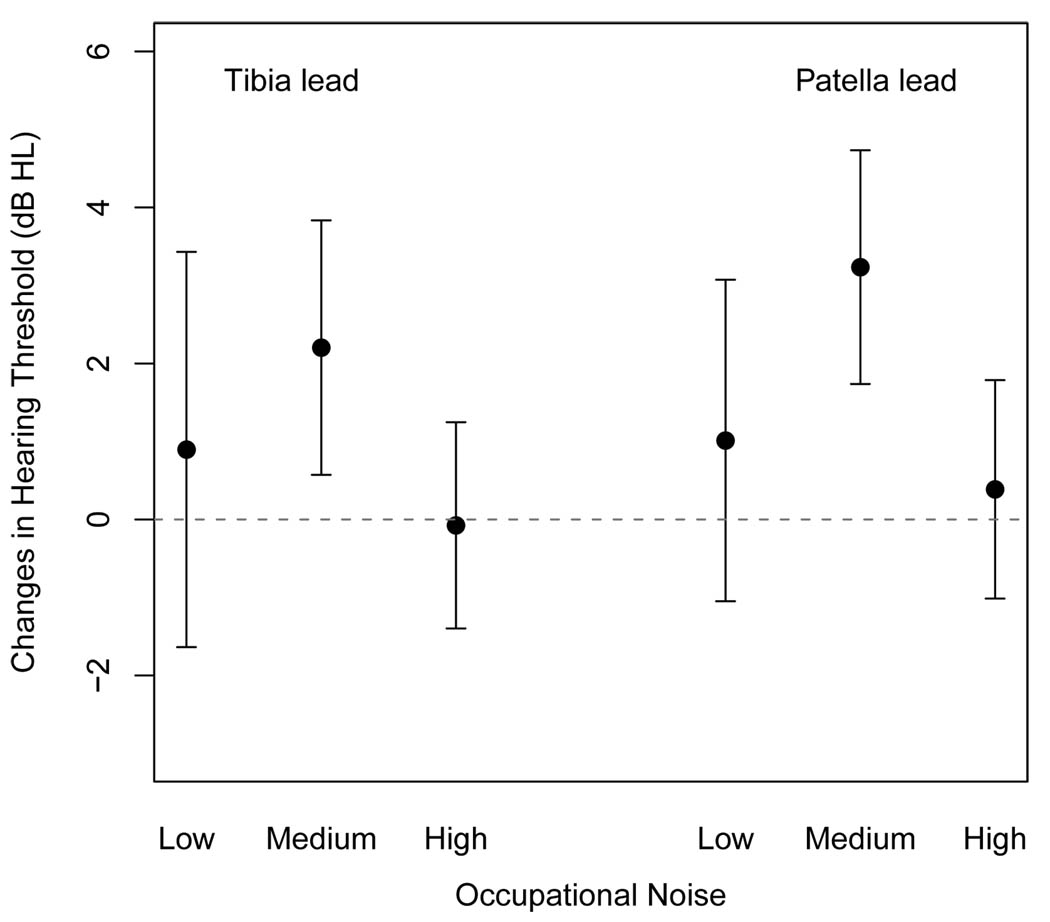
We evaluated whether the associations between bone lead levels and hearing outcomes (hearing thresholds and loss) were modified by occupational noise exposure. Figure 1 shows adjusted associations of air conduction PTA with lead markers by three occupational noise exposure groups. There were significant differences in the association of patella lead with PTA hearing thresholds between low and medium noise groups, but no differences were found between low and high noise groups. The associations in other high frequencies (2 kH and higher) and in the risk of hearing loss were similar to those in PTA (data not shown).
Figure 1.
Adjusted effect estimates and 95% confidence intervals in pure tone average hearing thresholds (dB) with one interquartile range increment in bone lead measure by occupational noise groups. The regression models were adjusted for age, race (white or other), education (>12 yr of education or not), body mass index (kg/m2), pack-years of cigarettes (0, <30, 30+), diabetes and hypertension.
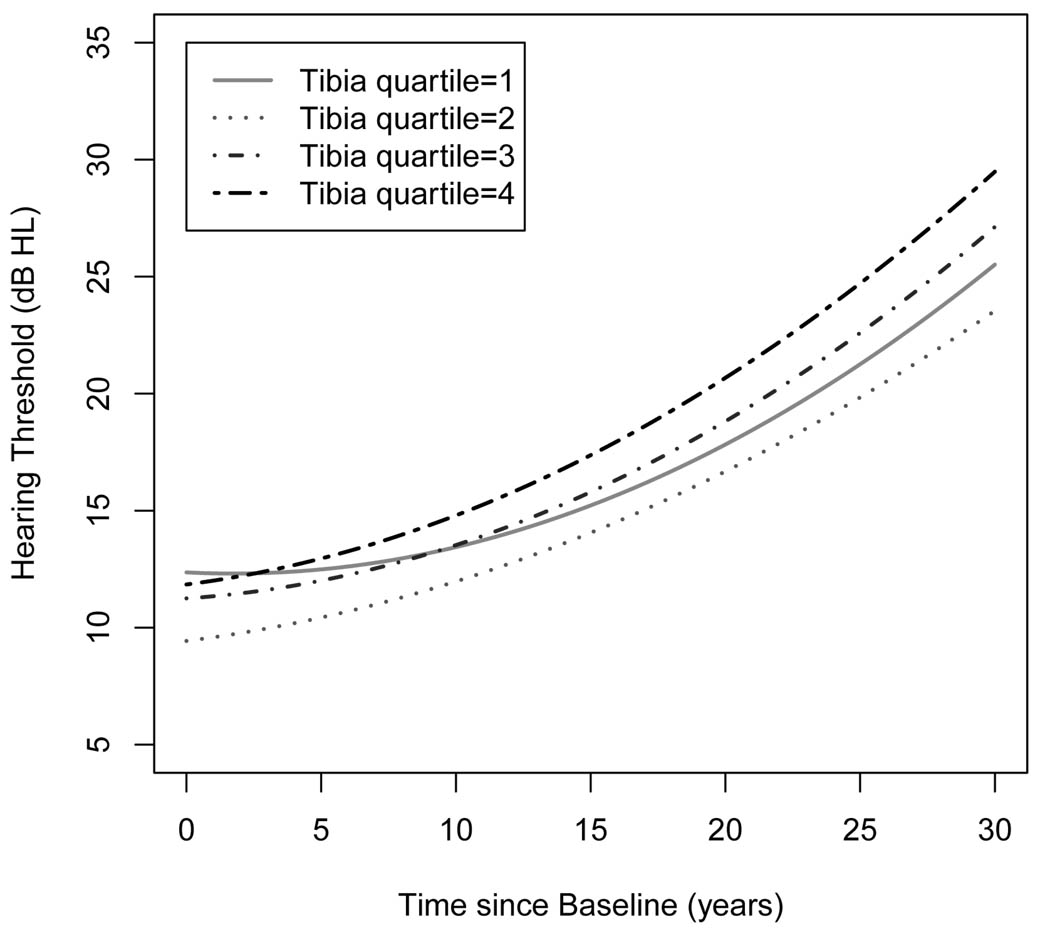
Table 5 shows adjusted associations of air conduction PTA with time, tibia lead and the interaction between time and tibia lead from the linear mixed effects models with a total of 2,264 observations (median observations/participant=5; median follow-up=23 years). We found that the interaction terms between time and tibia lead for the frequencies at 1, 2 and 8 kH and PTA were positive and statistically significant, suggesting that the higher tibia lead levels, the faster the rate of elevation in hearing thresholds. For air conduction PTA, for example, the annual rate of elevation in hearing thresholds for subjects with average tibia lead levels (23 µg/g) was 0.406 (SE=0.017) dB HL/yr and it increased by 0.05 dB HL/yr per one IQR increase (15 µg/g) in tibia lead after adjustment for potential confounders. We also examined if the changes of hearing thresholds over time were linear by adding linear and quadratic time terms into the model. Figure 2 shows that for subjects in the lowest quartile of tibia lead, hearing thresholds didn’t change until 10 years since baseline and then the rate increased gradually next 20 years. There were gradual increases in the rate of hearing thresholds over the follow-up across the upper three quartiles of tibia lead.
Table 5.
Adjusted effect estimates and standard errors in hearing thresholds with one interquartile range increment in tibia lead (15 µg/g) from linear mixed effects models (No=448; total observations=2264).
| Effect estimate [beta (SE)], dB HL | |||
|---|---|---|---|
| Frequency | Time | Tibia lead | Time×tibia lead |
| 0.25 kHz | 0.263 (0.019)§ | −0.129 (0.319) | 0.026 (0.018) |
| 0.5 kHz | 0.157 (0.019)§ | −0.067 (0.364) | 0.026 (0.018) |
| 1 kHz | 0.244 (0.017)§ | 0.429 (0.336) | 0.038 (0.017)† |
| 2 kHz | 0.468 (0.027)§ | 0.740 (0.543) | 0.082 (0.026)‡ |
| 3 kHz | 0.727 (0.031)§ | 1.250 (0.954) | 0.045 (0.030) |
| 4 kHz | 0.748 (0.028)§ | 1.767 (0.980) | 0.049 (0.026) |
| 6 kHz | 0.868 (0.034)§ | 0.913 (1.139) | 0.058 (0.033) |
| 8 kHz | 1.204 (0.036)§ | 0.452 (1.103) | 0.090 (0.035)† |
| PTA | 0.406 (0.017)§ | 0.756 (0.431) | 0.050 (0.017)‡ |
All linear mixed effects models included an interaction between time elapsed from the baseline and tibia lead along with the main effects, adjusting for baseline age, race (white or other), education (>12 yr of education or not), occupational noise (low, medium, high), time varying covariates of body mass index (kg/m2), pack-years of cigarettes (0, <30, 30+), noise notch (yes or no), diabetes and hypertension, and random intercepts and random slopes for time. The spatial exponential correlation structure was considered the covariance matrix.
p<0.05,
p<0.01,
p<0.001.
Figure 2.
The longitudinal changes in hearing thresholds over time by quartiles of tibia lead. The model was adjusted for baseline age, race (white or other), education (>12 yr of education or not), occupational noise (low, medium, high), time varying covariates of body mass index (kg/m2), pack-years of cigarettes (0, <30, 30+), noise notch (yes or no), diabetes and hypertension, and random intercepts and random slopes for time. The spatial exponential correlation structure was considered the covariance matrix. To capture nonlinear trajectories, linear and quadratic time terms were fit.
DISCUSSION
In this study, chronic cumulative lead exposure, as measured in bone by KXRF, was significantly associated with poorer hearing thresholds, especially in the frequency of 4 kHz, in a community-based cohort of men. Higher patella bone lead levels were also associated with an elevated risk of hearing loss: 48% higher odds of hearing loss in air conduction PTA. The odds were roughly equivalent to an increased risk of hearing loss associated with aging of about 4 years in our sample. We also found a significant positive interaction between tibia bone lead and age (time) in the longitudinal analysis followed up for up to 32 years. This finding suggests that cumulative lead exposure may accelerate age-related hearing loss. Adjustment for occupational noise exposure attenuated those associations, but the statistical significances were retained. There was no dose-dependent increasing trend in the association between bone lead and hearing loss by occupational noise exposure.
This is the first study to evaluate the impact of chronic lead exposure on age-related hearing loss in both cross-sectional and longitudinal settings in the general population. Furthermore, this study utilized an advanced exposure assessment technique for lead biomarkers, which measures lead levels in tibia bone and patella bone by KXRF. This method allows us to investigate age-related diseases, which take many years to develop. Approximately 95% of the total body burden of lead is present in the skeleton and, consequently, measurement of bone lead levels can provide an integrated picture of more long-term exposure (Hu, 1998; Hu et al., 2007). In epidemiologic studies, use of KXRF has revealed evidence of the impact of long-term low-level lead exposure on age-related diseases, such as hypertension (Cheng et al., 2001; Hu et al., 1996; Korrick et al., 1999), cardiac conduction,(Cheng et al., 1998) cognitive declines (Weisskopf et al., 2004; Wright et al., 2003), and cataract formation (Schaumberg et al., 2004). The present study is in line with the fact that cumulative lead exposure may be linked to age-related pathological changes.
It is difficult to compare our findings to other studies because no study of the association between environmental lead exposure and hearing loss in older adults has been published. Two early studies using the national survey data (NHANES-II and Hispanic Health and Nutrition Examination Survey (HHANES)) examined the association between blood lead and hearing thresholds among children and adolescents (6 to 19 years of age) and they found significant positive associations at all 4 frequencies (0.5, 1, 2, and 4 kHz) even at blood lead levels less than 10 µg/dL (Schwartz et al., 1987; Schwartz et al., 1991). These studies and our findings suggest that low-level lead exposure may have a detectable impact on hearing ability among persons in susceptible periods of age (the young and the old). In addition, our study also provides evidence that those associations were seen among young and middle-aged adults; we observed significant positive associations with tibia lead levels at the time of the first audiometric test when age ranged from 23 to 69 years (mean age 41 years) (data not shown). Therefore, the auditory effect of lead even at non-occupational low-lead exposures may not depend on age, but further investigation is needed to verify our results.
Most hearing loss results from degeneration of the cochlea, including degeneration of the organ of Corti, ganglion cell loss, strial atrophy and basilar membrane stiffness (Liu et al., 2007). Although significant progress has been made to identify the biological mechanisms of hearing loss, the exact mechanism is not conclusive. Among the various mechanisms, the hypothesis of oxidative stress in mitochondria and reduced blood flow in the cochlea is the most intriguing one (Le Prell et al., 2007; Seidman et al., 2004). With aging, hypoperfusion of the cochlear tissue occurs, which results in ischemia and the formation of reactive oxygen species (ROS). These ROS damage cochlea mitochondrial DNA, leading to the production of specific mtDNA deletion as well as decreased mitochondrial membrane potential. Lead exposure is known to generate ROS (Ahamed et al., 2007; Gurer et al., 2000): (a) inhibition of delta-aminolevulinic acid dehydratase by lead can cause accumulation of delta-aminolevulinic acid, which can be readily oxidized to generate ROS; (b) high affinity for sulfhydryl groups by lead may lead to depletion of glutathione and protein-bound sulfhydryl groups, resulting in the production of ROS; (c) lead can provoke ferrous ion-initiated membrane lipid peroxidation. Other potential mechanisms have been proposed, such as neuro-ototoxic effects of lead on the auditory brainstem and cochlea (Bertoni et al., 1988; Lasky et al., 1995; Yamamura et al., 1989). Lead also has the ability to inhibit ion flow through calcium channels (Audesirk, 1993; Garza et al., 2006), and disrupted plasma membrane calcium pump is associated with loss of sensory hair cells and hearing loss (Brini, 2009; Schultz et al., 2005; Shull et al., 2003; Spiden et al., 2008).
Occupational noise exposure is a well-known risk factor for noise-induced hearing loss. The National Institute for Occupational Safety and Health (NIOSH) estimates that 5–30 million workers who are exposed to noise levels at work are at risk of hearing loss (NIOSH, 1996). Although lead exposure in the study participants was mostly unrelated to their occupations, it is possible that people with high exposure to lead were more exposed to noise at their workplaces. We used occupation titles and the occupation-specific adjusted prevalence ratio of hearing difficulty from the National Health Interview Survey (Tak et al., 2008) to assess individual’s occupational noise exposure because direct measure of noise in workplaces or information on occupational noise exposure was not available. We also tried to identify hearing loss cases induced by noise using audiometric notch (noise notch) and controlled for it in the regression analyses. In the age-adjusted model, we found a significant dose-dependent association of hearing loss with the occupational noise variable (Table 2). In the analyses of bone lead-hearing outcomes, adjustment for this occupational noise variable slightly attenuated those associations (Tables 3 and 4). Despite its limitation (e.g. exposure misclassification), utilization of risk of hearing difficulty from the national survey and noise notch might be useful to control for the potential confounding effect of occupational noise in a study of ototoxicity other than noise where occupational noise information is not available.
A study conducted with Andean adults in the ceramic lead-glazing industry found that lead exposure alone is not the cause of the sensory-neural hearing impairment, whereas the intense occupational noise levels or the combination of lead intoxication and noise exposure may induce hearing loss (Counter et al., 2002). We, therefore, assessed effect modification by occupational noise, and found a positive significant association between patella lead and hearing loss only among the medium noise exposure group but not in the high noise group. The major occupations in the medium noise group include protective service occupations, sales occupations, and motor vehicle operators, whereas most blue collar jobs were included in the high noise group. In this cohort, those two groups had significantly higher air conduction PTA than the low noise group at the time of bone lead measurement, but there was no difference between the medium and the high groups (Table 2). There were similar patterns in bone lead levels among the occupational noise groups. It is possible that the observed differential associations by occupational noise groups indicate differential use of personal protective equipment, that is, subjects in the high noise group were more likely to use personal protective equipment than those in the medium group; however, no such information was available in this cohort to examine this possibility. If this were the case, subjects in the medium noise group might be exposed to higher biologically effective noise than those in the higher group, which would lead to a significant association between cumulative lead and hearing loss only in the medium noise group but not in the high group. Further investigation is needed in properly designed studies to confirm the interaction between noise and lead.
Important strengths of this study include the community-based longitudinal design with a large number of subjects, providing sufficient power to detect small changes in hearing thresholds. In longitudinal analyses, most subjects had at least 4 repeated measures of hearing thresholds to account for longitudinal changes over time and subject-specific variations in hearing thresholds. We also treated age, BMI, and pack-years of cigarette smoking as time-dependent confounding factors, affording better control for them relative to cross-sectional studies.
There are a number of limitations in this study. We cannot exclude a possibility of selection bias resulting from selective survival of participation in the bone lead measurement. If subjects who failed to follow-up (e.g., death) or declined to participate in the bone lead study were more or less susceptible to lead-induced hearing impairment, we could expect either underestimation or overestimation of the association between bone lead and hearing loss. Nonparticipants were older and more likely to be heavy smokers at the times of the first audiometric test and the bone lead measurement, but there was no difference in hearing threshold levels in all frequencies and other characteristics, such as education and occupational noise exposure (see supplemental table). In addition, there was no difference in blood lead levels despite many missing values on blood lead levels. This suggests that selection bias due to selective survival is unlikely.
Although we adjusted many important potential confounders in regression models, we cannot rule out a possibility of bias due to residual or unmeasured confounding factors. Because noise is present in every human activity at home, at leisure, during sleep, during traveling, and at work (Babisch, 2005), non-occupational noise, such as traffic noise, loud music and firearms, exposures to which are not available in this cohort, also contributes to decreased hearing ability. Other potential confounders that were not considered in the present study include use of ototoxic medication (e.g., aminoglycoside antibiotics, chemotherapy agents, loop diuretics), exposure history to ototoxic chemicals and metals (e.g., toluene, xylene, cadmium), use of personal protective equipment at work, and genetic variants.
The Normative Aging Study consists of all males of predominantly white race. Gender and race are important determinants of hearing loss. A recent study found that age-related hearing loss differs by gender and race (Helzner et al., 2005). Hence, these results may not be generalizable to women or other racial/ethnic groups.
Finally, it should be noted that although we conducted a prospective cohort study of age-related hearing loss in relation to lead exposure, our biomarker for bone lead did not come from the beginning of the period under study. Instead, our biomarker was measured at a point in time closer to the study terminus date; as a result, this level may not have closely reflected the body burden of lead present during the initial NAS subject visit. Nonetheless, despite these limitations, use of tibia bone lead of which concentrations in this population have been shown to decline by only 0.8% per year (t1/2 = 79 years) (Wilker et al., 2007) suggest that the bone lead levels likely served as a useful proxy for what the bone lead levels likely were at the beginning of the interval. In addition, a reverse causality scenario (i.e., that hearing loss or a process associated with hearing loss causes more lead to be retained in bone) seems highly unlikely.
In conclusion, our results provide evidence that long-term low-level lead exposure, such as that commonly experienced by adults in the United States, may be an important risk factor for age-related hearing loss. This research suggests that reduction of lead exposure could help prevent or delay development of age-related hearing loss.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: This work was supported by the National Institute of Environment Health Sciences (NIEHS) grants K01-ES016587, R01-ES05257, P42-ES05947 and P30-ES00002. This research was also supported in part by a pilot project research training grant from the Center for Occupational Health and Safety Engineering (COHSE) at the University of Michigan. COHSE an Education and Research Center, is supported by Training Grant No. 2T42OH008455 from the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The contents are solely the responsibility of the author(s) and do not necessarily represent the official views of the National Institute for Occupational Safety and Health. Dr. Spiro was supported by a VA Merit Review. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- IQR
interquartile range
- KXRF
K x-ray fluorescence
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PTA
pure tone average
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahamed M, Siddiqui MK. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383:57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Audesirk G. Electrophysiology of lead intoxication: effects on voltage-sensitive ion channels. Neurotoxicology. 1993;14:137–147. [PubMed] [Google Scholar]
- Babisch W. Noise and health. Environ Health Perspect. 2005;113:A14–A15. doi: 10.1289/ehp.113-a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6:179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- Bertoni JM, Sprenkle PM. Lead acutely reduces glucose utilization in the rat brain especially in higher auditory centers. Neurotoxicology. 1988;9:235–242. [PubMed] [Google Scholar]
- Brini M. Plasma membrane Ca(2+)-ATPase: from a housekeeping function to a versatile signaling role. Pflugers Arch. 2009;457:657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- Burger DE, Milder FL, Morsillo PR, Adams BB, Hu H. Automated bone lead analysis by K-x-ray fluorescence for the clinical environment. Basic Life Sci. 1990;55:287–292. doi: 10.1007/978-1-4613-1473-8_39. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Schwartz J, Vokonas PS, Weiss ST, Aro A, Hu H. Electrocardiographic conduction disturbances in association with low-level lead exposure (the Normative Aging Study) Am J Cardiol. 1998;82:594–599. doi: 10.1016/s0002-9149(98)00402-0. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol. 2001;153:164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- Chuang HY, Kuo CH, Chiu YW, Ho CK, Chen CJ, Wu TN. A case-control study on the relationship of hearing function and blood concentrations of lead, manganese, arsenic, and selenium. Sci Total Environ. 2007;387:79–85. doi: 10.1016/j.scitotenv.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Coles RR, Lutman ME, Buffin JT. Guidelines on the diagnosis of noise-induced hearing loss for medicolegal purposes. Clin Otolaryngol Allied Sci. 2000;25:264–273. doi: 10.1046/j.1365-2273.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Neuro-ototoxicity in andean adults with chronic lead and noise exposure. J Occup Environ Med. 2002;44:30–38. doi: 10.1097/00043764-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Discalzi G, Fabbro D, Meliga F, Mocellini A, Capellaro F. Effects of occupational exposure to mercury and lead on brainstem auditory evoked potentials. Int J Psychophysiol. 1993;14:21–25. doi: 10.1016/0167-8760(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Discalzi GL, Capellaro F, Bottalo L, Fabbro D, Mocellini A. Auditory brainstem evoked potentials (BAEPs) in lead-exposed workers. Neurotoxicology. 1992;13:207–209. [PubMed] [Google Scholar]
- Forst LS, Freels S, Persky V. Occupational lead exposure and hearing loss. J Occup Environ Med. 1997;39:658–660. doi: 10.1097/00043764-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12:RA57–RA65. [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–945. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, de Rekeneire N, Harris TB, Rubin SM, Simonsick EM, Tylavsky FA, Newman AB. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Hu H. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environ Health Perspect. 1998;106 Suppl 4:961–967. doi: 10.1289/ehp.98106s4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115:455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, Rotnitzky A. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996;275:1171–1176. [PubMed] [Google Scholar]
- Hwang YH, Chiang HY, Yen-Jean MC, Wang JD. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci Total Environ. 2009;408:43–49. doi: 10.1016/j.scitotenv.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Jain NB, Potula V, Schwartz J, Vokonas PS, Sparrow D, Wright RO, Nie H, Hu H. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men: the VA Normative Aging Study. Environ Health Perspect. 2007;115:871–875. doi: 10.1289/ehp.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrick SA, Hunter DJ, Rotnitzky A, Hu H, Speizer FE. Lead and hypertension in a sample of middle-aged women. Am J Public Health. 1999;89:330–335. doi: 10.2105/ajph.89.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky RE, Maier MM, Snodgrass EB, Hecox KE, Laughlin NK. The effects of lead on otoacoustic emissions and auditory evoked potentials in monkeys. Neurotoxicol Teratol. 1995;17:633–644. doi: 10.1016/0892-0362(95)02006-3. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- NIOSH. Preventing Occupational Hearing Loss - A Practical Guide. [verified Jan 25 2010];1996. [Online] http://www.cdc.gov/niosh/docs/96-110/pdfs/96-110.pdf.
- Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. National Center for Health Statistics. 2009 [PubMed] [Google Scholar]
- Rabinowitz PM, Galusha D, Slade MD, Dixon-Ernst C, Sircar KD, Dobie RA. Audiogram notches in noise-exposed workers. Ear Hear. 2006;27:742–750. doi: 10.1097/01.aud.0000240544.79254.bc. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Mendes F, Balaram M, Dana MR, Sparrow D, Hu H. Accumulated lead exposure and risk of age-related cataract in men. JAMA. 2004;292:2750–2754. doi: 10.1001/jama.292.22.2750. [DOI] [PubMed] [Google Scholar]
- Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Lead, blood pressure, and cardiovascular disease in men and women. Environ Health Perspect. 1991;91:71–75. doi: 10.1289/ehp.919171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Otto D. Blood lead, hearing thresholds, and neurobehavioral development in children and youth. Arch Environ Health. 1987;42:153–160. doi: 10.1080/00039896.1987.9935814. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Otto D. Lead and minor hearing impairment. Arch Environ Health. 1991;46:300–305. doi: 10.1080/00039896.1991.9934391. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Ahmad N, Joshi D, Seidman J, Thawani S, Quirk WS. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Otolaryngol Suppl. 2004:16–24. doi: 10.1080/03655230410017823. [DOI] [PubMed] [Google Scholar]
- Shull GE, Okunade G, Liu LH, Kozel P, Periasamy M, Lorenz JN, Prasad V. Physiological functions of plasma membrane and intracellular Ca2+ pumps revealed by analysis of null mutants. Ann N Y Acad Sci. 2003;986:453–460. doi: 10.1111/j.1749-6632.2003.tb07229.x. [DOI] [PubMed] [Google Scholar]
- Spiden SL, Bortolozzi M, Di Leva F, de Angelis MH, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, Mammano F, Steel KP. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet. 2008;4:e1000238. doi: 10.1371/journal.pgen.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S, Calvert GM. Hearing difficulty attributable to employment by industry and occupation: an analysis of the National Health Interview Survey--United States, 1997 to 2003. J Occup Environ Med. 2008;50:46–56. doi: 10.1097/JOM.0b013e3181579316. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Wright RO, Schwartz J, Spiro A, 3rd, Sparrow D, Aro A, Hu H. Cumulative lead exposure and prospective change in cognition among elderly men: the VA Normative Aging Study. Am J Epidemiol. 2004;160:1184–1193. doi: 10.1093/aje/kwh333. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation. 2009;120:1056–1064. doi: 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Korrick SA, Hu H, Schwartz J, Wright RO. Genetic Variation and Nonlinearity in the Decay of Bone Lead in an Aging Population. Epidemiology. 2007;18:S167. [Google Scholar]
- Wright RO, Tsaih SW, Schwartz J, Spiro A, 3rd, McDonald K, Weiss ST, Hu H. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology. 2003;14:713–718. doi: 10.1097/01.EDE.0000081988.85964.db. [DOI] [PubMed] [Google Scholar]
- Wu TN, Shen CY, Lai JS, Goo CF, Ko KN, Chi HY, Chang PY, Liou SH. Effects of lead and noise exposures on hearing ability. Arch Environ Health. 2000;55:109–114. doi: 10.1080/00039890009603396. [DOI] [PubMed] [Google Scholar]
- Yamamura K, Terayama K, Yamamoto N, Kohyama A, Kishi R. Effects of acute lead acetate exposure on adult guinea pigs: electrophysiological study of the inner ear. Fundam Appl Toxicol. 1989;13:509–515. doi: 10.1016/0272-0590(89)90287-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.